Abstract
The global eradication of poliomyelitis, believed to be achievable around the year 2000, relies on strategies which include high routine immunization coverage and mass vaccination campaigns, along with continuous monitoring of wild-type virus circulation by using the laboratory-based acute flaccid paralysis (AFP) surveillance. Israel and the Palestinian Authority are located in a geographical region in which poliovirus is still endemic but have been free of poliomyelitis since 1988 as a result of intensive immunization programs and mass vaccination campaigns. To monitor the wild-type virus circulation, environmental surveillance of sewage samples collected monthly from 25 to 30 sites across the country was implemented in 1989 and AFP surveillance began in 1994. The sewage samples were processed in the laboratory with a double-selective tissue culture system, which enabled economical processing of large number of samples. Between 1989 and 1997, 2,294 samples were processed, and wild-type poliovirus was isolated from 17 of them in four clusters, termed “silent outbreaks,” in September 1990 (type 3), between May and September 1991 (type 1), between October 1994 and June 1995 (type 1), and in December 1996 (type 1). Fifteen of the 17 positive samples were collected in the Gaza Strip, 1 was collected in the West Bank, and 1 was collected in the Israeli city of Ashdod, located close to the Gaza Strip. The AFP surveillance system failed to detect the circulating wild-type viruses. These findings further emphasize the important role that environmental surveillance can play in monitoring the eradication of polioviruses.
The last paralytic poliomyelitis cases occurred in Israel during an outbreak in 1988 (32). A general campaign of mass vaccination of the population under the age of 40 and enhanced combined inactivated and oral poliovirus vaccine vaccination programs for infants and young children, which have been implemented in the Israeli and the Palestinian populations since 1990, resulted in elimination of the disease. A high level of vaccination coverage has been continuously maintained since then (13, 33, 35).
Because Israel and the Palestinian Authority are both located in the same geographical region of endemicity (1, 2, 27, 37), the possibility of a new introduction of wild-type poliovirus always exists. The virus can circulate in a well-vaccinated population (7, 16, 19, 26, 27, 32, 33) with or without clinical cases. Therefore, eradication of the virus can only be proven if, in addition to a lack of poliomyelitis cases, the results of continuous surveillance for wild-type virus are negative. The World Health Organization (WHO) strategy for surveillance is to identify acute flaccid paralysis (AFP) cases and their contacts (6). However, this is not the only way to monitor poliovirus circulation in large populations. Isolation of wild-type polioviruses from sewage samples has been shown to reflect its circulation in the community both qualitatively and quantitatively and has been used for evaluation of the effectiveness of immunization or for epidemiological investigations (7, 15, 16, 19, 22, 25, 26, 34). Epidemiological links can be established by molecular analysis of the virus isolates, either by restriction fragment length polymorphosis analysis or by sequencing of the VP1 gene and/or the VP1-2A junction (1, 3, 9, 19, 23, 28, 36). As many countries are becoming free of poliomyelitis due to extensive vaccination programs (17), the monitoring of the inapparent circulation of the virus is becoming critical (6). AFP surveillance is not always applicable or effective (6, 29), and environmental surveillance offers an alternative (19).
Environmental surveillance for poliovirus in sewage samples has been implemented since 1989 in Israel, the Gaza Strip, and the West Bank (later the Palestinian Authority), following the outbreak of poliomyelitis which occurred in 1988. It involves the monthly collection of sewage samples in 25 to 30 locations throughout the country, which are then submitted to the laboratory for analysis. To overcome problems of high background from nonpolio enteroviruses and vaccine poliovirus we have developed a double-selective protocol, which includes propagation of virus isolates in HEp-2 cells (selection against nonpolio enteroviruses) at 40°C (selection against vaccine poliovirus). This protocol obviated the need to analyze irrelevant isolates and allowed us to effectively screen thousands of sewage samples without much effort (20, 31). In addition, AFP surveillance has been implemented since 1994. We report the results of the environmental surveillance program between February 1989 and February 1998, which detected four separate episodes of wild-type poliovirus circulation in the years 1990, 1991, 1994 to 1995, and 1996. During these years there were no AFP cases due to poliomyelitis.
MATERIALS AND METHODS
Collection of sewage samples.
Raw sewage samples were collected from the central sewage treatment facilities of 36 communities throughout the country, including 17 Jewish towns, 10 Arab towns, 6 towns with mixed population, and 3 Arab refugee camps.
