Abstract
Study Design:
Prospective pilot clinical safety study of novel treatment, consecutive case series from first human use in patients with early adolescent idiopathic scoliosis (AIS).
Objective:
The primary purpose was to determine the initial safety of a titanium clip-screw implant system for spine growth modulation. The secondary aim was to document curvatures to 2 years postoperatively.
Summary of Background Data:
Spinal growth modulation was documented in preclinical studies. A prospective pilot clinical safety study was then performed under an FDA Investigational Device Exemption (IDE) (www.clinicaltrials.gov Identifier: NCT01465295).
Methods:
Six subjects with early AIS underwent thoracoscopic placement of titanium clip-screw devices. Eligibility criteria included only patients at high risk for progression to 50°: single major thoracic curve 25°−40°, age ≥10 years, skeletally immature (Risser 0 plus open triradiate cartilages), and if female, premenarchal. Adverse events (AEs), clinical outcomes, and radiographic measures were documented using Good Clinical Practices.
Results:
Six consecutive subjects were enrolled, three females and three males, aged 12.1 years (±1.7). Adverse events included one device-related — mild device migration at 18 months in the most rapidly progressive curve. Procedure-related AEs were mostly pulmonary. A chylous effusion which met the clinical protocol definition of a serious AE resolved after minimally invasive interventions. Major thoracic curves were 34° (± 3°) preoperatively and 38° (±18°) at two years (intra-subject change 4° ±18°). At 24 months, curves in 3 patients were >45° and 3 were <40°.
Conclusions:
A spine growth modulation system undergoing study under an FDA IDE was determined to be safe. Variability in curve response to the implant was high, ranging from progression to correction. Investigational approval was granted by the US FDA for the next cohort of 30 subjects.
Keywords: Adolescent idiopathic scoliosis, Spine growth modulation, Fusionless, Safety, Titanium
Introduction
Optimal treatment of late juvenile and early adolescent idiopathic scoliosis (AIS) remains challenging. Progression depends primarily on skeletal immaturity and major curve magnitude. Left untreated, patients with curves 25°−40° prior to their adolescent growth spurt are highly likely to progress to indications for surgical fusion [1–5] and have relatively low rates of bracing efficacy [6–9]. In a prospective study, bracing decreased the progression of curves to the threshold for surgery (≥50°) in skeletally immature AIS patients Risser 0 to 2, with rate of treatment success 72% after bracing compared with 48% after observation [10]. So although largely successful, years of bracing may yet result in fusion surgery. Posterior spinal fusion (PSF) with segmental spinal instrumentation corrects the curve at the expense of spine flexibility.
Scoliosis progression has long been considered to be caused in part by the Hueter-Volkmann principle via asymmetrical compression of the vertebral body physes [11–15]. Treating scoliosis by counteracting these stresses has long been a goal [16]. Since a report of an early attempt using vertebral body staples [17], many related approaches [18–19] have been reported. In long bones, hemiepiphyseal stapling was shown to cause histomorphometric changes in rat physes [20]. Clinical radiographs of staple hemiepiphysiodesis in knees were used to determine the compressive force on the physes that corrected asymmetric growth in long bones [21]. Early attempts to apply these principles to the spine reported mixed results [22–26]. The preclinical studies that led to directly to the present study used stainless steel staple-screw constructs in a large animal model [27–30]. In a series of in vivo tests, particular implant design factors were found to be critical to altering spine growth, with one implant design consistently inducing spine curvatures [28]. Further, asymmetrical structural changes to the vertebral growth plates indicated reduced growth on the implant side [29].
To move from preclinical to clinical study, changes were made to the materials, device design, surgical tools and procedures for patient safety, implant flexibility, explant procedures, and compatibility with MRI. A prospective clinical safety study of the titanium clip-screw system was begun from first human use under a US Food and Drug Administration (FDA) Investigational Device Exemption (IDE) (www.clinicaltrials.gov Identifier: NCT01465295) [31]. The implant construct was designed as a fusionless growth modulation system for patients who were diagnosed in the early stages of AIS and who had a high probability of curve progression to PSF threshold. The objective was to evaluate the initial acute safety of the system in the spinal guided growth treatment of progressive AIS, and determine the design and methods of a larger pivotal study. Early clinical results have been presented [30,32]. The primary purpose of the present study was to determine initial safety results in this first pilot cohort. Secondarily, major curvatures at 24 months postoperative were compared to immediate pre-operative values.
Material and Methods
Six subjects with progressive AIS underwent thoracoscopic placement of a titanium clip-screw device (SpineForm, LLC, Cincinnati, OH) (Figs. 1 and 2) (institutional review board approved). Written informed parental consent and child assent were obtained. The study design was a prospective case series, pilot phase safety study. Two sites were approved with a combined allowable sample size of 4 to 6 patients. The endpoint was completion of surgery of at least 4 cases with documented follow-up of one month and data monitoring committee (DMC) recommendation to continue to a larger pivotal study. Subjects were scheduled for evaluation at least 8 times to 24 months, plus additional follow-up until each achieved skeletal maturity, defined as height velocity <1 cm/year calculated from two assessments approximately six months apart [8].
Fig. 1.
Titanium clip-screw implant construct (size 12 mm) is shown with preloaded screws in four views.
Fig. 2.
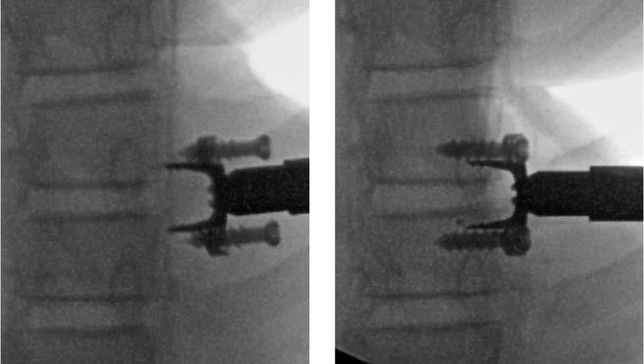
Intraoperative fluoroscopic images from the clinical study showing titanium clip with preloaded screws in insertion tool before (left) and after implantation (right). Disc wedging at implantation is shown, with disc height slightly decreased on the implant side and increased on the opposite side. [Reprinted from Reference 33, Figure 2: Wall EJ, Bylski-Austrow DI, Reynolds JE, et al. Chapter 45. Growth modulation techniques: titanium clip-screw implant system (HemiBridge). In: The growing spine. Management of spinal disorders in young children, 2nd ed, Part VI. Berlin: Springer; 2016:769—81]
Inclusion criteria were a diagnosis of idiopathic scoliosis, single major thoracic curve of Lenke Type 1A or 1B of magnitude 25°−40° measured by Cobb method, chronologic age ≥10 years, if female, pre-menarchal at screening examination, bone age as determined by radiographs of the left hand and wrist using the “Atlas Matching” (AM) method [33] for females ≥8 years+10 months and ≤13 years, and males ≥10 years and ≤15 years, skeletal immaturity classified as Risser grade 0 plus open triradiate cartilages (OTCs). Clinical diagnosis was determined from standing posterior- anterior (PA) radiographs with end vertebrae between or including T3 and L1. Subjects were to remove their braces, if worn, 24 hours before preoperative radiographs. Additional criteria included achievable anatomical fit of implants, body mass index <30, and ability to undergo surgery with single lung ventilation.
