Abstract
Social communication differences are seen in autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD), and obsessive–compulsive disorder (OCD), but the brain mechanisms contributing to these differences remain largely unknown. To address this gap, we used a data-driven and diagnosis-agnostic approach to discover brain correlates of social communication differences in ASD, ADHD, and OCD, and subgroups of individuals who share similar patterns of brain-behavior associations. A machine learning pipeline (regression clustering) was used to discover the pattern of association between structural brain measures (volume, surface area, and cortical thickness) and social communication abilities. Participants (n = 416) included children with a diagnosis of ASD (n = 192, age = 12.0[5.6], 19% female), ADHD (n = 109, age = 11.1[4.1], 18% female), or OCD (n = 50, age = 12.3[4.2], 42% female), and typically developing controls (n = 65, age = 11.6[7.1], 48% female). The analyses revealed (1) associations with social communication abilities in distributed cortical and subcortical networks implicated in social behaviors, language, attention, memory, and executive functions, and (2) three data-driven, diagnosis-agnostic subgroups based on the patterns of association in the above networks. Our results suggest that different brain networks may contribute to social communication differences in subgroups that are not diagnosis-specific.
Keywords: attention-deficit/hyperactivity disorder, autism spectrum disorder, obsessive–compulsive disorder
Introduction
Capacity for “sociality” can be quantified at different levels of social cognition (cognitive processes underlying social behavior), social behavior (observable interactions between individuals), and social functioning (contextualized ability to interact with others) (Kennedy and Adolphs 2012). Social behavior is a complex phenotype; its expression reflects the interaction of many neurobiological processes and sociodemographic factors. The neuroanatomical substrates of social behavior span multiple levels of regulation, from neurotransmitters to a system of interacting and functionally specialized neural networks regulating social and nonsocial processes (e.g., perception, interpretation, understanding the behavior and mental states of others, affective processing, executive function, and cognitive control) (Van Overwalle 2009; Kennedy and Adolphs 2012; Bickart et al. 2014; Lamblin et al. 2017; Alcalá-López et al. 2018; Baribeau et al. 2019; Porcelli et al. 2019). An emerging body of literature is beginning to delineate these networks (Kennedy and Adolphs 2012; Bickart et al. 2014; Alcalá-López et al. 2018; Porcelli et al. 2019). For example, the mirroring, mentalizing, and empathy networks are suggested to underlie different domains of social behavior (Van Overwalle 2009; Kennedy and Adolphs 2012; Bickart et al. 2014; Porcelli et al. 2019). A recent meta-analysis used a data-driven approach to extend these to 4 functionally segregated circuits: visual-sensory and limbic circuits (collectively responsive to biologically relevant cues, perception/action cycles in social cognition), an intermediate-level processing circuit (empathy and pain tasks, preprocessed sensory input and motor response preparation), and a high-level associative circuit (responsible for theory-of-mind, attention, executive functioning, memory, and spatial processes) (Alcalá-López et al. 2018).
The high complexity of the brain systems affecting social behaviors allows for susceptibility to pathology at different points, making social communication a final common pathway of many neuropathological processes. It is therefore not surprising that social communication differences are present in several psychiatric and neurodevelopmental conditions (Kennedy and Adolphs 2012). For example, these differences are a defining feature of autism spectrum disorder (ASD), and are frequently associated with other neurodevelopmental disorders (NDDs) including attention-deficit/hyperactivity disorder (ADHD) and obsessive–compulsive disorder (OCD) (Baribeau et al. 2019). This has motivated the study of social communication differences as a transdiagnostic dimension, the neurobiological correlates of which may transcend the boundaries of existing diagnostic labels (Ameis et al. 2016; Baribeau et al. 2019). To this end, a data-driven, diagnosis-agnostic approach was used in our previous work to examine neurobiological and phenotypic overlap across ASD, ADHD, and OCD (Kushki et al. 2019). The results revealed new groupings that contained participants from multiple diagnostic categories, highlighting shared phenotypes and cortical thickness patterns among the diagnostic groups. These findings are also consistent with studies reporting shared neurobiology among NDDs in terms of brain structure and function (Dajani et al. 2019).
Given that some overlap in neuroanatomical features has been reported in ASD, ADHD, and OCD (Ameis et al. 2016; Baribeau et al. 2019), it may be the case that shared brain differences contribute to social communication differences across these disorders. However, it is also possible that the diagnosis-specific neuropathological features may underlie social communication differences in these disorders. This is supported by the notion that different cognitive and affective processes may impact social abilities across the diagnoses. Very few studies have examined the association between brain features and social communication abilities in a dimensional manner and across ASD, ADHD, and OCD, revealing mixed results. For example, functional (Lake et al. 2019) and structural (Aoki et al. 2017) connectivity patterns have been associated with autistic-like social differences across ASD and ADHD. Looking at brain structure, both diagnosis-specific and shared patterns of association were revealed across ASD, ADHD, and OCD (Baribeau et al. 2019). However, large confidence intervals were reported, highlighting the high variability within and across disorders. This large within-diagnosis variability and between-diagnosis overlap motivates the search for other groupings that may have similar patterns of brain-behavior associations (Byrge et al. 2015; Ciarrusta et al. 2019). To examine this hypothesis, we used a data-driven, diagnosis-agnostic approach to discover subgroups of children with ASD, ADHD, and OCD who may share similar brain-behavior associations.
Social behaviors are modulated by many interacting cognitive and affective processes which may share neurobiological bases. Although we recognize the merit and importance of studying these processes individually, the focus of this study was investigating system-level neurobiological heterogeneity that ultimately results in variability in social behaviors. This approach provides some abstraction of complexities of interacting processes, compensatory mechanisms, and environmental contexts that may influence the outcomes of those with neurobiological risk factors. Our goal was to characterize neuroanatomical predictors of social communication across ASD, ADHD, and OCD and how these may differ across the empirically derived diagnosis-agnostic subgroups.
