Abstract
The neural mechanisms contributing to flexible cognition and behavior and how they change with development and aging are incompletely understood. The current study explored intrinsic brain dynamics across the lifespan using resting-state fMRI data (n = 601, 6–85 years) and examined the interactions between age and brain dynamics among three neurocognitive networks (midcingulo-insular network, M-CIN; medial frontoparietal network, M-FPN; and lateral frontoparietal network, L-FPN) in relation to behavioral measures of cognitive flexibility. Hierarchical multiple regression analysis revealed brain dynamics among a brain state characterized by co-activation of the L-FPN and M-FPN, and brain state transitions, moderated the relationship between quadratic effects of age and cognitive flexibility as measured by scores on the Delis-Kaplan Executive Function System (D-KEFS) test. Furthermore, simple slope analyses of significant interactions revealed children and older adults were more likely to exhibit brain dynamic patterns associated with poorer cognitive flexibility compared with younger adults. Our findings link changes in cognitive flexibility observed with age with the underlying brain dynamics supporting these changes. Preventative and intervention measures should prioritize targeting these networks with cognitive flexibility training to promote optimal outcomes across the lifespan.
Keywords: aging, central executive network, default mode network, executive function, salience network
Introduction
Flexible brain dynamics support cognition and behavior (Grady and Garrett 2014; Jia et al. 2014). However, little is known regarding brain dynamic changes across the lifespan associated with cognitive flexibility, a component of executive function (Diamond 2013) that supports the ability to adapt behavior to an ever-changing environment (Dajani and Uddin 2015). Cognitive flexibility is associated with positive academic, occupational, and social outcomes throughout life (Davis et al. 2010; Genet and Siemer 2011; Burt and Paysnick 2012; Yeniad et al. 2013; Colé et al. 2014). Understanding age-related changes in brain dynamics and their relationship with cognitive flexibility is crucial to identifying neural markers of risk and resilience across development and aging.
Across the lifespan, greater dynamic brain flexibility is increasingly being associated with younger adulthood and enhanced cognitive performance (Jia et al. 2014; Braun et al. 2015; Nomi et al. 2017a; Xia et al. 2019; Battaglia et al. 2020). A greater number of transitions among certain brain states has been found in younger adults compared with older adults (Xia et al. 2019) and children (Hutchison and Morton 2015). The dwell time, or the time spent within a brain state, has also been shown to differ across age, with shorter dwell times in certain states in young adulthood (Hutchison and Morton 2015) potentially underlying efficient cognitive control. Dwell time increases with older age (Xia et al. 2019), potentially underlying cognitive changes and reduced cognitive efficiency (i.e., perseveration) (Ridderinkhof et al. 2002). Lastly, the frequency of occurrence of highly variable brain states has also been associated with better performance on behavioral measures of executive function including cognitive flexibility (Nomi et al. 2017b). Although greater dynamic brain flexibility is increasingly being associated with younger age and enhanced cognitive performance, there is little known about variability in brain dynamics supporting cognition across age. For example, growing evidence suggests individuals have varying “brain ages,” resulting in differences in functional brain maturity among age-matched individuals (Dosenbach et al. 2010). Therefore, age-related changes associated with brain network dynamic variability and cognitive flexibility require further investigation (Cohen 2018) as they may provide potential markers of risk for, and resilience to, age-related cognitive decline across the lifespan.
Within and between network connectivity among the midcingulo-insular network (M-CIN; also known as salience), medial frontoparietal network (M-FPN; also known as default), and lateral frontoparietal network (L-FPN; also known as executive control) (Uddin et al. 2019) has also been shown to be important for aging (Ryali et al. 2016; Chand et al. 2017), and cognitive and neural flexibility (Uddin et al. 2011; Chen et al. 2016). The M-CIN is involved in interoceptive, affective, attention, and control processes associated with subjective salience; the L-PFN is involved in executive control and modulating goal-oriented behaviors and decisions; and the M-FPN is involved in self-related processes and social cognition (Uddin et al. 2019). Together, these networks support various functions important for adaptation across the lifespan (Masten and Obradovic 2006; Touroutoglou et al. 2018). A longer dwell time within certain states of the M-CIN, M-FPN, and L-FPN has been associated with less flexibility in children’s brain dynamic repertoires compared with young adults (Ryali et al. 2016). Greater flexibility within these networks may therefore account for improved behavioral performance across development. In older age, extant literature suggests weaker modulation occurs among the M-FPN and L-FPN, resulting in the greater reliance on crystallized knowledge, and weaker fluency skills (Turner and Nathan Spreng 2015; Spreng et al. 2018). Furthermore, temporal variability specifically of the M-CIN has been shown to uniquely predict individual differences in cognitive flexibility in young adults (Chen et al. 2016). Conversely, higher M-FPN and L-FPN functional dynamics during the resting-state have been associated with poorer cognitive flexibility (Douw et al. 2016). Overall, dynamic relationships among the M-CIN, M-FPN, and L-FPN appear to be important contributors to cognitive flexibility across the lifespan.
Despite its importance to optimal lifespan development, no previous studies have characterized brain network dynamics supporting cognitive flexibility from childhood to older adulthood. This study provides a novel framework for understanding the relationship between brain dynamics and cognitive flexibility and may lend insight into neuropsychiatric disorders and resilience in typical development and aging. Previous studies have found both linear and quadratic relationships across the lifespan related to cognitive flexibility and brain dynamics when examining within- and between-network associations (Grady et al. 2006; Wang et al. 2012; Betzel et al. 2014; Cao et al. 2014; Nomi et al. 2017a). To extend previous findings, we examined the hypotheses that between-network dynamics among the M-CIN, M-FPN, and L-FPN exhibit a quadratic trajectory across the lifespan. To examine if varying levels of brain dynamics supports optimal cognitive flexibility across the lifespan, we also tested the hypothesis that brain dynamics among these three large-scale networks interact with age to enable cognitive flexibility changes associated with healthy aging. Specifically, we hypothesized that greater brain dynamic flexibility as indexed by dwell time, frequency of occurrence, and transitions between states would be associated with greater cognitive flexibility across the lifespan.
Methods
Neuroimaging, phenotypic, and behavioral data collected from 601 healthy adult participants were downloaded from the Enhanced Nathan Kline Institute.
(NKI)-dataset (http://fcon_1000.projects.nitrc.org/indi/enhanced/). Participants were selected according to the following inclusion criteria: 1) availability of neuroimaging and behavioral data, 2) no current or past DSM-diagnosis for psychiatric disorders and/or attention deficit hyperactivity disorder, and (3) resting-state fMRI data head motion <0.5 mm. See Table 1 for participant information and Supplementary Figure S1 for information about the age distribution included in this study. The study was approved by the NKI institutional review board and all participants provided informed consent. Written consent and assent was collected from child participants and their legal guardian (Nooner et al. 2012)
Table 1.
Participant Demographics
| N = 601; mean ± sd (min—max) | |
|---|---|
| Age (year) | 37.22 ± 20.73 (6.18–85.62) |
| Gender | 239 M 361 F 1 NR |
| Mean FD (mm) | 0.25 ± 0.09 (0.08–0.50) |
| Ethnicity | 514 (not Hispanic or Latino) 86 (Hispanic or Latino) 1 NR |
| Race | 4 (1) 46 (2) 116 (3) 1 (4) 417 (5) 16 (6) 1(NR) |
| CWIT inhibition/switching total completion time | 62.80 ± 17.89 (32–146) |
| CWIT inhibition/switching total errors | 1.92 ± 2.24 (0–22) |
| TMT number-letter switching total completion time | 81.70 ± 38.79 (25–240) |
| VF switching total correct | 13.49 ± 3.20 (4–23) |
Note: SD, standard deviation; M, male; F, female; NR: no response; 1: American Indian or Native Alaskan; 2: Asian; 3: Black or African American; 4: Native Hawaiian or Other Pacific Islander; 5: White; 6: Other Race; CWIT, Color-Word Interference Test; TMT, Trail Making Test; VF, Verbal Fluency.