Exclusion criteria included any serious pulmonary or anatomic condition that would contraindicate an anterior thoracoscopic approach; prior thoracotomy, thorocostomy or any spine surgery; known history of, or existing, malignancy; any systemic or local infection; spinal cord abnormality requiring treatment; neurological deficit; reduced pulmonary function (pulmonary function test <60%) or moderate to severe ventilatory limitation; systemic disease; bleeding disorder; ataxia; family history of neurofibromatosis or Marfan syndrome; medical contraindication to anesthesia; known or suspected allergy to titanium; or lack of availability for interval visits and long-term follow-up examinations.
Study procedures complied with Good Clinical Practice and ISO 14155 (International Standards Organization) Clinical Investigation of Medical Devices for Human Subjects. Assistance with recording clinical and outcomes tests was provided by the base institution’s Clinical Trials Office. A data monitoring committee composed of three independent, pediatric orthopaedic spine surgeons determined if patients qualified under the radiographic eligibility criteria, independently measured Cobb angles, then reviewed and classified adverse events (AEs) by time, intensity, severity, and probability of relatedness to procedure or device.
An anesthesiologist performed single lung ventilation after bronchoscopically inserting a double lumen tube and deflating the lung on the convex side of the thoracic curve. With the goal of providing maximum gravity-assisted thoracic curve correction, the patient was placed on a radiolucent table in a lateral decubitus position, convex curve side up [34]. To improve spinal alignment, a padded axillary roll was placed just distal to the armpit, and the hip was elevated so as to allow the chest to hang freely, slightly contacting the table. Gas (CO2) insufflation was used to help deflate the lung on the operative side and improve the thoracoscopic view.
Using a thoracoscopic approach, segmental vessels were usually cauterized with a harmonic scalpel to minimize bleeding. Each clip, with two preloaded screws, was placed to encompass the disc and two vertebral body physes as viewed in the midvertebral body in a lateral view. After an acceptable position was confirmed with fluoroscopy, the clip was tamped into place and the screws were advanced. The remaining clips were similarly placed, in sequence, across adjacent discs, one at each level of the curve.
A chest tube was placed, then the deflated lung was expanded. A chest x-ray was obtained prior to extubation. In the first case, a mucus plug occurred on the ventilated side. Bronchoscopic lavage and suction were used to clear both lungs of mucus plugs immediately postoperatively in the remaining cases. Postoperatively protocol included aggressive pulmonary therapy with an incentive spirometer, cough, and deep breathing. The chest tube was removed when the output was approximately less than 50 ml over an eight-hour shift. No brace was used and no limitations on nonsports activities or cardiovascular conditioning were imposed. Team sports were restricted, conservatively, for 6 months in this pilot group.
Clinical and outcomes tests were administered at six-month intervals. Quality of life was determined using the Scoliosis Research Society (SRS)-22r questionnaire. Radiographically, standing PA with calibration standard, standing lateral, supine right and left side-bending, and left-hand PA radiographs were obtained at enrollment. Standing PA and standing lateral radiographs were taken postoperatively, and at 3, 6, 12, 18 and 24 months postoperation. Supine right and left side-bending radiographs were repeated at 24 months. At least two measurements for each Cobb angle were averaged. If the difference was >8°, measurement from a third reviewer was added. The levels used for the Cobb angles were not prescribed. Summary statistics were generated, with the caveat that the study was intentionally not powered to detect curve differences. Longitudinal curvatures were plotted at six-month intervals.
Results
The first consecutive six patients who met the eligibility criteria chose to enroll. Three were female, aged 11.9 years (±1.5), and three were male, aged 12.4 years (±2.2). Preoperative major thoracic curve was 34° (±3, range 30.5—38.5), all right-sided. Preoperative mean SRS-22r score was 4.4 (range 3.5—4.7). These six subjects underwent placement of the implants. Demographic, surgical, and pre-operative values were recorded (Table 1). Thoracoscopy under general anesthesia was used for all patients. Mean surgery time was 162 minutes (±31), implantation time 84 minutes (± 31), and estimated intraoperative blood loss ≤100 mL. Hospital stay following surgery was 3.7 days. In the first postoperative standing radiographs, the major curve averaged 24° (±8°).
Table 1.
Subject demographics, age at diagnosis, treatments previous to present surgery, immediately preoperative parameters, and surgical and implant parameters
| Subject ID | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Gender | F | F | M | M | F | M |
| Age at diagnosis / first standing radiograph | 12.3 | 10.3 | 10.1 / 11.2 | 7.2 | 6.9 | 13.0 |
| Curve magnitude at diagnosis / first standing (deg) | 23 | 11 | 26 by MRI / 39 # | 17 | 19 | 11 |
| Prior AIS treatment | None | None | None | Braced | None | Chiropractic |
| Age (years) at surgery | 13.0 | 12.6 | 11.7 | 10.6 | 10.1 | 14.9 |