Materials and Methods
Participants
Participants were recruited through the Province of Ontario (Canada) Neurodevelopmental Disorders Network (POND), a multicentre research network studying NDDs. Consent and assent was obtained as per institutional ethics board guidelines.
The included participants were 3–22 years old, had sufficient English comprehension to complete the testing protocols, and did not have contraindications for magnetic resonance imaging (MRI). Diagnoses for the clinical groups were confirmed using in-depth assessments (ASD: Autism Diagnostic Observation Schedule–2 (ADOS) (Lord et al. 2000) and Autism Diagnostic Interview–Revised (ADI-R) (Lord et al. 1994); ADHD: Parent Interview for Child Symptoms (PICS) (Ickowicz et al. 2006); OCD: Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) and the Children’s Yale–Brown Obsessive Compulsive Scale (CY-BOCS) (Scahill et al. 1997)). The typically developing (TD) controls did not have a neurodevelopmental, psychiatric and/or neurological diagnosis, were born after 35 weeks gestation, and did not have a first-degree relative with a neurodevelopmental condition. Having complete responses for the Social Communication Questionnaire (SCQ) (Rutter et al. 2003a) social communication questions was an inclusion criterion.
Behavioral Measures
Our analyses focused on “autism-like” social communication differences, quantified using the SCQ-life-time version. The SCQ is a 40-question parent questionnaire probing reciprocal social interaction, communication, and restricted, repetitive, and stereotyped patterns of behavior (Rutter et al. 2003a). The SCQ has acceptable psychometric properties including internal consistency (alpha 0.84–0.93), discriminant ADI-R (correlation coefficients 0.73–0.82; (Rutter et al. 2003b). Of the 40-items on the SCQ, the 28 questions relate to social communication differences and were used after correction for age and sex. The 28 questions were determined based on alignment with the ADI-R domains (Rutter et al. 2003b, p. 8). Additional measures of social cognition, behavior, and function used to validate the findings included scores on the Reading the Eyes in the Mind task (RMET) (Baron-Cohen et al. 2001), the Child Behavior Checklist (CBCL)—Social Problems subscale (Achenbach 1994), and the Adaptive Behavior Assessment System-II (ABAS)—the Social and General Adaptive Composite subscales (Oakland and Harrison 2008). Full-scale IQ was estimated using the age-appropriate Wechsler or Stanford–Binet scales.
Imaging Data
Measures of brain structure (volume, cortical thickness, and cortical/subcortical volume) were used in the analyses. These features have been consistently implicated in the neurobiology of NDDs (Van Rooij et al. 2018; Hoogman et al. 2019; Boedhoe et al. 2020), have been shown to be associated with differences in autistic-like traits (Van Rooij et al. 2018), and specifically social communication differences (Baribeau et al. 2019). Neuroanatomical features are also suggested to serve as an intermediate phenotype to link genetic variants such as those seen in NDDs (Ellegood et al. 2015).
Structural MRI data were collected at the Hospital for Sick Children, in Toronto, Ontario. Nearly half of the scans were obtained using a 3-Tesla Siemens Trio TIM (184 participants); the remaining participants were scanned after a hardware update to the Siemens PrismaFIT. T1-weighted images were acquired (Trio: TR/TE: 2300/2.96 ms; FA: 9°; FOV: 192 × 240 × 256mm; 1.0 mm isotropic voxels; Prisma: TR/TE: 1870/3.14 ms, FA: 9°, FOV: 192 × 240 × 256mm, 0.8 mm isotropic voxels; both scan times: 5 min). Cortical volume, surface area, and cortical thickness were extracted from the T1-weighted images using the CIVET pipeline (version 2.1.0) (Sled et al. 1998). The pipeline applies a nonuniformity correction on the images (Sled et al. 1998) followed by stereotaxic registration to the Montreal Neurologic Institute (MNI ICBM152) template (nonlinear sixth generation target) (Collins et al. 1994; Grabner et al. 2006). Next, brains were masked, extracted, and classified into gray matter, white matter, and cerebrospinal fluid. Tissue classification images were used to generate gray and white matter surfaces (Zijdenbos et al. 1998; MacDonald et al. 2000; Smith 2002; Tohka et al. 2004; Kim et al. 2005) and surfaces were registered to the automated anatomical labelling (AAL) atlas (Robbins 2003; Lyttelton et al. 2007; Boucher et al. 2009). Cortical thickness was computed for each region of interest in the AAL atlas as the average t-link distance between the gray matter and white surfaces extracted by CIVET distance (30 mm bandwidth heat-kernel smoothing). Volumes for subcortical structures were determined based on segmentations using the multiple automatically generated templates (MAGeT) (Pipitone et al. 2014). Quality assurance was carried out using CIVET’s automatic quality control pipeline, relaxed slightly such that scans that produced surfaces with fewer than 150 surface–surface intersections and self-intersections per hemisphere were included. This cutoff was chosen to represent a reasonable trade-off between including manually identified high quality scans and excluding manually identified low quality scans in a subset of the data. Further scans with gross subcortical segmentation failures were excluded where at least one atlas structure was not identified in the segmentation.
The above measurements were linearly regressed against age, sex, total gray matter volume, and scanner type in a sequential manner and the z-scored residuals were used in subsequent analyses.
Analysis
Pipeline
We used a machine learning pipeline (Supplementary Fig. 1) for regression clustering. The pipeline entails three steps. First, a Gaussian mixture model is used to cluster participants using a given brain measure (volume, cortical thickness, surface area) and SCQ. A Gaussian mixture model is a probabilistic model that assumes the data points in a sample are generated from a mixture of a finite number of Gaussian distributions with unknown parameters. The model parameters are estimated using the expectation–maximization (EM) algorithm. We used the Bayesian Information Criterion (BIC) to estimate the number of clusters (mixtures) within the range of one to five clusters (range determined based on visual inspection of the data). Next, linear regression analysis was used to test the association of SCQ and each brain measure within each cluster. Each time a significant regression coefficient was found, the respective entry in a feature-participant matrix was incremented. To improve stability and generalizability of our results, bagging (Breiman 1996) was used. Bagging is a method for generating and aggregating decisions based on multiple random subsets of data to improve the accuracy, stability, and generalizability of machine learning algorithms (Dudoit and Fridlyand 2003). For this study, a full run of the pipeline consisted of 125 000 subsamples (bootstrapping), each using a random subset of 63% of participants (Breiman 1996).