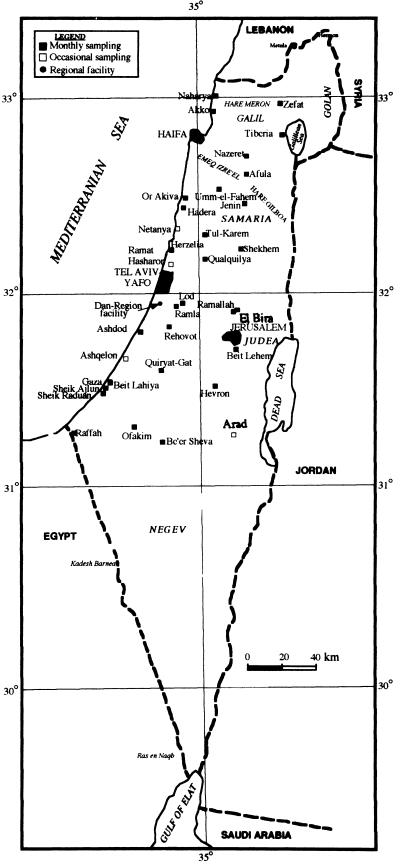
The samples were collected by local authorities by either a 24-h automatic composite sampling or manual “grab” sampling every half hour during peak capacity. Alternatively, a gauze pad was placed in the sewage stream for 24 to 48 h. The sewage (1.5 to 2.0 liters) or the soaked gauze pads were transferred to the laboratory and kept at 4°C until they were treated. Table 1 shows a list of the sampling sites, and Fig. 1 shows their locations.
TABLE 1.
Sewage sampling sites, population type, and sampling methods used
| Site | District | Population | Sampling methoda |
|---|---|---|---|
| Akko | North | Jewish and Arab town | a |
| Zefat | North | Jewish town | b |
| Naharya | North | Jewish town | a |
| Tiberia | North | Jewish town | b |
| Nazeret | North | Jewish and Arab town | b |
| Afula | North | Jewish town | a |
| Haifa | Haifa | Jewish and Arab town | a |
| Umm-El-Fahem | Haifa | Arab town | b |
| Hadera | Haifa | Jewish town | b |
| Or-Akiva | Haifa | Jewish town | b |
| Netanya | Center | Jewish town | b |
| Lod | Center | Jewish and Arab town | c |
| Ramla | Center | Jewish and Arab town | c |
| Rehovot | Center | Jewish town | c |
| Herzlia | Tel Aviv | Jewish town | a |
| Ramat-Hasharon | Tel Aviv | Jewish town | b |
| Dan region facility | Dan metropolitan | Jewish town | a |
| Ashdod | Ashquelon | Jewish town | c |
| Ashquelon | Ashquelon | Jewish town | b |
| Quiryat-Gat | Ashquelon | Jewish town | c |
| Ofakim | South | Jewish town | b, c |
| Beer-Sheva | South | Jewish town | b |
| Arad | South | Jewish town | b |
| Jenin | Samaria | Arab town | b |
| Tul-Karem | Samaria | Arab town | b |
| Shekhem | Samaria | Arab town | b |
| Qualquilya | Samaria | Arab town | b |
| Jerusalem | Jerusalem | Jewish and Arab town | a, b |
| Ramallah | Judea | Arab town | b |
| Beit-Lehem | Judea | Arab town | b |
| Hebron | Judea | Arab town | b |
| Elbira | Judea | Refugee camp | b |
| Beit-Lahiya | Gaza | Arab town and refugee camp | b |
| Sheik-Raduan | Gaza | Arab town | b |
| Sheik-Ajlun | Gaza | Arab town and refugee camp | b |
| Raffah | Gaza | Arab town and refugee camp | b |
a, automatic composite sample; b, manual composite sample; c, gauze pad sample (24 to 48 h).
FIG. 1.
Sites and frequency of sewage sampling in Israel and the Palestinian Authority. ■, monthly sampling; □, occasional sampling; ●, regional sewage treatment facility (monthly sampling).
Extraction of virus particles from sewage samples.
Sewage samples were allowed to settle for at least 24 h at 4°C. Most of the top aqueous phase was discarded, and the bottom 250 ml, including sediment, was retained. Virus extraction was performed as initially described by Berg et al. (4, 5). Twenty milliliters of Freon 113, 30 ml of glycin buffer (pH 9.0), and 0.5 g of bentonite were added to each sewage sample, which was then homogenized in a blender for 1 to 2 min at low speed and sedimented for 20 min in a cooled centrifuge at 4,000 rpm (6,000 × g). One hundred milliliters of the supernatant was mixed with 4 ml of 10× concentrated tissue culture medium M199 (final medium concentration, 0.4×) supplemented with antibiotics (final concentrations, 400 U of penicillin/ml, 0.8 mg of streptomycin/ml 50 U of micostatin/ml, and 0.5 mg of neomycin/ml). These 100-ml samples were retained and used to inoculate tissue cultures (see below), while the rest of the sample was discarded. If no viruses were isolated, a concentration step was added. Forty milliliters of the sample was ultracentrifuged at 4°C in a Beckman L7 ultracentrifuge, using a SW28 rotor at 27,000 rpm (150,000 × g). The pellet was resuspended in 2 ml of M199 medium containing antibiotics and was used to reinoculate tissue cultures. Sewage samples collected on gauze pads were placed in containers with 100 to 200 ml of saline (enough to cover the pad), 20 ml of Freon 113, 30 ml of Glycin buffer (pH 9.0), and 0.5 g of bentonite. The containers were shaken vigorously for 30 min, the gauze pads were removed, and the solutions were transferred into other containers. One hundred milliliters was then treated in the same way as the other types of samples following the homogenization.
