Abstract
AKS-452 is a biologically-engineered vaccine comprising an Fc fusion protein of the SARS-CoV-2 viral spike protein receptor binding domain antigen (Ag) and human IgG1 Fc (SP/RBD-Fc) in clinical development for the induction and augmentation of neutralizing IgG titers against SARS-CoV-2 viral infection to address the COVID-19 pandemic. The Fc moiety is designed to enhance immunogenicity by increasing uptake via Fc-receptors (FcγR) on Ag-presenting cells (APCs) and prolonging exposure due to neonatal Fc receptor (FcRn) recycling. AKS-452 induced approximately 20-fold greater neutralizing IgG titers in mice relative to those induced by SP/RBD without the Fc moiety and induced comparable long-term neutralizing titers with a single dose vs. two doses. To further enhance immunogenicity, AKS-452 was evaluated in formulations containing a panel of adjuvants in which the water-in-oil adjuvant, Montanide™ ISA 720, enhanced neutralizing IgG titers by approximately 7-fold after one and two doses in mice, including the neutralization of live SARS-CoV-2 virus infection of VERO-E6 cells. Furthermore, ISA 720-adjuvanted AKS-452 was immunogenic in rabbits and non-human primates (NHPs) and protected from infection and clinical symptoms with live SARS-CoV-2 virus in NHPs (USA-WA1/2020 viral strain) and the K18 human ACE2-trangenic (K18-huACE2-Tg) mouse (South African B.1.351 viral variant). These preclinical studies support the initiation of Phase I clinical studies with adjuvanted AKS-452 with the expectation that this room-temperature stable, Fc-fusion subunit vaccine can be rapidly and inexpensively manufactured to provide billions of doses per year especially in regions where the cold-chain is difficult to maintain.
Keywords: Infectious disease, Coronavirus, Prophylaxis, Pandemic, COVID-19, Fc-fusion
Abbreviations: ACE2, angiotensin converting enzyme-2; Ag, antigen; APCs, antigen-presenting cells; ELISA, Enzyme Linked Immunosorbent Assay; EUA, Emergency Use Authorization; HCS, Human convalescent serum; ID50, Inhibitory Dilution 50%; MFI, mean fluorescent intensity; nAb, neutralizing antibody; NHP, non-human primate; OD, optical density; P, passage; PFU, plaque-forming units; PRNT, plaque reduction neutralization test; PRNT50, maximum dilution with greater than 50% inhibition; SP, Spike Protein; SP/RBD, spike protein receptor binding domain
1. Background
The COVID-19 pandemic has resulted in significant cases of infection and deaths globally due to the novel SARS-CoV-2 coronavirus [1], despite public health measures such as social-distancing and mask-wearing. Therefore, development of a variety of vaccine formats and modalities is critical to the implementation of more effective large-scale public health measures to control the pandemic [2], [3]. The most advanced vaccine platforms include live viral vectors carrying SARS-CoV-2 Ag-encoding genetic material, liposome-encapsulated mRNA encoding the Spike Protein (SP), and inactivated whole virus that have reported significant protective efficacy in relatively short Phase III studies [4], [5], [6], [7], [8], [9], [10], [11] leading to Emergency Use Authorization (EUA) [12]. While short-term safety of these EUA vaccines has been deemed acceptable based on outcomes of clinical studies and safety monitoring of general population vaccination programs, there remains a need for an improved safety profile upon multiple dosing (reviewed in [13]) and for optimization of the cost, scale, and speed of manufacturing and distribution, especially to underdeveloped geographic regions of globally-diverse populations [14], [15].
To address these obstacles, an adjuvanted recombinant subunit vaccine, AKS-452, is in development, comprising the fusion protein Ag, SP/RBD-Fc, formulated in the water-in-oil adjuvant, Montanide™ ISA 720. The RBD Ag focuses the neutralizing antibody (nAb) response to the most effective SP region required for virus binding to the host target protein, angiotensin converting enzyme-2 (ACE2) [16], thus avoiding Antibody-Dependent Enhancement (ADE) of viral infectivity mediated by non-RBD-binding Abs to SP, as was shown with SARS and MERS coronavirus vaccine candidates [17]. The AKS-452 Fc moiety is designed to enhance immunogenicity by increasing uptake of the SP/RBD Ag via FcγRs on APCs [18] and prolonging exposure via FcRn recycling such that immunogenicity might be achieved with a single dose. Immunogenicity is further enhanced via delivery with the demonstrably-safe and well-tolerated ISA 720 adjuvant [19] to ensure amplification and durability of neutralizing IgG titers and the desired T helper 1 (Th1) response. Importantly, the RBD fused with Fc has demonstrated at least a 10-fold greater production yield relative to that of the whole SP Ag expression (<0.1 g/L for SP compared to >1 g/L for SP/RBD-Fc in the same expression system). Here we describe the development of AKS-452 in animals, which has supported the initiation of a Phase I/II safety and immunogenicity clinical trial (ClinicalTrials.gov: NCT04681092).