Clustering
The above pipeline yielded a feature-participant significance frequency matrix. Each entry (b,p) of this matrix indicated how frequently a participant, p, appeared in a subsample where a brain measure b was significantly associated with the SCQ social communication score. Hierarchical clustering was then used to cluster this matrix, with the Euclidean distance as the distance function. Scores for each brain measure were computed as the median of the scores across all participants in consideration of the skewed nature of the distributions. The scores can be interpreted as the frequency with which a given brain measure has a significant linear association with SCQ score across 125 000 randomly chosen subsets of the sample.
Cluster Validity
To confirm that the clustering result was indicative of true connections between participants, the analyses were run on 100 sets created by randomly permuting the SCQ values. Feature-participant entries were retained only if they were different than those from the randomly permuted data at a significance the level of 0.05.
Post-hoc Statistical Analyses
Phenotypic and behavioral characteristics were compared using the Kruskal–Wallis test given the non-normal nature of the data. False discovery rate (FDR) or Bonferroni corrections were used where multiple comparisons were performed.
Regression clustering and statistical analyses were conducted using Python 3.6.1 and R 3.3.3.
Results
Participants
The dataset used in the analyses was pulled from POND database in December 2019. Of the 613 scans available, 197 were excluded due to failed quality control (QC; n = 89) and 108 were excluded due to missing or incomplete SCQ (Supplementary Table 1). Demographic information for the remaining participants is shown in Table 1. The list of medications used by participants in the study is provided in Supplementary Table 2. The diagnostic groups in our sample were not statistically different in age, but the ASD and ADHD groups had a higher proportion of males and lower IQ compared to OCD and TD groups (P < 0.0001). As expected, there was a significant effect of diagnosis on total SCQ score (P < 0.0001); despite the differences in median values, the distributions of SCQ scores were highly overlapping among groups (Supplementary Fig. 2; Supplementary Fig. 3).
Table 1.
Participant demographics. Reported values are median (interquartile range (IQR)). P values are not corrected for multiple comparisons (6 comparisons)
| ASD (n = 192) | ADHD (n = 109) | OCD (n = 50) | TD (n = 65) | Group effect (uncorrected P value) | |
|---|---|---|---|---|---|
| Age | 12.0(5.6) | 11.1(4.1) | 12.3(4.2) | 11.6(7.5) | 0.06 |
| Sex (m:f) | 155:37 | 89:20 | 29:21 | 34:31 | <0.0001 Male ASD, ADHD > OCD, TD |
| Full-scale IQ | 96.5(29.7) | 102.5(20.0) | 115.0(15.5) | 110.0(18.0) | <0.0001 ASD < ADHD<OCD, TD |
| SCQ | 20.0(10.0) | 5.0(8.0) | 4.5(6.8) | 1.0(3.0) | <0.0001 TD < OCD, ADHD< ASD |
| SCQ—social communication | 14.0(9.0) | 3.0(6.0) | 2.0(4.0) | 1.0(3.0) | <0.0001 TD < OCD < ADHD < ASD |
| SWAN—inattention | 5.0(5.0) | 7.0(4.0) | 0.5(3.7) | 0(0) | <0.0001 TD < OCD < ASD < ADHD |
| SWAN—hyperactivity | 3.0(5.0) | 3.0(6.0) | 0(1.7) | 0(0) | <0.0001 TD < OCD < ASD < ADHD |
| TOCS | −3.0(29.5) | −17.0(45.0) | 20.5(24.0) | −42.0(53.5) | <0.0001 TD < ADHD<ASD < OCD |
| CBCL—anxiety problems | 67.5(17.0) | 60.0(16.0) | 70.0(9.5) | 51.0(4.0) | <0.0001 TD < ADHD<ASD,OCD |
| CBCL—internalizing problems | 65.0(12.7) | 65.0(14.5) | 68.0(12.5) | 48.0(12.0) | <0.0001 TD < ASD, ADHD, OCD |
| CBCL—externalizing problems | 60.0(15.0) | 61.0(15.0) | 53.0(12.0) | 40.0(14.0) | <0.0001 TD,OCD < ASD,ADHD |
Note: Fifty-seven of the 416 participants were missing IQ data.
CBCL, Child Behavior Checklist; SWAN, The Strengths and Weaknesses of Attention-Deficit/Hyperactivity Disorder Symptoms and Normal Behavior Scale (SWAN); TOCS, Toronto Obsessive Compulsive Scale.
Clusters
The optimal number of clusters was determined to be two based on the Silhouette Coefficient and the Calinski-Harabasz and the Davies-Bouldin metrics. However, in the interest of generalizability, we present the results of hierarchical clustering as the number of clusters, C, increases from 2 to 8. As seen in Figure 1, two large groups of participants are evident regardless of the number of clusters used: a cluster of participants with NDDs (NDD cluster) and a cluster containing a mix of participants from all groups (mixed cluster). The NDD and mixed clusters were not significantly different in age, but the NDD cluster had a higher proportion of males (NDD: 0.80, mixed cluster: 0.67; χ2(1) = 8.34, P = 0.004). As seen in Figure 2, the NDD cluster is also characterized by significantly higher degrees of social differences, both in measures used in clustering (SCQ-social communication: NDD: 14.0(8.0), mixed: 2.0(3.0), χ2(1) = 307.0, P < 0.0004) and those not used in clustering. The latter include measures of social cognition (RMET: NDD: 17.0(7.0), mixed: 19.0(6.0), χ2(1) = 24.9, P < 0.0001), social behavior (CBCL social problems subscale: NDD: 65.0(12.0), mixed: 54.0(11.5), χ2(1) = 57.19, P < 0.0001), and social function (ABAS-social: NDD: 68.0(19.25), mixed: 96.0(27.0), χ2(1) = 8.34, P < 0.0001). This group also had significantly decreased full-scale IQ (NDD: 97.0(27.5), mixed: 108.0(18.0), χ2(1) = 41.98, P < 0.0001) and general adaptive functioning (ABAS-General Ability Composite: NDD: 66.0(20.0), mixed: 91.0(29.0), χ2(1) = 128.4, P < 0.0001) scores.