Cell lines. (i) BGM.
(Buffalo green monkey) cells (10) were maintained in M199 medium containing 10% fetal calf serum (FCS). Cell monolayers were grown either in 10-cm-diameter dishes or in Bellco glass tissue culture tubes and were used for virus isolation or neutralization assays.
(ii) HEp-2.
(Human larynx carcinoma) cells (38) were plated at 2 × 105/ml in tissue culture tubes and grown in Eagle’s minimal essential medium containing 10% FCS at 37°C to form monolayers. The final concentrations of antibiotics added to all tissue cultures were as follows: penicillin, 160 U/ml; streptomycin, 0.32 g/ml; and mycostatin, 20 U/ml.
Virus strains.
Wild-type 1 (Mahoney), 2 (MEF-1), and 3 (Saukett) and the vaccine (Sabin) poliovirus strains were obtained from Radu Crainic, Pasteur Institute, Paris, France. The enteroviruses coxsackie A21, coxsackie B3, echo 3, echo 7, echo 9, and echo 30 were field isolates obtained from sewage samples and identified with Lim-Benyesh-Melnick antibody pools (12) by microneutralization assays (see below).
Isolation of wild-type poliovirus by the double-selection protocol.
Three 10-cm-diameter tissue culture plates with BGM monolayers were used for each sewage sample. The medium was removed and replaced with 4.5 ml of the treated sewage sample per plate. Following adsorption for 1 h in a 37°C incubator under 5% CO2, the samples were removed and the monolayers were covered with Bactoagar (L11; Oxoid) containing 2× M199, antibiotics, and 2% FCS. The plates were incubated at 37°C under 5% CO2 for 24 h, after which growth medium containing 0.02% neutral red was added for 40 to 60 min. The medium with stain was removed, and the plates were further incubated at 37°C and 5% CO2 for an additional 24 h, after which virus plaques were visualized.
Plaques were collected in 0.2 ml of M199 medium and transferred into HEp-2 cells in tissue culture tubes for immediate selective growth conditions or stored at −20°C for future analysis.
To select for wild-type poliovirus, HEp-2 cells, which are highly permissive for polioviruses but much less so for other enteroviruses (18, 38), were incubated for 5 days with 5% CO2 at 40°C, a temperature which selects against growth of vaccine poliovirus (24). Tubes showing cytopathic effect were then subpassaged in BGM cultures at 37°C and 5% CO2 to obtain high-titer viral stocks for characterization by microneutralization assays.
This double-selective system was developed during 1991 (20). Before its introduction we used BGM cells at 40°C and selected only against vaccine poliovirus, not against other enteroviruses. The selective power of the new protocol reduced the number of plaques requiring analysis by microneutralization assays by 88%. The sensitivity of this protocol was assessed by spiking 10 sewage samples with known amounts of wild-type poliovirus and processing them. The recovery rates obtained ranged between 5 and 14%, and the detection limit calculated per sewage sample ranged between 18 and 50 PFU per sewage sample. Toxicity or inhibitory effects were rare, as judged by the appearance of numerous enterovirus plaques in every sample. Continuous parallel use of the same BGM and HEp-2 cells for poliovirus titration and neutralization assays with standard preparations assured the sensitivity of the cultures in use.
Microneutralization assays.
The microneutralization assays were carried out on BGM cells in 96-well tissue culture plates according to standard protocols recommended by the WHO (38). Polyclonal antibodies were used for virus typing, and monoclonal antibodies to vaccine poliovirus types were used for intratypic differentiation between wild-type and vaccine-type strains. The antibodies were supplied by Radu Crainic (9).
Sequence analysis of poliovirus isolates.