2. Methods
2.1. Vaccine components
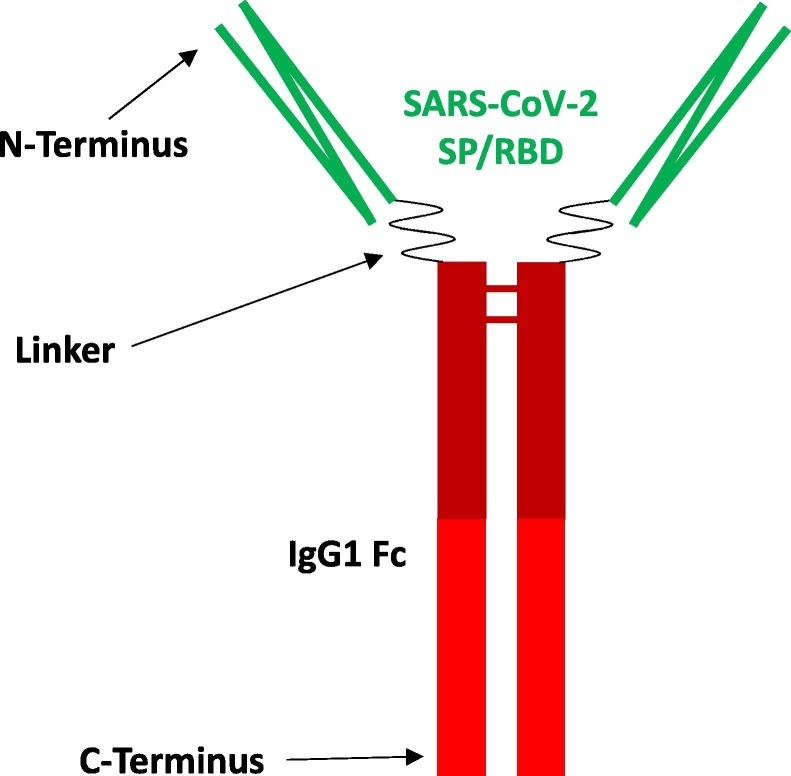
AKS-452 is a recombinant fusion protein comprising SP/RBD and an Fc fragment containing a portion of the hinge region, in which the full CH2 and CH3 domains of the human IgG1 Fc fragment are connected via a covalent peptide linker sequence, all encoded by a single nucleic acid molecule expressed in CHO-K1 cells (Fig. 1 ; #700452, 522 µg/ml; Akston Biosciences, Beverly, MA; see PCT/US21/26577 for details). AKS-452 (Batch #: MDS0001) was expressed in a CHO-K1 cell line derivative (LakePharma, Belmont, CA), harvested via depth filtration (Pall Corporation, Port Washington, NY), purified via Protein-A affinity chromatography (MabSelect Sure, Cytiva Life Sciences, Marlborough, MA) followed by buffer exchange, further purified via anion exchange chromatography (Q-HP resin, Cytiva) with final buffer exchange, and concentrated via ultrafiltration-diafiltration (TengenX SIUS 30 kDa, Repligen, Waltham, MA) to 522 µg/mL confirmed by A280. Final drug substance was identified via an observed mass of 112,950.3 Da versus an expected mass of 112,943.4 Da (<0.01% difference) via LC-MS with glycan removal. The batch was >98% with respect to molecular aggregates via SEC-HPLC and fragments via capillary electrophoresis-sodium dodecyl sulfate (CE-SDS) analysis (see Supplemental Methods and Supplemental Tables 1, 2, and 3 for production and characterization details). The expression yield was 0.75 g/L for material used in this study and has since been optimized to approximately 3 g/L, compared to less than 0.1 g/L for non-Fc modified, full length SP produced in the same expression system. For adjuvanted immunizations, sterile aqueous solutions of AKS-452 were emulsified in the water-in-oil adjuvant, Montanide™ ISA 720 (#2624653, Seppic S.A., Paris, France; 30%/70% aqueous Ag/adjuvant emulsification) [19] and injected into animals within 16 h of preparation. A murine IgG2a Fc fusion protein with the SP/RBD was expressed in HEK293 cells and was also used for immunizations (muIgG2a-Fc-SP/RBD; SPD-C5259, 600 µg/ml; AcroBiosystems Inc., Newark, DE).