Figure 1 .
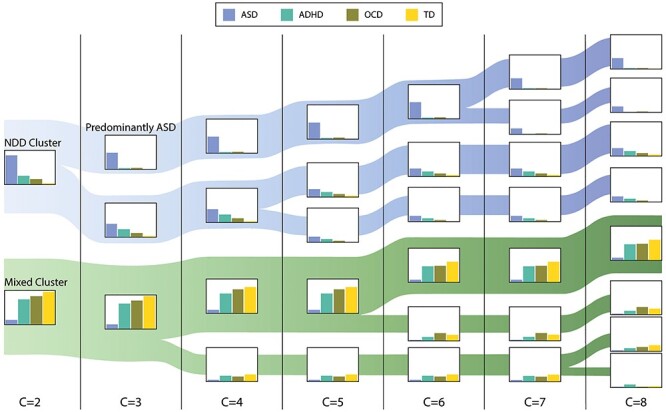
Results of hierarchical clustering. Bar plots represent percent participants from each diagnostic group in the cluster. Width of each band represents the number of participants in the respective cluster. The NDD and mixed clusters and their subdivisions are shaded in blue and green, respectively.
Figure 2 .
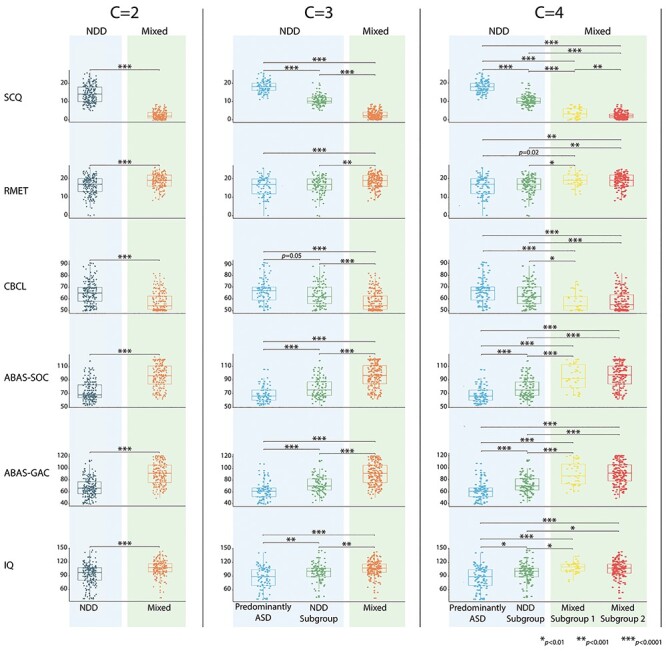
Demographic characterization of clusters for the 2, 3, and 4 cluster solutions. Demographic characterization of clusters for the 2, 3, and 4 cluster solutions. ASD, autism spectrum disorder; ABAS-GAC, Adaptive Behavior Assessment System—General Adaptive Composite; CBCL, Child Behavior Checklist; IQ, Intelligence Quotient; RMET, Reading the Mind in the Eyes Test; SCQ, Social Communication Questionnaire.
NDD Cluster
Looking at the two-cluster solution (C = 2; Fig. 1), the first cluster contains participants with neurodevelopmental diagnoses and predominantly those with ASD (NDD cluster: 86% ASD, 26% ADHD, 16% OCD, and 3% TD). As the number of clusters increases, this cluster further differentiated into a “predominantly-ASD” and an NDD cluster (C = 3; predominantly-ASD cluster: 48% ASD, 3% ADHD, 4% OCD, 0% TD; NDD cluster: 38% ASD, 23% ADHD, 12% OCD, and 3% TD), and these groupings remain stable as the number of clusters increases to 8. The predominantly-ASD subgroup was not significantly different than the other participants in the NDD cluster in age or sex proportion, but as seen in Figure 2 (C = 3), was more impaired on measures of social communication (SCQ-social communication, P < 0.0001), social behavior (CBCL social, P = 0.05), social function (ABAS-social, P < 0.0001), general adaptive functioning (ABAS-General Ability Composite, P < 0.0001), and IQ (P = 0.008).
Mixed Cluster
Looking at the two-cluster solution (C = 2), the second cluster houses the majority of participants in the ADHD, OCD, and TD groups as well as a small subgroup of those with a diagnosis of ASD (mixed cluster: 13% ASD, 74% ADHD, 84% OCD, and 97% TD). This cluster continues to differentiate into clusters with a mix of participants from the ADHD, OCD, and TD groups. The subgroups of the mixed cluster are not statistically different on age, sex, proportions, or any measures of social behavior and function except for the SCQ-social communication score (C = 4–6; P < 0.01). Across the various cluster numbers, none of the subgroups separated controls from the ADHD and OCD groups.
