Abstract
Background
Neuropathic pain (NP) is one of the main complications of leprosy, and its management is challenging. Infrared thermography (IRT) has been shown to be effective in the evaluation of peripheral autonomic function resulting from microcirculation flow changes in painful syndromes. This study used IRT to map the skin temperature on the hands and feet of leprosy patients with NP.
Methodology/Principal findings
This cross-sectional study included 20 controls and 55 leprosy patients, distributed into 29 with NP (PWP) and 26 without NP (PNP). Thermal images of the hands and feet were captured with infrared camera and clinical evaluations were performed. Electroneuromyography (ENMG) was used as a complementary neurological exam. Instruments used for the NP diagnosis were visual analog pain scale (VAS), Douleur Neuropathic en 4 questions (DN4), and simplified neurological assessment protocol. The prevalence of NP was 52.7%. Pain intensity showed that 93.1% of patients with NP had moderate/severe pain. The most frequent DN4 items in individuals with NP were numbness (86.2%), tingling (86.2%) and electric shocks (82.7%). Reactional episodes type 1 were statistically significant in the PWP group. Approximately 81.3% of patients showed a predominance of multiple mononeuropathy in ENMG, 79.6% had sensory loss, and 81.4% showed some degree of disability. The average temperature in the patients’ hands and feet was slightly lower than in the controls, but without a significant difference. Compared to controls, all patients showed significant temperature asymmetry in almost all points assessed on the hands, except for two palmar points and one dorsal point. In the feet, there was significant asymmetry in all points, indicating a greater involvement of the lower limbs.
Conclusion
IRT confirmed the asymmetric pattern of leprosy neuropathy, indicating a change in the function of the autonomic nervous system, and proving to be a useful method in the approach of pain.
Author summary
Pain has been shown to be a significant problem for leprosy patients and may be of nociceptive origin due to tissue inflammation, which occurs during reactional episodes mediated by the immune system, or neuropathic due to leprosy affecting the somatosensory system. It is important to differentiate neuropathic pain from chronic neuritis pain because the clinical implications and treatment are different. Multidrug therapy does not seem to prevent the occurrence of neuropathic pain, which is associated with low indices of quality of life and the general state of health. Infrared thermography is a complementary imaging test that is still growing and can be used in monitoring and determining the prognosis of patients in the health area. The thermographic examination records the abnormal thermal distribution and temperature differences in circulation alterations, which are not noticeable in the subjective evaluation of pain. We believe that the use of thermography as a complementary exam in the study of pain in leprosy neuropathy can assist in the definition of conduct protocols, which will enable the tracking and referral of patients to specific sectors in specialized health units, thereby changing the direction of the disease.
Introduction
Leprosy is a chronic infectious disease caused by the bacillus Mycobacterium leprae [1], which, due to its high affinity for peripheral nerves, has been reported as one of the most common causes of treatable peripheral neuropathy in the world [2]. Peripheral nervous system involvement occurs by two main factors, including the bacillus’s predilection for Schwann’s cell and reactions mediated by the host immune system [3]. This neural impairment often leads to changes in sensory, motor, and autonomic function [4]. Leprosy peripheral neuropathy may occur before, during, or after treatment with multidrug therapy (MDT) [5].
Pain has been shown to be a significant problem in leprosy neuropathy, and may be of nociceptive origin due to neuritis, which occurs during reactional episodes, neuropathic due to the involvement of the somatosensory system, or mixed (nociceptive and neuropathic) [6,7]. According to studies, neuropathic pain (NP) has been shown to be one of the most common late complications of leprosy [8,9], which may clinically manifest continuously or intermittently and occurs in a single or in several locations [10]. The prevalence of NP in leprosy has been described as 45% in China, 21% in India, 11% in Ethiopia, and 56% in Brazil; this variation in prevalence is due to the use of different study models, clinical forms of the selected patients, and screening tools [6,10–12]. As pain is a subjective symptom, difficult to measure, and involves physical and psychic aspects, making the diagnosis is sometimes a challenge [7]. It is important that NP is well diagnosed and adequately treated, since it is associated with low indices of quality of life and general health status [8,5].
In the absence of biomarkers or other gold standard examination, the diagnosis is initially clinical [13]. Validated clinical instruments for pain screening, such as Neuropathic Symptoms and Signs (LANSS), Douleur neuropathique en 4 questions (DN4), and painDETECT are often used [14].
There are some quantitative assessment methods available that can assist in the approach of NP, such as infrared thermography (IRT), which allows the mapping of the skin surface temperature, capturing changes in the microcirculation blood flow [15]. According to the previous study, there is a good correlation between skin temperature and cutaneous nervous activity, showing that skin temperature is a good predictor of sympathetic activity [16]. The sympathetic nervous system is the main regulator of cutaneous thermal emission [17]. Therefore, IRT allows to evaluate the abnormal thermal distribution and temperature differences caused by changes in the cutaneous peripheral circulation in various pathologies [18]. IRT has been used for the early and differential diagnosis of several pathological syndromes, as well as in painful syndromes, such as Complex Regional Pain Syndrome, Myofascial, Post Traumatic, Fibromyalgia, and neuropathic pain, and in inflammatory diseases of the skeletal muscle system [18,19].
The present study used IRT to assess the function of the peripheral autonomic nervous system by mapping the skin temperature of the hands and feet of leprosy patients who were undergoing treatment for relapses or treatment failure. Considering the magnitude of neuropathic pain in leprosy, this study aims to describe thermographic findings in leprosy patients, evaluating the association of these findings with the presence of neuropathic pain, as autonomic impairment may accompany this clinical condition.
Methods
Ethics statement
The Committee of Ethics in Research from the Federal University of Uberlandia (UFU)/MG approved this study (CAAE: 60427816.4.0000.5152). The researchers explained the aim of the study to the participants, and those who agreed to participate in the study signed the free and informed consent form.
Study design and subjects
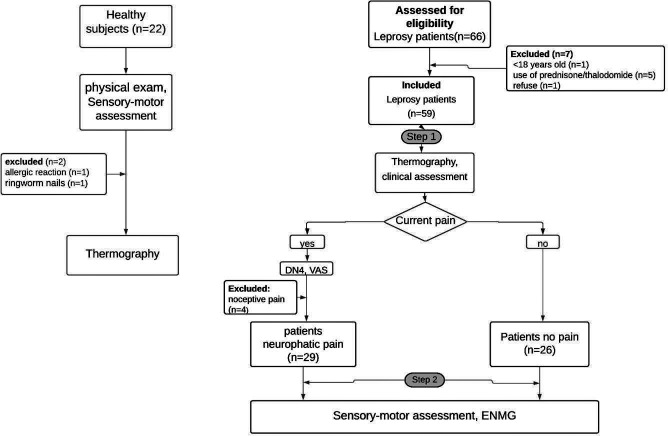
This cross-sectional study was conducted from January 2018 to December 2019 at the National Reference Center for Sanitary Dermatology and Leprosy (CREDESH), Clinical Hospital, Federal University of Uberlandia, located in Uberlandia/MG, Brazil. Patients (>18 years old) diagnosed with leprosy relapse or failure of previous treatment were eligible for this study. Clinical criteria were used for the diagnosis of leprosy relapse or treatment failure as follows: 1) Presence of active skin lesions; 2) New areas with change in sensitivity; 3) Neurological abnormalities, persistent neuritis, or reactional outbreak unresponsive to clinical treatment. Laboratory criteria used included: 1) Presence and characteristics of bacillus according to the bacilloscopic index in dermal scraping and skin biopsy; 2) Presence and quantification of bacillary DNA by qPCR (threshold cycle result) in dermal scraping and skin biopsy; 3) Maintenance or increase of the ELISA IgM anti-PGL-I index. Regarding the time to relapse, it was considered as greater than or equal to five years from the time of discharge from the last treatment. Regarding treatment failure, patients who had no clinical and laboratory improvement were defined as stated above, at the time of discharge from MDT for 24 months or alternative ROM regimen (rifampicin 600 mg + ofloxacin 400 mg + minocycline 100 mg) in monthly supervised doses for 24 months [20]. This study excluded patients who had reactional states, plantar ulcers, vasculopathies, and/or peripheral neuropathies of other etiologies (diabetes mellitus, HIV, alcoholism, etc.), including toxic and dapsone-related neuropathy. A total of 55 leprosy patients were selected and distributed into two groups: Patients with NP (PWP) and patients without pain (PNP). Patients with nociceptive pain were excluded from this study. The selection of the sample and the study design are illustrated in Fig 1.