Fig. 1.
Structure of AKS-452, an Fc fusion protein of SP/RBD and human IgG Fc (SP/RBD-Fc). SARS-CoV-2 SP/RBD Ag (green), Linker of amino acid sequence creating the fusion between the SP/RBD and the Fc fragment, human IgG1 Fc fragment that directs antigen presenting cells to take up and process the SP/RBD Ag via FcγRs and enhances residence time via FcRn recycling (red). There is one N-linked glycosylation site on the “N297” site (using the well-known Kabat amino acid numbering scheme for antibodies, which for AKS-452 is at position 358 from the N-terminus) in the Fc fragment, along with two N-linked glycosylation sites in the SP/RBD region of the molecule at positions 14 and 25 from the N-terminus. The molecular weight of each monomer of the homodimer is 97,365.48 + 10.0 Da (post deglycosylation).
2.2. Ethics statement and animal exposure (for all animal studies)
All animal studies (at different institutions) were performed with protocols approved by their respective Animal Care and Use Committees (IACUCs) in accordance with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978), and with veterinary care in accordance with the testing facility standard operating procedures and regulations outlined in the applicable sections of the Final Rules of the Animal Welfare Act regulations (9 CFR).
2.3. Immunogenicity studies in BALB/c mice
Six- to eight-week old female BALB/c mice (Jackson laboratories, Bar Harbor, ME, USA) were acclimated for at least 7 days prior to randomization into study groups. Mice were administered one, two, or three doses of vaccine with or without adjuvant (100 µL, unless otherwise stated) 21 days apart via s.c. injection. In some experiments, titers were allowed to resolve below 500 µg/mL SP/RBD-specific IgG for a few weeks before receiving a booster injection of AKS-452 without adjuvant. Blood was collected via submandibular venipuncture into micro-vacutainer tubes 7 days prior to the first dose and then 14 days after each dose, and allowed to clot before serum was separated by centrifugation and stored at −20 °C.
2.4. Immunogenicity studies in NHPs
An immunization study to evaluate the effectiveness of different vaccine formulations was performed at Biomere, Inc (Worcester, MA) with cynomolgus monkeys of Chinese and Indonesian orgin (purpose bred, Macaca fascicularis). Male and female animals 2- to 4-years of age weighing 2 to 3 kg were purchased from Alpha Genesis (Yemassee, SC). Animals were acclimated for at least 14 days prior to randomization into study groups and were administered vaccine in one, two, or three doses without adjuvant at 100 µg (100 μL) or 1,000 µg (1,000 μL) or emulsified in ISA 720 at 10 µg (67 µL) or 30 µg (200 μL) on days 0, 21, and 100 (or day 150 as indicated) via s.c. injection. Animals were observed for 1–3 h after each vaccination dose, then daily for general health. Blood was collected via peripheral vein venipuncture into vacutainer tubes and allowed to clot prior to serum separation via centrifugation and stored at −20 °C.
2.5. SP/RBD-specific serum IgG titer ELISAs for mouse, NHP, human samples
Serum anti-SP/RBD IgG Ab titers were determined by several types of ELISAs. Serum samples were diluted in sample dilution buffer (1:100–1:10,000) prior to addition to microtiter wells coated with wild-type SARS-CoV-2 SP/RBD protein (#200080; Lake Pharma, Inc., San Mateo, CA) and incubated for one hour. Reference immune sera from the appropriate species (animals treated with AKS-452 emulsified in ISA 720) were used to generate sample titer values expressed as a dilution of the reference curve in Reference Titer Units. Human convalescent serum (HCS) samples from subjects known to have acquired COVID-19 that recovered to an asymptomatic state for at least 14 days prior to serum collection were purchased or obtained from BioIVT (Westbury, NY), Invent Diagnostica (Hennigsdorf, Germany), and locally sourced donors under informed consent. After washing plates, bound SP/RBD specific IgG serum Abs were detected by a goat anti-species-specific IgG (anti-mouse total IgG, IgG1, IgG2a, IgG2b, IgG3, Southern Biotech #1073-05, 1083-05, 1093-05, 1103-05, respectively; anti-NHP IgG, Southern Biotech #4700-05; anti-human IgG, Southern Biotech #2046-05) conjugated to horseradish peroxidase (HRP) followed by TMB chromogenic substrate. After stopping the reaction with a stop reagent (1% H2SO4), optical density (OD) was measured via a microplate spectrophotometer at 450 nm, and Reference Titer Units of each OD450 value were calculated from the reference curve using the 4-parameter standard curve fitting algorithm of SoftMax Pro (Molecular Devices, San Jose, CA).