Neuroanatomical Correlates of Social Behavior
Scores were computed for brain measures based on how frequently each measure was found to be significantly correlated with the SCQ social communication score (Fig. 3). Overall, the regions whose volume, cortical thickness, or surface area were associated with SCQ scores came from distributed networks of cortical and subcortical regions commonly implicated in the social brain (Kennedy and Adolphs 2012; Alcalá-López et al. 2018) and other cognitive processes that underlie social behavior and function (e.g, attention, language, memory), as well as thalamocortical networks for visual and somatosensory processing. Components of the default mode network (ventromedial prefrontal cortex (vmPFC)/dorsomedial prefrontal cortex (dmPFC), posterior cingulate cortex (PCC), precuneus) also featured in the top scoring regions. Across all groups, association between SCQ and cortical thickness were mainly seen in the frontal and occipital lobes, whereas associations with surface area were more widespread across the cortex.
Figure 3 .
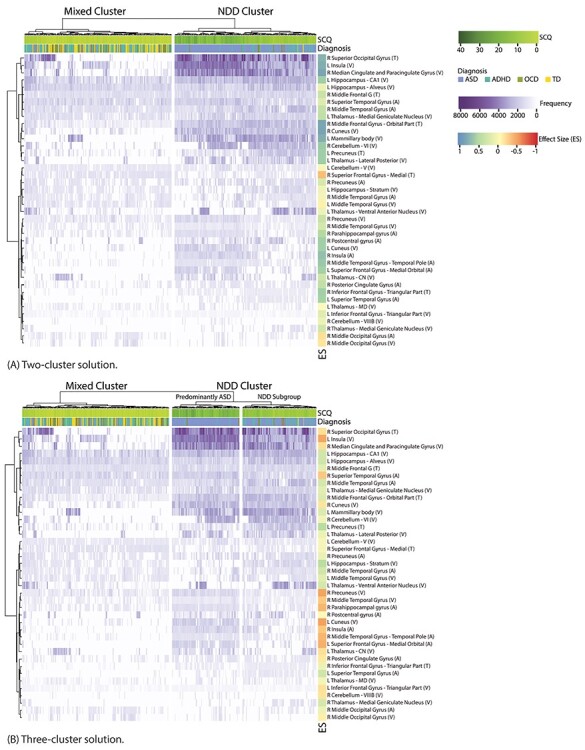
Frequency of association of brain measures with SCQ scores for the 2 (A) and 3 (B) cluster solutions. Only measures with association frequency significantly higher than chance are shown. Side bar shows effect size.
With the two-clustering solution (Fig. 3A), compared to the mixed cluster, the NDD group was characterized by more frequent associations between SCQ scores and brain measures, predominantly featuring right/bilateral cortical and left subcortical regions. Medium to large positive effect sizes (r > 0.3) were seen in regions implicated in the social behavior, attention, memory, and thalamocortical networks involved in sensory processing. There was also increased association between SCQ and components of the default mode network in the NDD group.
With reference to the three-cluster solution (Fig. 3B), within the NDD cluster, the predominantly-ASD subgroup showed increased frequency of association between SCQ and the brain measures in the social brain regions, as well as the left cuneus and the right parahippocampal gyrus (medium effect sizes). Decreased association frequencies were found in the left hippocampus, superior temporal gyrus, and precuneus.
To quantify the direction of the effects and differences between clusters in SCQ-brain associations, traditional linear regression analyses were used (Supplementary Fig. 4). Comparing the mixed and NDD subclusters, a significantly larger negative slope was found for the NDD subgroup in the left hippocampus (CA1 volume, P = 0.03; stratum volume, P = 0.02) and thalamus (medial geniculate nucleus (MGN) volume, P = 0.03; CN volume, P = 0.02; LP volume, P = 0.02), although the differences did not survive FDR correction for multiple comparisons.
Compared to the mixed cluster, a significantly larger positive slope was found for the predominantly-ASD group in the bilateral superior temporal gyrus (surface area, P = 0.002), the right temporal pole of the middle temporal gyrus (surface area, P = 0.04), right parahippocampal gyrus (surface area, P = 0.01), bilateral insula (right surface area, P = 0.02; left volume, P = 0.003), and the left hippocampus (subcortical volume; CA1, P = 0.003; stratum, P = 0.04; alveus, P = 0.04). After FDR correction, only differences in the bilateral superior temporal gyrus, the left insula, and the left hippocampus (CA1) survived.
Discussion
Subgroups
In this study, we used a data-driven, diagnosis-agnostic approach to examine the association of brain morphology and social behavior. In contrast to traditional statistical approaches which use a priori group labels (e.g., diagnosis), the proposed approach allowed for multiple types of linear associations to emerge from the data. To increase the generalizability of the results and decrease the risk of overfitting, we reported on patterns that emerged consistently when different numbers of clusters were used. Overall, our results suggest that there may be subgroups of neurodevelopmental conditions defined by unique patterns of brain-social behavior association. In particular, two groups of participants emerged: a mixed group comprised of TD participants together with the majority of those with ADHD and OCD, but very few participants with ASD. The second cluster was comprised of participants with NDDs; within this cluster, a subgroup with predominantly-ASD diagnosis emerged.
The composition of these groups supports the conceptualization of social communication differences as a transdiagnostic dimension with neurobiological correlates that transcend the boundaries of existing labels of ASD, ADHD, OCD, and typical development (Ameis et al. 2016; Aoki et al. 2017; Baribeau et al. 2019; Lake et al. 2019). For example, the predominantly-ASD cluster contained fewer than half of those with a diagnosis of ASD, with the others spread across the mixed and NDD groups. Our data-driven groups were differentiated by behavioral and demographic profiles not used in the clustering, with the predominantly-ASD group showing increased impairment in a number of domains including social cognition, behavior, and function, attention, IQ, and adaptive functioning. Given the pervasiveness and lack of specificity of impairments, this group may represent a “severe neurodevelopmental difference” group, and likely not a subtype unique to ASD.
Neuroanatomical Correlates
Overall, our results support the idea that transdiagnostic neuropathological features may underlie social deficits in ASD, ADHD, and OCD. This is consistent with the substantial etiological overlap between these disorders and typical variation in social communication ability (Robinson et al. 2016). These results also support the notion of a continuous dimension of ASD-like social differences, with NDDs at the extremes of this continuum (Plomin et al. 2009; van der Meer et al. 2017). Our findings suggest that these transdiagnostic features may not lie in a continuous spectrum, but that different neuropathological signatures may exist depending on the level of impairment, regardless of the diagnostic label.