Fig 1. Flowchart of data collection and subject selection.
To compare epidemiologic data and thermal measurements, 22 healthy individuals who had no contact with leprosy patients were invited to participate in this study as a control group. Their health status was confirmed by physical exam, and those with a normal sensory and motor assessment were included, while individuals with suspected systemic or localized diseases were excluded. The final sample consisted of 20 healthy individuals (Fig 1).
Thermal assessment
The thermal images of the hands and feet of healthy individuals and leprosy patients were captured using a thermal camera. To perform the IRT, the patients were instructed to prepare according to the protocol of the American Academy of Thermology [21], which consists of not performing physiotherapy, acupuncture, physical activity, or an electrodiagnostic test in the 24-hour period prior to the exam; not consuming caffeine products or nicotine in the 4-hour period prior to imaging, and not using cosmetics (skin lotions, sun screens, deodorants, etc.) on the hands or feet on the day of the exam.
On the day of the examination, the individuals were placed in an air-conditioned room without air currents or a heat source. The lighting of the room was made with fluorescent lamps to prevent the heating of the room. The temperature and humidity were monitored using the digital thermo-hygrometer Incoterm model: 7666.02.0.00. The room temperature was kept at 23°C ± 1°C and relative humidity at 55.5% ± 3.5%, and the participants remained seated and barefoot, resting in this environment for 15 minutes for acclimatization. A qualified physician captured the images using an infrared camera, with high spatial resolution of 320 x 240 pixels, calibrated with thermal sensitivity from 0.045°C to 30°C, frequency of 60 Hz, and having the ability to adjust the emissivity to 0.98. The camera was positioned on a tripod at a distance of 67–70 cm from the participant, and IT was captured from the hands (palm and dorsum) and feet (dorsum and sole) bilaterally. The IT obtained were saved in Joint Photographic Experts Group (JPEG) format and analyzed using thermography software.
Regions of interest (ROIs) were defined for quantitative temperature evaluation, 24 in the hands, back, and palm, and 17 in the feet, plant, and back (Fig 2). The ROIs coincided with the areas of innervation of the peripheral nerves frequently affected in leprosy, which are the ulnar, median, radial, common fibular, and tibial nerves. To assess the temperature in the peripheral innervation territories of the skin, ROIs were analyzed separately and grouped by neural area. Regarding the measurements in the neural area (grouped ROIs), the mean of the points referring to the dermatome of each peripheral nerve was calculated.
Fig 2. Region of interest of hands and feet.
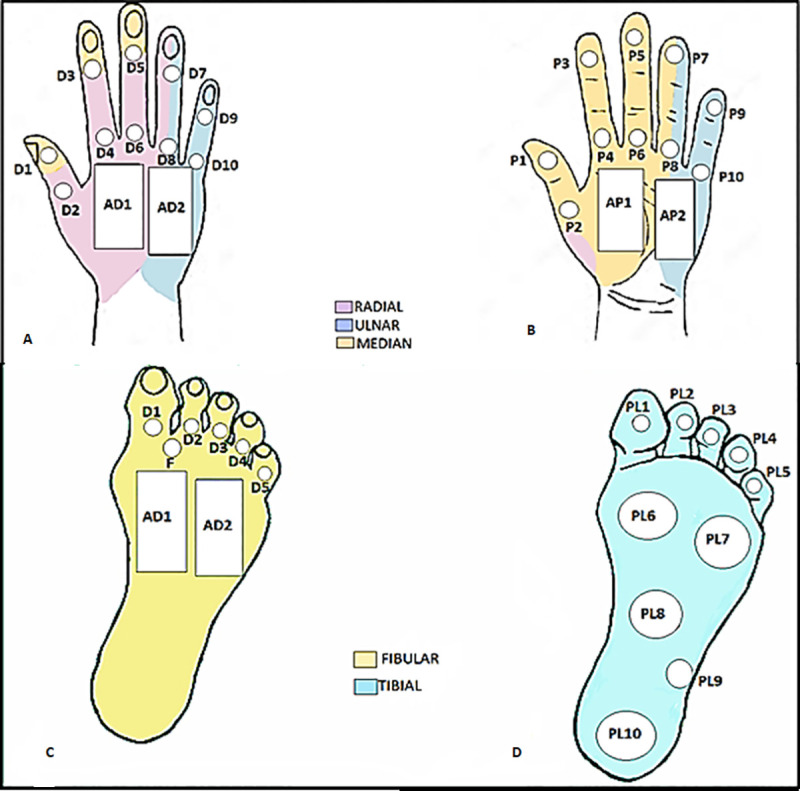
(A) Dorsum of the hand; (B) Palm of the hand; (C) Dorsum of foot; (D) Plantar of foot.
The ROIs defined in each neural area were: 1) Median nerve: P1, P2, P3, P4, P5, P6, AP1, D1, D3, and D5; 2) Ulnar nerve: P9, P10, AP2, D9, D10, and AD2; 3) Radial nerve: D2, D4, D6, and AD1; 4) Tibial nerve: PL1, PL2, PL3, PL4, PL5, PL6, PL7, PPL8, PL9, and PL10; 5) Fibular nerve: D1, D2, D3, D4, D5, F, AD1, and AD2 (Fig 2). Points P7, P8, D7, and D8 were not included in the neural areas due to the mixed innervation.
For statistical comparison, the average temperatures of the ROIs (isolated and grouped) and the temperature difference (ΔT = Tright—Tleft) between the contralateral hands and feet were calculated. The ΔT was used to assess asymmetry and detect possible dysfunctions.
Clinical evaluation
The patients underwent clinical evaluation during which sociodemographic (age, gender) and clinical data were collected: World Health Organization (WHO) and the Ridley-Jopling classification [22], previous MDT treatment, presence of pain, time of onset of pain, and previous leprosy reactions. In the control group, only sociodemographic data were collected.
Participants in the PWP group were asked to locate the anatomical region of their pain, and the degree of pain intensity was assessed using the visual analog pain scale (VAS), where 0 = no pain and 10 = maximum pain [23]. The intensity of pain was interpreted and classified as: 0: no pain, 1–3: mild pain, 4–7: moderate, 8–10: severe. The presence of NP was evaluated using the Douleur Neuropathique en 4 Questions (DN4) [24], a universal instrument that has been validated in Portuguese [25], composed of seven items related to symptoms and three related to the clinical examination, totaling ten points. Each positive response was given a point. When the total score was greater than or equal to 4, the pain was classified as NP, and below this value, it was classified as nociceptive.