A Luminex 200 multiplex microbead immunoassay format (Luminex Corp., Austin, TX) was also used to detect NHP serum Abs specific for different SARS-CoV-2 Ags (performed at the University of California at Davis, Primate Assay Laboratory, Davis, CA) [20], [21]. The following antigens were conjugated to carboxylated magnetic beads: SARS-CoV-2 Nucleocapsid His-tag (Sino Biological, Wayne, PA), BetaCoV/Wuhan/IVDC‐HB‐05/2019 SP trimers His-tag (aa 1‐1208; GIAID# EPI_ISL_402121; Immune Technology Corp, New York, NY), SP/RBD His-tag (GenScript, Piscataway, NJ), and gamma-irradiated viral lysate, SARS-Related Coronavirus 2, Isolate USA-WA1/2020, Gamma-Irradiated, NR-52287 (deposited by the CDC, obtained through BEI Resources) [22]. The four conjugated beads were mixed in wells of a 96-well microtiter plate, incubated with test sera or control IgG (Positive Control SARS-CoV-2 NHP immune-serum-derived IgG and Negative Control IgG) diluted in PBS-0.05% Tween20 with 2% Prionex blocking agent, washed, and detected via PE-conjugated goat anti-human IgG (that also binds macaque IgG; Jackson ImmunoResearch, West Grove, PA). Results were reported as mean fluorescent intensity (MFI) units in which a sample was considered positive if it was above the Positive Control IgG MFI.
2.6. ACE2 binding inhibition ELISA
This competitive inhibition ELISA is comprised of serially diluted serum sample addition to recombinant SP/RBD protein bound to plastic wells of a 96-well plate. After washing away unbound proteins, biotinylated-human ACE2 (biotin-huACE2; R&D Systems, Minneapolis, MN) was added for binding to any free SP/RBD. After washing, streptavidin-labelled HRP was added to bind to bound biotin-huACE2. The assay was developed as described above for ELISAs. Inhibitory Dilution 50% (ID50), a potency value per sample that represents the reciprocal dilution at which 50% of the total ACE2 binding signal occurred, was calculated using GraphPad Prism software via a 4-parameter curve fit algorithm, log-inhibitor vs. response, variable slope.
2.7. Plaque reduction neutralization test (PRNT)
nAb titers were determined by the PRNT as previously described [23] and performed at the Feigin ABSL3 Facility (Texas Children’s Hospital, Houston, TX) using the SARS-CoV-2 viral strain, USA-WA1/2020 (# NR-52281; BEI Resources, Manassas, VA). Positive and negative control nAbs (Sino Biological, Wayne, PA) were used as 100% and 0% cell viability values. The serum neutralization titer is the reciprocal of the highest dilution resulting in an infection reduction of >50% (PRNT50).
2.8. NHP infection model of SARS-CoV-2 live virus challenge
2.8.1. Cynomolgus monkeys and vaccination
A total of 10 cynomolgus monkeys of Chinese and Indonesian origin (purpose bred, Macaca fascicularis) were used in the study, in which the five vaccinated animals were from the immunized NHP group at Biomere, Inc. described above for the immunization segment. These animals were shipped to BIOQUAL (Rockville, MD) and housed in BSL3 conditions for at least two weeks prior infection. An additional five naïve animals were purchased from PrimGen, Inc. (Lehigh Acres, FL) by BIOQUAL. Prior to all blood collections, animals were anesthetized using ketamine. At the end of the study, animals were euthanized with an intravenous overdose of sodium pentobarbital.
