Abstract
Objectives:
Chronic hepatitis B virus infection is a major cause of morbidity and mortality. The aim of the study is to describe the hepatic histology in children chronically infected with hepatitis B virus living in the United States and Canada.
Methods:
Liver biopsies of 134 treatment-naïve children with chronic hepatitis B virus infection were scored for inflammation, fibrosis, and other histological features, and correlated with clinical and laboratory data.
Results:
Sixty percentage of subjects acquired the infection vertically, 51% were male, and 69% were hepatitis B e antigen-positive at the time of the biopsy. Hepatitis B DNA levels were generally high (mean 7.70 log IU/mL), as was serum alanine aminotransferase (median 120 U/L). Using the Ishak-modified histology activity index scoring system, interface hepatitis was mild in 31%, moderate in 61%, and severe in 6%. Lobular inflammation was mild in 54%, moderate in 29%, and marked in 7%. Portal inflammation was mild in 38% and moderate in 62% of subjects. Eighteen percentage had no fibrosis, 59% had portal expansion without bridging fibrosis, 19% had bridging fibrosis, and 4% had cirrhosis. Alanine aminotransferase positively correlated with inflammation and fibrosis. Neither age, duration of infection, nor Hepatitis B virus DNA levels correlated with fibrosis. Fibrosis-4 index did not correlate with fibrosis but correlated with inflammation. Aspartate aminotransferase/platelet ratio index correlated with both inflammation and fibrosis.
Conclusions:
Chronic hepatitis B virus infection results in significant inflammation and fibrosis during childhood. Serum alanine aminotransferase is a strong indicator of the severity and extent of hepatic inflammation and fibrosis.
Keywords: fibrosis, inflammation, Ishak, liver biopsy, Metavir
Hepatitis B virus (HBV) is a major cause of liver disease worldwide. Approximately 350 million people are chronically infected with HBV (1). The evolution of HBV infection is a dynamic process that is influenced by several factors, such as host immune response, age, viral load, HBV genotype, and duration of disease (2,3). Patients with chronic infection who have elevated hepatitis B virus deoxyribonucleic acid (HBV DNA) and serum alanine aminotransferase (ALT) levels are at increased risk of developing complications, such as cirrhosis, liver failure, and hepatocellular carcinoma (4). Liver biopsy plays an important role in the management of patients with chronic hepatitis B. It helps establish the extent and severity of hepatic inflammation and fibrosis (2) and to diagnose other co-existent liver conditions that may influence disease progression, such as nonalcoholic steatohepatitis. The histological impact of chronic HBV infection in children has revealed variable severity of disease. In 1 Italian study (5) of 57 children ages 2 to 18 years, with a mean duration of infection of approximately 7 years, who underwent pretreatment liver biopsy, most patients (57.9%) had minimal hepatitis, 33.3% had moderate hepatitis, 7.1% had severe hepatitis, and 1.7% had cirrhosis. Most reports of hepatic histology have, however, originated from Asian and European countries. There is a paucity of data from HBV-infected children living in the United States and Canada where environmental risk factors may differ from those in Asian and European countries.
The Hepatitis B Research Network (HBRN) (6), a multicenter collaborative initiative to conduct research on chronic hepatitis B, provided a unique opportunity to describe the pretreatment hepatic histology of a large, well-characterized cohort of children chronically infected with HBV and residing in the United States and Canada. In this study, we correlated the histopathological findings with demographic, clinical, and virologic characteristics at the time of the liver biopsy for children with chronic hepatitis B.
METHODS
Study Design and Oversight
The HBRN consists of 21 adult and 7 pediatric clinical sites throughout the United States and Canada, a Data Coordinating Center and an Immunology Laboratory. The pediatric cohort includes subjects seen at sites in the United States located in California, Maryland, Minnesota, Missouri, Texas, and Washington, and a Canadian site in Ontario. Criteria for inclusion into the pediatric cohort are: ≥6 months and <18 years old, hepatitis B surface antigen (HBsAg) positive, and willing to provide informed consent/assent. Patients were excluded if they: had a history of hepatic decompensation, had a history of hepatocellular carcinoma (HCC), had a history of liver transplantation, had known human immunodeficiency virus (HIV) co-infection, were receiving antiviral therapy for HBV at the time of enrollment, were unable/unwilling to return for routine follow-up, or had a history or other evidence of severe illness or other medical or social condition that would make the patient, in the opinion of the investigator, unsuitable for the study. Visits occurred at enrollment, 6 months after enrollment, and annually thereafter. Clinical research forms were completed by the physician or the study coordinator. The Institutional Review Board at each site approved the study.