MRI and Behavior Protocol
Participants were assessed during a 1- or 2-day examination by trained experts. Details of the MRI and behavioral assessment procedures can be found at http://fcon_1000.projects.nitrc.org/indi/enhanced/mri_protocol.html, and http://fcon_1000.projects.nitrc.org/indi/enhanced/assessments.html, respectively. Some participants were missing behavioral data for certain measures and were omitted when necessary. Additionally, children below the age of 8 years (n = 7) were not administered the executive function tests, as the test battery is only valid in 8–89 year olds (Delis et al. 2001). These children were excluded from the analyses with behavioral measures of executive function.
Cognitive Flexibility Measures
Participants were administered the Delis-Kaplan Executive Function System (D-KEFS), a series of neuropsychological tests designed to measure executive functions in children and adults between the ages of 8–89 (Delis et al. 2001). The commonly used cognitive flexibility tests within the D-KEFS include the Color-Word Interference Task (CWIT), the Trail Making Test (TMT), and the Verbal Fluency (VF) Task.
The CWIT is a modified Stroop task (Stroop and Ridley Stroop 1992) and consists of four conditions. The first two conditions are similar to the Stroop interference task, and the last condition involves Inhibition/Switching and is a commonly used cognitive flexibility task (Bohnen et al. 1992; Mattson et al. 1999). In the Inhibition/Switching condition, participants are presented with a page containing the words “red,” “green,” and “blue,” written in red, green, or blue ink. Some of the words are contained in a box and the subject must switch between saying the color of the ink (word is not inside a box) or the color of the word (word inside a box). Participants are told to complete the task as quickly as possible. Raw scores include the time to complete the Inhibition/Switching condition in seconds and the total number of errors made during the task. Higher scores indicate poorer cognitive flexibility.
The TMT was created to isolate set-shifting abilities by including baseline conditions such as visual scanning, number sequencing, letter sequencing and motor speed (Fine et al. 2011). TMT also includes a Number-Letter Switching condition, a commonly used cognitive flexibility task (Kleinhans et al. 2005; Mcdonald et al. 2005; Yochim et al. 2007). During the Number-Letter Switching condition, participants switch back and forth between connecting numbers and letters (i.e., 1, A, 2, B etc.,) (Yochim et al. 2007). They are instructed to connect the numbers and letters as quickly as possible. The raw score measure for the Number-Letter Switching task is the total time to complete the task in seconds. Higher scores indicate poorer cognitive flexibility.
The VF test requires participants to generate words beginning with a letter (phonemic fluency) or from a category (category fluency). The VF task also includes a Category Switching condition where participants alternate between saying words from two different semantic categories. The Category Switching condition is a commonly used task to study cognitive flexibility (de Paula et al. 2015; Ramanan et al. 2015). In the switching condition, participants are told to produce as many words within 60 seconds. The VF category switching raw score is the total correct number of responses and a higher score indicates better cognitive flexibility.
MRI Data Acquisition
A Siemens Trio 3.0 T scanner was used to obtain the functional images. Multiband (factor of 4) echo-planarimage (EPI) sequenced resting-state images (rsfMRI; TR = 1400 ms, TE = 30 ms, flip angle 65°, field of view (FOV) 224 mm, voxel size = 2x2x2 mm, 64 interleaved slices, 404 volumes) were applied for the acquisition of the functional images. Participants were instructed to keep their eyes open and fixate on a cross in the center of the screen during the 9-min 19-s rsfMRI scan. For detailed MRI protocol see: http://fcon_1000.projects.nitrc.org/indi/enhanced/mri_protocol.html.
Neuroimaging Data Preprocessing and Postprocessing
The resting-state fMRI data were preprocessed using the Data Preprocessing Assistant for Resting-State fMRI Advanced edition (DPARSF-A, Yan and Zang, 2016), which uses FSL, SPM-12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/), and AFNI https://afni.nimh.nih.gov (Cox 1996). The preprocessing steps were the following: removal of the first 5 volumes to allow scanner signal to reach equilibrium, despiked using AFNI 3dDespike, realignment, normalization to 3 mm MNI template, and smoothing (6 mm FWHM) (Espinoza et al. 2019).
Independent component analysis (ICA) was conducted using FSL’s MELODIC by means of automatic dimensionality estimation (Nomi et al. 2017a; Espinoza et al. 2019). The ICA-FIX classifier was trained on hand-classified independent components separated into noise and non-noise categories using randomly chosen participants (n = 24) across the lifespan (Griffanti et al. 2014; Nomi et al. 2017b). The ICA-FIX classification algorithm was applied to the data (FSL’s ICA-FIX; (Griffanti et al. 2014) to classify noise and non-noise components from individual subject data before conducting nuisance regression of classified noise components from the resting-state scans in MNI space. The ICA-FIX fMRI data then underwent nuisance covariance regression (linear detrend, Friston 24 motion parameters (6 motion parameters of each volume, the preceding volume, and the 12 corresponding squared items) (Friston et al. 1996), global mean signal, followed by bandpass filtering (0.01–0.10 Hz) (Damoiseaux et al. 2006). Preprocessing and postprocessing were additionally conducted without global mean signal regression (GSR) to assess the effect of this step on subsequently derived metrics, as there is yet no consensus regarding the extent to which this step removes neural signal in addition to noise (Uddin 2020a).
Nine regions-of-interest (ROIs) representing the three large-scale networks (Uddin et al. 2011) were selected (Table 2), including the right and left fronto-insular cortex (rFIC) and anterior cingulate cortex (ACC) of the M-CIN; right and left dorsolateral prefrontal cortex (rDLPFC) and right and left posterior parietal cortex (rPPC) of the L-FPN; and the ventromedial prefrontal cortex (VMPFC) and posterior cingulate cortex (PCC) of the M-FPN. These networks and regions were chosen because of previous work demonstrating their functional roles in flexible cognition (Uddin et al. 2011) and aging (Ryali et al. 2016; Chand et al. 2017). Additionally, these ROIs have long been recognized as critical nodes in the three neural networks (Seeley et al. 2007; Menon and Uddin 2010; Chand et al. 2017) and as evidenced by recent ICA group analyses (Marshall et al. 2020; Kupis et al. 2021). A trained research assistant examined all ROIs in older participants (≧ 70–85 years), the years where the most marked changes in brain atrophy can occur (Scahill et al. 2003), to ensure the masks were within the cerebral cortex for each individual subject.
Table 2.
Coordinates of M-CIN, M-FPN, and L-FPN regions
| Network | Region | BA | Peak MNI coordinates (mm) |
|---|---|---|---|
| M-CIN | rFIC | 47 | 39, 23, −4 |
| lFIC | 47 | −34, 20, −8 | |
| ACC | 24 | 6, 24, 32 | |
| L-FPN | rDLPFC | 9 | 46, 20, 44 |
| lDLPFC | 9 | −46, 20, 44 | |
| rPPC | 40 | 52, −52, 50 | |
| lPPC | 40 | −40, −56, 44 | |
| M-FPN | VMPFC | 11 | −2, 38, −12 |
| PCC | 23/30 | −6, −44, 34 |
Co-Activation Pattern Analysis
For each individual subject, time series extracted from the nine ROIs were converted to z-statistics and then concatenated into one matrix containing all subjects [(399 TR × 601 subjects) × 9 ROIs], following previous studies (Hutchison and Morton 2015; Kupis et al. 2020). Both children and adults were included due to prior evidence suggesting the brain’s repertoire of states are generally preserved across age (Hutchison and Morton 2015). The matrix was then subjected to k-means clustering to determine the optimal number of clusters. The elbow criterion was applied to the cluster validity index (the ratio between within-cluster to between-cluster distance) for values of k = 2–20 to determine the optimal value of k = 5 (Supplementary Figure S2) (Liu et al. 2013).