The VP1-2A junction region of the poliovirus type 1 genome was amplified by reverse transcription (RT)-PCR as follows: poliovirus RNA was prepared by the guanidinium thiocyanate method (8) from 250 μl of cell-free tissue culture supernatant from poliovirus-infected HEp-2 cells with TRI-LS reagent (MRC, Cincinnati, Ohio) according to the manufacturer’s instructions. This RNA was amplified by a single-step RT-PCR with generic degenerate primers Y7 (5′-GGI TTT GTG TCA GCI TGC AAT GA-3′) and Q8 (5′-AAG AGG TCT CTR TTC CAC AT-3′), which recognize sequences upstream of VP1 and downstream of the VP1-2a junction, respectively (28). Amplification reactions were done in 100-μl reaction mixtures containing RNA templates in 15 mM Tris (pH 9.0); 50 mM KCl; 2.5 mM MgCl2; 2.5 mM dithiothreitol; 0.1% Triton X-100 (Sigma); 2 mM (each) dATP, dTTP, dGTP, and dCTP (Pharmacia); 40 U of placental RNase inhibitor (Promega, Madison, Wis.); 5 to 25 U of avian myeloblastosis virus reverse transcriptase (Boehringer Mannheim); 10 U of Taq DNA polymerase (Boehringer Mannheim); and 200 ng each of sense and antisense primers. The reaction mixtures were overlaid with mineral oil (Sigma) and incubated in a thermal cycler at 45°C for 60 min for RT, denatured at 94°C for 3 min, and then amplified by 40 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 2 min. After a final elongation step at 72°C for 10 min, 10 μl of the RT-PCR mixture was analyzed by electrophoresis on horizontal 1% agarose gels (low-electroosmosis pronarose D1; Hispanagar s.a., Burgos, Spain) with ethidium bromide staining for the presence of the 1,100-bp sequence amplified with Y7 and Q8. The amplified cDNA was purified from the remainder of the reaction mixture by using QIAquick columns (Qiagen, Dusseldorph, Germany). For the highest yield, the RNA template and the antisense primer were premixed in 35 μl of aqueous solution, heated under oil at 95°C for 5 min, and slowly cooled to below 30°C before the addition of the remaining reaction mixture components. Both strands of the VP1-2A region of the amplified cDNA were directly sequenced on an Applied Biosystems (Foster City, Calif.) model 373 DNA automatic sequencing system (Biological Services, Weizman Institute, Rehovot, Israel) by using separate PRISM dye deoxy terminator cycle sequencing reactions (Applied Biosystems) with Q8 and a new internal primer, S14F (5′-GTG GTT AAT GAT CAC AAC CC-3′). The 150-bp VP1-2A sequences internal to primers S14F and Q8 were determined by comparative analysis of both complementary DNA strands with the SeqEd program (Applied Biosystems). The University of Wisconsin Genetics Computer Group gene analysis programs (11) were used for calculating the percent homology of nucleic acid and computer-generated amino acid sequences.
RESULTS
Isolation and identification of wild-type poliovirus from sewage between 1989 and 1997.
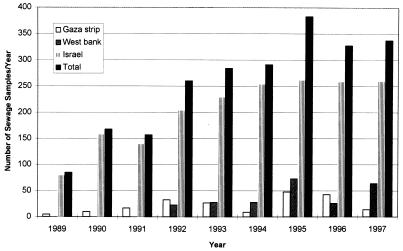
Between February 1989 and February 1997, a total of 2,294 sewage samples were submitted to the laboratory for analysis. The locations of the sampling sites, including the Gaza Strip and the West Bank, are shown in Fig. 1. The type of community, sewage system, and sampling method are shown in Table 1. The distribution of sewage samples collected each year from Israel, the West Bank (Samaria and Judea), and the Gaza Strip is shown in Fig. 2, which demonstrates the steady increase of the total number of samples over the years. The samples were processed as described in Materials and Methods, and wild-type poliovirus isolates were identified.
FIG. 2.
Annual distribution of sewage samples, 1989 to 1997. The numbers of sewage samples collected from three regions, the Gaza Strip, the West Bank (Judea and Samaria), and Israel, are shown separately, as well as the total number of samples collected.
A summary of all of the sewage samples from which wild-type poliovirus was isolated is shown in Table 2. In October 1990, poliovirus type 3 was isolated from Beit-Lahiya, an Arab town and refugee camp in the Gaza Strip. In May 1991 and again in September and October 1991, poliovirus type 1 was isolated from Beit-Lahiya. Three years later, between October 1994 and June 1995, wild-type virus was isolated from 13 samples. The first one was from Qualquilya (West Bank), followed by 11 positive samples, which were obtained monthly from various sites in the Gaza Strip (Beit-Lahiya, Raffah, Sheik Ajlun, and Sheik-Raduan) between December 1994 and April 1995, and, finally, a sample collected in June 1995 in the Jewish city of Ashdod, located not far from the Gaza Strip (Fig. 1). Eighteen months later, in December 1996, wild-type poliovirus type 1 was isolated from a sewage sample from Raffah, in the southern part of the Gaza Strip. Thus, there were four clusters of virus isolation between 1989 and 1997, which consequently were termed “silent outbreaks” because they were not followed by paralytic poliomyelitis cases.
TABLE 2.
Wild-type poliovirus isolates from sewage by date and location
| Date (mo/yr) | Site | District | Type of communitya | Polio type | Isolates sequenced |
|---|---|---|---|---|---|
| 10/90 | Beit-Lahiya | Gaza | AT + RC | 3 | |
| 5/91 | Beit-Lahiya | Gaza | AT + RC | 1 | |
| 9/91 | Beit-Lahiya | Gaza | AT + RC | 1 | isr91 |
| 10/91 | Beit-Lahiya | Gaza | AT + RC | 1 | |
| 10/94 | Qualquilya | Samaria | AT | 1 | isr94 |
| 12/94 | Beit-Lahiya | Gaza | AT + RC | 1 | |
| 12/94 | Rafah | Gaza | AT + RC | 1 | isr94b, -94c |
| 12/94 | Sheik-Ajlun | Gaza | AT + RC | 1 | |
| 1/95 | Beit-Lahiya | Gaza | AT + RC | 1 | |
| 1/95 | Sheik-Raduan | Gaza | AT | 1 | |
| 2/95 | Beit-Lahiya | Gaza | AT + RC | 1 | isr95c |
| 2/95 | Raffah | Gaza | AT + RC | 1 | isr95 |
| 3/95 | Beit-Lahiya | Gaza | AT + RC | 1 | isr95b |
| 4/95 | Beit-Lahiya | Gaza | AT + RC | 1 | |
| 6/95 | Ashdod | Ashquelon | JT | 1 | isr95d |
| 12/96 | Rafah | Gaza | AT + RC | 1 | isr96a, -96b |
AT, Arab town; RC, refugee camp; JT, Jewish town.