| Bone age at surgery (years) Site / DMC | 12 / 11 | 12 / 11 | 13.5 / 12 | 10 / 10 | 11.5 / 12 | 13.5 / 13 |
| Height (cm) | 151 | 141 | 174 | 134 | 139 | 164 |
| Weight (kg) | 40.2 | 33.9 | 54.0 | 26.5 | 52.7 | 50.9 |
| BMI | 17.7 | 17.0 | 17.9 | 14.7 | 27.4 | 19 |
| BMI (%-ile age, gender) | 35 | 28 | 55 | 8 | 98 | 39 |
| Risser grade | 0 | 0 | 0 | 0 | 0 | 0 |
| Triradiate status | Open | Open | Open | Open | Open | Open |
| Menarchal status | Pre | Pre | NA | NA | Pre | NA |
| Clinical outcome score (SRS-22r) | 4.4 | 4.5 | 4.7 | 3.5 | 4.6 | 4.5 |
| Back pain, VAS (cm) | 0.8 | 4.3 | 0.2 | 0.2 | 1.5 | 0.5 |
| Pre-op curve parameters | ||||||
| Curve type (Lenke) | 1A | 1A | 1A | 1A | 1A | 1B |
| Curve side | Right | Right | Right | Right | Right | Right |
| Major thoracic curvature (deg) | 35 | 30.5 | 35 | 36 | 31 | 38.5 |
| Levels, Major curve | T6 - L1 | T6 - T12 | T5 - T10, T6-T11 | T5 - T11, T6-T11 | T5 - T10, T5T11 | T7 - T12 |
| Bend angle, major thoracic, (deg), R / L | 15 / 38 | 2 / 25 | 18.5 / 40 | 4 / 37 | 10 / 29 | 12 / 36 |
| Flexibility (%) = 100*[1 - CobbRtBend / CobbStanding pre-op] | 57 | 93 | 47 | 89 | 68 | 69 |
| Thoracolumbar/lumbar curvature (deg) | 24 | 26 | 18.5 | 27.5 | 18.5 | 28 |
| Levels, TL/L | L1-L4, L1-L5 | T12-L4, L1L4 | T10-L4, T11L4 | T11-L4 | T10-L3, T11L4 | T12-L4 |
| Bend angle, TL/L, (deg), R / L | 28 / 3 | 31 / -2.5 | 30 / 1 | 31 / 5.5 | 24 / 0 | 30 / 3 |
| Proximal thoracic curvature (deg) | 14 | 12 | 25.5 | 17 | 19 | 22 |
| Levels, PT | T1-T5, T2-T6 | T2-T6 | T1-T5, T2T5 | T2-T5, T2T6 | T1-T5, T2-T5 | T3-T6, T3T7 |
| Bend angle, PT, (deg), R / L | 22 / 4 | 20 / 1 | 35 / 11 | 14.5 / 4.5 | 16 / 4 | 25 / 6 |
| Thoracic kyphosis (deg) | 33.5 | 27.7 | 10.5 | 22.5 | 25.5 | 18.0 |
| Levels, kyphosis | T3-T12 | T3-T12, T1-T12, T3-L1 | T4-T12, T5T12 | T4-T12, T3T12 | T3-T12, T2T12 | T3-T12, T2T12 |
| Apical trunk rotation (deg) | 10 | 13 | 12 | 12 | 9 | 8 |
| Height velocity (cm/year), based on immediately pre-op to 6 months post-op times | 8.8 | 5.8 | 4.8 | 8.0 | 7.8 | 10.8 |
| Surgical parameters | ||||||
| Surgery time (minutes) | 203 | 173 | 162 | 123 | 182 | 129 |
| Implantation time (minutes) | 120 | 86 | 63 | 57 | 124 | 55 |
| Fluoroscopy time (s) | 140 | 120 | 95 | 106 | 157 | 102 |
| Anesthesia | General / SLV | General / SLV | General / SLV | General / SLV | General / SLV | General / SLV |
| Procedure | VATS | VATS | VATS | VATS | VATS | VATS |
| Estimated blood loss (ml) | 100 | <50 | 100 | <50 | 100 | 100 |
| Length of stay (days) | 5 | 4 | 3 | 3 | 4 | 3 |
| Suctioning of lung performed | No | Yes (lightly) | Yes (thoroughly R & L) | Yes | Yes (aggressively) | Yes |
| Intraoperative bronchoscopy | No (but performe d PO D4) | Yes | No | Yes (removed mucus plugs) | No | Yes (removed mucus plugs) |
| Pneumothorax on immediate post-op radiograph | No | Yes (resolved PO D1) | No | No | No | No |
| Number of portals | 3 | 3 | 3 | 2 | 2 | 2 |
| Implant parameters | ||||||
| Number of implants | 7 | 6 | 5 | 6 | 6 | 6 |
| Levels instrumented | T6–7 to T12-L1 | T6–7 to T11–12 | T5–6 to T910 | T5–6 to T10–11 | T5–6 to T1011 | T6–7 to T11–12 |
| Size of implants (mm), cranial to caudal | 12, 10, 10, 10, 12, 14, 14 | 10, 10, 12, 12, 12, 12 | 12, 14, 14, 14, 14 | 10, 12, 12, 12, 14, 14 | 10, 12, 12, 12, 14, 14 | 14, 14, 14, 14, 14, 14 |
| Initial post-op major thoracic curve at first standing radiograph (deg) | 27 | 13.5 | 35.5 | 20 | 26.5 | 19 |
AIS: Adolescent idiopathic scoliosis; BMI, body mass index; DMC, data monitoring committee; MRI magnetic resonance imaging; post-op, postoperative; SLV: Single lung ventilation; SRS-22r, Scoliosis Research Society – 22 Patient Questionnaire, revised; VAS, visual analog scale; VATS, video-assisted thoracoscopic spine surgery.
Patient from external institution; out of protocol for documented progression
Initial safety results showed no unanticipated adverse events. The one device-related AE was mild device migration at 18 months. Procedure-related AEs up to the first month postoperation were few, mostly pulmonary. A chylous effusion, which met the definition of a serious AE, was resolved with pigtail catheter and nonfat diet. A procedure-related mucous plug secondary to single lung ventilation in one patient resolved after bedside bronchoscopy. Minor procedure-related AEs immediately postoperation included nausea, dizziness, pain, atelectasis, small pneumothorax, and pleural effusion that resolved with observation. No device misplacement in spinal canal or disc space, no neuromonitoring changes or neurological deficits, and no device breakage, gross loosening or migration were reported.
Radiographically, major thoracic curvatures immediately preoperation, immediately postoperation, at 3, 6, 12, 18, and 24 months postoperation were: 34° (±3°), 24° (±8°), 26° (±9°), 28° (±9°), 30° (±13°), 36° (±15°), and 38° (±18°), respectively (Fig. 3) (Table 2). Mean intrasubject change in thoracic curve from pre-operative to 24 months was 3.8° (±18°). Mean lumbar curvatures at the same time points were 24° (±4°), 17° (±4°), 21° (±3°), 22° (±2°), 23° (±5°), 26° (±9°), 28° (±7°) (Fig. 4). The mean intrasubject change in lumbar curve from pre-operative to 24 months was 3.8° (±8°). Upper thoracic to cervical curvatures were 18° (±5°), 16° (±6°), 19° (±3°), 22° (±8°), 22° (±8°), 22.5° (±9.5°), 22° (±8°); mean intra-subject change: 4.2° (± 7°). Curve flexibilities based on right bend radiographs (Tables 1, 2) ranged from 47% to 93% preoperatively and from 10% to 70% at 24 months postoperation.
Fig. 3.