Sensory-motor assessment
The leprosy patients were submitted to the assessment of the peripheral sensory-motor function by the physiotherapy team at the CREDESH. In the motor assessment, functional tests were performed in the muscle groups of abductor pollicis brevis and lumbricals (first and second) for the median nerve; first dorsal interosseous, abductor digiti minimi, and lumbricals (third and fourth).for the ulnar nerve; common extensor of the fingers and radial carpal for the radial nerve; and the muscles tibial anterior, extensor digitorum longus, and extensor hallucis longus innervated by the deep fibular nerve. The Medical Research Council Scale was used with a graduation of 0–5, considered as grade 0 = paralysis and 5 = normal strength, and any change in function < 4 in one or more muscle groups was considered abnormal [26].
The sensory evaluation was performed using the six Semmes-Weinstein filaments, which exert forces of 0.05 g, 0.2 g, 2g, 4g, 10g, and 300 g when applied to the skin, and the tested points coincided with the ROIs evaluated. Sensory loss was considered as the absence of positive response to filaments of 0.2 g in the hands and 2g in the feet [27].
Electroneuromyography
Electroneuromyography was performed on the participants in the PWP and PNP groups to assess neuropathy, by the neurophysiologist of CREDESH. The device used in the examination was the MEB 4200K (NIHON-KODHEN) and the techniques and configurations of the examination were standardized in the previous study. The sensory nerves evaluated were the median, ulnar, dorsal cutaneous of the hand, radial, sural, superficial fibular, and medial plantar bilaterally, and the motor nerves were the median, ulnar, deep fibular, and tibial bilaterally. The electroneuromyography was classified according to the neurophysiological pattern in mononeuropathy: presence of only one altered nerve or asymmetric multiple mononeuropathy and presence of two or more altered nerves [28].
Sample size calculation
It was calculated the sample size of this study using the software G*Power (version 3.1.9.2, for windows). One-way ANOVA fixed effects were performed by means of priori analysis with the effect size of 0.37, obtained from a pilot study concerning thermography (not published), alpha err probability of 5% (0.05), power of test (1-β err probability) 0.80 (80%) and the number of 3 groups. Thus, the total sample size, considering the above parameters, was 75 individuals.
Statistical analysis
The D’Agustino-Pearson test was used to evaluate the normality of the values referring to the temperatures of each cutaneous region and the values of ΔT. The nonparametric distribution variables were normalized by the Log 10 transformation when necessary. The Binomial test evaluated the association between clinical/sociodemographic variables and patient groups with or without pain.
The student’s t-test, for paired samples, was used in the comparison between the average temperatures of the upper limbs right and left. Mann-Whitney test was used in the comparisons between the sum of the average of the stations for each ΔT referring to the differences in temperatures by cutaneous region obtained for different groups.
The statistical program used was the Statistical Package for Social Sciences version 22 (IBM, Armonk, NY, USA), with a significance level of 5% for all analyses.
Results
Fifty-five leprosy patients were evaluated, with 29 patients in the PWP group and 26 patients in the PNP group. There was no statistical difference between the PWP group (46.75 ± 11.4 years; 58.6% (17/29) male), PNP group (48 ± 13.44 years: 61.5% (16/26) male), and healthy subjects (43.05 ± 14.74 years; 40% (8/20) male) in relation to age and gender (p>0.05). The control group presented normal sensory-motor function, being the inclusion criteria for this group. The clinical data of the PWP and PNP groups are shown in Table 1. There was also no significant difference between the leprosy patient groups in relation to the number of relapses or treatment failure of the disease, operational classification, date of onset, and type of current treatment.
Table 1. Clinical characteristics of the patients with and without neuropathic pain.
| Variables | PWP | PNP | |
|---|---|---|---|
| n = 29 | n = 26 | p-value | |
| Relapse | 23 (79.3%) | 20 (76.9%) | |
| Treatment Failure | 6 (20.7%) | 6 (23.1%) | 0.8305 |
| Operational | |||
| classification | |||
| MB | 23 (79.3%) | 22 (84.6%) | |
| PB | 6 (20.7%) | 4 (15.4%) | 0.6106 |
| Clinical Form | |||
| Tuberculoid | 1 (3.5%) | 0 | |
| Borderline-tuberculoid | 9 (31%) | 10 (38.5%) | 0.5631 |
| Borderline-borderline | 6 (20.7%) | 1 (3.8%) | 0.0613 |
| Borderline-lepromatous | 4 (13.8%) | 0 | |
| Lepromatous | 9 (31%) | 15 (57.7%)* | 0.0466 |
| Current treatment | |||
| ROM | 29 (100%) | 25 (96.2%) | |
| Other | 0 | 1 (3.8%) | 0.2865 |
| Relapse time | |||
| at 6 months | 20 (69%) | 19 (73.1%) | |
| < 6 months | 9 (31%) | 7 (26.9%) | 0.7375 |
| Leprosy reactions | |||
| Type 1 | 18 (57.6%)* | 8 (30.8%) | 0.0203 |
| Type 2 | 11 (42.4%) | 14 (53.8%) | 0.2366 |
| No | 0 | 4 (15.4%) | |
| Electroneuromyography | |||
| normal | 0 | 6 (23.1%) | |
| mononeuropathy | 2 (6.9%) | 1 (3.8%) | 0.6189 |
| multiple mononeuropathy | 27(93.1%)* | 19 (73.1%) | 0.0450 |
| sensory function | |||
| normal | 3 (10.4%) | 8 (30.8%) | |
| abnormal | 26 (89.6%) | 18 (69.2%) | 0.0587 |
| motor function | |||
| normal | 9 (31%) | 19 (73.1%) | |
| abnormal | 20 (69%)* | 7 (26.9%) | 0.0018 |
| Who disability grade | |||
| 0 | 6 (20.7%) | 11 (42.3%) | |
| 1 | 12 (41.4%) | 11 (42.3%) | 0.0833 |
| 2 | 11 (37.9%) | 4 (15.4%) | 0.9444 |
PWP: patients with pain, PNP: patients no pain, MB: multibacillary, PB: paucibacillary.
n is a total number of cases; % is a percentage of cases.
WHO: World Health Organization, ROM: rifampin, ofloxacin, minocycline.
*p was considered significant when p< 0.05.
The previous reactional episodes type 1 were statistically significant in the PWP group (p = 0.020). The ENMG showed a predominance of multiple mononeuropathy in the patient groups, with a significant difference in the PWP and PNP groups (p < 0.045). Most individuals presented sensory loss and some degree of disability, but there was no significant difference between the PWP and PNP groups. There was a greater loss of motor function in the PWP group (p = 0.001) (Table 1).
In the PWP group, 93.1% (27/29) of patients had NP and 6.9% (2/29) mixed pain. The prevalence of NP was 52.7% (29/55), considering individuals who had mixed pain. The most frequent DN4 items in individuals with NP were numbness and tingling 86.2% each (25/29), electric shocks 82.7% (24/29), hypoesthesia to touch 75.8% (22/29), pins and needles 72.4% (21/29), and burning 68.9% (20/29), and the least frequent were brushing/painful cold with 37.9% each (11/29), hypoesthesia to prick 34.5% (10/29), and Itching 27.6% (8/29). All patients with NP had chronic pain, and as for the periodicity of pain, 62.1% (18/29) of the individuals reported that the pain was intermittent and 37.9% (11/29) reported that the pain was continuous.