K-means clustering using a squared Euclidean distance was then applied to the matrix using the optimal k = 5 to produce 5 co-activation pattern (CAP) “brain states.” The CAP metrics included: a) dwell time, calculated as the average number of continuous TRs that a participant stayed in a given brain state, b) frequency of occurrence of brain states, calculated as an overall percentage that the brain state occurred throughout the duration of the scan compared with other brain states, and c) the number of transitions, calculated as the number of switches between any two brain states.
In the processing pipeline including the data without GSR, k-means analysis was again conducted to obtain the optimal k, determined to be k = 5.
Statistical Analysis
To test our first hypothesis that the dynamic network integration among networks important for cognitive flexibility differs across age, linear and quadratic regressions were conducted with Age and Age2, predicting the dynamic brain state metric (dwell time, frequency, and transitions) for each CAP. Covariates included head motion and sex. Age2 was included due to prior evidence revealing age has a quadratic or curvilinear relationship with certain brain regions and networks (DuPre and Nathan Spreng 2017; Chen et al. 2018). Overall, this model was conducted to extend prior “static” results by using dynamic brain network states.
Ŷ = B0 + B1(Age) + B2(Age2) + Bn(Covariates).
To test our second hypothesis that brain dynamics moderate the relationship between age and cognitive flexibility, hierarchical multiple regressions were conducted. Hierarchical multiple regression analysis includes adding variables into the model in separate steps (Francis et al. 1975). In the first step, Age and Age2 were included as predictors of cognitive flexibility, with sex and mean FD included as covariates. This tested for quadratic relationships between age and cognitive flexibility before the moderation analysis were conducted. In the second step, the brain dynamic metric (dwell time, frequency, and transitions) for each CAP was included as a predictor. In the last step, the interaction between Age and the dynamic metric and the interaction between Age2 and the dynamic metric were included into the regression analysis. Brain dynamics were tested as the moderator in this study due to the idea that there may be variability in brain functioning among subjects of the same age (Dosenbach et al. 2010). This approach supports assessing variability in brain dynamics associated with cognitive flexibility across the lifespan, while still revealing age-related changes. The cognitive flexibility measures used were the CWIT Inhibition/Switching, the TMT Color/Number Switching, and the VF Category Switching raw scores. Following significant interactions, the simple slopes were examined to aid interpretation. Simple slopes were computed to explore the effect of Age2 on the cognitive flexibility measure at three different levels of the moderator as represented by the brain dynamic metric (i.e., at −1 SD below the mean, at the mean, and at +1 SD above the mean). All analyses were conducted using R (Computing and Others 2013) (https://www.R-project.org/) and all analyses are publicly available (https://github.com/lkupis/lifespan_Dynamics). Additional analyses were also conducted with more ROIs using the Schaefer parcellation (Schaefer et al. 2018), and are available in the Supplementary Materials.
Ŷ = B0 + B1(Age) + B2(Age2) + Bn(Covariates) [Step 1]
Ŷ = B0 + B1(Age) + B2(Age2) + B3(Brain Dynamic) + Bn(Covariates) [Step 2]
Ŷ = B0 + B1(Age) + B2(Age2) + B3(Brain Dynamic) + B1(Age × Brain Dynamic) + B2(Age2 × Brain Dynamic) + Bn(Covariates) [Step 3]
Results
Recurrent CAP Analysis
Results from the CAP analysis among the M-CIN, L-FPN, and M-FPN are presented in Figure 1. The first brain state (CAP 1) was characterized by stronger co-activation among the M-FPN nodes relative to the L-FPN and M-CIN. The second brain state (CAP 2) was characterized by co-activation among the M-CIN nodes. The third brain state (CAP 3) was characterized by co-activation among the M-CIN and the M-FPN. The fourth brain state (CAP 4) was characterized by co-activation among the L-FPN and M-CIN. The last brain state (CAP 5) was characterized by co-activation among the L-FPN and M-FPN.
Figure 1 .
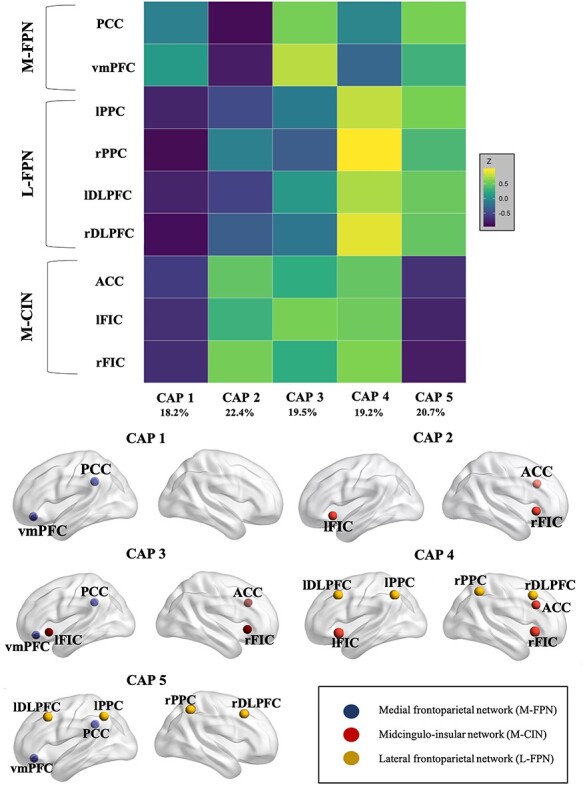
Top: CAPs or brain states from dynamic CAP analysis. CAP 1 was characterized by stronger co-activation among the M-FPN nodes relative to the L-FPN and M-CIN. CAP 2 was characterized by co-activation among the M-CIN nodes. CAP 3 was characterized by co-activation among the M-CIN and the M-FPN. CAP 4 was characterized by co-activation among the L-FPN and M-CIN. Lastly, CAP 5 was characterized by co-activation among the L-FPN and M-FPN. Bottom: Graphical brain representation of each CAP as demonstrated by the ROIs. Note: PCC, posterior cingulate cortex; vmPFC, ventromedial prefrontal cortex; lPPC, left posterior parietal cortex; rPPC, right posterior parietal cortex; lDLPFC, left dorsolateral prefrontal cortex; rDLPFC, right dorsolateral prefrontal cortex; ACC, anterior cingulate cortex; lFIC, left fronto-insular cortex; rFIC, right fronto-insular cortex.
CAP Analysis without Global Signal Regression
Results from the CAP analysis using data without GSR are presented in Supplementary Figure S3. The resulting CAPs revealed the influence of the global signal, notably in CAPs 1 and 2. CAP 1 shows all nodes with inactivity and CAP 2 shows all nodes with activity representing the global signal across all nodes. Prior work suggests that the decision to remove the global signal or not depends on the scientific question, and should be considered when interpreting the results (Murphy and Fox 2017). The removal of the global signal as a preprocessing step significantly mitigates artifacts from a variety of sources (Power et al. 2017; Ciric et al. 2018). Although in some cases the global signal can represent neuronal signal (Hyder and Rothman 2010; Schölvinck et al. 2010); in the current dataset, removal of the global signal was beneficial to revealing CAPs associated with cognition. Therefore, all statistical analyses and results presented are derived from data that was preprocessed with GSR.