Subjects and Specimens
All subjects participating in the study underwent extensive evaluation including detailed history, physical, and laboratory examinations. Non-HBV causes of liver disease were excluded. Clinical and demographical information including sex, age at time of biopsy, duration of infection to biopsy (years), mode of transmission, genotype, platelet count, alanine aminotransferase (ALT) level, HBV DNA level, hepatitis B surface antigen (HBsAg), hepatitis B envelope antigen (HBeAg), hepatitis B envelope antibody (HBeAb), and antibody to hepatitis delta virus (anti-HDV) coinfection were collected. The aspartate aminotransferase (AST)/platelet ratio index (APRI score) and Fibrosis-4 (FIB-4) index were calculated. Liver biopsies were done at the discretion of the investigator/clinician. A total of 151 liver biopsy specimens performed between 2004 and 2014 inclusive were collected for analysis (Fig. 1). Fifty-two liver biopsy specimens were from children enrolled in the HBRN. Fifteen of them were excluded as patients were previously treated for hepatitis B. Ninety-nine liver biopsy specimens from treatment-naïve children obtained within the last 10 years in the participating pediatric centers but not enrolled in the HBRN, were also included. These biopsies were also obtained under a protocol approved by the Institutional Review Board at each site, which waived the need for signed consent for the stored biopsies. All biopsies were de-identified and bar coded and read by a single expert histopathologist (D.E.K). Two biopsies were excluded from the latter group because of inadequate tissue for assessment. At the end, 134 liver biopsy specimens were used for the final analysis.
FIGURE 1.
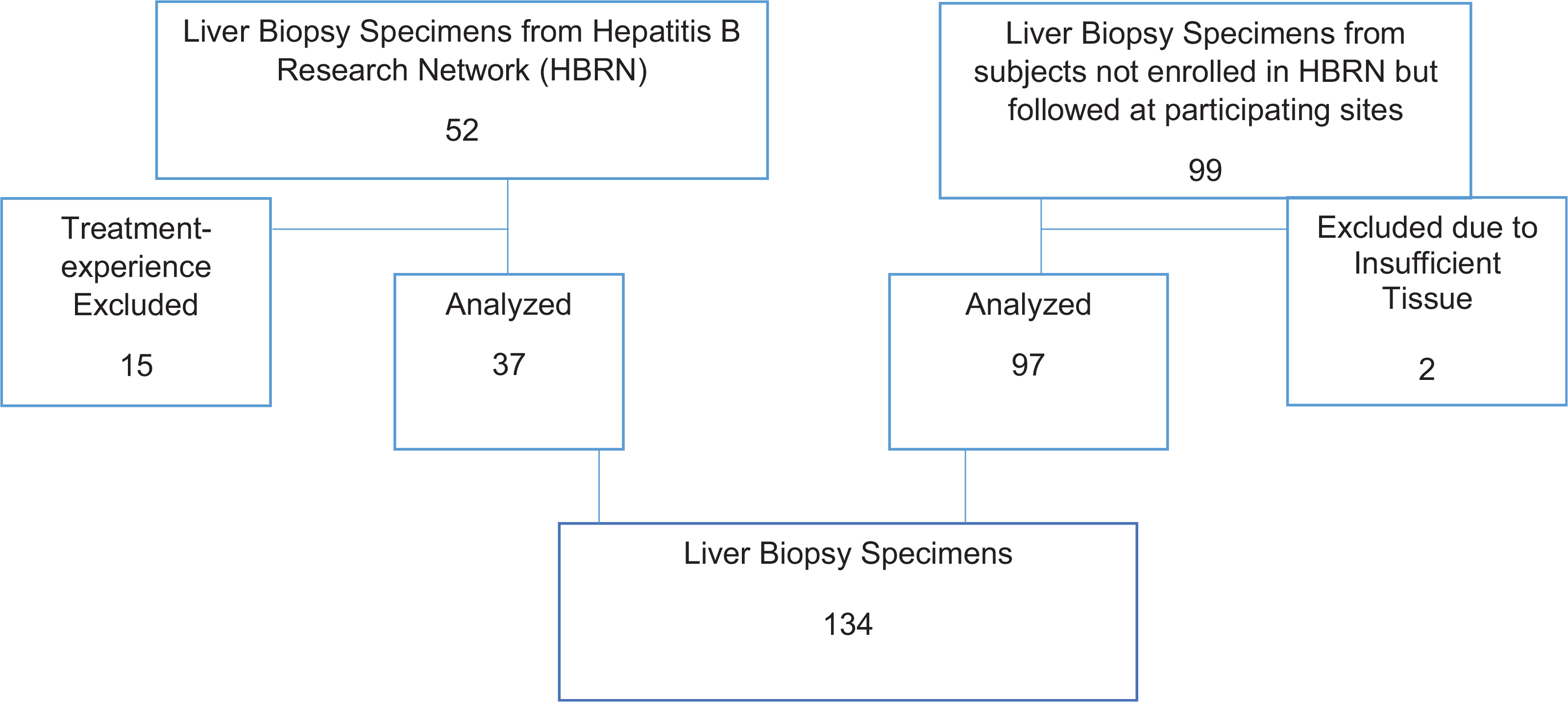
Liver biopsy specimens.