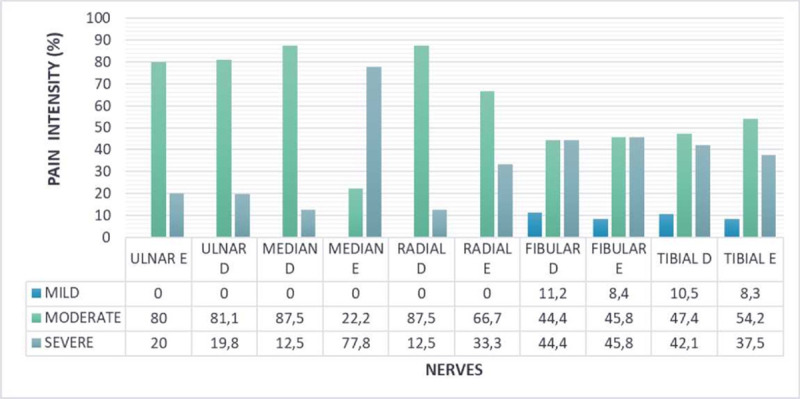
The patients reported NP in the innervation territory of the following nerves: 89.6% (26/29) left tibial, 75.9% (22/29) left fibular, 58.6% (17/29) right tibial, 62.1% (18/29) right fibular, 55.2% (16/29) left ulnar, 41.4% (12/29) right ulnar, 34.5% (10/29) left radial and median, and 31.03% (9/29) right radial and median. Only 13.8% (4/29) of the patients with NP reported pain in only one nerve, and 86.2% (25/29) had two or more nerve involvement. Pain intensity assessed by VAS showed that 93.1% (27/29) of patients with NP had moderate/severe pain. The intensity of peripheral nerve topography is represented in Fig 3.
Fig 3. Percentage of pain intensity (VAS) in nerves of patients with neuropathic pain.
Comparisons of the mean temperature of each individual with that of the contralateral side was performed, and the ΔT measurements were compared between the group of patients (PWP and PNP) and healthy controls. The average temperatures of the ROIs and the neural areas ranged from 31.69°C to 33.74°C in the hands and 29.5°C to 32.06°C in the feet, in the group of patients (PWP and PNP). There was a significant mean temperature difference in P4, P5 and P10 in the hands and PL7, PL8, D2, and D3 in the feet of the group of leprosy patients. In the healthy controls, the average temperature was between 32.34°C to 33.71°C in the hands and 30.38°C to 32.15°C in the feet, but only ROI P5 had a significant difference in this group (S1 Table). In all groups, the average temperature in the lower limbs was lower than that of the upper limbs (Table 2). To assess symmetry, the ΔT was also calculated and compared among the groups of patients and healthy controls. There was a significant difference between the ΔT of almost all the evaluated points (p < 0.05) in hands, including in the neural areas, except for the palm points (AP1 and AP2) and one point on the back of the hands (AD1) (Table 2). In the feet, there was a significant difference in all the ROIs and neural areas analyzed, indicating a greater involvement in the lower limbs. In the hands of all patients, the median ΔT ranged from 0.2°C to 0.7°C, and in the feet, the median ranged from 0.4°C to 0.7°C. In the hands of the control group, the median ΔT ranged from 0.15°C to 0.3°C, and in the feet, it ranged from 0.15°C to 0.25°C. IRT detected the asymmetry of the patients’ hands and feet when compared with subjects in the control group (Fig 4).
Table 2. Comparison of ΔT in ROIs and neural areas of the hands between the group of patients and healthy controls.
| LEPROSY PATIENTS (n = 55) | HEALTHY CONTROLS (n = 20) | ||
|---|---|---|---|
| ROI | ΔT | ΔT | |
| median (Q1-Q3) | median (Q1-Q3) | p value | |
| HANDS | |||
| P1 | 0.6 (0.3–0.9) | 0.2 (0.1–0.2) | <0.001 |
| P2 | 0.5 (0.2–0.7) | 0.2 (0.1–0.3) | 0.006 |
| P3 | 0.4 (0.2–0.9) | 0.2 (0.1–0.4) | <0.001 |
| P4 | 0.4 (0.2–0.7) | 0.2 (0.1–0.3) | 0.008 |
| P5 | 0.5 (0.3–0.9) | 0.15 (0.1–0.2) | <0.001 |
| P6 | 0.4 (0.2–0.7) | 0.2 (0.1–0.3) | 0.007 |
| P7 | 0.5 (0.2–0.9) | 0.25 (0.1–0.4) | 0.002 |
| P8 | 0.5 (0.2–0.9) | 0.2 (0.2–0.3) | 0.002 |
| P9 | 0.6 (0.2–1.1) | 0.2 (0.12–0.3) | <0.001 |
| P10 | 0.3 (0.2–1.0) | 0.2 (0.1–0.3) | 0.004 |
| AP1 | 0.2 (0.1–0.5) | 0.2 (0.12–0.3) | 0.525 |
| AP2 | 0.3 (0.1–0.7) | 0.25 (0.2–0.3) | 0.154 |
| D1 | 0.4 (0.2–1.0) | 0.3 (0.2–0.37) | 0.025 |
| D2 | 0.4 (0.1–0.9) | 0.2 (0.2–0.3) | 0.013 |
| D3 | 0.4 (0.1–1.0) | 0.2 (0.1–0.3) | 0.007 |
| D4 | 0.4 (0.2–0.8) | 0.25 (0.2–0.3) | 0.009 |
| D5 | 0.4 (0.2–0.8) | 0.2 (0.1–0.3) | 0.001 |
| D6 | 0.3 (0.1–0.6) | 0.2 (0.1–0.3) | 0.022 |
| D7 | 0.4 (0.2–1.0) | 0.3 (0.2–0.37) | 0.013 |
| D8 | 0.4 (0.2–0.8) | 0.2 (0.1–0.3) | 0.001 |
| D9 | 0.5 (0.3–1.1) | 0.2 (0.2–0.3) | <0.001 |
| D10 | 0.6 (0.3–0.9) | 0.25 (0.1–0.3) | <0.001 |
| AD1 | 0.3 (0.1–0.5) | 0.2 (0.1–0.3) | 0.138 |
| AD2 | 0.3 (0.1–0.6) | 0.2 (0.1–0.3) | 0.005 |
| RADIAL | 0.7 (0.2–0.8) | 0.2 (0.1–0.3) | <0.001 |
| ULNAR | 0.5 (0.2–0.9) | 0.2 (0.1–0.3 | <0.001 |
| MEDIAN | 0.4 (0.1–0.7) | 0.2 (0.1–0.3) | <0.001 |
| FEET | |||
| PL1 | 0.5 (0.3–1.3) | 0.2 (0.1–0.3) | <0.001 |
| PL2 | 0.7 (0.4–1.3) | 0.2 (0.02–0.3) | <0.001 |
| PL3 | 0.8 (0.3–1.3) | 0.2 (0.1–0.3) | <0.001 |
| PL4 | 0.6 (0.3–1.0) | 0.25 (0.1–0.4) | 0.001 |
| PL5 | 0.7 (0.4–1.3) | 0.2 (0.1–0.3) | <0.001 |
| PL6 | 0.5 (0.2–0.8) | 0.2 (0.2–0.3) | 0.003 |
| PL7 | 0.5 (0.3–0.8) | 0.2 (0.1–0.3) | <0.001 |
| PL8 | 0.4 (0.2–0.8) | 0.2 (0.1–0.3) | 0.001 |
| PL9 | 0.5 (0.2–0.8) | 0.2 (0.1–0.3) | <0.001 |
| PL10 | 0.4 (0.2–0.7) | 0.2 (0.1–0.3) | <0.001 |
| PF | 0.4 (0.2–0.8) | 0.2 (0.1–0.3) | <0.001 |
| D1 | 0.5 (0.2–1.3) | 0.15 (0.1–0.3) | <0.001 |
| D2 | 0.4 (0.2–1.0) | 0.2 (0.1–0.3) | <0.001 |
| D3 | 0.5 (0.2–0.8) | 0.2 (0.2–0.3) | <0.001 |
| D4 | 0.4 (0.2–0.9) | 0.2 (0.1–0.3) | 0.005 |
| D5 | 0.7 (0.3–1.3) | 0.2 (0.1–0.3) | <0.001 |
| AD1 | 0.4 (0.1–0.9) | 0.15 (0.1–0.3) | 0.009 |
| AD2 | 0.4 (0.1–0.7) | 0.15 (0.1–0.3 | 0.005 |
| FIBULAR | 0.6 (0.3–1.1) | 0.2 (0.1–0.3) | <0.001 |
| TIBIAL | 0.5 (0.2–1.0) | 0.2 (0.1–0.3) | <0.001 |
ROI: Region of interest, *p-value comparison between ΔTs of patients and controls
Mann- Whitney test. * p was considered significant when p < 0.05.