Associations between Brain Dynamics and Quadratic Effects of Age
Curvilinear regressions were conducted with Age and Age2 predicting the brain dynamic metric for each brain state (CAP 1–5), while controlling for sex and mean FD. There was a positive quadratic effect of age when predicting the frequency of CAP 3, characterized by co-activation among the M-CIN and M-FPN, β = 0.42, b = < 0.001, SE = < 0.001, P = 0.030, uncorrected. CAP 3 occurred less frequently as age increased, but increased in occurrence with older age (see Fig. 2A). There was also a negative quadratic effect of age when predicting the frequency of CAP 5, characterized by co-activation among the L-FPN and M-FPN, β = −0.40, b = <0.001, SE = < 0.001, P = 0.037, uncorrected. CAP 5 occurred more frequently as age increased; however, it decreased with older age (see Fig. 2B). Lastly, there was a positive quadratic effect of age predicting the dwell time of CAP 4, characterized by co-activation among the M-CIN and L-FPN, β = 0.37, b = < 0.001, SE = < 0.001, P = 0.053, uncorrected. The dwell time of CAP 4 decreased with age, and increased with older age (see Fig. 2C).
Figure 2 .
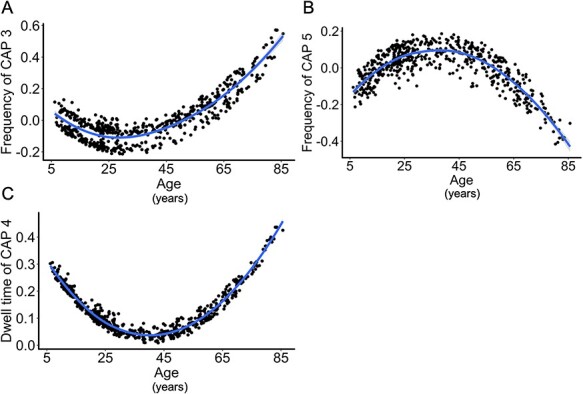
Positive and negative quadratic effects of age (years) predicting dynamic brain metrics for specific CAPs. For all graphs, regression coefficients from the regression lines of quadratic effects of age predicting each dynamic brain state were plotted. Y-axes were z-scored to facilitate interpretation across graphs. For A and B, a negative value represents lower frequency of occurrence compared with the average, whereas positive values represent greater frequency of occurrence compared with the average. (A) A positive quadratic relationship among age and CAP 3 frequency of occurrence. CAP 3 occurred frequently during childhood, decreased in frequency during young adulthood, and increased in frequency again throughout middle- to older adulthood. CAP 3 consisted of co-activation among the M-CIN (salience) and the M-FPN (default). (B) A negative quadratic relationship among age and CAP 5 frequency of occurrence. CAP 5 occurred less frequently during childhood, increased in frequency during young- and middle-adulthood, and decreased again in frequency in older adulthood. CAP 5 consisted of co-activation among the L-FPN (executive) and M-CIN. Lastly, (C) A positive quadratic relationship among age and CAP 4 dwell time. CAP 4 exhibited longer dwell times during childhood, shorter dwell times during young- and middle-adulthood, and longer dwell times again in older adulthood. CAP 4 consisted of co-activation among the L-FPN and M-FPN. In A, B, and C, children and older adults had similar brain dynamic patterns for each CAP, whereas young adults had different brain dynamic patterns. For example, in B, CAP 5 occurred less frequently in early childhood and older adulthood, but occurred more frequently in early adulthood.
Main Effects of age 2 and Brain Dynamics Predicting Cognitive Flexibility
Age, Age2, and the brain dynamic metric were included in steps 1 and 2 of the hierarchical regression analyses. There were significant quadratic effects of age for the cognitive flexibility measures including the CWIT total errors raw score, TMT completion time raw score, and VF total correct number of responses raw score (P’s < 0.001), but not for CWIT completion time raw score (P’s > 0.05). The brain dynamic metrics were not significant predictors of cognitive flexibility when included into the regression equations (P’s > 0.05).
Interactions between Age and Brain Dynamics Predicting Cognitive Flexibility
There were multiple significant interactions between the dynamic brain states and Age2 predicting cognitive flexibility (Supplementary Table S1). Only the significant interactions that survived Bonferroni correction ((.05/10) = 0.005) will be discussed. The dwell time of CAP 5, characterized by co-activation among the L-FPN and M-FPN, moderated the relationship between the quadratic effect of age and cognitive flexibility (TMT switching completion time), b = 0.02, SE = 0.01, P = 0.002. Simple slope analyses indicated there was a significant slope between Age2 and TMT switching completion time at low (−1 SD), b = 0.02, SE = 0.01, P = 0.003, average, b = 0.03, SE = 0.004, P = < 0.001, and high (+1 SD), b = 0.04, SE = 0.01, P < 0.001 CAP 5 dwell times. A low CAP 5 dwell time was associated with improved cognitive flexibility across the lifespan; Average CAP 5 dwell time consisted of slightly poorer cognitive flexibility at young and older ages and improved cognitive flexibility mid age. A higher CAP 5 dwell time was associated with poorer cognitive flexibility at younger and older ages and improved cognitive flexibility performance during mid-age. Although the simple slopes were significant at low, average, and high levels of CAP 5 dwell time, examination of the slopes in Figure 3A further revealed the effect was minimal at a low level (Li 2018). Overall, the dynamics of a brain state consisting of co-activation among the L-FPN and M-FPN moderated the relationship between cognitive flexibility with Age2 (see Fig. 3A).
Figure 3 .
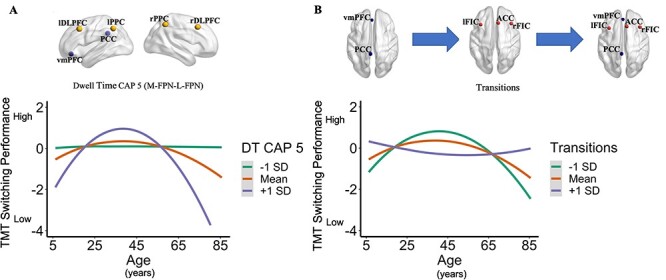
Brain dynamics moderate the relationship between age and cognitive flexibility: simple slopes. The interactions presented in A and B were between Age2 and the brain dynamic metrics for CAP 5 and brain state transitions; however, they are presented across age (years) for visual purposes. Additionally, the simple slopes for both interactions are presented to visually determine the effect of age on the cognitive flexibility measure across three different levels of the moderator as represented by the brain dynamic metric (i.e., −1 SD below the mean, at the mean, and + 1 SD above the mean). Additionally, the y-axes were reversed and standardized so better cognitive flexibility is at higher ends (the top) and poorer cognitive flexibility is at lower ends (the bottom) of the y-axes. (A) The CAP 5 dwell time (DT) moderated the relationship between Age2 and the TMT Switching condition (total time to complete the task) as represented by the simple slopes. CAP 5 is characterized by co-activation among the L-FPN (executive control) and M-FPN (default). Children and older adults who spent a longer time in CAP 5 had poorer cognitive flexibility, whereas younger adults had optimal cognitive flexibility regardless of their CAP 5 brain dynamics. Similar findings were seen at average levels of CAP 5 dwell time. Children and older adults who spent less time in CAP 5 had optimal cognitive flexibility relative to those with average and greater time spent in CAP 5, whereas younger adults had poorer, yet still optimal, cognitive flexibility. (B) The number of transitions moderated the relationship between Age2 and the TMT switching condition (total time to complete the task) as represented by the simple slopes. Children and older adults who had fewer brain state transitions had poorer cognitive flexibility, whereas younger adults had optimal cognitive flexibility at average and fewer transitions. Similar findings were seen across individuals with average numbers of brain state transitions. Children and older adults with greater brain state transitions had optimal cognitive flexibility relative to those with average and fewer brain state transitions, whereas young adults had poorer cognitive flexibility.