2.8.2. Propagation of SARS-CoV-2 virus and infection of animals
VERO-E6 cells (ATCC; #CRL 1586) were grown in Dulbecco’s 260 modified essential media (DMEM; Gibco) with 10% heat-inactivated fetal bovine serum (FBS; Gibco) at 37 °C with 5% CO2. SARS-CoV-2 passage (P) 4 isolate USA-WA1/2020 (BEI resources, NR-52281, GenBank accession number MN985325.1) was used to generate the animal exposure stock (P5). The stock was generated by infecting VERO-E6 cells at a multiplicity of infection (MOI) of 0.002 in DMEM containing 2% FBS; viral supernatant was harvested at four days post infection. The stock was confirmed to be SARS-CoV-2 via deep sequencing and confirmed to be free of adventitious agents. Animals were infected with a total of 1.1 × 105 plaque-forming units (PFU) of the SARS-CoV-2 viral strain, USA-WA1/2020 (NR-52281; BEI Resources, Manassas, VA) in 2 mL; i.e., 1 mL drop-wise with a 3 mL syringe via the intranasal route (0.5 mL into each nostril) and 1 mL with a French rubber tube via the intratracheal route. Viral titers were collected by nasal swabs (two flocked swabs per animal, FLOQSwabs®, Copan, Carlsbad, CA, placed into one vial each containing 1 mL PBS) and bronchioalveolar lavage (BAL) was collected using 10 mL saline administered and aspirated using a rubber feeding tube. Collected swabs and BAL aliquots were stored at −80 °C until viral load analysis. The amounts of viral RNA copies per mL of bodily fluid or per gram of tissue were determined using a qRT-PCR assay as previously described [24] using the following primer and probe sequences:
Genomic RNA (gRNA): 2019-nCoV_N1-F:5′-GAC CCC AAA ATC AGC GAA AT-3′; 2019-nCoV_N1-R: 5′-TCT GGT TAC TGC CAG TTG AAT CTG-3′; 2019-nCoV_N1-P: 5′-FAM-ACC CCG CAT TAC GTT TGG TGG ACC-BHQ1-3′;
Subgenomic RNA (sg-RNA): SG-F: CGATCTTGTAGATCTGTTCCTCAAACGAAC; SG-R: ATATTGCAGCAGTACGCACACACA; PROBE: FAM-ACACTAGCCATCCTTACTGCGCTTCG-BHQ.
2.9. huACE2-Tg mouse infection model of SARS-CoV-2 live virus (B.1.351 variant)
2.9.1. Mice and vaccination
All experimental procedures were performed in the Feigin ABSL3 Facility (Texas Children’s Hospital). Specific-pathogen-free, 4–5-weeks-old, female and male B6.Cg-Tg(K18- ACE2)2Prlmn/J (Stock No: 034860, K18 hACE2) hemizygotes were purchased from The Jackson Laboratory (Bar Harbor, ME). Ten K18-huACE2-Tg mice per group (5 male, 5 female) received one or two doses of vaccine 21 days apart after which blood samples were obtained 4 days prior to viral challenge on day 35 after the initial vaccine dose.
2.9.2. Propagation of SARS-CoV-2 virus and infection of mice
SARS-CoV-2 virus, Beta variant in lineage B.1.351 was obtained as a stock (NR-54009 BEI Resources, Source from the Africa Health Research Institute) and used to generate a master seed stock by infecting at low MOI of 0.01 Vero CCL-81 cells, with titrations performed on Vero-E6 cells as previously described [25]. This viral stock genetic sequence was verified using Oxford Nanopore sequencing technologies using the NEBNext ARCTIC protocol (New England Biolabs, Ipswich, MA). K18-huACE2-Tg mice were infected intranasally with 104 PFU of virus in a final volume of 20 µL following isoflurane sedation. Mice were monitored daily for morbidity (i.e., body weight and clinical scoring) and mortality. After signs of weight loss, daily monitoring occurred 2–3 times per day. Mice showing >20% loss of initial body weight or a clinical score of ≥4 more than once per day were defined as reaching the experimental endpoint and humanely euthanized, and all mice were euthanized at the end of study on day 11.
2.10. Statistical analyses
Group means were compared using either 1-way or 2-way analysis of variance (ANOVA) tests, as appropriate, on the log transformed data using either SAS (SAS Institute, Cary, NC) or GraphPad Prism (GraphPad, San Diego, CA) software. The Log-Rank (Mantel-Cox) Survival Curve test was applied to the data in Fig. 9C. Because the statistical analysis is exploratory in nature, no adjustments were made for multiple comparisons. Specific statistical analyses and results are described in detail in each of the figure captions.