Fig 4. Infrared thermal imaging.
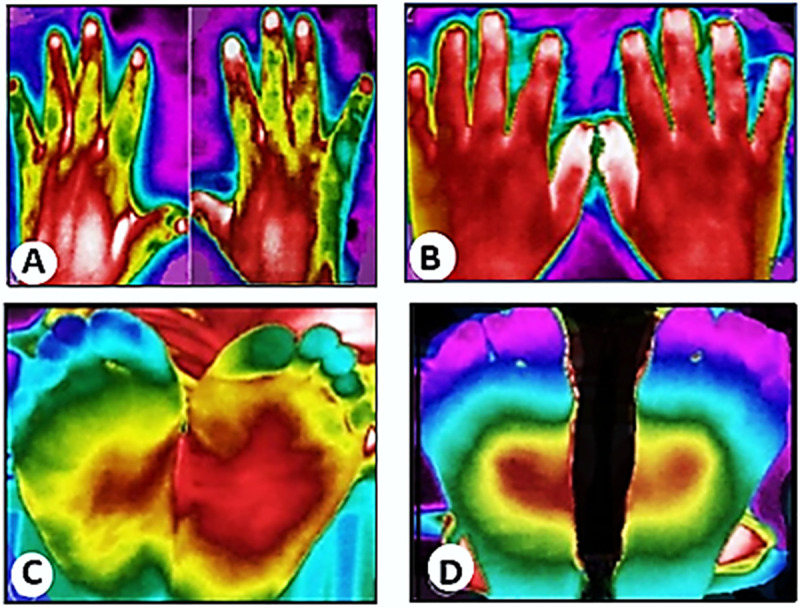
(A) Thermal image of the dorsal region of the hands of patient with leprosy; (B) Thermal image of dorsal region of the hands of a healthy individual; (C) Thermal image of feet of a patient with leprosy; (D) Thermal image of the plantar region of feet of a healthy individual.
When the groups of patients were compared to each other, the average temperature in the hands of the PWP group ranged from 31.38°C to 33.63°C and in the PNP group from 31.71°C to 33.6°C. In the feet, it varied from 29.62°C to 32.01°C in the PWP group and 29.35°C to 32.11°C in the PNP group. In the PWP group, there was a significant difference in the mean temperature on the right side with the left in two ROIs (P2 and AD2) in the hands and two ROIs (PL2 and PL7) in the feet (p < 0.05). In the PNP group, there was no significant difference between the average temperatures (p > 0.05) (S2 Table). There was also no significant difference of ΔT in the ROIs and neural areas of the patients’ hands and feet. (Table 3). In the hands of the PWP group, the median of ΔT varied from 0.3°C to 0.7°C in the hands and 0.3 to 0.8°C in the feet. In the PNP group, there was a median variation from 0.2°C to 0.6°C in hands and 0.3°C to 0.75°C in the feet. Although the asymmetry is slightly higher in the PWP group, the values were not significant (Table 3).
Table 3. Comparation of ΔT in the ROIs and neural areas of hands and feet in the PWP and PNP groups.
| PWP (n = 29) | PNP (n = 26) | ||
|---|---|---|---|
| ROI | ΔT | ΔT | |
| median (Q1-Q3) | median (Q1-Q3) | p value | |
| HANDS | |||
| P1 | 0.7 (0.35–1.15) | 0.5 (0.3–1.15) | 0.191 |
| P2 | 0.5 (0.2–0.8) | 0.4 (0.2–0.7) | 0.551 |
| P3 | 0.6 (0.35–1.3) | 0.3 (0.2–0.7) | 0.677 |
| P4 | 0.5 (0.2–1.0) | 0.3 (0.2–0.5) | 0.295 |
| P5 | 0.6 (0.25–1.0) | 0.5 (0.3--0.75) | 0.878 |
| P6 | 0.6 (0.3–1.2) | 0.2 (0.2–0.8) | 0.060 |
| P7 | 0.6 (0.2–1.05) | 0.45 (0.2–1.15) | 0.754 |
| P8 | 0.5 (0.25–0.85) | 0.5 (0.2–1.07) | 0.388 |
| P9 | 0.6 (0.4–1.15) | 0.5 (0.2–1.15) | 0.869 |
| P10 | 0.4 (0.3–1.05) | 0.35 (0.2–1.1) | 0.883 |
| AP1 | 0.3 (0.15–0.8) | 0.2 (0.1–0.75) | 0.116 |
| AP2 | 0.3 (0.2–0.65) | 0.3 (0.1–0.75) | 0.638 |
| D1 | 0.6 (0.25–1.2) | 0.35 (0.1–0.85) | 0.162 |
| D2 | 0.5 (0.3–1.3) | 0,25 (0.1–0.5) | 0.061 |
| D3 | 0.4 (0.15–1.2) | 0.4 (0.1–0.9) | 0.708 |
| D4 | 0.3 (0.15–0.7) | 0.45 (0.4–0.9) | 0.876 |
| D5 | 0.6 (0.2–0.9) | 0.45 (0.2–0.7) | 0.398 |
| D6 | 0.3 (0.1–0.6) | 0.4 (0.1–0.6) | 0.303 |
| D7 | 0.5 (0.2–1.05) | 0.4 (0.2–0.9) | 0.334 |
| D8 | 0.4 (0.2–1.0) | 0.35 (0.2–0.7) | 0.324 |
| D9 | 0.5 (0.25–1.2) | 0.55 (0.3–1.1) | 0.871 |
| D10 | 0.6 (0.25–1.0) | 0.6 (0.5–0.9) | 0.876 |
| AD1 | 0.2 (0.1–0.5) | 0.3 (0.2–0.6) | 0.644 |
| AD2 | 0.6 (0.25–1.0) | 0.3 (0.2–0.7) | 1 |
| RADIAL | 0.4 (0.2–0.65) | 0.35 (0.2–0.6) | 0.760 |
| ULNAR | 0.2 (0.3–0.7) | 0.45 (0.2–0.8) | 0.684 |
| MEDIAN | 0.5 (0.2–1.0) | 0.4 (0.3–1.0) | 0.618 |
| FEET | |||
| PL1 | 0.5 (0.25–1.4) | 0.6 (0.4–1.15) | 0.848 |
| PL2 | 0.8 (0.4–1.4) | 0.4 (0.3–1.1) | 0.064 |
| PL3 | 0.8 (0.4–1.2) | 0.7 (0.30–1.4) | 0.896 |
| PL4 | 0.7 (0.3–1.1) | 0.55 (0.20–1.0) | 0.486 |
| PL5 | 0.7 (0.45–1.25) | 0.6 (0.3--1.3) | 0.640 |
| PL6 | 0.6 (0.2–0.95) | 0.45 (0.2–0.7) | 0.613 |
| PL7 | 0.6 (0.3–0.85) | 0.4 (0.3–0.9) | 0.908 |
| PL8 | 0.5 (0.25–0.8) | 0.4 (0.2–0.8) | 0.753 |
| PL9 | 0.4 (0.25–1.05) | 0.5 (0.2–0.7) | 0.976 |
| PL10 | 0.5 (0.2–0.75) | 0.3 (0.2–0.6) | 0.369 |
| PF | 0.6 (0.35–1.15) | 0.4 (0.2–0.7) | 0.165 |
| D1 | 0.7 (0.2–1.55) | 0.4 (0.2–0.9) | 0.207 |
| D2 | 0.4 (0.2–1.05) | 0.4 (0.3–1.0) | 0.495 |
| D3 | 0.5 (0.2–1.1) | 0.45 (0.3–0.8) | 0.878 |
| D4 | 0.3 (0.1–0.9) | 0.5 (0.3–1.1) | 0.098 |
| D5 | 0.7 (0.3–1.35) | 0.75 (0.3–1.3) | 0.713 |
| AD1 | 0.6 (0.1–1.1) | 0.4 (0.1–0.6) | 0.477 |
| AD2 | 0.5 (0.2–0.6) | 0.45 (0.1–0.8) | 0.485 |
| FIBULAR | 0.5 (0.15–1.0) | 0.4 (0.3–0.7) | 0.903 |
| TIBIAL | 0.6 (0.3–1.0) | 0.45 (0.2–0.8) | 0.516 |
ROI: Region of interest, PWP: patients with pain, PNP: patients no pain.