The number of brain state transitions also moderated the relationship between the quadratic effect of age and cognitive flexibility for TMT switching completion time, b = −0.001, SE = < 0.001, P = 0.005. Simple slope analyses indicated there was a significant slope between Age2 and TMT switching completion time at low (−1 SD), b = 0.04, SE = 0.01, P = < 0.001, average, b = 0.03, SE = 0.004, P = < 0.001, and high (+1 SD), b = 0.02, SE = 0.01, P = 0.001, transitions. Simple slopes analyses indicated that greater numbers of transitions were associated with stable/good cognitive flexibility throughout the lifespan, with a reduction in cognitive flexibility around mid-age. In both average and low transitions, cognitive flexibility was poorer in younger and older ages, but peaked during mid-age. Overall, transitions moderated the relationship between cognitive flexibility and Age2 (see Fig. 3B).
Discussion
Cognitive flexibility is an important executive function enabling optimal outcomes in academic achievement, transitions into adulthood, quality of life, and resilience to negative life events (Uddin 2021). Examining brain dynamic changes across the lifespan aids the understanding of the neural mechanisms underlying optimal and flexible cognition (Grady and Garrett 2014) and may inform studies of cognitive (Zhang et al. 2020a) and neuropsychiatric disorders (Rabany et al. 2019; Uddin 2020b). The large-scale networks known as the M-CIN (salience), L-FPN (executive), and M-FPN (default), are thought to be important for flexible cognition (Uddin et al. 2011; Qin et al. 2015) across aging (Chand et al. 2017; Adnan et al. 2019a). The present study examined brain dynamics among the M-CIN, L-FPN, and M-FPN as they relate to lifespan development, and as a moderator between age and cognitive flexibility.
The present study revealed five recurring CAP (CAPs or “brain states”) involving the M-CIN, L-FPN, and M-FPN across the lifespan. Quadratic relationships were observed between age and the brain dynamic metrics, primarily within hybrid brain states characterized by between-network coupling. Furthermore, brain dynamics moderated the relationship between a quadratic effect of age and cognitive flexibility. We demonstrate differences in intrinsic brain network dynamics across aging associated with cognitive flexibility, specifically within the M-FPN/L-FPN co-activation state (CAP 5), and brain network transitions. We found that a greater M-FPN/L-FPN dwell time in children and older adults was associated with poorer cognitive flexibility. Furthermore, greater brain state transitions in children and older adults was associated with better cognitive flexibility, consistent with prior observations (Grady and Garrett 2014; Battaglia et al. 2020). Mid-adulthood, however, was associated with different dynamic patterns associated with optimal cognitive flexibility. This age represents a change in cognition from greater fluid to semantic abilities (Park et al. 2001). Our findings suggest children and older adults are most vulnerable to cognitive flexibility deficits, however, a “deficit” in children is defined by having worse cognitive flexibility compared with age-matched peers, with the potential of improvement in adulthood. Cognitive inflexibility in children and adults was associated with brain dynamic alterations among the M-CIN, M-FPN, and L-FPN based on time spent in the hybrid M-FPN/L-FPN state and variability in state transitions.
U-Shaped Trajectories of between-Network Dynamics
Previous studies have demonstrated quadratic effects of age associated with between-network connections (Betzel et al. 2014; Cao et al. 2014). Prior studies are consistent with our findings of quadratic or U-shaped trajectories in between-network dynamics among three large-scale brain networks of the M-CIN, L-FPN, and M-FPN (Chen et al. 2018). We found the brain state consisting of co-activation of the M-CIN and M-FPN (CAP 3) decreased in frequency of occurrence during middle adulthood but increased during both childhood and older adulthood. Functional connectivity between the M-CIN and M-FPN has been previously shown to be associated with greater cognitive control (Jilka et al. 2014), behavioral performance on cognitive tasks (Putcha et al. 2016), and memory in older adults (Zhang et al. 2020b). Additionally, there is evidence that coupling between the M-FPN and M-CIN may be an intermediary “switching mechanism” prior to later M-FPN and L-FPN coupling (Beaty et al. 2016), potentially underlying greater use of semantic or crystallized knowledge (Spreng and Turner 2019).
Previous work examined M-CIN and M-FPN connections using static functional connectivity approaches, whereas we explored the relationship using dynamic or time-varying methods. Therefore, dynamic interactions between the M-CIN and M-FPN may be critical to further assess in relation to previous static functional connectivity findings. Furthermore, we expand upon previous findings by demonstrating increased dynamic interactions or frequency of occurrence of the M-CIN and M-FPN state is associated with older age and development. This may be due to its role as an intermediary switching mechanism prior to M-FPN and L-FPN connections, which is greater in older adults (Spreng and Turner 2019). Thus, M-CIN/M-FPN coupling may occur more frequently prior to M-FPN/L-FPN coupling. Within- and between-brain network integration increases with age, therefore, brain network variability between certain brain networks may be greater in children due to less integration (Gu et al. 2015; Kundu et al. 2018). Furthermore, connectivity with the M-FPN is important for brain network development (Dosenbach et al. 2010). Together, the M-CIN/M-FPN hybrid state exhibits a quadratic trend across the lifespan, and children and older adults may be more likely to enter this state prior to engaging other functional configurations.
Similarly, we found the co-activation between the L-FPN and M-CIN (CAP 4) decreased in dwell time during middle adulthood and increased during childhood and older adulthood. The effect size for this finding was moderate (β = 0.37) (Schäfer and Schwarz 2019). Previous work demonstrates the M-CIN may independently act as a switching mechanism between the M-FPN and L-FPN (Goulden et al. 2014). In children and older adults, a longer time was spent in the L-FPN/M-CIN state during a task-free environment, suggesting the M-CIN related switching mechanism may not be fully developed in children (Uddin et al. 2011), and may be “stickier” or less efficient in older adults. Conversely, middle-aged-adults dwelled less in this state, potentially due to having greater brain state transitions and variability than children and older adults (Grady and Garrett 2014; Ryali et al. 2016; Xia et al. 2019).
Lastly, we found the connection between the L-FPN and M-FPN decreased in frequency of occurrence during childhood and older adulthood and increased during middle adulthood. Although reliance on semantic knowledge and subsequently greater M-FPN/L-FPN connections is not as prevalent in mid-adulthood, evidence suggests mid-adulthood is characterized by the intersection of greater reliance on semantic knowledge while fluency abilities are still retained (Park et al. 2001; Spreng and Turner 2019). Therefore, the M-FPN/L-FPN state may still occur in middle adulthood and may occur more frequently due to there being more flexible brain dynamics compared with older adults and children.
Together, our results demonstrate that hybrid between-network dynamics in certain brain states exhibit quadratic relationships across age, and may underlie the cognitive changes observed through development and aging. Our results are in line with behavioral studies of cognitive flexibility, which reveal cognitive flexibility takes an inverted U-shaped trend across the lifespan (Cepeda et al. 2001; Zelazo et al. 2014). Cognitive flexibility increases throughout childhood and into adulthood, and declines in older age (Cepeda et al. 2001; Zelazo et al. 2014). Although the frontoparietal regions are overall thought to support these changes (Gogtay et al. 2004; Luna et al. 2010), we extend this prior work by revealing between-network dynamic coupling among the M-CIN, M-FPN, and L-FPN may also facilitate changes associated with cognitive flexibility across aging.
Brain Dynamics as a Moderator of Age and Cognitive Flexibility: L-FPN and M-FPN
We examined brain dynamics as a moderator between quadratic effects of age and cognitive flexibility to directly examine how brain dynamics among networks impact the relationship between aging and cognitive flexibility (Dajani and Uddin 2015). First, the brain state characterized by co-activation among the L-FPN and M-FPN moderated the relationship between the quadratic effect of age and cognitive flexibility as measured by the TMT. This finding was also replicated using more regions of interest within the M-FPN, L-FPN, and M-CIN (see Supplementary Materials). Emerging evidence suggests that greater connectivity between the M-FPN and L-FPN is a central feature of neurocognitive aging (Spreng and Schacter 2012; Turner and Nathan Spreng 2015; Spreng et al. 2018; Adnan et al. 2019a; Adnan et al. 2019b), termed the “default-executive coupling hypothesis of aging” (DECHA) (Turner and Nathan Spreng 2015; Spreng et al. 2018). Relatedly, at each end of the lifespan, behavioral evidence suggests that cognitive flexibility performance is poorer in both childhood and older adulthood (Cepeda et al. 2001; Ridderinkhof et al. 2002; Wasylyshyn et al. 2011; Dajani and Uddin 2015).