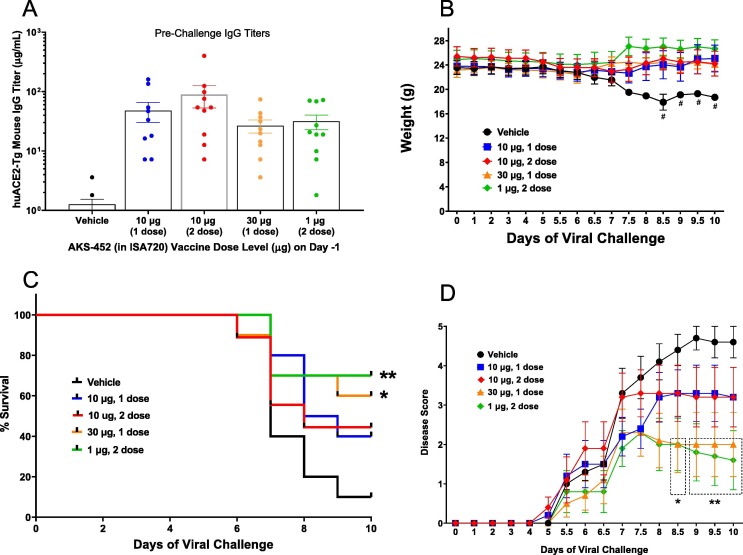
Fig. 9.
AKS-452-induced protection from viral challenge in K18-huACE2-Tg mice with Montanide ISA 720 adjuvant. Animals were treated with PBS vehicle or one dose of AKS-452 in ISA 720 adjuvant at dose levels of 10 or 30 µg on Day 0 or two doses at dose levels of 1 µg and 10 µg on Days 0 and 21 prior to challenge on Day 35 via intranasal and intratracheal split dose of live SARS-CoV-2 virus (B1.351 variant, total of 105 PFU). (A) Serum samples were obtained three days prior to challenge and assessed for SP/RBD-specific IgG titers (ELISA using a mouse non-specific polyclonal IgG standard curve). Mice were (B) weighed, (C) assessed for mortality, and (D) evaluated for disease score at daily intervals after viral challenge (disease scores of euthanized animals were included in longitudinal means; Clinical Scoring System included the following 0–5 scale; 0, Healthy, no signs of disease, body tone appropriate, weight is consistent or increasing from the previous day, normal activity level; 1, ∼5% weight loss and/or no signs or very mild signs of disease, no hunching, normal to slightly decreased activity level; 2, 5–10% weight loss and/or mild signs of disease, decreased activity; 3, 10–15% weight loss and/or onset of breathing abnormalities such as rapid shallow breaths, some hunching, mildly lethargic; 4, 15–20% weight loss and/or rapid breathing abnormalities, hunching, lethargy; 5, >20% weight loss, respiratory distress or moribund condition, immediate euthanasia required). Panel B, # N < 3 due to reduction in survival that negated statistical analyses. Panel C, * p < 0.05, ** p < 0.01 for comparison to “Vehicle” group via Log-Rank (Mantel-Cox) Survival Curve test with no adjustment for multiple comparisons. Panel D, * p < 0.05, ** p < 0.01 for comparison between “Vehicle” and individual “AKS-452/ISA720-treated” groups on each day using a parametric mixed model ANOVA of untransformed data with adjustment for multiple comparisons with Dunnett-Hsu correction (model: treatment, day and random animal effects, dotted-lined box denotes significant values). All statistical analyses were performed using SAS software.
3. Results
3.1. Immunogenicity in mice and rabbits
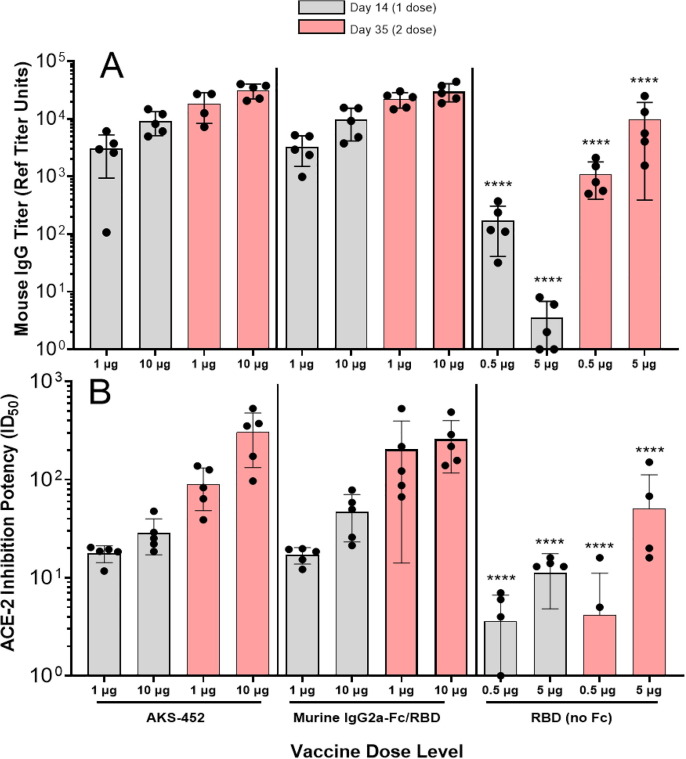