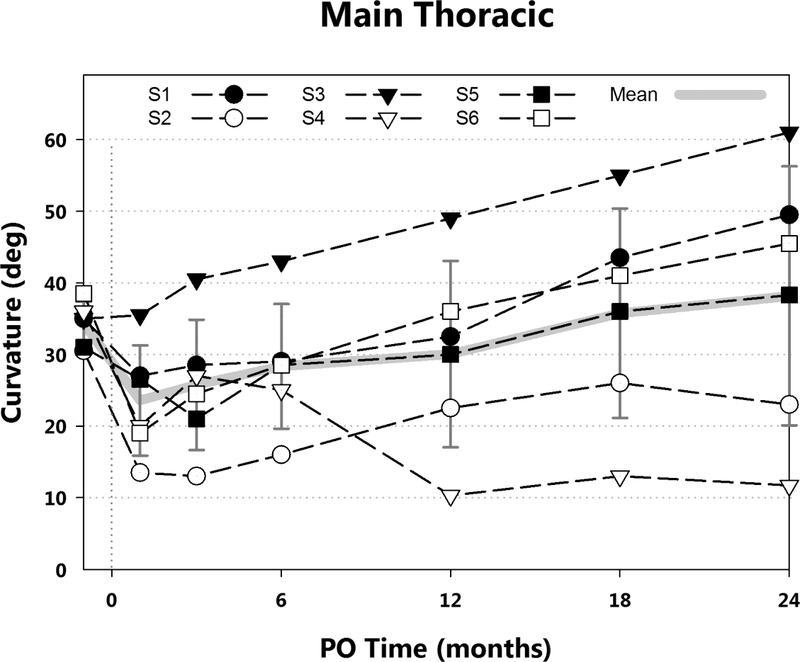
Major thoracic curvatures by postoperative (PO) time are shown. Mean and standard deviation (gray), and curvatures for each subject (black) are presented. Variability was high, however, the major curvature at 2 years postoperative remained below surgical fusion (PSF) indications (45° - 50°) in a cohort chosen specifically to have a high probability of progression. Mean intrasubject curvature increase from immediately preoperation to 24 months was less than 5°.
Table 2.
Postoperative time 24 months, subject demographics, clinical outcome scores, and radiographic parameters
| Subject ID | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|
| Demographics at 24 month follow-up | ||||||
| Age (years) | 15.1 | 14.6 | 13.5 | 12.3 | 12.1 | 16.9 |
| Height (cm) | 160 | 149 | 184 | 149 | 148 | 175 |
| Weight (kg) | 58.5 | 45.6 | 56.8 | 40.2 | 70.7 | 62.9 |
| Menarchal status | Post-menarchal (at 12 mo po) | Post-menarchal (at 12 mo po) | NA | NA | Post-menarchal (at 24 mo po) | NA |
| Risser grade | 2 | 3 | 4–5 | 0 | 3 | 2 |
| Triradiates | Closed | Closed | Closed | Open | Closed | Closed |
| Clinical outcome score (SRS-22r) | 3.7 | 4.8 | 3.4 | 3.9 | 4.5 | 3.7 |
| Back pain, VAS (cm) | 6.5 | 0.2 | 0.3 | 4.9 | 0.5 | 1.0 |
| Post-op 24 months curve parameters | ||||||
| Major thoracic curvature (deg) | 49.5 | 23 | 61 | 11.7 | 38.3 | 45.5 |
| Levels, Major curve | T5-L1, T6-L1 | T6-T12, T7T12 | T5-T10 | T5-T11, T6-T11 | T5-T11, T5-T10 | T6-T12 |
| Change in curvature from pre-op (deg) | 14.5 | −7.5 | 26 | −24.3 | 7.3 | 7 |
| Bend angle, major thoracic, (deg), R / L | 31 / 60 | 13 / 28 | 55 / 58 | 3.5/ 12.5 | 29 / 38.5 | 25 / 46 |
| Flexibility (%) = 100*[1 - CobbRtBend / CobbStanding pre-op] | 37 | 43 | 10 | 70 | 24 | 45 |
| Thoracolumbar/lumbar curvature (deg) | 27.3 | 29.5 | 21.5 | 17.5 | 31.5 | 38 |
| Proximal thoracic curvature (deg) | 30 | 13.5 | 31 | 14 | 18 | 28 |
| Thoracic kyphosis (deg) | 28.5 | 32.5 | 16.7 | 30.5 | 25 | 29 |
| Levels, kyphosis | T3 - T12 | T3 - T12 | T3 – T12, T4-T12, T2-L2 | T3 – T12 | T3-T12, T2T12 | T3-T12, T2T12 |
| Apical trunk rotation (deg) | 14 | 10 | 9 | 8 | 7 | 10.5 |
| Height velocity (cm/year), based on PO time 18 to 24 months | 2.8 | 0 | 7.4 | 7.6 | 1.4 | 3.0 |
| Rate of change of curvature from immediate PO to 24 months post-op (deg/year) | 11.3 | 4.8 | 12.8 | −4.2 | 5.9 | 13.3 |
| Time from menarche to 24 mo visit (months) | 18 | 14 | NA | NA | 0.4 | NA |
NA, not applicable; post-op, postoperative; SRS-22r, Scoliosis Research Society – 22 Patient Questionnaire, revised; VAS, visual analog scale.
Fig. 4.
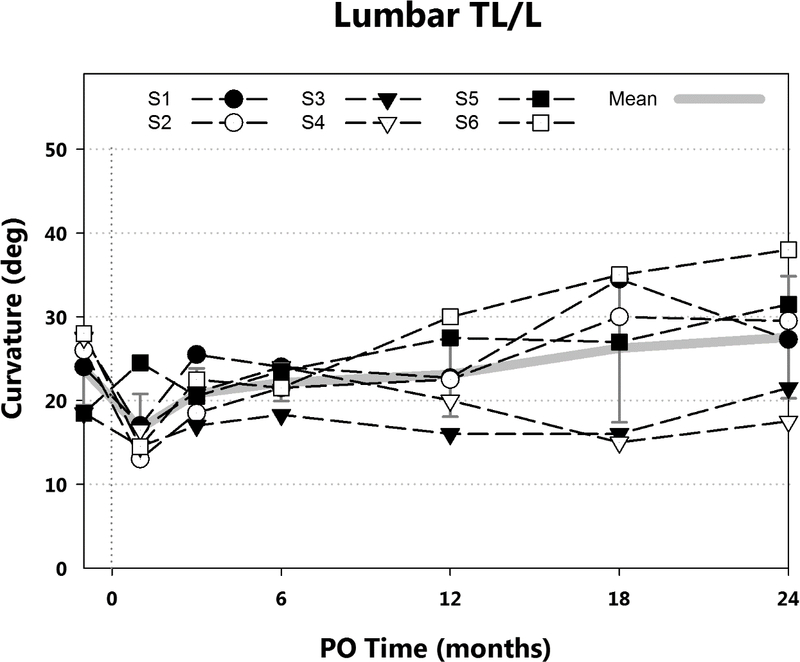
Lumbar curvatures by postoperative time are shown. Mean and standard deviation (gray), and curvatures for each subject (black) are presented. Mean intrasubject curvature increase from immediately preoperation was less than 5°.
In the sagittal plane, thoracic kyphosis values immediately preoperation and postoperation, and at 3, 6, 12, 18, and 24 months postoperation were 23° (±8°), 19° (±11°), 21° (±6°), 26° (±6°), 28° (±11°), 28° (±8°), and 27° (±6°). Mean intrasubject change from preoperation to 24 months was 4.1° (±5.9) (Fig. 5). For quality of life outcomes at 2 years, the SRS-22r mean score was 4.0 (3.4—4.8), with the lowest score occurring after the posterior fusion. Height velocities from 0 to 6 months and from 18 to 24 months were 7.7 cm/year (±2) and 3.8 cm/year (±3), respectively.
Fig. 5.