Specimens stained with hematoxylin-eosin and Masson’s trichrome were examined in all cases and scored for inflammation and fibrosis using the Ishak (7) and Metavir (8) scoring systems. Other histological features, such as the presence of ground glass cells were noted when identified.
Statistical Analysis
Variables were assessed for normality using histograms and Quantile-Quantile plots (QQ plots). Associations between continuous functions and histological data, such as inflammation, fibrosis, and presence of ground glass cells were assessed using Spearman correlation. All P values were 2-sided, with P < 0.05 considered statistically significant. SAS 9.4 (SAS Institute Inc., Cary, NC) was used for all analysis.
RESULTS
Patient Characteristics
Of the 134 subjects whose liver biopsies were reviewed, 51% were male (Table 1). The mode of transmission was assigned by the site investigator based on the information obtained from caregivers: 60% of subjects were assessed to have acquired their infection vertically, and in 37%, the mode of transmission was unclear. In our HBRN cohort of 371 subjects, 190 were adopted (51%). Information regarding mode of transmission is unclear for some adopted subjects, which account for the large number of unknown. At the time of the biopsy, the subjects ranged from 1 to 22 years of age, with a mean of 9.1. Similarly, the mean duration of infection in relation with the time when the liver biopsy was obtained was 9 years (range: 1–22 years) for those in whom mode of transmission was known. Sixty-nine percentage of the subjects were HBeAg-positive, 8% were HBeAg-negative, and in 23% the HBeAg status was unknown at the time of biopsy. HBsAg was positive in 72% of the subjects, negative in 1%, and unknown in 27%. Subjects with unknown HBeAg and HBsAg had confirmation of hepatitis B infection based on the identification of the HBV genotypes and/or HBV DNA levels. There was a wide distribution of HBV genotypes (A: 4%: B: 47%; C: 31%; D: 11%; E: 7%). Mean HBV DNA levels were 7.70, (range 0–9.85 log IU/mL). At the time of the biopsy, serum ALT ranged from 16 to 2878 U/L (median 120, interquartile range 68–199). The normal ranges for ALT and AST used in this study were as follows: ALT (13–25) and AST (13–25) for both male and female individuals.
TABLE 1.
Patient characteristics, N = 134
| Frequency (%) or median [25th to 75th percentile] | |
|---|---|
| Age at biopsy, years | 8 [4–13] |
| Male, % | 68 (51) |
| Mode of transmission | |
| Vertical | 81 (60) |
| Unknown | 50 (37) |
| Horizontal | 2 (2) |
| Medical/surgical | 1 (1) |
| Duration of infection, years, N = 71 | 9 [4–13] |
| HBeAg status | |
| Positive | 93 (69) |
| Negative | 10 (8) |
| Unknown* | 31 (23) |
| HBsAg status | |
| Positive | 97 (72) |
| Negative | 1 (1) |
| Unknown* | 36 (27) |
| HBV genotypes, N = 55 | |
| A | 2 (4) |
| B | 26 (47) |
| C | 17 (31) |
| D | 6 (11) |
| E | 4 (7) |
| HBV DNA (×108), N = 89 | 1.1 [0.5–2.4] |
| ALT, IU/L, N = 126 | 120 [68–199] |
| APRI, N = 78 | 0.9 [0.5–1.5] |
| FIB-4, N = 78 | 0.3 [0.2–0.5] |
AST/APRI score, aspartate aminotransferase/platelet ratio index, FIB-4, fibrosis-4 index; HBeAg, hepatitis B envelope antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HBV DNA, hepatitis B virus deoxyribonucleic acid; ALT, alanine aminotransferase.
Subjects with unknown HBeAg and HBsAg had confirmation of hepatitis B infection based on the identification of HBV genotypes and/or HBV DNA levels.