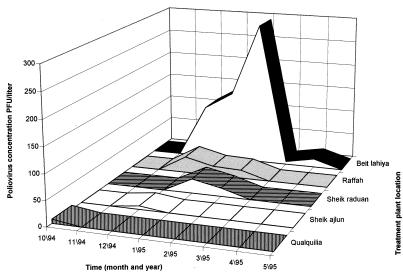
Quantitative evaluation of the virus circulation in 1994 and 1995.
Beit-Lahiya was the site from which wild-type poliovirus was most frequently isolated. In 1990 and 1991 it was the only site, and in 1994 and 1995 it was the source for 5 of the 12 positive samples. A quantitative analysis of the 1994–1995 silent outbreak is shown in Fig. 3. The number of PFU per liter of sewage sample from 12 of 13 positive samples from the 1994–1995 silent outbreak was calculated from the number of plaques obtained for each site. The amount of virus circulating in Beit-Lahiya was found to be substantially greater than that at all other sites. In Ashdod, the amount of virus was the least (data not shown).
FIG. 3.
Monthly number of wild-type polio plaques per liter of sewage sample (PFU/liter) determined for 12 positive samples collected in the Gaza Strip and Qualquilya during the 1994–1995 silent outbreak.
Characterization of the isolates by sequence analysis.
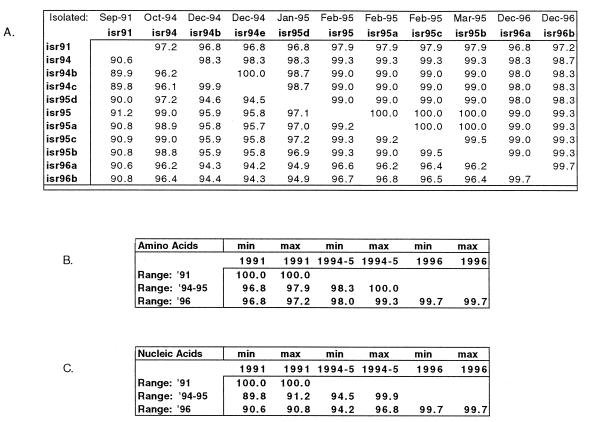
The wild-type poliovirus type 1 isolates were further analyzed by molecular methods to try to link them to each other and to previous local isolates. We have used the method developed by Rico-Hesse et al. (28) described in Materials and Methods. For the purpose of analysis, isolates were chosen from different years and months and from different locations throughout the 1994–1995 silent outbreak. In addition, isolates from the same site and date but from different plaques (Raffah 12/94 and Raffah 12/96) were also analyzed. The VP1-2A junction regions of all isolates were amplified by RT-PCR and sequenced by the method developed by Rico-Hesse et al. (28). The nucleic acid and amino acid sequences of the isolates were aligned, and the degree of homology was calculated (Fig. 4A). The differences in nucleotide sequence between poliovirus type 1 isolates of the 1991 and later outbreaks were in the range of 9 to 10%, the differences between the 1994 to 1995 and the 1996 isolates were in the range of 3 to 5%, and the differences among various isolates of the 1994–1995 outbreak were in the range of 0.1 to 5% (Fig. 4C). The differences in amino acid sequence ranged from 0 to 3.2% among all isolates analyzed (Fig. 4B). These data suggest that the 1994 to 1995 and 1996 viruses were distinct from the 1991 virus but could be related to each other.
FIG. 4.
Homology between the VP1-2A genes in various isolates. The sampling time (month-year) and location of collection of each isolate are indicated in Table 2. (A) Above diagonal, amino acid sequence homology; below diagonal, nucleic acid sequence homology. (B) Range of amino acid homologies among various isolates. (C) Range of nucleic acid homologies among various isolates. min, minimum; max, maximum.
DISCUSSION
Continuous surveillance of sewage for the presence of wild-type polioviruses has been performed throughout the country for the last 8 years in Israel and the Palestinian Authority. We have developed an efficient and economic selective system for virus isolation and identification which avoids the need for neutralization analysis of many enteroviral or vaccine poliovirus isolates. Currently we process hundreds of sewage samples per year, which yield thousands of plaques. The success of our system is evidenced by the identification of the four silent outbreaks between 1990 and 1996. The virus circulation was not detected during this time in any other way, including by active AFP surveillance, which has been implemented since 1994. This is in contradiction to the WHO approach, which relies on AFP surveillance as a sufficient means for detecting wild-type virus circulation. We are currently testing new concentration and detoxification protocols, as suggested by Shieh et al. (30), and working on improvement of our isolation and virus identification protocols.