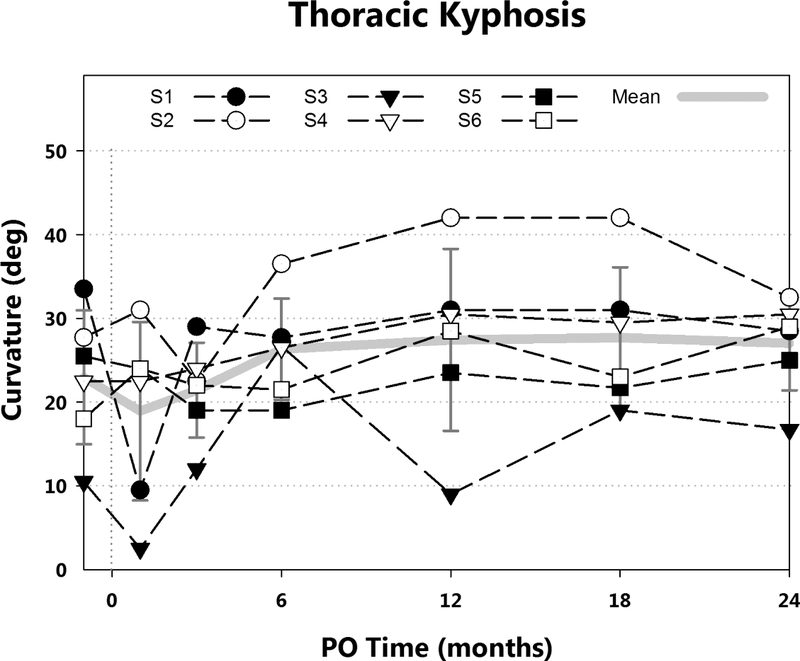
Thoracic kyphosis curvatures by postoperative time are shown. Mean and standard deviation (gray), and curvatures for each subject (black) are presented. Mean intrasubject curvature increase from immediately preoperation was less than 5°.
In two patients, the curve progressed to spinal fusion indications within 24 months. In the first, no immediate postoperative correction was achieved and the curve then increased by 1°/month. This subject, a male with the lowest curve flexibility, underwent PSF without removal of the clips at 22 months (Fig. 6) when the curve was 61°. In a female patient, the curve was reduced immediately postoperation from 35° to 27°. This curve then also increased at a rate of 1°/ month to 49.5° at 24 months. At 39 months, this patient underwent PSF without clip removal. In the midrange of response, three subjects (two female, one male) were each reduced immediately postoperatively, then lost some initial correction. At 24 months, differences from preoperative curvatures ranged from −8° to 7° in these three. In the most successful case, a male, the curve was corrected by 67% in 24 months, from 36° to 12° (Fig. 7a–d), with improvement in the rib hump (Fig. 7e–h).
Fig. 6.
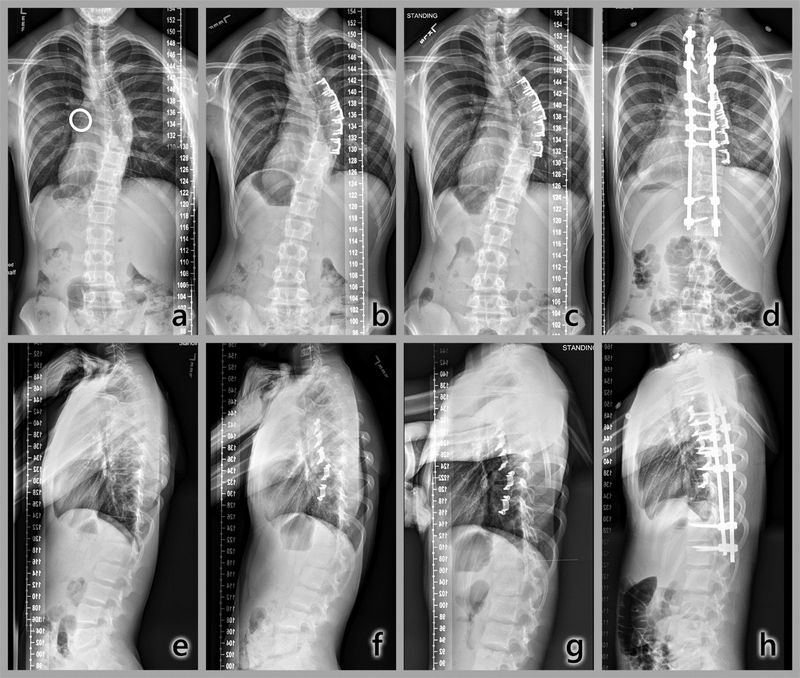
Radiographic time sequence of most rapidly progressing curvature. Top: Coronal plane (posteroanterior, PA). a) Immediately preoperative, with calibration ring, major thoracic curve, 35°; b) 3 months postoperation, 40.5°; c) 12 months, 49°; d) 22 months, after PSF without clip removal. Bottom: Sagittal plane. e) Immediate preoperative; f) 3 months postoperation; g) 12 months; h) 22 months postoperation. Thoracic hypokyphosis and rib hump are evident throughout the time sequence.
Fig. 7.
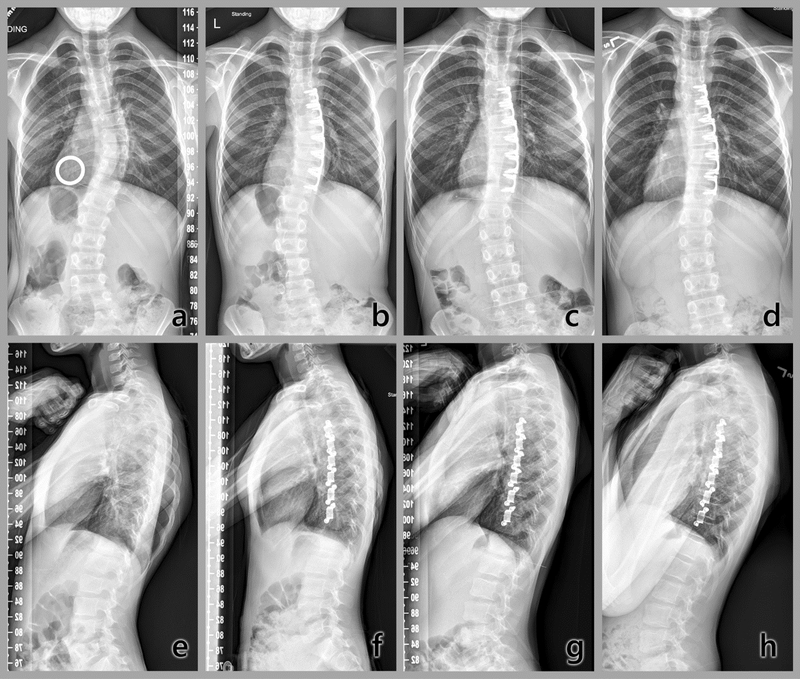
Radiographic time sequence of most successful subject results to date. Top: Coronal plane (posteroanterior, PA). a) Immediately preoperative, with calibration ring, major thoracic curve 36°; b) First postoperative standing radiograph, 20°; c) 1 year, 10°; d) 2 years, 12°. This time course showed that the method corrected a thoracic curvature, and altered the progression of a lumbar compensatory curvature, providing evidence of growth modulation in humans by this method. Bottom: Sagittal plane. e) Immediately preoperative; f) First postoperative standing; g) 1 year; h) 2 years. Note improvement in the symmetry of the posterior aspect of the ribs with reduction of rib hump. [Figs 6a—d reprinted from Figure 9, Chapter 45 in Wall EJ, Bylski-Austrow DI, Reynolds JE, et al. Chapter 45. Growth modulation techniques: titanium clip-screw implant system (HemiBridge). In: The growing spine. Management of spinal disorders in young children, 2nd ed, Part VI. Berlin: Springer; 2016:769–81]
Discussion
In an initial safety study of an experimental spine growth modulation system using titanium implants in very skeletally immature AIS patients, blood loss was minimal, surgical times low, and no device failure or misplacement occurred. Variability in major curve response to the implant was high. At 24 months, major thoracic curves in three patients were >45°, and three were <40°. In the subject with the greatest curve reduction, a male with a previously braced 36° curve, the time course indicated that the method may not only gradually correct a thoracic curve beyond initial correction, but may also alter progression of compensatory curves and coronal alignment. Therefore, this study demonstrated both the safety of the system and provided evidence of the principle of growth modulation by a gradual decrease in curvature after treatment in one patient. In those who subsequently underwent PSF, the effect on the second surgery was judged by the surgeon as not discernibly more difficult with the clips left in place.