Across our entire sample, common neuroanatomical correlates of social behaviors were found to be distributed across networks of cortical and subcortical networks previously implicated in social cognition/behavior as well as attention, language, memory, and executive control. This is not a surprising finding given that social behavior is a complex phenotype that reflects the downstream effect of many cognitive processes. These findings resonate with those of a recent meta-analysis (Alcalá-López et al. 2018) which found that social behaviors may not be realized by a single region or network, as well as other cross-diagnosis investigations of neurobiological social behavior correlates (Aoki et al. 2017; Baribeau et al. 2019; Kushki et al. 2019; Lake et al. 2019).
We found three data-driven subgroups which showed uniqueness in some neuroanatomical correlates of social communication behaviors. The largest and most pervasive differences were seen between the predominantly-ASD and the other two groups, in cortical and subcortical regions implicated in social behaviors and memory. These results reflect the nature of social behaviors as the final common pathway of many interacting cognitive and affective processes whose neurobiological bases are distributed across the brain. We found more frequent associations between neuroanatomical features commonly related to social function (e.g., the social brain) in the predominantly-ASD group. These were apparent across the same range of the neuroanatomical measures as in the other groups, but only existed at higher degrees of impairment. This may suggest higher sensitivity to differences in neuroanatomical features as impairment increases and may reflect differences in underlying biological mechanisms. Future studies are needed to further investigate this.
Relative to the mixed cluster group, the predominantly-ASD group seemed to be differentiated by a pattern of a positive association between social communication impairments and surface area of regions implicated in social function (superior temporal gyrus and insula) as well as hippocampal volume. In this context, our results support the notion that different mechanisms may underlie social differences across the subgroups. Moreover, our results suggested a prominent role for cortical surface area in contributing to social differences in the predominantly-ASD group. Surface area is one determinant of cortical volume, the other being cortical thickness which was less prominently featured in our findings. Surface area and cortical thickness are suggested to have distinct genetic determinants and may quantify different aspects of the underlying neural structure (number of cells within cortical columns vs. number of columns in a cortical region) (Raznahan et al. 2011). The literature is generally mixed on the contribution of cortical thickness and surface area to the neuropathology of ASD (Ecker et al. 2013; Mensen et al. 2017; Van Rooij et al. 2018). Our results may offer some explanation for this variability and highlight the role of impairment severity as an important stratification variable in future studies.
Implications
Overall, the findings of this study are consistent with the previous literature reporting misalignment between the existing diagnostic labels of ASD, ADHD, and OCD and underlying neurobiology (Aoki et al. 2017; Baribeau et al. 2019; Kushki et al. 2019; Lake et al. 2019). Collectivity, this emerging literature raises questions about the validity of our existing diagnostic categories, and whether alternative groupings should be used for neurobiological studies of NDDs. Specifically, the diagnosis-agnostic subgroups can provide an alternative stratification approach for examination of biomarkers and brain-behavior associations.
From a clinical perspective, the lack of alignment between diagnostic labels and underlying biology questions our existing focus on these labels for provision of services and interventions, and motivates a needs-based approach for supporting children with neurodevelopmental conditions.
Limitations
Our findings should be interpreted in the context of several limitations. First, the association between brain structure and social communication abilities may vary by age, and complex developmental trajectories have been observed for the different regions (Van Rooij et al. 2018). Our analyses statistically controlled for the effect of age, and it is encouraging that the data-driven clusters did not differ significantly in age. It is important that future studies further extend this to explore the interaction of brain and age effects on social communication. In our sample, the majority of participants in the high SCQ range had a diagnosis of ASD. Future studies focused on ranges where significant overlap in SCQ scores occurs can help to further understand the impact of diagnoses on neural correlates of social behaviors. Furthermore, differences in IQ can play an important role in interpreting differences across the neurodevelopmental groups studied here. Despite the lower IQ in our ASD group, the mean IQ score is relatively high in our sample, and inclusion of a broader range of IQ in future samples is needed. In addition, the inclusion of a developmental delay control group could further clarify the role of overall cognitive ability in driving the group differences found in this study. Finally, the sample size used for the analyses reported in this paper was limited, with unequal distribution of participants across the diagnostic groups. A larger control group may help to further characterize the variability in the nonspecific group.
Conclusion
We examined the neuroanatomical correlates of social abilities across ASD, ADHD, OCD, and typical development. Our results revealed associations with social communication symptoms in distributed cortical and subcortical networks implicated in social behaviors, language, attention, memory, and executive functions. Based on these associations, we found a group of participants with predominantly-ASD diagnoses which showed high impairments across a number of functional domains, a neurodevelopmental group which contained participants with diagnoses of ASD, ADHD, and OCD, and a mixed group which included TD participants as well as the majority of those with ADHD, and OCD.