Almost all of the isolates of wild-type virus were found in sewage of semiurban communities with low sanitary and socioeconomic conditions. However, a conclusion that the virus circulated only in such communities may be misleading. The isolation of virus in these communities but not in large urban communities with high socioeconomic conditions, such as large Israeli cities (Tel Aviv, Haifa, etc.) may be associated with the type of sewage system and the sewage content: in large treatment facilities there is high dilution of domestic sewage in other types of sewage, mixing of sewage from several communities, and toxicity due to industrial waste. It has been estimated (16a) that in industrialized communities it would be necessary for about 1% of the population to excrete virus in order for it to be detected in an unconcentrated sewage sample. Thus, in order to be effective, analysis of samples obtained from such facilities should include concentration and detoxification steps, which we did not do. In one case we isolated the virus from the sewage of a small Jewish city (Ashdod) following the extensive circulation of the virus in the neighboring Gaza strip. This case proves that the virus can be transmitted and can circulate not only in communities with low socioeconomic and sanitary conditions but also in those with high standards of living. It is not known why there were silent outbreaks in 1990 (type 3) and 1991 (type 1) when the population immunity was high, as was shown by serological surveys (14). However, the appearance and massive circulation of type 1 virus in 1994, which lasted throughout the first half of 1995, was coincidental with the return of thousands of Palestinians to the Gaza Strip from countries where the virus is endemic, some with low rates of vaccination coverage, following the establishment of the Palestinian Authority. The amount of circulating virus was very high, as was reflected by the large number of positive samples containing high concentrations of virus and thus yielding large numbers of plaques.
During the extensive circulation in the Gaza Strip, the virus spread into two additional sites, Qualquilya in the West Bank and Ashdod in southern Israel, but it was present in the sewage of these two cities for only a short period. National immunization days held by WHO in the Gaza Strip in 1995 apparently intercepted the virus circulation, leading to its temporary disappearance from the sewage.
Comparison of the nucleotide sequences of viruses between and within outbreaks suggests that the 1991 outbreak was a separate episode, while the link between the 1994 to 1995 outbreak and the 1996 isolate is not clear. The possibility that in 1994 and 1995 there were several separate introductions of wild-type virus has also been raised (31). A more detailed molecular analysis of the outbreak viruses has been performed (unpublished data) to resolve these questions. Interestingly, we found that the population immunity against the 1994 to 1995 strains is better than that against the 1988 strain (13), which suggests that the 1994 to 1995 virus is antigenically more similar to the vaccine strain and partially explains the lack of clinical cases. A comparative molecular analysis of the 1988 and 1991 to 1996 strains and the vaccine strain may shed light on this question, and it is under way.
Our experience demonstrates the high value of the sewage surveillance system as a tool in the global initiative to eradicate poliomyelitis. Today there are many more countries with high vaccination coverage and no clinical polio cases but which are located in regions of endemicity or where the virus has been endemic until recently (37). In these countries the circulation of wild-type virus must be carefully monitored to ensure that the chain of transmission has been broken. In addition, such surveillance could be used to monitor the circulation of vaccine-derived polioviruses when the goal of eradication has been achieved and vaccination with oral poliovirus vaccine has been terminated. The question of whether vaccine-derived strains can circulate indefinitely is still unanswered. Such strains may revert to virulence (21) and cause large outbreaks in the absence of high immunization coverage. Environmental surveillance may supply both qualitative and quantitative information on the circulation of such potentially hazardous strains.
REFERENCES
- 1.Afif H, Sutter R W, Kew O M, Fontaine T E, Pallansch M A, Goyal M K, Cochi S L. Outbreak of poliomyelitis in Gizan, Saudi Arabia: circulation of wild type 1 polioviruses from three separate origins. J Infect Dis. 1997;175(Suppl. 1):S71–S75. doi: 10.1093/infdis/175.supplement_1.s71. [DOI] [PubMed] [Google Scholar]