The primary mechanism of curve progression, as well as loss of initial curve correction, was an increase in vertebral bone height between the implants (Fig 8a, b), likely due to micromotion at the bone—implant interface. By contrast, early results of disc and vertebral height ratios [35] as well as the results of the present study indicate that this system may counteract effects of scoliosis by disc compression on the convex side and decompression of the concave side (Fig. 8c, d, e). Previous studies have shown that scoliosis begins with disc wedging followed by vertebral wedging [36–38].
Fig. 8.
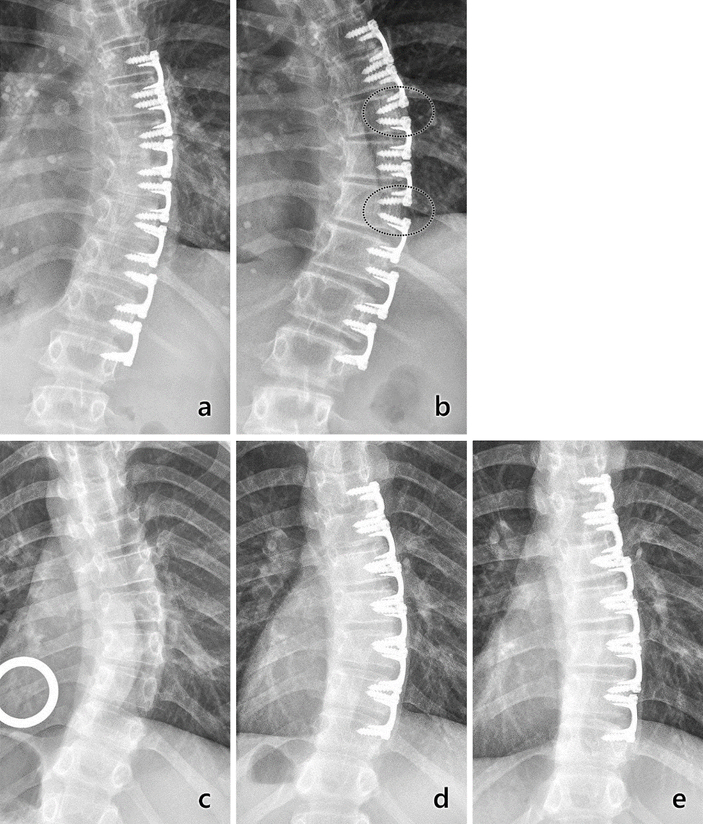
Radiographs (PA) of major thoracic curvatures from two subjects, one whose curvature progressed (top) and one whose curvature corrected (bottom). Top: Progression. a) Three-month postoperatively radiograph showing implant placement with 29° curve. b) At 18 months postoperatively, two pairs of adjacent implants clearly moved apart from each other, with apparent bone growth between the implants (circled areas) and increase in curvature. Bottom: Correction. a) Radiograph showing immediately preoperative curvature of 36°. b) Six-month postoperative radiograph showing implant placement and curvature reduction to 25°. c) At 2 years postoperatively, curvature was corrected to 12°. In this case, implants remained tightly grouped.
Limitations include that this was an early-phase clinical safety case series. As a Phase I FDA IDE study, the number of subjects was intentionally small, with no expectation of statistical power to detect differences in curvature. The strengths include that it was a prospective study with stringent eligibility criteria designed to include only subjects most likely to progress. Comparisons may now begin to be made to studies of observed, braced, or surgically treated patients that include longitudinal curvature results on subjects with the same eligibility criteria, with open triradiate cartilages and thoracic curves, especially prospective studies conducted under similar regulatory processes, and after the studies have outcomes at or beyond skeletal maturity. One bracing study [39] reported that patients with OTC had a 54% failure rate, more than twice the 21% failure rate of patients with closed triradiates. In other studies, most (83%) patients’ braced curves progressed to 45° or a magnitude requiring surgery when their curves were ≥30° prior to peak growth velocity [40,41]. Notably, in a study of Risser stage on bracing outcome in AIS, for curves 30°−39°, the rate of surgery (progression to ≥50°) for the 30 children with OTC, all curve types combined, was 70% [42]. At ≥15 hours of daily wear, 54% of patients with OTC needed surgery. Even at ≥18 hours of measured wear, 7 of 10 curves progressed to ≥50°. In those 7, the curve averaged 36.4° at brace prescription and wear time was 19.3 hours/day [42].
Factors that may affect the performance of this system include those related to subject, implant design, the relationship between them, and the surgical process. Specifically, these include: anthropometry, gender, curve magnitude, rotation, and flexibility; blade size, implant flexibility; screw purchase, relative fit to motion segments, surface characteristics at the bone-implant interface, and intraoperative curve correction. With respect to the process of preparing for the present study, preclinically, the constructs were characterized mechanically and with computational models. In vivo tests were repeated in the porcine model using an independent testing facility accredited for Good Laboratory Practices (GLP). The divergently angled blades combined with implant screw fixation were shown to promote initial disc wedging (Fig. 2) and to allow for thoracoscopic explantation by reversal of implantation procedures. Biomechanical tests were used to determine disc compressive stresses in vitro and in vivo, computational models were used to estimate disc stresses and growth reductions [43–46]. Biomechanical tests [47,48] also showed that partial spine flexibility was retained, due in part to material properties of titanium plus the geometry of the bridge and blades. These preclinical studies combined with the results of the present clinical study allowed for inferences to guide improvements to the system.