Histological Findings
The degree of inflammation seen in the liver biopsies ranged from none to severe inflammation whereas the presence of fibrosis stage ranged from none to cirrhosis (Fig. 2). Using the Ishak modified histology activity index scoring system, interface hepatitis was mild in 31% (score 1), moderate in 61% (score 2, 39%; score 3, 22%), severe in 6% (score 4), and not seen in 2% (score 0). Confluent necrosis was absent in 85% of cases, 3% had focal necrosis, 9% had zone 3-limited necrosis, and 3% had necrosis extending beyond zone 3.
FIGURE 2.
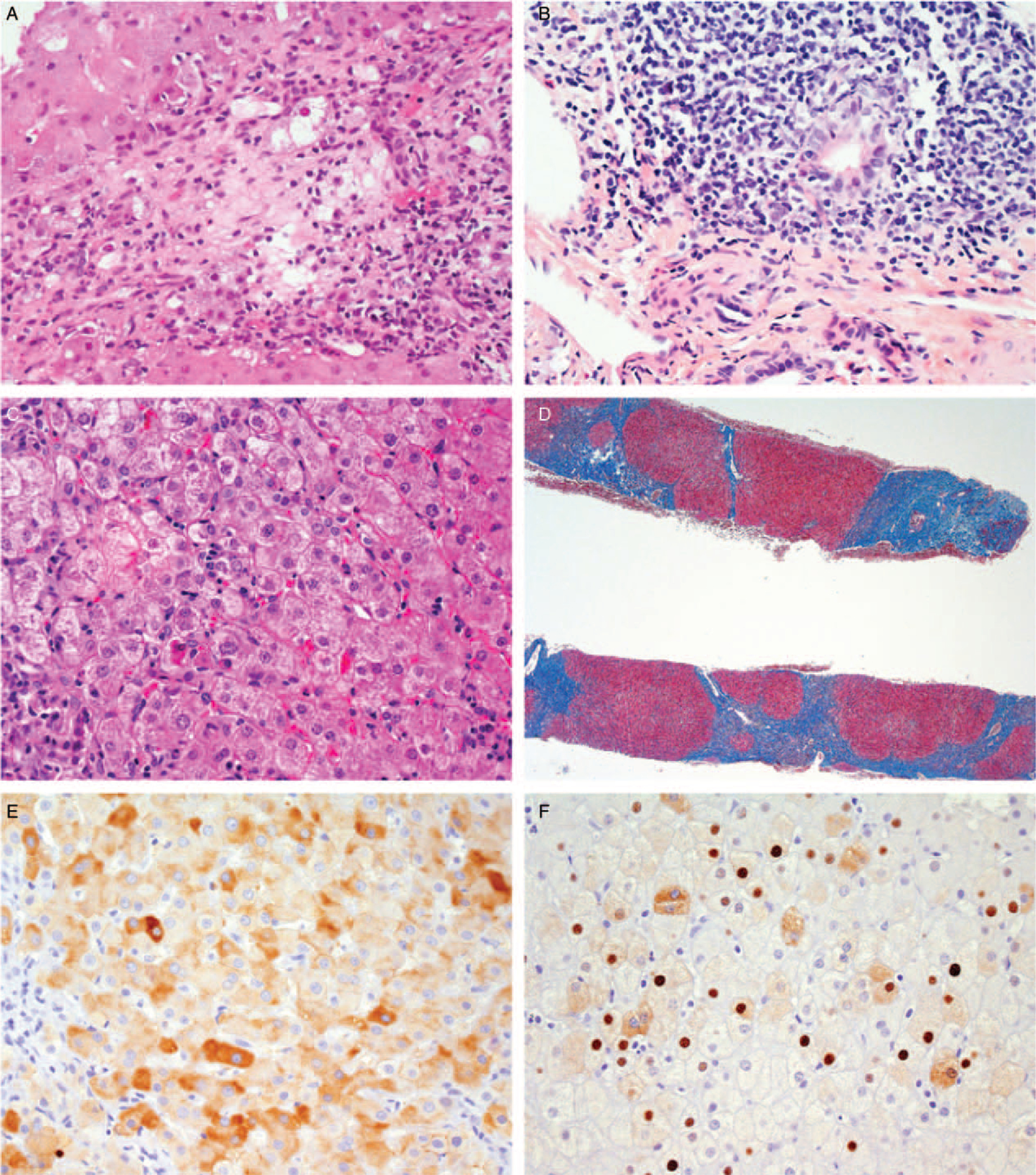
Features of chronic hepatitis B in children. A. Portal inflammation with extensive interface hepatitis (H&E, 400x). B. Portal lymphoid aggregate with Poulsen-type duct lesion. (H&E, 400x). C. Lobular inflammation with acidophil bodies. (H&E, 400x). D. Cirrhosis with small regenerative nodules. (Masson trichrome, 40x). E. Immunohistochemical stain for hepatitis B surface antigen showing cytoplasmic and membrane staining (400x). F. Immunohistochemical stain for hepatitis B core antigen showing nuclear and cytoplasmic staining. (400x).