Notes
This research was conducted with the support of the Ontario Brain Institute (POND, PIs: Anagnostou, Lerch), an independent non-profit corporation, funded partially by the Ontario government. The opinions, results and conclusions are those of the authors and no endorsement by the Ontario Brain Institute is intended or should be inferred. Conflict of Interest: AK reports grants from New Frontiers in Research Fund – Exploration Grant (ID: NFRFE–2018–00482) during the conduct of the study and personal fees from Shaftesbury outside the submitted work. AK is the inventor of a software called the ``Anxiety Meter'' (US 9,844,332 B2, US 16/276,208 pending). The software is used for detection and management of emotion dysregulation. She is involved in commercializing the Anxiety Meter and will financially benefit from its sales. AK also served on the board of advisors for Shaftesbury, a media company developing virtual reality products for children with autism spectrum disorder and was compensated financially for this role. RS reports grants from Ontario Brain Institute during the conduct of the study. JC reports grants from New Frontiers Research Fund – Exploration Grant (ID: NFRFE–2018–00482) during the conduct of the study. EK reports grants from Ontario Brain Institute during the conduct of the study and grants from Freemason's Society of Ontario outside the submitted work. RN reports grants from Ontario Brain Institute during the conduct of the study and grant funding from Hoffman – La Roche Canada. EA reports grants from Ontario Brain Institute (grant IDS–11–02) during the conduct of the study. EA also reports grants from Roche, consultation fees from Roche, consultation fees from Quadrant, editorial honaria from Wiley, book royalties from Springer, book royalties from APPI, and non-financial support from AMO Pharma outside the submitted work. In addition, EA has a patent US 9,844,332 B2 with royalties from Awake Labs. EA holds a patent on the software called the ``Anxiety Meter'' that is being commercialized and she will financially benefit from its sales. PA receives funding from the Alberta Innovates Translational Health Chair in Child and Youth Mental Health. MA receives funding from National Institute of Mental Health (grant number 1R01MH112904–01), including a component of his salary. The other authors report no financial interests or potential conflicts of interest.
Funding
New Frontiers in Research Fund (grant NFRFE–2018–00482; PI: Kushki) and the Ontario Brain Institute (grant IDS–11–02; PIs: Anagnostou, Lerch).
Supplementary Material
References
- Achenbach TM. 1994. Child Behavior Checklist and related instruments. In: The use of psychological testing for treatment planning and outcome assessment. Hillsdale, NJ, US: Lawrence Erlbaum Associates, Inc., pp. 517–549. [Google Scholar]
- Alcalá-López D, Smallwood J, Jefferies E, van Overwalle F, Vogeley K, Mars RB, Turetsky BI, Laird AR, Fox PT, Eickhoff SB, et al. 2018. Computing the social brain connectome across systems and states. Cereb Cortex. 28(7):2207–2232. [DOI] [PubMed] [Google Scholar]
- Ameis SH, Lerch JP, Taylor MJ, Lee W, Viviano JD, Pipitone J, Nazeri A, Croarkin PE, Voineskos AN, Lai M-C. 2016. A diffusion tensor imaging study in children with ADHD, autism spectrum disorder, OCD, and matched controls: distinct and non-distinct white matter disruption and dimensional brain-behavior relationships. Am J Psychiatry. 173(12):1213–1222. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Yoncheva YN, Chen B, Nath T, Sharp D, Lazar M, Velasco P, Milham MP, Di Martino A. 2017. Association of white matter structure with autism spectrum disorder and attention-deficit/hyperactivity disorder. JAMA Psychiat. 74(11):1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baribeau DA, Dupuis A, Paton TA, Hammill C, Scherer SW, Schachar RJ, Arnold PD, Szatmari P, Nicolson R, Georgiades S. 2019. Structural neuroimaging correlates of social deficits are similar in autism spectrum disorder and attention-deficit/hyperactivity disorder: analysis from the POND network. Transl Psychiatry. 9:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Spong A, Scahill V, Lawson J. 2001. Studies of theory of mind: are intuitive physics and intuitive psychology independent. J Dev Learn Disord. 5:47–78. [Google Scholar]
- Bickart KC, Dickerson BC, Barrett LF. 2014. The amygdala as a hub in brain networks that support social life. Neuropsychologia. 63:235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedhoe PS, Van Rooij D, Hoogman M, Twisk JW, Schmaal L, Abe Y, Alonso P, Ameis SH, Anikin A, Anticevic A. 2020. Subcortical brain volume, regional cortical thickness, and cortical surface area across disorders: findings from the ENIGMA ADHD, ASD, and OCD working groups. Am J Psychiatry. 177(9):834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher M, Whitesides S, Evans A. 2009. Depth potential function for folding pattern representation, registration and analysis. Med Image Anal. 13(2):203–214. [DOI] [PubMed] [Google Scholar]
- Breiman L. 1996. Bagging predictors. Mach Learn. 24(2):123–140. [Google Scholar]
- Byrge L, Dubois J, Tyszka JM, Adolphs R, Kennedy DP. 2015. Idiosyncratic brain activation patterns are associated with poor social comprehension in autism. J Neurosci. 35(14):5837–5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarrusta J, O’Muircheartaigh J, Dimitrova R, Batalle D, Cordero-Grande L, Price A, Hughes E, Steinweg JK, Kangas J, Perry E. 2019. Social brain functional maturation in newborn infants with and without a family history of autism spectrum disorder. JAMA Netw Open. 2(4):e191868–e191868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. 1994. Automatic 3d intersubject registration of mr volumetric data in standardized talairach space. J Comput Assist Tomogr. 18(2):192–205. [PubMed] [Google Scholar]
- Dajani DR, Burrows CA, Odriozola P, Baez A, Nebel MB, Mostofsky SH, Uddin LQ. 2019. Investigating functional brain network integrity using a traditional and novel categorical scheme for neurodevelopmental disorders. NeuroImage Clin. 21:101678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudoit S, Fridlyand J. 2003. Bagging to improve the accuracy of a clustering procedure. Bioinformatics. 19(9):1090–1099. [DOI] [PubMed] [Google Scholar]
- Ecker C, Ginestet C, Feng Y, Johnston P, Lombardo MV, Lai MC, Suckling J, Palaniyappan L, Daly E, Murphy CM, et al. 2013. Brain surface anatomy in adults with autism: the relationship between surface area, cortical thickness, and autistic symptoms. Arch Gen Psychiatry. 70(1):59–70. [DOI] [PubMed] [Google Scholar]