- 2.Aylward R B, Mansour E, El-Said A O, Haridi A, Abu El Kheir A, Hassan A. The eradication of poliomyelitis in Egypt: critical factors affecting progress to date. J Infect Dis. 1997;175(Suppl. 1):S56–S61. doi: 10.1093/infdis/175.supplement_1.s56. [DOI] [PubMed] [Google Scholar]
- 3.Balanant J, Guillot S, Candera A, Delpeyroux F, Crainic R. The natural genomic variability of poliovirus analyzed by a restriction fragment length polymorphism assay. Virology. 1991;184:645–654. doi: 10.1016/0042-6822(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 4.Berg G, Dahling D R. Methods for recovering viruses from river water solids. Appl Environ Microbiol. 1980;39:850. doi: 10.1128/aem.39.4.850-853.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg G, Safferman R S, Dahling D R, Berman D, Hurt C J. USEPA manual of methods for virology. EPA-600/4-84-013. U.S. Washington, D.C: Environmental Protection Agency; 1984. Methods for recovering viruses from toxic sludges and solids; pp. 8-1–8-14. [Google Scholar]
- 6.Birmingham M F, Linkins R W, Hull B P, Hull H F. Poliomyelitis surveillance, the compass for eradication. J Infect Dis. 1997;175(Suppl. 1):S146–S150. doi: 10.1093/infdis/175.supplement_1.s146. [DOI] [PubMed] [Google Scholar]
- 7.Bottinger M, Herrstrom E. Isolation of polioviruses from sewage and their characteristics. Experience over two decades in Sweden. Scand J Infect Dis. 1992;24:151–155. doi: 10.3109/00365549209052605. [DOI] [PubMed] [Google Scholar]
- 8.Chomczynski P. A reagent for the single step simultaneous isolation of RNA, DNA and proteins from cell and tissue culture samples. BioTechniques. 1993;15:532–537. [PubMed] [Google Scholar]
- 9.Crainic R, Couillin P, Blondel B, Cabau N, Boue A, Horodniceanu F. Natural variation of poliovirus neutralization epitope. Infect Immunol. 1983;41:1217–1225. doi: 10.1128/iai.41.3.1217-1225.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahling D R, Berg G, Berman D. BGM, a continuous cell line more sensitive than primary Rhesus and African Green for recovery of viruses from water. Health Lab Sci. 1974;11:275–282. [PubMed] [Google Scholar]
- 11.Genetics Computer Group. Program manual for the Wisconsin package version P, September 1994. Madison, Wis: Genetics Computer Group; 1994. [Google Scholar]
- 12.Grandien M, Forsgren M, Ehrnst A. Enteroviruses and reoviruses. In: Schmidt N J, Emmons R W, editors. Diagnostic procedures for viral rickettsial and chlamidial infections. 6th ed. Washington, D.C: American Public Health Association; 1988. pp. 513–579. [Google Scholar]
- 13.Green M S, Handsher R, Cohen D, Melnick J, Slepon R, Mendelson E, Danon Y. Age differences in immunity against wild and vaccine strains of poliovirus prior to the 1988 outbreak in Israel and response to booster immunization. Vaccine. 1993;11:75–81. doi: 10.1016/0264-410x(93)90342-u. [DOI] [PubMed] [Google Scholar]
- 14.Handsher R, Neuman M, Abramovitz B, Mendelson E, Swartz T. Annual Meeting of the Israeli Society for Microbiology. 1997. Polio neutralizing antibody in selected young Israeli age groups in the 90’s, abstr. 11; p. 18. [Google Scholar]
- 15.Horstmann D M, Emmons J, Gimpel L, Subrahmanyan T, Riordan J T. Enterovirus surveillance following community-wide oral poliovirus vaccination program: a seven year study. Am J Epidemiol. 1973;97:173–186. doi: 10.1093/oxfordjournals.aje.a121498. [DOI] [PubMed] [Google Scholar]
- 16.Hovi T, Huolainen A, Kuronen T, et al. Outbreak of paralytic poliomyelitis in Finland: widespread circulation of antigenically altered poliovirus type 3 in a vaccinated population. Lancet. 1986;ii:1427–1432. doi: 10.1016/s0140-6736(86)91566-7. [DOI] [PubMed] [Google Scholar]
- 16a.Hovi, T. Personal communication.
- 17.Hull H F, Birmingham M E, Melgaard B, Lee J W. Progress toward global polio eradication. J Infect Dis. 1997;175(Suppl. 1):S4–S9. doi: 10.1093/infdis/175.supplement_1.s4. [DOI] [PubMed] [Google Scholar]
- 18.Johnston S L G, Siegel C S. Presumptive identification of enteroviruses with RD, HEp-2, and RMK cell lines. J Clin Microbiol. 1990;28:1049–1050. doi: 10.1128/jcm.28.5.1049-1050.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kew O M, De L, Yang C F, Nottay B, Pallansch M. The role of virologic surveillance in the global initiative to eradicate poliomyelitis. In: Kustak E, editor. Control of virus diseases. New York, N.Y: Marcel Dekker; 1993. pp. 215–246. [Google Scholar]