Consistent with DECHA and behavioral evidence associated with cognitive flexibility across aging, we show individuals with greater M-FPN/L-FPN dwell time, or individuals with less modulation of the M-FPN and L-FPN, perform worse on cognitive flexibility tasks than older individuals with average or shorter CAP 5 (M-FPN/L-FPN) dwell time. Although the DECHA model has primarily been applied to older individuals, we additionally found evidence that a greater CAP 5 dwell time is associated with cognitive inflexibility during childhood. This may contribute to the poorer performance on cognitive flexibility tasks observed during childhood (Dick 2014; Buttelmann and Karbach 2017). Additionally, previous evidence suggests there is less flexibility among the M-CIN, M-FPN, and L-FPN during childhood (Ryali et al. 2016). Our results extend this finding by relating reduced network flexibility (M-FPN and L-FPN) with reduced cognitive flexibility. Furthermore, our findings suggest that older adults are more severely impacted by reduced M-FPN/L-FPN modulation than children. Overall, our findings support the DECHA model of aging, and extend previous work by revealing M-FPN/L-FPN coupling is associated with cognitive flexibility during both childhood and aging.
Furthermore, our results demonstrate different neural patterns associated with cognitive flexibility during mid-adulthood compared with older adults and children. This finding suggests that a greater M-FPN/L-FPN dwell time may be beneficial to cognitive flexibility during mid-adulthood. Additionally, average and reduced M-FPN/L-FPN dwell time during mid-adulthood were also associated with higher levels of cognitive flexibility. Mid-adulthood has previously been shown as a turning point of declining cognitive control and increased reliance on semantic (crystallized) knowledge (Park et al. 2001). However, there is evidence that fluid skills are declining yet intact, while semantic knowledge is increasing, and may actually bolster cognition (Li et al. 2015; Samanez-Larkin and Knutson 2015). Therefore, mid-adulthood has been seen as an optimal period for decision-making (Samanez-Larkin and Knutson 2015; Spreng and Turner 2019) due to the ability to integrate both fluid and semantic knowledge. Overall, our results reflect this idea and demonstrate mid-adulthood is associated with optimal cognitive flexibility that may additionally be aided by semantic knowledge.
Brain Dynamics as a Moderator of Age and Cognitive Flexibility: Transitions
We found that the number of brain state transitions moderated the relationship between a quadratic effect of age and cognitive flexibility. This finding was also replicated using additional regions of interest within the M-FPN, L-FPN, and M-CIN (see Supplementary Materials). Specifically, a greater number of brain state transitions was associated with stable or high cognitive flexibility across the lifespan. As expected, average and lower number of brain state transitions were associated with poorer cognitive flexibility during childhood and older adulthood, consistent with the literature (Hutchison and Morton 2015; Xia et al. 2019; Battaglia et al. 2020). Our findings suggest that the childhood and the older adulthood stages of life are most vulnerable to reduced brain state transitions associated with poorer cognitive flexibility compared with mid-adulthood. This finding has implications for development during both childhood and older adulthood. Overall, our findings demonstrate direct relationships between brain dynamics associated with age and cognitive flexibility changes across the lifespan (Uddin 2021).
Conclusion
Using CAP analysis, we identified brain states characterized by between- and within-network connectivity of neural networks important for cognitive flexibility. We discovered that between-network dynamics of a state characterized by co-activation among the M-FPN and L-FPN, and brain state transitions, moderated the relationship between aging and cognitive flexibility. Our results reveal dynamic brain mechanisms contributing to poorer cognitive flexibility in youth and older individuals. Preventative measures and interventions should prioritize strategies targeting brain dynamics among the M-CIN, M-FPN, and L-FPN, and focus on cognitive flexibility training to promote optimal outcomes across the lifespan.
Funding
The Canadian Institute for Advanced Research, a Gabelli Senior Scholar Award from the University of Miami; R01MH107549 from the National Institute of Mental Health (NIMH) (to L.Q.U.); an NIMH award (R03MH121668); a NARSAD Young Investigator Award (to J.S.N.).
Notes
Conflict of Interest: None declared.
Supplementary Material
Contributor Information
Lauren Kupis, Department of Psychology, University of Miami, Coral Gables, FL 33124, USA.
Zachary T Goodman, Department of Psychology, University of Miami, Coral Gables, FL 33124, USA.
Salome Kornfeld, Department of Psychology, University of Miami, Coral Gables, FL 33124, USA.
Stephanie Hoang, Department of Psychology, University of Miami, Coral Gables, FL 33124, USA.
Celia Romero, Department of Psychology, University of Miami, Coral Gables, FL 33124, USA.
Bryce Dirks, Department of Psychology, University of Miami, Coral Gables, FL 33124, USA.
Joseph Dehoney, Department of Psychology, University of Miami, Coral Gables, FL 33124, USA.
Catie Chang, Department of Electrical Engineering and Computer Science, Vanderbilt University, Nashville, TN 37235, USA; Department of Biomedical Engineering, Vanderbilt University, Nashville, TN 37235, USA; Vanderbilt University Institute of Imaging Science, Vanderbilt University, Nashville, TN 37232, USA.
R Nathan Spreng, Montreal Neurological Institute, Department of Neurology and Neurosurgery, McGill University, Montreal, QC H3A 2B4, Canada.
Jason S Nomi, Department of Psychology, University of Miami, Coral Gables, FL 33124, USA.
Lucina Q Uddin, Department of Psychology, University of Miami, Coral Gables, FL 33124, USA; Neuroscience Program, University of Miami Miller School of Medicine, Miami, FL 33136, USA.