ΔT: right/left temperature difference; * p was considered significant when p < 0.05 (Mann-Whitney test).
Discussion
NP in leprosy has been underdiagnosed and poorly treated. Therefore, new tools are needed to help in the correct approach to the diagnosis of pain, because the presence of pain greatly impacts the quality of life of patients [8]. Patients with leprosy relapse and treatment failure have a greater chance of neural complications such as NP, because they are a group of patients chronically affected by the disease [29]. The population of this study was composed mostly of individuals in the economically active age group, with a mean age of 46.75 (±11.4) years in the PWP group and 48 (±13.4) years in the PNP group, showing the high social impact of leprosy [30,31]. There was a predominance of males among the patients. According to a systematic review study, men are at a higher risk of acquiring leprosy, while women are probably more concerned about their health and are diagnosed early, and/or there are different levels of leprosy exposure risk factors in men and women [32]. According to the WHO operational classification, 81.8% (45/55) were multibacillary patients, and the clinical forms with the highest incidence were lepromatous and borderline-tuberculoid, as the region is highly endemic, and this result corroborates the epidemiological results [33].
In the present study, 92.7% (51/55) of patients had previous reactional episodes, and the PWP group had a higher number of type 1 reactions than the PNP group (p = 0.02). Multibacillary patients have a higher chance of developing leprosy reactions regardless of the type [34]. Type 1 reaction is the main cause of neural involvement in leprosy [35] and is accompanied by neuritis associated with sensory and motor changes. Leprosy reactions are significant risk factors for the triggering of NP [36].
It has been described that sensory and autonomic fibers (small-fiber neuropathy) are the first to be affected in leprosy [37], therefore, when motor changes occur, it is a sign that the disease is in an advanced phase with involvement of several nerve trunks [2], leading to the classic pattern in the electroneurophysiological examination of multiple sensori-motor asymmetric mononeuritis [38]. In this study, most patients were already in an advanced stage of the disease, especially those in the PWP group, with significant motor loss (p = 0.001) and a predominance of multiple asymmetric mononeuritis (p = 0.045). The highest degree of disability was related to pain and the general poor perception of health [5]; although no significant difference was shown between the groups, the prevalence of disability grade II was high (37.9%) in the PWP group. These findings confirm that NP is a late complication of leprosy [39].
In this study, the prevalence of NP was 52.7% (29/55). The high prevalence of NP in patients with relapse and treatment failure could be caused by chronic inflammation around the nerve, the existence of persistent M leprae antigens [37], or resistant drugs [29]. In the Reference Center, all cases of relapse and therapeutic failure were investigated for drug resistance, and no drug resistance was found. The early diagnosis of neuropathy in leprosy relapse has been defiant due to the long incubation period of the bacillus and the presence of sequelae that hinder the differential diagnosis [28].
Regarding pain localization, this study showed a higher incidence in the tibial and fibular nerves, a fact that differed from other studies that reported a higher incidence in the ulnar nerves [5,40]. Tibial nerve neuropathy may be underdiagnosed, as tibial neuritis may develop silently [41]. Chronic pain is defined as persistent or recurrent for more than or equal to 3 months [42], and it is known that the neuropathic component in chronic pain impacts more on the quality of life [10] than pain without the neuropathic component [43]. All individuals with NP presented chronic pain intermittently or continuously and 93% reported moderate to severe pain. These findings show the chronic suffering to which patients are submitted and the lack of diagnosis of NP [42]. Given that the sampling of this study was randomly obtained, it was not possible to compare individuals with neuropathic and nociceptive pain, which is a limitation of the study.
The IRT has been used as an auxiliary tool in the triage and monitoring of neuropathic painful syndromes, but its use should be judicious because it reflects the emission of heat determined by physiological and pathological neural processes or not [44]. The skin temperature difference in symmetrical areas of the body may indicate a pathologic process [45]. In neural lesions, hypothermic or hyperthermic variations that are reflected in thermal imaging may occur and can be valuable in the diagnosis and proper treatment of pain [46].
In this study, the average temperature of the ROIs showed almost no significant difference between the control group and leprosy patients (PWP and PNP), which due to the subtle variation in the temperature distribution [46] or because of leprosy, neuropathy occurs asymmetrically and to varying degrees between patients, and it is not possible to determine a single temperature pattern. The temperatures of the hands and feet varied throughout the day between individuals and in the individual himself, being less stable than the other parts of the body, but the ΔT remained constant in the same individual [47].
In this study, the method used was the static method, where the temperature measurement was performed in a single moment and the main way of evaluating the temperatures in this method was by ΔT, which evaluates the temperature difference on one side of the body compared to its contralateral side [48]. There is a thermal symmetry between the areas of the human body, where the temperature difference of < 0.3°C between the areas of the body is considered normal [47,49]. Asymmetry (ΔT) was significant in all ROIs (hands and feet) evaluated when all leprosy patients were compared with the controls, except for the palm and dorsal points, indicating once again the asymmetric characteristic of leprosy and the greater distal neural involvement of the hands.
There was no statistical difference in the ΔT between the PWP and PNP groups in the ROIs and neural areas evaluated, probably because most patients were already presenting an advanced neural disease. The static thermography was able to diagnose autonomic dysfunction in leprosy, which is not always accompanied by NP; therefore, thermal images should always be analyzed based on clinical evaluation. It should be considered that thermography captures acute or chronic sympathetic nervous system dysfunction, and as chronic neurological disease progresses, sympathetic thermal dysfunction becomes bilateral [49]. Thermography may indicate a change in abnormal physiology, but it is nonspecific, and its thermal patterns must be properly interpreted in order not to lead to diagnostic error [48].
IRT seems to be a promising method to approach pain, as it is safe and non-invasive. By tracking changes in the microcirculation of the hands and feet of patients with leprosy, sensorimotor neural assessments can be correlated with the dysautonomia demonstrated by the skin’s thermal pattern.
The creation of a database with individual thermograms can assist in monitoring the progression of neuropathies through temperature changes. These repeated accompanying measures during treatment will clarify the link between asymmetric temperature distributions and pathophysiological changes on the skin surface and the extent of the neural injury.
Given its limitations, IRT cannot be considered as a substitute for clinical examination and motor-sensory evaluation, but it complements this peripheral neural evaluation and can assist in therapeutic approaches to pain, impacting measures to prevent leprosy deficiencies.