Lobular inflammation was mild (score 1, 22%; score 2, 32%) in 54%, moderate (score 3) in 29%, and marked (score 4) in 17% (Supplemental Figure 1, Supplemental Digital Content, http://links.lww.com/MPG/B808). Portal inflammation was not present in 1% (score 0), mild in 38% (score 1), and moderate in 61% (score 2, 53%; score 3, 8%) of subjects. No fibrosis was seen in 18%, portal expansion was present in 59%, and 23% had advanced fibrosis consisting of bridging fibrosis in 19% and cirrhosis in 4%. Steatosis was not observed in this population (96% with no or <5% of hepatocytes that stained for fat). Ground glass cells were observed in only 28% of the biopsies. Immunostaining was positive for core antigen in 80% and for surface antigen in 77% of biopsies.
Association Between Histology and Clinical Variables
Serum ALT was moderately correlated with hepatic inflammation (ρ = 0.53, P < 0.0001) and was loosely associated with fibrosis stage (ρ = 0.33, P = 0.001). The same association was observed with APRI (inflammation: ρ = 0.61, P < 0.0001; fibrosis: ρ = 0.34, P = 0.005) (Fig. 3). FIB4 had a low correlation with inflammation (ρ = 0.34, P = 0.005) and no correlation with fibrosis. No associations were found between fibrosis stage and duration of infection, age at biopsy, or HBV DNA level. For the association between fibrosis and duration of infection, our correlation coefficient was 0.041, P = 0.748. For the association between fibrosis and age at biopsy, our correlation coefficient was 0.009, P = 0.925 (Supplemental Figure 2, Supplemental Digital Content, http://links.lww.com/MPG/B808). There was no association between genotype and inflammation or fibrosis.
FIGURE 3.
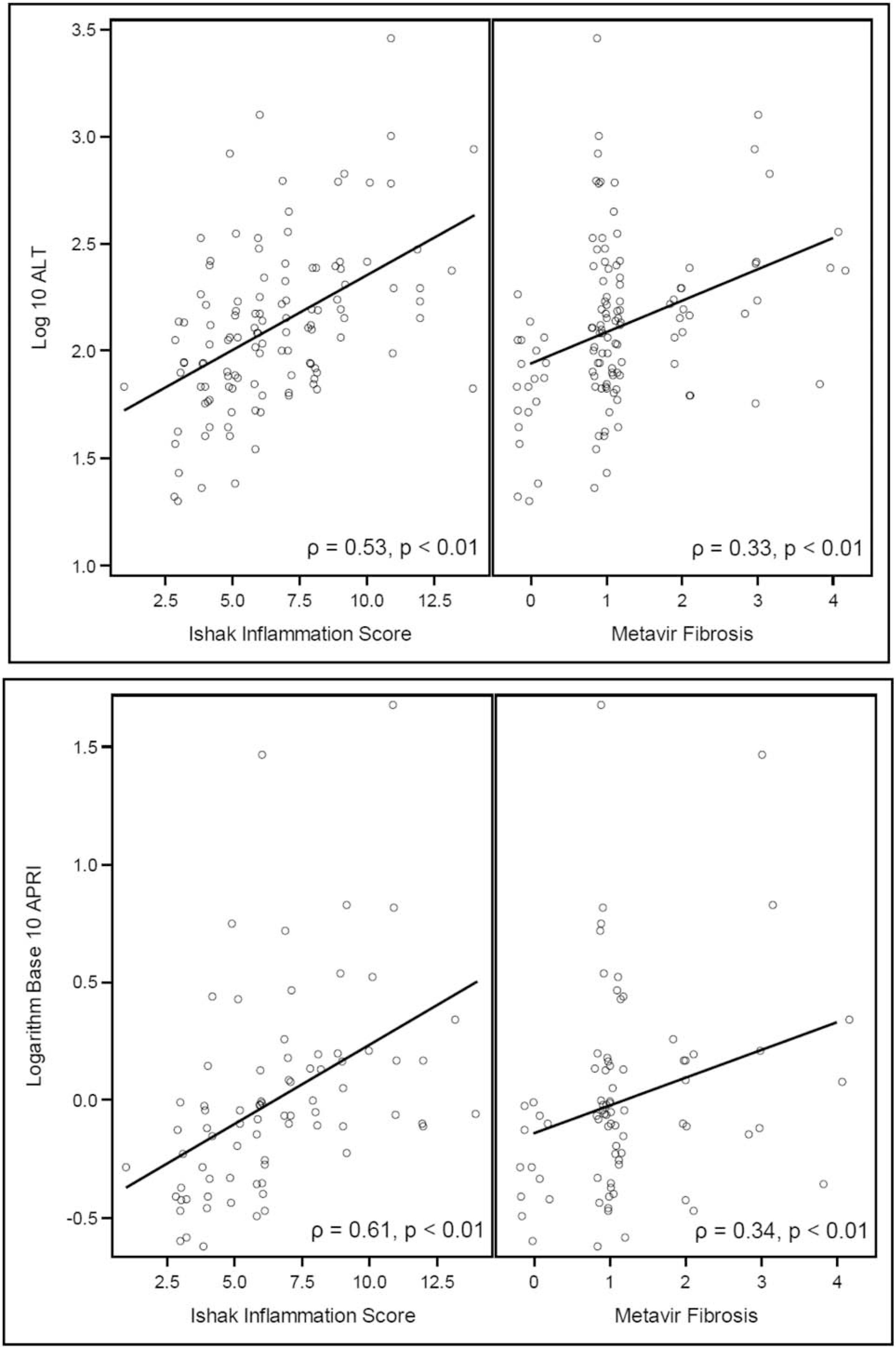
Correlation between alanine aminotransferase (ALT) and aspartate aminotransferase-to-platelet ratio index score (APRI) with inflammation, and fibrosis.
DISCUSSION
This large cohort of children with chronic hepatitis B infection in the United States and Canada revealed that among children undergoing liver biopsy for clinical indications, moderate inflammation is seen in a large number of the children chronically infected with HBV living in North America. Greater hepatic inflammation was found to correlate with higher serum ALT levels. There was, however, not significant correlation between the presence and degree of inflammation and subject age or the duration of infection. This is in contrast with findings in adults in which studies have shown an association of age over 40 years and duration of disease with advanced fibrosis and