To demonstrate enhancement of immunogenicity by the Fc moiety of the AKS-452, BALB/c mice received two s.c. injections 21 days apart of AKS-452 (containing the human IgG1 Fc moiety), mouse IgG2a Fc-SP/RBD, or SP/RBD lacking an Fc moiety at different dose levels, and serum was collected 14 days after each dose (i.e., days 14 and 35, respectively) and assessed for anti-SP/RBD-specific titers (Fig. 2 ). (Note that one SP/RBD molecule has approximately half the molecular weight of the SP/RBD-Fc molecule.) Both dose levels of the human and mouse IgG Fc antigenic molecules induced significantly greater SP/RBD-specific IgG (Fig. 2 A) and ACE2-RBD binding inhibitory (Fig. 2 B) titers at both timepoints than did immunization with molar equivalent dose levels of unmodified SP/RBD, demonstrating the Fc-enhancing effect. Furthermore, human and mouse Fc-based Ags induced similar titers demonstrating that foreign sequences of the human IgG1 Fc did not appear to play an enhancing role in this BALB/c mouse immunogenicity model. The human IgG1 Fc moiety of AKS-452 was confirmed to bind mouse FcγRs in addition to those of NHP and rabbit at similar affinities (data not shown).
Fig. 2.
Adjuvant role of the Fc moiety in the IgG-Fc-SP/RBD vaccine antigen. BALB/c mice 6 to 8 weeks of age were immunized with two doses of AKS-452 (human IgG1 Fc-SP/RBD fusion protein), murine IgG2a-Fc SP/RBD fusion protein, or SP/RBD that lacks an IgG Fc moiety, and serum was collected on Days 14 and 35 after the first (administered on Day 0) and second (administered on Day 21) doses, respectively, and evaluated for (A) anti-SP/RBD IgG titers via ELISA (using a mouse serum reference curve) and (B) functional potency to inhibit huACE2 from binding to SP/RBD (ID50 values represent the reciprocal of the dilution at which 50% of ACE2 binding was achieved by a serum sample). Group means were compared using a parametric mixed model ANOVA of the log transformed data conducted in SAS software (model effects: treatment, dose, day and random animal); the effect of human (AKS-452) vs. murine Fc was not significant for both IgG titer and ACE-2 inhibition potency. However, p < 0.0001 (****) for RBD (no Fc) vs. AKS-452 or murine IgG2a-Fc/RBD respective dose groups for both IgG titer and ACE-2 inhibition potency; note that 0.5 µg RBD dose ∼ 1 µg AKS-452 or murine IgG2a-FC/RBD dose and that 5 µg RBD dose ∼ 10 µg AKS-452 or murine IgG2a-FC/RBD dose.