PN in leprosy needs to be better understood to be treated properly, as it directly impacts the individual’s quality of life. Further IRT studies are needed, evaluating the patient early before the installation of permanent neural damage and using dynamic methods associated with thermal stress tests that can increase sensitivity for the detection of NP dysautonomia in leprosy, thereby allowing for the better understanding and applicability of thermal tests.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
To the team from CREDESH/HC/UFU, for the assistance of quality and support to the research committed to improve the life conditions of this population affected by leprosy.
Data Availability
All relevant data are within the manuscript and its Supporting Information file.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Lockwood DN, Saunderson PR. Nerve damage in leprosy: a continuing challenge to scientists, clinicians and service providers. Int Health. 2012Jun;4(2):77–85. doi: 10.1016/j.inhe.2011.09.006 . [DOI] [PubMed] [Google Scholar]
- 2.Nascimento OJ. Leprosy neuropathy: clinical presentations. Arq Neuropsiquiatr. 2013Sep;71(9B):661–6. doi: 10.1590/0004-282X20130146 . [DOI] [PubMed] [Google Scholar]
- 3.Ooi WW, Srinivasan J. Leprosy and the peripheral nervous system: basic and clinical aspects. Muscle Nerve. 2004Oct;30(4):393–409. doi: 10.1002/mus.20113 . [DOI] [PubMed] [Google Scholar]
- 4.Wagenaar I, Post E, Brandsma W, Ziegler D, Rahman M, Alam K et al. Early detection of neuropathy in leprosy: a comparison of five tests for field settings. Infect Dis Poverty. 2017Sep1;6(1):115. doi: 10.1186/s40249-017-0330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haroun OMO, Vollert J, Lockwood DN, Bennett DLH, Pai VV, Shetty V et al. Clinical characteristics of neuropathic pain in leprosy and associated somatosensory profiles: a deep phenotyping study in India. Pain Rep. 2019Dec6;4(6): e743. doi: 10.1097/PR9.0000000000000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haroun OMO, Hietaharju A, Bizuneh E, Tesfaye F, Brandsma WJ, Haanpää M, et al. Investigation of neuropathic pain in treated leprosy patients in Ethiopia: a cross-sectional study. Pain. 2012Aug;153(8):1620–1624. doi: 10.1016/j.pain.2012.04.007 Epub 2012 Jun 22. . [DOI] [PubMed] [Google Scholar]
- 7.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010Aug;9(8):807–19. doi: 10.1016/S1474-4422(10)70143-5 . [DOI] [PubMed] [Google Scholar]
- 8.Ramos JM, Alonso-Castañeda B, Eshetu D, Lemma D, Reyes F, Belinchón I, et al. Prevalence and characteristics of neuropathic pain in leprosy patients treated years ago. Pathog Glob Health. 2014Jun;108(4):186–90. doi: 10.1179/2047773214Y.0000000140 Epub 2014 Jun 3. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lasry-Levy E, Hietaharju A, Pai V, Ganapati R, Rice AS, Haanpää M, et al. Neuropathic pain and psychological morbidity in patients with treated leprosy: a cross-sectional prevalence study in Mumbai. PLoS Negl Trop Dis. 2011Mar8;5(3): e981. doi: 10.1371/journal.pntd.0000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S, Qu J, Chu T. Prevalence and characteristics of neuropathic pain in the people affected by leprosy in China. Lepr Rev. 2012Jun;83(2):195–201. . [PubMed] [Google Scholar]
- 11.Stump PR, Baccarelli R, Marciano LH, Lauris JR, Teixeira MJ, Ura S, et al. Neuropathic pain in leprosy patients. Int J Lepr Other Mycobact Dis. 2004Jun;72(2):134–8. doi: . [DOI] [PubMed] [Google Scholar]
- 12.Toh HS, Maharjan J, Thapa R, Neupane KD, Shah M, Baral S, Hagge DA, Napit IB, Lockwood DNJ. Diagnosis and impact of neuropathic pain in leprosy patients in Nepal after completion of multidrug therapy. PLoS Negl Trop Dis. 2018Jul2;12(7):e0006610. doi: 10.1371/journal.pntd.0006610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulvey MR, Boland EG, Bouhassira D, Freynhagen R, Hardy J, Hjermstad MJ, et al. Neuropathic pain in cancer: systematic review, performance of screening tools and analysis of symptom profiles. Br J Anaesth. 2017Oct1;119(4):765–774. doi: 10.1093/bja/aex175 . [DOI] [PubMed] [Google Scholar]
- 14.Bouhassira D. Neuropathic pain: Definition, assessment and epidemiology. Rev Neurol (Paris). 2019Jan-Feb;175(1–2):16–25. doi: 10.1016/j.neurol.2018.09.016 Epub 2018 Oct 29. . [DOI] [PubMed] [Google Scholar]
- 15.Chojnowski M. Infrared thermal imaging in connective tissue diseases. Reumatologia. 2017;55(1):38–43. doi: 10.5114/reum.2017.66686 Epub 2017 Mar 22. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niehof SP, Huygen FJ, van der Weerd RW, Westra M, Zijlstra FJ. Thermography imaging during static and controlled thermoregulation in complex regional pain syndrome type 1: diagnostic value and involvement of the central sympathetic system. Biomed Eng Online. 2006May12(5):30. doi: 10.1186/1475-925X-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc. 2003May;78(5):603–12. doi: 10.4065/78.5.603 . [DOI] [PubMed] [Google Scholar]
- 18.Nahm FS. Infrared thermography in pain medicine. Korean J Pain. 2013Jul;26(3):219–22. doi: 10.3344/kjp.2013.26.3.219 Epub 2013 Jul 1. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ring EF, Ammer K. Infrared thermal imaging in medicine. Physiol Meas. 2012Mar;33(3):R33–46. doi: 10.1088/0967-3334/33/3/R33 Epub 2012 Feb 28. . [DOI] [PubMed] [Google Scholar]
- 20.Ministério da Saúde. Diretrizes para a vigilância, atenção e eliminação da Hanseníase como problema de saúde pública: manual técnico-operacional. [Internet]. Ministério da Saúde. 2016. 58 p. Brasil. Available from: http://lproweb.procempa.com.br/pmpa/prefpoa/cgvs/usu_doc/diretrizes_hanseniase.pdf.
- 21.American Academy of Thermology. Guidelines for Neuromusculoskeletal Infrared Thermography Sympathetic Skin Response (SSR) Studies. Pan American Journal Of Medical Thermology, 2015. 2(1), 35–43. Available from https://www.abraterm.com.br/revista/index.php/PAJMT/article/view/26. [Google Scholar]
- 22.Scollard DM. Classification of leprosy: a full color spectrum, or black and white? Int J Lepr Other Mycobact Dis. 2004Jun;72(2):166–8. doi: . [DOI] [PubMed] [Google Scholar]
- 23.Katz J, Melzack R. Measurement of pain. Surg Clin North Am. 1999Apr;79(2):231–52. doi: 10.1016/s0039-6109(05)70381-9 . [DOI] [PubMed] [Google Scholar]
- 24.Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005Mar;114(1–2):29–36. doi: 10.1016/j.pain.2004.12.010 Epub 2005 Jan 26. . [DOI] [PubMed] [Google Scholar]
- 25.Santos JG, Brito JO, de Andrade DC, Kaziyama VM, Ferreira KA, Souza I, Teixeira MJ, Bouhassira D, Baptista AF. Translation to Portuguese and validation of the Douleur Neuropathique 4 questionnaire. J Pain. 2010May;11(5):484–90. doi: 10.1016/j.jpain.2009.09.014 Epub 2009 Dec 16. . [DOI] [PubMed] [Google Scholar]
- 26.Compston A Aids to the investigation of peripheral nerve injuries. Medical Research Council: Nerve Injuries Research Committee. His Majesty’s Stationery Office: 1942; pp. 48 (iii) and 74 figures and 7 diagrams; with aids to the examination of the peripheral nervous system. By Michael O’Brien for the Guarantors of Brain. Saunders Elsevier: 2010; pp. [8] 64 and 94 Figures. Brain. 2010 Oct;133(10):2838–44. doi: 10.1093/brain/awq270 . [DOI] [PubMed] [Google Scholar]
- 27.Ministério da saúde. Cadernos de prevenção e reabilitação em hanseníase: normas e manuais técnicos, Manual de Prevenção de Incapacidades.3 ed. Brasília 2008.