significant inflammation (9). Fibrosis was present in most specimens (82%) and correlated with hepatic inflammation and serum ALT, but not with subject age, duration of infection, or HBV DNA levels. The lack of correlation of fibrosis with age or duration of infection may be because of the relatively short duration of infection, or to the dynamic nature of HBV with patients transiently experiencing a flare of hepatitis with long periods of inactivity. Although cirrhosis is generally believed to be rare in children with chronic HBV, it does occasionally develop during childhood. In one of the largest studies, 292 children with HBsAg-positive HBV infection and elevated serum ALT levels (10), cirrhosis was found in 10 patients (3%) at a mean age of 4.0 ± 3.3 years. The children with cirrhosis in this series (10), however, had a higher prevalence of HDV co-infection compared with those without cirrhosis, which may have contributed to disease progression. In our cohort, only 5 children had cirrhosis (4%), and none of them had coinfection with HDV or HCV. Additionally, development of moderate or severe fibrosis is relatively common in childhood. In a series of 76 HBeAg-positive HBV children and elevated serum ALT, at least 50% had moderate-to-severe fibrosis (11). These findings suggest that many individuals with perinatally acquired chronic HBV are at risk for developing cirrhosis in the long-term, even during childhood. In our cohort, most of the subjects were vertically infected and had at least some degree of fibrosis.
HBsAg retained in hepatocytes may cause the cytoplasm to have a “ground-glass” appearance (3), and this feature is considered a sign of chronic infection. The presence of ground glass cells was seen in less than 1/3 of our subjects and, as expected, correlated with HBV DNA levels and positive staining for HBsAg, but inversely with ALT in this pediatric cohort. Furthermore, there was no correlation between ground glass cells and fibrosis. The inverse correlation of ground glass cells/HBV DNA with ALT likely reflects what is commonly seen in a majority of pediatric patients with chronic HBV: normal ALT and high viral levels.
Steatosis was not a predominant feature in the biopsies in our cohort. This finding contrasts with what has been described in children with chronic hepatitis C infection (12). One of the limitations of our study is that we were not able to establish a correlation between the presence of steatosis in liver biopsy and obesity as we only have BMI z-scores for a small number of subjects (37/134).