Conclusions
In this initial safety study of a spinal growth modulation system, comprised of titanium clip-screw implant constructs, in a small prospective cohort of very skeletally immature AIS patients at very high risk of scoliosis progression, blood loss was minimal, surgical times low, and no device misplacement occurred. FDA IDE approval was granted for the next 30 subjects. Whereas the number of subjects was intentionally small, and radiographic results were highly variable, evidence of growth modulation was provided by a subject whose thoracic curve gradually decreased. The larger proposed study of 30 patients may allow for subgroup analysis to categorize patients who may benefit the most from this procedure. If eventually proven efficacious, the system may obviate PSF or years of brace wear for select patients with late juvenile to early adolescent idiopathic scoliosis.
Highlights:
A spine growth modulation system undergoing study under an FDA IDE was determined to be safe.
Variability in curvature change was high, ranging from progression to surgical posterior spinal fusion to curve stability to curve correction.
Gradual curve correction provided evidence of growth modulation by this method.
Investigational approval was granted by the US FDA for the next cohort of 30 subjects.
Device Status Statement:
The devices that are the subject of this manuscript are being evaluated as part of an ongoing FDA-approved investigational protocol (IDE) for the intended use of guided spinal growth treatment of progressive idiopathic scoliosis (IS). The test article is intended for anterior-lateral fixation across the growth plates from T3 to L1 with placement through video-assisted thoracoscopic surgery.
Acknowledgements:
The authors thank the following individuals: Rich Grant, Kevyn Irving, Rena Irving, David L. Glos, Jose A. Herrera-Soto, MD, Courtney W. Brown, MD, Mark A. Erickson, MD, Robert M. Campbell Jr., MD, Richard E. McCarthy, MD, Peter F. Sturm, MD. Regulatory status: IRB approved, FDA Investigational Device Exemption (IDE), http://clinicaltrials.gov /show/ NCT01465295. U.S. Food and Drug Administration. Evaluate Initial Safety of the HemiBridge System in Guided Spinal Growth Treatment of Progressive Idiopathic Scoliosis. 2013 [cited 2015 Nov 5]. Device approved for use in European Union (CE Mark) for the labeled indications. Financial activities of authors relevant to the submitted work include: board memberships, advisories, consultancies, intellectual property/patent rights, grants/grants pending/royalty, travel/ accommodations/meeting expenses, equity/stock/stock options/ownership interest, and employment.
Funding:
Grant funding to support this study was awarded to sponsor SpineForm, LLC, by the State of Ohio, Ohio Third Frontier (2010–13) and USA FDA R01 1-R01 FD004144–01 (2012–13).
References
- 1.Ponseti IV, Friedman B. Prognosis in idiopathic scoliosis. J Bone Joint Surg Am. 1950:32:381–95. [PubMed] [Google Scholar]
- 2.Lonstein JE, Carlson JM. The prediction of curve progression in untreated idiopathic scoliosis during growth. J Bone Joint Surg Am. 1984September1;66:1061–71. [PubMed] [Google Scholar]
- 3.Charles YP, Daures JP, de Rosa V, et al. Progression risk of idiopathic juvenile scoliosis during pubertal growth. Spine. 2006;31:1933–42. [DOI] [PubMed] [Google Scholar]
- 4.Sanders JO, Browne RH, McConnell SJ, Margraf SA, Cooney TE, Finegold DN. Maturity assessment and curve progression in girls with idiopathic scoliosis. J Bone Joint Surg Am. 2007;89:64–73. [DOI] [PubMed] [Google Scholar]
- 5.Sanders JO, Khoury JG, Kishan S, et al. Predicting scoliosis progression from skeletal maturity: a simplified classification during adolescence. J Bone Joint Surg Am. 2008;90:540–553. [DOI] [PubMed] [Google Scholar]
- 6.Karol LA, Johnston CE, Browne RH, et al. Progression of the curve in boys who have idiopathic scoliosis. J Bone Joint Surg Am. 1993;75:1804–1810. [DOI] [PubMed] [Google Scholar]
- 7.Karol LA. Effectiveness of bracing in male patients with idiopathic scoliosis. Spine. 2001;26:2001–2005. [DOI] [PubMed] [Google Scholar]
- 8.Richards BS, Bernstein RM, D’Amato CR, et al. Standardization of criteria for adolescent idiopathic scoliosis brace studies: SRS Committee on Bracing and Nonoperative Management. Spine. 2005;30:2068–75. [DOI] [PubMed] [Google Scholar]
- 9.Katz DE, Herring JA, Browne RH, et al. Brace wear control of curve progression in adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2010;92:1343–1352. [DOI] [PubMed] [Google Scholar]
- 10.Weinstein SL, Dolan LA, Wright JG, et al. Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med. 2013;369:1512–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arkin AM. The mechanism of the structural changes in scoliosis. J Bone Joint Surg Am. 1949;31:519–28. [PubMed] [Google Scholar]
- 12.Roaf R Vertebral growth and its mechanical control. J Bone Joint Surg Br. 1960;42-B:40–59. [DOI] [PubMed] [Google Scholar]
- 13.Schultz AB. Biomechanical factors in the progression of idiopathic scoliosis. Annals Biomed Engineering 1984;12:621–630. [DOI] [PubMed] [Google Scholar]
- 14.Stokes IAF, Aronsson DD, Urban JPG. Biomechanical factors influencing progression of angular skeletal deformities during growth. Eur J Exp Musculoskel Res. 1994;3:51–60. [Google Scholar]
- 15.Stokes IA, Burwell RG, Dangerfield PH, IBSE: Biomechanical spinal growth modulation and progressive adolescent scoliosis—a test of the ‘vicious cycle’ pathogenic hypothesis: summary of an electronic focus group debate of the IBSE. Scoliosis. 2006;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrington PR. Is Scoliosis Reversible?: In vivo observations of reversible morphological changes in the production of scoliosis in mice. Clinical orthopaedics and related research. 1976;116:103–11. [PubMed] [Google Scholar]
- 17.Smith AD, Von Lackum WH, Wylie R. An operation for stapling vertebral bodies in congenital scoliosis. J Bone Joint Surg Am. 1954;36-A:342–8. [PubMed] [Google Scholar]
- 18.MacEwen GD. Experimental scoliosis. Clin Orthop. 1973;93:69–74. [DOI] [PubMed] [Google Scholar]
- 19.Piggott H Growth modification in the treatment of scoliosis. Orthopedics. 1987;10(6):945–952. [DOI] [PubMed] [Google Scholar]