References
- Adnan A, Beaty R, Lam J, Nathan Spreng R, Turner GR. 2019a. Intrinsic default—executive coupling of the creative aging brain. Soc Cogn Affect Neurosci. 14:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adnan A, Beaty R, Silvia P, Spreng RN, Turner GR. 2019b. Creative aging: functional brain networks associated with divergent thinking in older and younger adults. Neurobiol Aging. 75:150–158. [DOI] [PubMed] [Google Scholar]
- Battaglia D, Boudou T, Hansen ECA, Lombardo D, Chettouf S, Daffertshofer A, McIntosh AR, Zimmermann J, Ritter P, Jirsa V. 2020. Dynamic functional connectivity between order and randomness and its evolution across the human adult lifespan. Neuroimage. 222:117156. [DOI] [PubMed] [Google Scholar]
- Beaty RE, Benedek M, Silvia PJ, Schacter DL. 2016. Creative cognition and brain network dynamics. Trends Cogn Sci. 20:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel RF, Byrge L, He Y, Goñi J, Zuo X-N, Sporns O. 2014. Changes in structural and functional connectivity among resting-state networks across the human lifespan. Neuroimage. 102(Pt 2):345–357. [DOI] [PubMed] [Google Scholar]
- Bohnen N, Jolles J, Twijnstra A. 1992. Modification of the stroop color word test improves differentiation between patients with mild head injury and matched controls. Clin Neuropsychol. 6:178–184. [DOI] [PubMed] [Google Scholar]
- Braun U, Schäfer A, Walter H, Erk S, Romanczuk-Seiferth N, Haddad L, Schweiger JI, Grimm O, Heinz A, Tost H, et al. 2015. Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc Natl Acad Sci U S A. 112:11678–11683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt KB, Paysnick AA. 2012. Resilience in the transition to adulthood. Dev Psychopathol. 24:493–505. [DOI] [PubMed] [Google Scholar]
- Buttelmann F, Karbach J. 2017. Development and plasticity of cognitive flexibility in early and middle childhood. Front Psychol. 8:1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Wang J-H, Dai Z-J, Cao X-Y, Jiang L-L, Fan F-M, Song X-W, Xia M-R, Shu N, Dong Q, et al. 2014. Topological organization of the human brain functional connectome across the lifespan. Dev Cogn Neurosci. 7:76–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda NJ, Kramer AF, Gonzalez de Sather JC. 2001. Changes in executive control across the life span: examination of task-switching performance. Dev Psychol. 37:715–730. [PubMed] [Google Scholar]
- Chand GB, Wu J, Hajjar I, Qiu D. 2017. Interactions of the salience network and its subsystems with the default-mode and the central-executive networks in normal aging and mild cognitive impairment. Brain Connect. 7:401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Cai W, Ryali S, Supekar K, Menon V. 2016. Distinct global brain dynamics and spatiotemporal Organization of the Salience Network. PLoS Biol. 14:e1002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhao X, Zhang X, Y’nan L, Zhou P, Ni H, Ma J, Ming D. 2018. Age-related early/late variations of functional connectivity across the human lifespan. Neuroradiology. 60:403–412. [DOI] [PubMed] [Google Scholar]
- Ciric R, Rosen AFG, Erus G, Cieslak M, Adebimpe A, Cook PA, Bassett DS, Davatzikos C, Wolf DH, Satterthwaite TD. 2018. Mitigating head motion artifact in functional connectivity MRI. Nat Protoc. 13:2801–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR. 2018. The behavioral and cognitive relevance of time-varying, dynamic changes in functional connectivity. Neuroimage. 180:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colé P, Duncan LG, Blaye A. 2014. Cognitive flexibility predicts early reading skills. Front Psychol. 5:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Computing R, Others . 2013. R: A language and environment for statistical computing. Vienna: R Core Team. [Google Scholar]
- Cox RW. 1996. AFNI: software for analysis and visualization of functional magnetic resonance Neuroimages. Comput Biomed Res. 29:162–173. [DOI] [PubMed] [Google Scholar]
- Dajani DR, Uddin LQ. 2015. Demystifying cognitive flexibility: implications for clinical and developmental neuroscience. Trends Neurosci. 38:571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. 2006. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 103:13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JC, Marra CA, Najafzadeh M, Liu-Ambrose T. 2010. The independent contribution of executive functions to health related quality of life in older women. BMC Geriatr. 10:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. 2001. Delis-Kaplan Executive Function System. [DOI] [PubMed]
- de Paula JJ, Paiva GC de C, Costa D de S. 2015. Use of a modified version of the switching verbal fluency test for the assessment of cognitive flexibility. Dement Neuropsychol. 9:258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. 2013. Executive functions. Annu Rev Psychol. 64:135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick AS. 2014. The development of cognitive flexibility beyond the preschool period: an investigation using a modified flexible item selection task. J Exp Child Psychol. 125:13–34. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, et al. 2010. Prediction of individual brain maturity using fMRI. Science. 329:1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douw L, Wakeman DG, Tanaka N, Liu H, Stufflebeam SM. 2016. State-dependent variability of dynamic functional connectivity between frontoparietal and default networks relates to cognitive flexibility. Neuroscience. 339:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPre E, Nathan Spreng R. 2017. Structural covariance networks across the life span, from 6 to 94 years of age. Network Neuroscience. 1:302–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza FA, Vergara VM, Damaraju E, Henke KG, Faghiri A, Turner JA, Belger AA, Ford JM, McEwen SC, Mathalon DH, et al. 2019. Characterizing whole brain temporal variation of functional connectivity via zero and first order derivatives of sliding window correlations. Front Neurosci. 13:634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine EM, Delis DC, Holdnack J. 2011. Normative adjustments to the D-KEFS trail making test: corrections for education and vocabulary level. Clin Neuropsychol. 25:1331–1344. [DOI] [PubMed] [Google Scholar]
- Francis JD, Kerlinger FN, Pedhazur EJ. 1975. Multiple regression in Behavioral research. Teach Sociol. 3:103. [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RSJ, Turner R. 1996. Movement-related effects in fMRI time-series. Magn Reson Med. 35:346–355. [DOI] [PubMed] [Google Scholar]
- Genet JJ, Siemer M. 2011. Flexible control in processing affective and non-affective material predicts individual differences in trait resilience. Cognit Emot. 25:380–388. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF 3rd, Herman DH, Clasen LS, Toga AW, et al. 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulden N, Khusnulina A, Davis NJ, Bracewell RM, Bokde AL, McNulty JP, Mullins PG. 2014. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage. 99:180–190. [DOI] [PubMed] [Google Scholar]
- Grady CL, Garrett DD. 2014. Understanding variability in the BOLD signal and why it matters for aging. Brain Imaging Behav. 8:274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. 2006. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 18:227–241. [DOI] [PubMed] [Google Scholar]
- Griffanti L, Salimi-Khorshidi G, Beckmann CF, Auerbach EJ, Douaud G, Sexton CE, Zsoldos E, Ebmeier KP, Filippini N, Mackay CE, et al. 2014. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. Neuroimage. 95:232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Satterthwaite TD, Medaglia JD, Yang M, Gur RE, Gur RC, Bassett DS. 2015. Emergence of system roles in normative neurodevelopment. Proc Natl Acad Sci U S A. 112:13681–13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Morton JB. 2015. Tracking the Brain’s functional coupling dynamics over development. J Neurosci. 35:6849–6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Rothman DL. 2010. Neuronal correlate of BOLD signal fluctuations at rest: err on the side of the baseline. Proc Natl Acad Sci U S A. 107:10773–10774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Hu X, Deshpande G. 2014. Behavioral relevance of the dynamics of the functional brain connectome. Brain Connect. 4:741–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka SR, Scott G, Ham T, Pickering A, Bonnelle V, Braga RM, Leech R, Sharp DJ. 2014. Damage to the salience network and interactions with the default mode network. J Neurosci. 34:10798–10807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans N, Akshoomoff N, Delis DC. 2005. Executive functions in autism and Asperger’s disorder: flexibility, fluency, and inhibition. Dev Neuropsychol. 27:379–401. [DOI] [PubMed] [Google Scholar]
- Kundu P, Benson BE, Rosen D, Frangou S, Leibenluft E, Luh W-M, Bandettini PA, Pine DS, Ernst M. 2018. The integration of functional brain activity from adolescence to adulthood. J Neurosci. 38:3559–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupis L, Goodman ZT, Kircher L, Romero C, Dirks B, Chang C, Nomi JS, Uddin LQ. 2021. Altered patterns of brain dynamics linked with body mass index in youth with autism. Autism Res. 14:873–886. [DOI] [PubMed] [Google Scholar]
- Kupis L, Romero C, Dirks B, Hoang S, Parlade MV, Beaumont AL, Cardona SM, Alessandri M, Chang C, Nomi JS, et al. 2020. Evoked and intrinsic brain network dynamics in children with autism spectrum disorder. Neuroimage: Clinical. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JCH. 2018. Curvilinear moderation—a more complete examination of moderation effects in Behavioral sciences. Frontiers in Applied Mathematics and Statistics. 4:7. [Google Scholar]