The FIB-4 index (13), a noninvasive panel initially developed to stage liver disease in individuals with chronic hepatitis C virus/HIV co-infection, has been validated for other liver diseases, such as non-alcoholic fatty liver disease (14). It has also been validated in adult patients with chronic HBV infection. A retrospective study of 1168 Chinese patients with chronic HBV infection showed that FIB-4 had a sensitivity of 94% and specificity of 46% distinguishing between patients with mild fibrosis and extensive fibrosis (15). FIB-4 has been studied in pediatric patients including those with cystic fibrosis liver disease (16). Contrary to the results seen in adults with liver disease including those with HBV, in our study, FIB-4 did not correlate with fibrosis, but it did correlate with inflammation (Supplemental Figure 3, Supplemental Digital Content, http://links.lww.com/MPG/B808). This correlation with inflammation could be because most of our subjects had a high ALT, which is one of the parameters used to determine this index. FIB-4 has a strong correlation in the presence of cirrhosis, and children generally have mild fibrosis.
The APRI score was developed to predict significant fibrosis and cirrhosis in HCV (17). APRI has been studied in children infected with hepatitis B and C (18–20), biliary atresia (21,22), and cystic fibrosis liver disease (16). In our pediatric HBV-chronically infected cohort, APRI correlated with both inflammation and fibrosis (Fig. 3). Additionally, on the basis of our results, APRI appears to be superior to FIB-4 in predicting fibrosis in children with chronic HBV infection. This finding is similar to the one reported in children with cystic fibrosis liver disease (16). Even though most of the liver biopsies were obtained from children with persistent elevation of serum ALT, it is important to recognize that there is a significant correlation of inflammation and fibrosis with serum ALT at time of the biopsy. Providing close monitoring of patients in the immunetolerant phase is recommended as they can develop elevation of ALT at any time.
In a study from the HBRN, our group described the distribution of HBV genotypes in children in the United States and Canada (23). Among the 343 children and adolescents with chronic HBV (age range of 1–18 years), the majority had HBV genotypes B or C (43% and 32%, respectively). The others had genotype A (5%), D (16%), or E (4%). This wide distribution of genotypes is similar to the genotype distribution in this report. No correlation was identified between genotype and degree of inflammation or fibrosis.
CONCLUSIONS
Immune-active chronic HBV may result in significant inflammation and fibrosis in children with elevated ALT and HBV DNA. Cirrhosis can be seen in childhood supporting close monitoring of HBV-infected children. APRI was shown to correlate with inflammation and fibrosis and may be a useful scoring system to monitor children with chronic HBV. Although FIB-4 correlated with inflammation, it did not correlate with fibrosis; therefore, it is not recommended for monitoring in children. Serum ALT is a good indicator of the severity of hepatic inflammation and extent of fibrosis.
Supplementary Material
What Is Known.
Chronic hepatitis B infection can result in hepatic inflammation and fibrosis and lead to end-stage liver disease.
What Is New?
Our large cohort identified serum alanine aminotransferase as a good indicator of the severity of hepatic inflammation and the extent of fibrosis in children chronically infected with hepatitis B virus.
Aspartate aminotransferase/platelet ratio index is a useful scoring system that correlates with inflammation and fibrosis in children with chronic hepatitis B infection.
Although cirrhosis is rare in children with chronic hepatitis B infection, it does occasionally develop during childhood.
Acknowledgments:
The authors would like to acknowledge the Hepatitis B Research Network (HBRN) Pathology committee and the study coordinators at the HBRN pediatric sites. The authors also acknowledge the use of HBRN samples and data as the sole contribution of the HBRN.
The Hepatitis B Research Network (HBRN) is funded by a U01 grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to the following investigators: U01 DK082916 (to PIs K.B.S., K.F.M., and N.R.-B.); U01 DK082874 (Harry Janssen; PI: S.C.L.); U01 DK082944 (to Norah Terrault; PI: P.R.); U01 DK082843 (to Lewis R. Roberts; PI: S.J.S.), U01 DK082871 (to Adrian Di Bisceglie; PI: J.H.T.). This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Conflicts of Interests: N.R.-B. receives research funding from Gilead. K.F.M. consults for Gilead and Albireo. S.C.L. receives research funding from Abbvie and Bristol-Myers Squibb. P.R. receives research funding from Abbvie, Gilead, Bristol-Myers Squibb, Roche/Genentech, Merck, Retrophin, and consults for Gilead, Abbvie, Intercept, Retrophin, Albireo, Audentes, Dicerna, and Mirum. K.B.S. receives research funding from Gilead, Bristol-Myers Squibb, Roche/Genentech, and consults for Gilead, Roche/Genentech, and Up to Date. S.J.S. receives research funding from the Cystic Fibrosis Therapeutic Disease Network, and the Cystic Fibrosis Foundation. J.T. receives research funding from Gilead, Alnylam Inc., Arrowhead Pharmaceuticals and Dicerna Inc., and consultant relationships with BioMarin, Editas, Proteostasis, and Retrophin. The remaining authors report no conflicts of interest.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