- 20.Farnum CE, Nixon A, Lee AO, et al. Quantitative three-dimensional analysis of chondrocytic kinetic responses to short-term stapling of the rat proximal tibial growth plate. Cells Tissues Organs. 2000;167:247–58. [DOI] [PubMed] [Google Scholar]
- 21.Bylski-Austrow DI, Wall EJ, Rupert MP, et al. Growth plate forces in adolescent human knees: Radiographic and mechanical study of epiphyseal staples. J Pediatr Orthop. 2001;21:817–23. [PubMed] [Google Scholar]
- 22.Nachlas IW, Borden JN. The cure of experimental scoliosis by directed growth control. J Bone Joint Surg Am. 1951;33-A:24–34. [PubMed] [Google Scholar]
- 23.Wynarsky G, Schultz A. Effects of age and sex on the external induction of scoliosis in rats. Spine 1987;12:974–77. [DOI] [PubMed] [Google Scholar]
- 24.Sarwark JF, Dabney KW, Salzman SK, et al. Experimental scoliosis in the rat. I. Methodology, anatomic features and neurologic characterization. Spine. 1988;13:466–71. [DOI] [PubMed] [Google Scholar]
- 25.Mente PL, Aronsson DD, Stokes IAF, et al. Mechanical modulation of growth for the correction of vertebral wedge deformities. J Orthop Res. 1999;17.4:518–24. [DOI] [PubMed] [Google Scholar]
- 26.Stokes IA, Mente PL, Iatridis JC, et al. Enlargement of growth plate chondrocytes modulated by sustained mechanical loading. J Bone Joint Surg Am. 2002;84:1842–48. [DOI] [PubMed] [Google Scholar]
- 27.Bylski-Austrow DI, Wall EJ, Kolata RJ, et al. Endoscopic nonfusion spinal hemiepiphysiodesis. Preliminary studies in a porcine model. Research into Spinal Deformities 2. 1999;2:270–3. [Google Scholar]
- 28.Wall EJ, Bylski-Austrow DI, Kolata RJ, et al. Endoscopic mechanical spinal hemiepiphysiodesis modifies spine growth. Spine. 2005; 30:10:1148–1153. [DOI] [PubMed] [Google Scholar]
- 29.Bylski-Austrow DI, Wall EJ, Glos DL, et al. Spinal hemiepiphysiodesis decreases the size of vertebral growth plate hypertrophic zone and cells. J Bone Joint Surg Am. 2009;91:584–593. [DOI] [PubMed] [Google Scholar]
- 30.Wall EJ, Bylski-Austrow DI. Growth modulation techniques for non-idiopathic early onset scoliosis. In: Yazici M, editor. Non-idiopathic spine deformities in young children. Heidelberg: Springer; 2011. p. 133–144. [Google Scholar]
- 31.Clinicaltrials.gov [Internet]. Identifier: NCT01465295: Evaluate initial safety of the HemiBridge™ System in guided spinal growth treatment of progressive idiopathic scoliosis. [updated 2013 Aug 28]. Available from: http://clinicltrials.gov/ct2/show/NCT01465295.
- 32.Wall EJ, Bylski-Austrow DI, Reynolds JE, et al. Chapter 45. Growth Modulation Techniques: Titanium Clip-Screw Implant System (HemiBridge). The Growing Spine. Management of Spinal Disorders in Young Children” Edition 2, Part VI pp 769–781. Springer Berlin Heidelberg, 2016. 769–81. [Google Scholar]
- 33.Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist, Stanford, Stanford University Press; 21, 1959:266–270. [Google Scholar]
- 34.Jain V, Lykissas M, Trobisch P, et al. Surgical Aspects of Spinal Growth Modulation in Scoliosis Correction. Am Acad Orthop Surg Instr Course Lect, 2014;63:335–44. [PubMed] [Google Scholar]
- 35.Bylski-Austrow DI, Entsuah N, Glos DL, et al. Spine growth modulation using titanium clip/screw device: Curvature, vertebral and disc height changes at 1 year. Trans Orthop Res Soc. 2015:682. [Google Scholar]
- 36.Grivas TB, Vasiliadis E, Malakasis M, Mouzakis V, Segos D. Intervertebral disc biomechanics in the pathogenesis of idiopathic scoliosis. Studies in health technology and informatics. 2006;123:80. [PubMed] [Google Scholar]
- 37.Volz R, Dolan LA, Masrouha F, et al. The effect of radiographic vertebral body and intervertebral disc wedging on curve progression in idiopathic scoliosis. Scoliosis. 2013;8(Suppl 1):O38. [Google Scholar]
- 38.Will RE, Stokes IA, Qiu X, et al. Cobb angle progression in adolescent scoliosis begins at the intervertebral disc. Spine. 2009;34:2782–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryan PM, Puttler EG, Stotler WM, et al. Role of the triradiate cartilage in predicting curve progression in adolescent idiopathic scoliosis. Journal of Pediatric Orthopaedics. 20071;27(6):671–6. [DOI] [PubMed] [Google Scholar]
- 40.Little DG, Song KM, Katz D, et al. Relationship of peak height velocity to other maturity indicators in idiopathic scoliosis in girls. J Bone Joint Surg Am 2000May1;82(5):685–93. [DOI] [PubMed] [Google Scholar]
- 41.Song KM, Little DG. Peak height velocity as a maturity indicator for males with idiopathic scoliosis. Journal of Pediatric Orthopaedics. 2000May1;20(3):286–8. [PubMed] [Google Scholar]
- 42.Karol LA, Virostek D, Felton K, et al. The Effect of the Risser Stage on Bracing Outcome in Adolescent Idiopathic Scoliosis. J Bone Joint Surg Am. 2016August3;98(15):1253–9. [DOI] [PubMed] [Google Scholar]
- 43.Glos DL, Sauser FE, Papautsky I, et al. Implantable MEMS compressive stress sensors: Design, fabrication and calibration with application to the disc annulus. J Biomechanics. 2010;43:2244–48. [DOI] [PubMed] [Google Scholar]
- 44.Bylski-Austrow DI, Glos DL, Sauser FE, et al. In vivo dynamic compressive stresses in the disc annulus: a pilot study of bilateral differences due to hemiepiphyseal implant in quadruped model. Spine. 2012;37:E949–56. [DOI] [PubMed] [Google Scholar]
- 45.Kumar B, Bylski-Austrow DI, Liu Y. Finite element model of spinal hemiepiphysiodesis: Effect of contact conditions, initial conditions, and growth. Studies in health technology and informatics. 2011;176:99–103. [PubMed] [Google Scholar]
- 46.Bylski-Austrow DI, Kumar B, Wall EJ. Vertebral growth reductions after spinal hemiepiphysiodesis as determined by histomorphometry are lower than predicted by finite element model with linear stress-growth relationship, Trans Orthop Res Soc. 2013:293. [Google Scholar]
- 47.Coombs MT, Glos DL, Wall EJ, et al. Biomechanics of spinal hemiepiphysiodesis for fusionless scoliosis treatment using titanium implant. Spine. 2013;38:E1454–60. [DOI] [PubMed] [Google Scholar]
- 48.Coombs MT, Glos DL, Carvalho MF, et al. Biomechanics of spinal hemiepiphysiodesis for early adolescent idiopathic scoliosis using clinically relevant construct. Trans Orthop Res Soc. 2014:1593. [Google Scholar]