- 28.Santos DFD, Mendonça MR, Antunes DE, Sabino EFP, Pereira RC, Goulart LR, et al. Revisiting primary neural leprosy: Clinical, serological, molecular, and neurophysiological aspects. PLoS Negl Trop Dis. 2017Nov27;11(11):e0006086. doi: 10.1371/journal.pntd.0006086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diniz LM, Moreira MV, Puppin MA, Oliveira MLWDR. Retrospective study on leprosy relapse in the State of Espírito Santo. Rev Soc Bras Med Trop. 2009; Aug42(4): 420–4. doi: 10.1590/s0037-86822009000400012 [DOI] [PubMed] [Google Scholar]
- 30.Gonçalves FG, Belone AFF, Rosa PS, Laporta GZ. Underlying mechanisms of leprosy recurrence in the Western Amazon: a retrospective cohort study. BMC Infect Dis. 2019May22;19(1):460. doi: 10.1186/s12879-019-4100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nobre ML, Illarramendi X, Dupnik KM, Hacker MA, Nery JA, Jerônimo SM, Sarno EN. Multibacillary leprosy by population groups in Brazil: Lessons from an observational study. PLoS Negl Trop Dis. 2017Feb13;11(2): e0005364. doi: 10.1371/journal.pntd.0005364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Araújo AERA Aquino DMC, Goulart IMB Pereira SRF, Figueiredo IA Serra HO, et al. Neural complications and physical disabilities in leprosy in a capital of northeastern Brazil with high endemicity. Rev Bras Epidemiol. 2014; oct-dec17(4): 899–910. doi: 10.1590/1809-4503201400040009 [DOI] [PubMed] [Google Scholar]
- 33.Santos DF, Mendonça MR, Antunes DE, Goulart LR, Goulart IMB. Epidemiologic, clinical, and laboratory aspects of leprosy neural relapses. Neurol Clin Pract. 2019Dec;9(6):468–471. doi: 10.1212/CPJ.0000000000000661 ; PMCID: PMC6927427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranque B, Nguyen VT, Vu HT, Nguyen TH, Nguyen NB, Pham XK, Schurr E, Abel L, Alcaïs A. Age is an important risk factor for onset and sequelae of reversal reactions in Vietnamese patients with leprosy. Clin Infect Dis. 2007Jan1;44(1):33–40. doi: 10.1086/509923 Epub 2006 Nov 28. . [DOI] [PubMed] [Google Scholar]
- 35.Giesel LM, Pitta IJR, da Silveira RC, Andrade LR, Vital RT, Nery JADC, Hacker MAVB, Sarno EN, Rodrigues MMJ. Clinical and Neurophysiological Features of Leprosy Patients with Neuropathic Pain. Am J Trop Med Hyg. 2018Jun;98(6):1609–1613. doi: 10.4269/ajtmh.17-0817 Epub 2018 Mar 29. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lund C, Koskinen M, Suneetha S, Lockwood DN, Haanpää M, Haapasalo H, Hietaharju A. Histopathological and clinical findings in leprosy patients with chronic neuropathic pain: a study from Hyderabad, India. Lepr Rev. 2007Dec;78(4):369–80. . [PubMed] [Google Scholar]
- 37.Garbino JA, Heise CO, Marques W Jr. Assessing nerves in leprosy. Clin Dermatol. 2016Jan-Feb;34(1):51–8. doi: 10.1016/j.clindermatol.2015.10.018 Epub 2015 Nov 6. . [DOI] [PubMed] [Google Scholar]
- 38.Saunderson P, Bizuneh E, Leekassa R. Neuropathic pain in people treated for multibacillary leprosy more than ten years previously. Lepr Rev. 2008Sep;79(3):270–6. . [PubMed] [Google Scholar]
- 39.Tiago LMP, Barbosa MFF, Santos DF, et al. Late follow-up of peripheral neural decompression in leprosy: functional and clinical outcomes. Arq Neuro-psiq. (internet). 10.1590/0004-282X-ANP-2020-0032. Forthcoming 2021. [DOI] [PubMed] [Google Scholar]
- 40.Garbino JA, Nery JA, Virmond M et al. Hanseníase: Diagnóstico e Tratamento da Neuropatia. In. Alves ED, Ferreira TL, Ferreira, editors. Projeto Diretrizes. Brasília. 2003;1–13. [Google Scholar]
- 41.Nugraha B, Gutenbrunner C, Barke A, Karst M, Schiller J, Schäfer P, et al. IASP Taskforce for the Classification of Chronic Pain. The IASP classification of chronic pain for ICD-11: functioning properties of chronic pain. Pain. 2019Jan;160(1):88–94. doi: 10.1097/j.pain.0000000000001433 . [DOI] [PubMed] [Google Scholar]
- 42.Raicher I, Stump PRNAG, Harnik SB, Oliveira RA, Baccarelli R, Marciano LHSC, et al. Neuropathic pain in leprosy: symptom profile characterization and comparison with neuropathic pain of other etiologies. Pain Rep. 2018Feb23;3(2): e638. doi: 10.1097/PR9.0000000000000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neves EB, Vilaça-Alves J, Rosa C, Reis VM. Thermography in Neurologic Practice. Open Neurol J. 2015Jun26; 9:24–7. doi: 10.2174/1874205X01509010024 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Etehadtavakol M, Ng EYK. An Overview of Medical Infrared Imaging in Breast Abnormalities Detection. In Application of Infrared to Biomedical Sciences. Singapore: Springer; 2017. 45–57 p. [Google Scholar]
- 45.Herry CL, Frize M. Quantitative assessment of pain-related thermal dysfunction through clinical digital infrared thermal imaging. Biomed Eng Online. 2004Jun28;3(1):19. doi: 10.1186/1475-925X-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uematsu S. Thermographic imaging of cutaneous sensory segment in patients with peripheral nerve injury. Skin-temperature stability between sides of the body. J Neurosurg. 1985May;62(5):716–720. doi: 10.3171/jns.1985.62.5.0716 . [DOI] [PubMed] [Google Scholar]
- 47.Jiang LJ, Ng EY, Yeo AC, Wu S, Pan F, Yau WY, Chen JH, Yang Y. A perspective on medical infrared imaging. J Med Eng Technol. 2005Nov-Dec;29(6):257–267. doi: 10.1080/03091900512331333158 . [DOI] [PubMed] [Google Scholar]
- 48.Feldman F, Nickoloff EL. Normal thermographic standards for the cervical spine and upper extremities. Skeletal Radiol. 1984;12(4):235–249. doi: 10.1007/BF00349505 . [DOI] [PubMed] [Google Scholar]
- 49.Hooshmand H, Hashmi M, Phillips E. Infrared thermal imaging as a tool in pain management-An 11-year study, Part II: Clinical Applications. Thermol Int. 2001Jan;11(2): 117–129. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information file.