- Liu X, Chang C, Duyn JH. 2013. Decomposition of spontaneous brain activity into distinct fMRI co-activation patterns. Frontiers in Systems Neuroscience. 7:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gao J, Enkavi AZ, Zaval L, Weber EU, Johnson EJ. 2015. Sound credit scores and financial decisions despite cognitive aging. Proc Natl Acad Sci U S A. 112:65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O’Hearn K. 2010. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 72:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall E, Nomi JS, Dirks B, Romero C, Kupis L, Chang C, Uddin LQ. 2020. Co-activation pattern analysis reveals altered salience network dynamics in children with autism spectrum disorder. Network Neuroscience. 4:1219–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS, Obradovic J. 2006. Competence and resilience in development. Ann N Y Acad Sci. 1094:13–27. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. 1999. Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 23:1808–1815. [PubMed] [Google Scholar]
- Mcdonald CR, Delis DC, Norman MA, Tecoma ES, Iragui-Madoz VJ. 2005. Is impairment in set-shifting specific to frontal-lobe dysfunction? Evidence from patients with frontal-lobe or temporal-lobe epilepsy. J Int Neuropsychol Soc. 11:477–481. [PubMed] [Google Scholar]
- Menon V, Uddin LQ. 2010. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Fox MD. 2017. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage. 154:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi JS, Bolt TS, Ezie CEC, Uddin LQ, Heller AS. 2017a. Moment-to-moment BOLD signal variability reflects regional changes in neural flexibility across the lifespan. J Neurosci. 37:5539–5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi JS, Vij SG, Dajani DR, Steimke R, Damaraju E, Rachakonda S, Calhoun VD, Uddin LQ. 2017b. Chronnectomic patterns and neural flexibility underlie executive function. Neuroimage. 147:861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooner KB, Colcombe SJ, Tobe RH, Mennes M, Benedict MM, Moreno AL, Panek LJ, Brown S, Zavitz ST, Li Q, et al. 2012. The NKI-Rockland sample: a model for accelerating the pace of discovery science in psychiatry. Front. Neuroscience. 6:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Polk TA, Mikels JA, Taylor SF, Marshuetz C. 2001. Cerebral aging: integration of brain and behavioral models of cognitive function. Dialogues Clin Neurosci. 3:151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Plitt M, Laumann TO, Martin A. 2017. Sources and implications of whole-brain fMRI signals in humans. Neuroimage. 146:609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putcha D, Ross RS, Cronin-Golomb A, Janes AC, Stern CE. 2016. Salience and default mode network coupling predicts cognition in aging and Parkinson’s disease. J Int Neuropsychol Soc. 22:205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Chen S-G, Hu D, Zeng L-L, Fan Y-M, Chen X-P, Shen H. 2015. Predicting individual brain maturity using dynamic functional connectivity. Front Hum Neurosci. 9:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabany L, Brocke S, Calhoun VD, Pittman B, Corbera S, Wexler BE, Bell MD, Pelphrey K, Pearlson GD, Assaf M. 2019. Dynamic functional connectivity in schizophrenia and autism spectrum disorder: convergence, divergence and classification. Neuroimage Clin. 24:101966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan S, Narayanan J, D’Souza TP, Malik KS, Ratnavalli E. 2015. Total output and switching in ategory fluency successfully iscriminates Alzheimer’s disease from mild cognitive impairment, but not from frontotemporal dementia. Dementia & neuropsychologia. 9:251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Span MM, van der Molen MW. 2002. Perseverative behavior and adaptive control in older adults: performance monitoring, rule induction, and set shifting. Brain Cogn. 49:382–401. [DOI] [PubMed] [Google Scholar]
- Ryali S, Supekar K, Chen T, Kochalka J, Cai W, Nicholas J, Padmanabhan A, Menon V. 2016. Temporal dynamics and developmental maturation of salience, default and central-executive network interactions revealed by Variational Bayes hidden Markov Modeling. PLoS Comput Biol. 12:e1005138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Knutson B. 2015. Decision making in the ageing brain: changes in affective and motivational circuits. Nat Rev Neurosci. 16:278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. 2003. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 60:989. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo X-N, Holmes AJ, Eickhoff SB, Yeo BTT. 2018. Local-global Parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex. 28:3095–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer T, Schwarz MA. 2019. The meaningfulness of effect sizes in psychological research: differences between sub-disciplines and the impact of potential biases. Front Psychol. 10:813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schölvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA. 2010. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci U S A. 107:10238–10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Nathan Spreng R, Lockrow AW, DuPre E, Setton R, Spreng KAP, Turner GR. 2018. Semanticized autobiographical memory and the default – executive coupling hypothesis of aging. Neuropsychologia. 110:37–43. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Schacter DL. 2012. Default network modulation and large-scale network interactivity in healthy young and old adults. Cereb Cortex. 22:2610–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Turner GR. 2019. The shifting architecture of cognition and brain function in older adulthood. Perspect Psychol Sci. 14:523–542. [DOI] [PubMed] [Google Scholar]
- Stroop JR, Ridley Stroop J. 1992. Studies of interference in serial verbal reactions. J Exp Psychol Gen. 121:15–23. [Google Scholar]
- Touroutoglou A, Zhang J, Andreano JM, Dickerson BC, Barrett LF. 2018. Dissociable effects of aging on salience subnetwork connectivity mediate age-related changes in executive function and affect. Front Aging Neurosci. 10:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner GR, Nathan Spreng R. 2015. Prefrontal engagement and reduced default network suppression co-occur and are dynamically coupled in older adults: the default–executive coupling hypothesis of aging. J Cogn Neurosci. 27:2462–2476. [DOI] [PubMed] [Google Scholar]
- Uddin LQ. 2020a. Bring the noise: Reconceptualizing spontaneous neural activity. Trends Cogn Sci. 24:734–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ. 2020b. Brain mechanisms supporting flexible cognition and behavior in adolescents with autism spectrum disorder. Biol Psychiatry. 89:172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ. 2021. Cognitive and behavioural flexibility: neural mechanisms and clinical considerations. Nat Rev Neurosci. 22:167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, Menon V. 2011. Dynamic reconfiguration of structural and functional connectivity across Core neurocognitive brain networks with development. J Neurosci. 31:18578–18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Yeo BTT, Spreng RN. 2019. Towards a universal taxonomy of macro-scale functional human brain networks. Brain Topogr. 32:926–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Su L, Shen H, Hu D. 2012. Decoding lifespan changes of the human brain using resting-state functional connectivity MRI. PLoS One. 7:e44530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyshyn C, Verhaeghen P, Sliwinski MJ. 2011. Aging and task switching: a meta-analysis. Psychol Aging. 26:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Chen Q, Shi L, Li M, Gong W, Chen H, Qiu J. 2019. Tracking the dynamic functional connectivity structure of the human brain across the adult lifespan. Hum Brain Mapp. 40:717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C-G, Wang X-D, Zuo X-N, Zang Y-F. 2016. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics. 14:339–351. [DOI] [PubMed] [Google Scholar]
- Yeniad N, Malda M, Mesman J, van IJzendoorn MH, Pieper S. 2013. Shifting ability predicts math and reading performance in children: a meta-analytical study. Learn Individ Differ. 23:1–9. [Google Scholar]
- Yochim B, Baldo J, Nelson A, Delis DC. 2007. D-KEFS Trail making test performance in patients with lateral prefrontal cortex lesions. J Int Neuropsychol Soc. 13:704–709. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Anderson JE, Richler J, Wallner-Allen K, Beaumont JL, Conway KP, Gershon R, Weintraub S. 2014. NIH toolbox cognition battery (CB): validation of executive function measures in adults. J Int Neuropsychol Soc. 20:620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Andreano JM, Dickerson BC, Touroutoglou A, Barrett LF. 2020a. Stronger functional connectivity in the default mode and salience networks is associated with youthful memory in Superaging. Cereb Cortex. 30:72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zuo X-N, Ng KK, Chong JSX, Shim HY, Ong MQW, Loke YM, Choo BL, Chong EJY, Wong ZX, et al. 2020b. Distinct BOLD variability changes in the default mode and salience networks in Alzheimer’s disease spectrum and associations with cognitive decline. Nature Scientific Reports. 10:6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