- Ellegood J, Anagnostou E, Babineau B, Crawley J, Lin L, Genestine M, DiCicco-Bloom E, Lai J, Foster J, Penagarikano O. 2015. Clustering autism: using neuroanatomical differences in 26 mouse models to gain insight into the heterogeneity. Mol Psychiatry. 20(1):118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL. 2006. Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv. 9:58–66. [DOI] [PubMed] [Google Scholar]
- Hoogman M, Muetzel R, Guimaraes JP, Shumskaya E, Mennes M, Zwiers MP, Jahanshad N, Sudre G, Wolfers T, Earl EA. 2019. Brain imaging of the cortex in ADHD: a coordinated analysis of large-scale clinical and population-based samples. Am J Psychiatry. 176(7):531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickowicz A, Schachar RJ, Sugarman R, Chen SX, Millette C, Cook L. 2006. The parent interview for child symptoms: a situation-specific clinical research interview for attention-deficit hyperactivity and related disorders. Can J Psychiatry. 51(5):325–328. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Adolphs R. 2012. The social brain in psychiatric and neurological disorders. Trends Cogn Sci. 16(11):559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab’bagh Y, MacDonald D, Lee JM, Kim SI, Evans AC. 2005. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 27(1):210–221. [DOI] [PubMed] [Google Scholar]
- Kushki A, Anagnostou E, Hammill C, Duez P, Brian J, Iaboni A, Schachar R, Crosbie J, Arnold P, Lerch JP. 2019. Examining overlap and homogeneity in ASD, ADHD, and OCD: a data-driven, diagnosis-agnostic approach. Transl Psychiatry. 9(1):318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake EMR, Finn ES, Noble SM, Vanderwal T, Shen X, Rosenberg MD, Spann MN, Chun MM, Scheinost D, Constable RT. 2019. The functional brain organization of an individual allows prediction of measures of social abilities transdiagnostically in autism and attention-deficit/hyperactivity disorder. Biol Psychiatry. 86(4):315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamblin M, Murawski C, Whittle S, Fornito A. 2017. Social connectedness, mental health and the adolescent brain. Neurosci Biobehav Rev. 80:57–68. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M. 2000. Autism diagnostic observation schedule (ADOS). J Autism Dev Disord. 30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. 1994. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 24(5):659–685. [DOI] [PubMed] [Google Scholar]
- Lyttelton O, Boucher M, Robbins S, Evans A. 2007. An unbiased iterative group registration template for cortical surface analysis. Neuroimage. 34(4):1535–1544. [DOI] [PubMed] [Google Scholar]
- MacDonald D, Kabani N, Avis D, Evans AC. 2000. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 12(3):340–356. [DOI] [PubMed] [Google Scholar]
- Mensen VT, Wierenga LM, van Dijk S, Rijks Y, Oranje B, Mandl RCW, Durston S. 2017. Development of cortical thickness and surface area in autism spectrum disorder. NeuroImage Clin. 13:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakland T, Harrison PL. 2008. Adaptive behavior assessment system-II. San Diego: Elsevier Science & Technology.
- Pipitone J, Park MTM, Winterburn J, Lett TA, Lerch JP, Pruessner JC, Lepage M, Voineskos AN, Chakravarty MM, Alzheimer’s Disease Neuroimaging Initiative . 2014. Multi-atlas segmentation of the whole hippocampus and subfields using multiple automatically generated templates. Neuroimage. 101:494–512. [DOI] [PubMed] [Google Scholar]
- Plomin R, Haworth CMA, Davis OSP. 2009. Common disorders are quantitative traits. Nat Rev Genet. 10:872–878. [DOI] [PubMed] [Google Scholar]
- Porcelli S, Van Der Wee N, van der Werff S, Aghajani M, Glennon JC, van Heukelum S, Mogavero F, Lobo A, Olivera FJ, Lobo E. 2019. Social brain, social dysfunction and social withdrawal. Neurosci Biobehav Rev. 97:10–33. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Clasen L, Gogtay N, Giedd JN. 2011. How does your cortex grow? J Neurosci. 31(19):7174–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins S. 2003. Anatomical standardization of the human brain in Euclidean 3-space and on the cortical 2-manifold [PhD thesis] Montreal: McGill University. [Google Scholar]
- Robinson EB, St Pourcain B, Anttila V, Kosmicki JA, Bulik-Sullivan B, Grove J, Maller J, Samocha KE, Sanders SJ, Ripke S, et al. 2016. Genetic risk for autism spectrum disorders and neuropsychiatric variation in the general population. Nat Genet. 48(5):552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. 2003. The Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, Cicchetti D, Leckman JF. 1997. Children’s Yale-Brown obsessive compulsive scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 36(6):844–852. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans a C. 1998. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 17(1):87–97. [DOI] [PubMed] [Google Scholar]
- Smith SM. 2002. Fast robust automated brain extraction. Hum Brain Mapp. 17(3):143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohka J, Zijdenbos A, Evans A. 2004. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 23(1):84–97. [DOI] [PubMed] [Google Scholar]
- van der Meer JMJ, Lappenschaar MGA, Hartman CA, Greven CU, Buitelaar JK, Rommelse NNJ. 2017. Homogeneous combinations of ASD–ADHD traits and their cognitive and behavioral correlates in a population-based sample. J Atten Disord. 21(9):753–763. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. 2009. Social cognition and the brain: a meta-analysis. Hum Brain Mapp. 30(3):829–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rooij D, Anagnostou E, Arango C, Auzias G, Behrmann M, Busatto GF, Calderoni S, Daly E, Deruelle C, Di Martino A. 2018. Cortical and subcortical brain morphometry differences between patients with autism spectrum disorder and healthy individuals across the lifespan: results from the ENIGMA ASD working group. Am J Psychiatry. 175(4):359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijdenbos A, Forghani R, Evans A. 1998. Automatic quantification of MS lesions in 3D MRI brain data sets: validation of INSECT. In: Wells WM, Colchester A, Delp S, editors. Medical Image Computing and Computer-Assisted Intervention – MICCAI'98. Berlin, Heidelberg: Springer Berlin Heidelberg, p. 439–448. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