REFERENCES
- 1.MacLachlan JH, Locarnini S, Cowie BC. Estimating the global prevalence of hepatitis B. Lancet 2015;386:1515–7. [DOI] [PubMed] [Google Scholar]
- 2.Das P, Ahuja A, Gupta SD. Overview of the histopathology of chronic hepatitis B infection. Hepatitis B Annual 2012;9:49–85. [Google Scholar]
- 3.Mani H, Kleiner DE. Liver biopsy findings in chronic hepatitis B. Hepatology 2009;49(5 Suppl):S61–71. [DOI] [PubMed] [Google Scholar]
- 4.Perz JF, Armstrong GL, Farrington LA, et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006;45:529–38. [DOI] [PubMed] [Google Scholar]
- 5.Iorio R, Giannattasio A, Cirillo F, et al. Long-term outcome in children with chronic hepatitis b: a 24-year observation period. Clin Infect Dis 2007;45:943–9. [DOI] [PubMed] [Google Scholar]
- 6.Ghany MG, Perrillo R, Li R, et al. Characteristics of adults in the Hepatitis B Research Network in North America reflect their country of origin and hepatitis B virus genotype. Clin Gastroenterol Hepatol 2015;13:183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishak KG, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696–999. [DOI] [PubMed] [Google Scholar]
- 8.Bedossa P, Poynard T. The French METAVIR Cooperative Study Group. An algorithm for the grading of activity in chronic hepatitis C.Hepatology 1996;24:289–93. [DOI] [PubMed] [Google Scholar]
- 9.Cadranel JF, Lahmek P, Causse X, et al. Epidemiology of chronic hepatitis B infection in France: risk factors for significant fibrosis–results of a nationwide survey. Aliment Pharmacol Ther 2007;26:565–76. [DOI] [PubMed] [Google Scholar]
- 10.Bortolotti F, Calzia R, Cadrobbi P, et al. Liver cirrhosis associated with chronic hepatitis B virus infection in childhood. J Pediatr 1986;108: 224–7. [DOI] [PubMed] [Google Scholar]
- 11.Godra A, Perez-Atayde AR, Jonas MM. Histologic features of chronic hepatitis B in children. Hepatology 2005;42(Suppl 1):478–9. [Google Scholar]
- 12.Goodman ZD, Makhlouf HR, Liu L, et al. Pathology of chronic hepatitis C in children: liver biopsy findings in the Peds-C Trial. Hepatology 2008;47:836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterling RK, Lissen E, Clumeck N, et al. , APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43: 1317–25. [DOI] [PubMed] [Google Scholar]
- 14.McPherson S, Stewart SF, Henderson E, et al. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010;59:1265–9. [DOI] [PubMed] [Google Scholar]
- 15.Ma J, Jiang Y, Gong G. Evaluation of seven noninvasive models in staging liver fibrosis in patients with chronic hepatitis B virus infection. Eur J Gastroenterol Hepatol 2013;25:428–34. [DOI] [PubMed] [Google Scholar]
- 16.Leung DH, Khan M, Minard CG, et al. Aspartate aminotransferase to platelet ratio and fibrosis-4 as biomarkers in biopsy-validated pediatric cystic fibrosis liver disease. Hepatology 2015;62:1576–83. [DOI] [PubMed] [Google Scholar]
- 17.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518–26. [DOI] [PubMed] [Google Scholar]
- 18.Jin W, Lin Z, Xin Y, et al. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis B-related fibrosis: a leading meta-analysis. BMC Gastroenterol 2012;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology 2011;53: 726–36. [DOI] [PubMed] [Google Scholar]
- 20.McGoogan KE, Smith PB, Choi SS, et al. Performance of the AST-to-platelet ratio index as a noninvasive marker of fibrosis in pediatric patients with chronic viral hepatitis. J Pediatr Gastroenterol Nutr 2010;50:344–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grieve A, Makin E, Davenport M. Aspartate aminotransferase-to-platelet ratio index (APRI) in infants with biliary atresia: prognostic value at presentation. J Pediatr Surg 2013;48:789–95. [DOI] [PubMed] [Google Scholar]
- 22.Kim SY, Seok JY, Han SJ, et al. Assessment of liver fibrosis and cirrhosis by aspartate aminotransferase-to-platelet ratio index in children with biliary atresia. J Pediatr Gastroenterol Nutr 2010;51:198–202. [DOI] [PubMed] [Google Scholar]
- 23.Schwarz KB, Cloonan YK, Ling SC, et al. Children with Chronic Hepatitis B in the United States and Canada. J Pediatr 2015;167:1287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