- 20.Manor Y, Handsher R, Halmut T, Neuman M, Abramovitz B, Mates A, Mendelson E. A double-selective tissue culture system for isolation of wild-type poliovirus from sewage applied in a long-term environmental surveillance. Appl Environ Microbiol. 1999;65:1794–1797. doi: 10.1128/aem.65.4.1794-1797.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melnick J L. Population genetics applied to live poliovirus vaccine. Am J Public Health. 1962;52:472–483. doi: 10.2105/ajph.52.3.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyamura K, Yamashita K, Yamadera S, Kato N, Akatsuka M, Hara M, Inouye S, Yamazaki S. Poliovirus surveillance: isolation of poliovirus in Japan 1980–1991. A report of the national epidemiological surveillance agents in Japan. Japan J Med Sci Biol. 1992;45:203–214. doi: 10.7883/yoken1952.45.203. [DOI] [PubMed] [Google Scholar]
- 23.Mulders M N, Lipskaya G, van der Avoort H G A M, Koopmans M P G, Kew O M, van Loon A M. Molecular epidemiology of wild poliovirus type 1 in Europe, the Middle East, and the Indian subcontinent. J Infect Dis. 1995;171:1399–1405. doi: 10.1093/infdis/171.6.1399. [DOI] [PubMed] [Google Scholar]
- 24.Nakano J H, Milford M H, Thieme M L, Nottay B. Parameters for differentiating vaccine derived and wild poliovirus strains. In: Melnick J L, editor. Progress in Medical Virology. S. New York, N.Y: Karger; 1978. pp. 178–206. [PubMed] [Google Scholar]
- 25.Nelson D B, Circo R, Evans A S. Strategic viral surveillance of sewage during and following an oral poliovirus vaccine campaign. Am J Epidemiol. 1967;86:641–652. doi: 10.1093/oxfordjournals.aje.a120773. [DOI] [PubMed] [Google Scholar]
- 26.Poyry T, Stenvik M, Hovi T. Viruses in sewage waters during and after a poliomyelitis outbreak and subsequent nationwide oral poliovirus vaccination campaign in Finland. Appl Environ Microbiol. 1988;54:371–374. doi: 10.1128/aem.54.2.371-374.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reichler M R, Abbas A, Kharabsheh S, Mahafzah A, Alexander J P, Jr, Rhodes P, Faouri S, Ptoum H, Bloch S, Abdel-Magid M, Mulders M, Aslanian R, Hull H F, Pallansch M, Patriarca P A. Outbreak of paralytic poliomyelitis in a highly immunized population in Jordan. J Infect Dis. 1997;175(Suppl. 1):S62–S70. doi: 10.1093/infdis/175.supplement_1.s62. [DOI] [PubMed] [Google Scholar]
- 28.Rico-Hesse R, Pallansch M A, Nottay B K, Kew O M. Geographic distribution of wild poliovirus type genotypes. Virology. 1987;160:311–322. doi: 10.1016/0042-6822(87)90001-8. [DOI] [PubMed] [Google Scholar]
- 29.Salisbury D M, Ramsay M E, White J M, Brown D W. Polio eradication: surveillance implications for the United Kingdom. J Infect Dis. 1997;175(Suppl. 1):S156–S159. doi: 10.1093/infdis/175.supplement_1.s156. [DOI] [PubMed] [Google Scholar]
- 30.Shieh Y-S C, Wait D, Tai L, Sobsey M D. Methods to remove inhibitors in sewage and other fecal wastes for enterovirus detection by the polymerase chain reaction. J Virol Methods. 1995;54:51–66. doi: 10.1016/0166-0934(95)00025-p. [DOI] [PubMed] [Google Scholar]
- 31.Shulman L M, Manor Y, Handsher R, Vonsover A, Kew O M, Mendelson E. Abstracts of the Xth International Congress of Virology. 1996. Molecular analysis of wild type 1 poliovirus isolated from patients and sewage samples collected in Israel, Gaza and the West Bank reveals at least three independent introductions of the virus into this region, abstr. W54-6; p. 79. [Google Scholar]
- 32.Slater P E, Orenstein W A, Morag A, et al. Poliomyelitis outbreak in Israel in 1988: a report with two commentaries. Lancet. 1990;335:1192–1198. doi: 10.1016/0140-6736(90)92705-m. [DOI] [PubMed] [Google Scholar]
- 33.Swartz T A, Handsher R. Israel in the elimination phase of poliomyelitis. Public Health Rev. 1993;21:99–106. [PubMed] [Google Scholar]
- 34.Tambini G, Andrus J K, Marques E, Boshell J, Pallansch M, de Quadros C A, Kew O M. Direct detection of wild poliovirus circulation by stool survey of healthy children and analysis of community wastewater. J Infect Dis. 1993;168:1510–1514. doi: 10.1093/infdis/168.6.1510. [DOI] [PubMed] [Google Scholar]
- 35.Tulchinsky T, et al. Successful control of poliomyelitis by a combined OPV/IPV polio vaccine program in the West-Bank and Gaza 1978–93. Israel J Med Sci. 1994;30:489–494. [PubMed] [Google Scholar]
- 36.Vonsover A, Handsher R, Neuman M, Guillot S, Ballanat J, Rudich H, Mendelson E, Swart T, Crainic R. Molecular epidemiology of type 1 polioviruses isolated in Israel and defined by restriction fragment length polymorphism assay. J Infect Dis. 1993;167:199–203. doi: 10.1093/infdis/167.1.199. [DOI] [PubMed] [Google Scholar]
- 37.Wahdan M H, Aslanian T, Reichler M R, Gaafar M T. Progress toward poliomyelitis eradication in the eastern Mediterranean region of the World Health Organization. J Infect Dis. 1997;175(Suppl. 1):S50–S55. doi: 10.1093/infdis/175.supplement_1.s50. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. Manual for the virological investigation of poliomyelitis. Global poliomyelitis eradication by the year 2000. Geneva, Switzerland: World Health Organization; 1990. [Google Scholar]