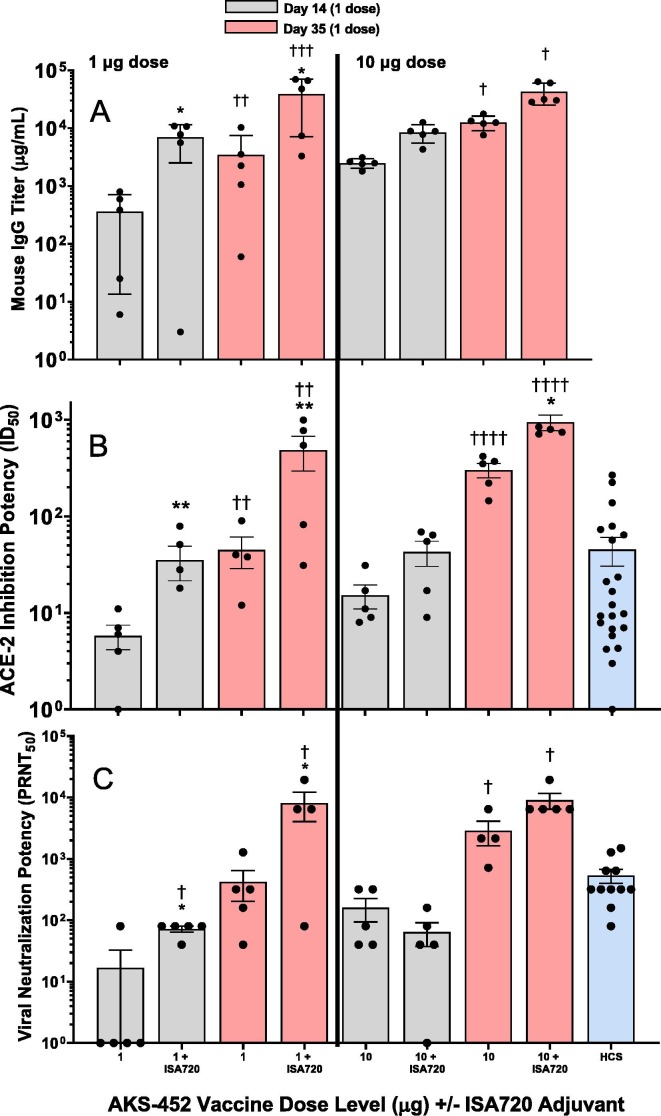
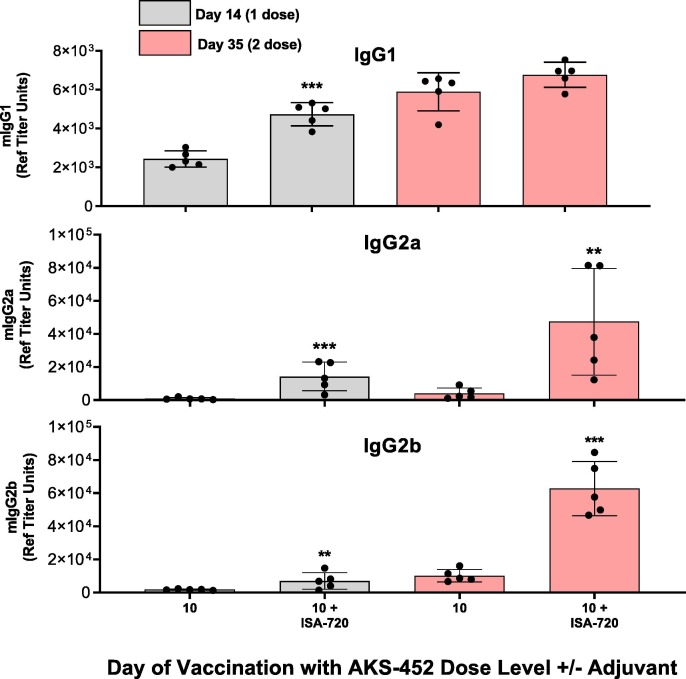
To further enhance immunogenicity of the SP/RBD-Fc Ag, the ISA 720 adjuvant was selected for its potent immunogenicity and its demonstrated favorable safety profile and low reactogenicity in humans in many clinical trials [19], [26], [27], [28], [29]. 1 and 10 µg dose levels were prepared with and without ISA 720 and administered in two doses 21 days apart. ISA 720 significantly enhanced serum IgG (Fig. 3 A) and ACE2-inhibition potency (Fig. 3 B) titers by approximately 4- to 20-fold at both dose levels after the first and second doses. Note that the ACE2-inhibitory potency titers were similar to or greater than those titers of HCS, demonstrating relevancy to human COVID-19 infection. Accordingly, the IgG and ACE2-inhibitory potency titers induced by ISA 720 correlated to significant enhancement of serum potency to neutralize live SARS-CoV-2 virus from infecting live VERO-E6 cells, which were also similar to or greater than those of HCS (Fig. 3 C). ISA 720 also induced significant Th1 type responses (i.e., greater IgG2a/b isotypes relative to IgG1) compared to those induced without adjuvant (Fig. 4 ; IgG2a/IgG1 isotype mean titer ratios at day 35 without and with ISA-720 were 0.7 and 7.0, respectively), demonstrating the favorable protective Th1 response expected from a COVID-19 vaccine.
Fig. 3.
Immunogenic enhancement by adjuvants added to AKS-452. BALB/c mice 6 to 8 weeks of age were immunized with two doses of AKS-452 (human IgG1 Fc-SP/RBD fusion protein) at 1 µg and 10 µg doses (100 µL) formulated with or without the adjuvant Montanide ISA 720. Serum was collected on Days 14 and 35 after the first (administered on Day 0) and second (administered on Day 21) doses, respectively, and evaluated for (A) anti-SP/RBD IgG titers via ELISA (using a mouse serum reference curve), (B) functional potency to inhibit huACE2 from binding to SP/RBD (ID50 values that represent the reciprocal of the dilution at which 50% of the ACE2 binding was achieved), and (C) neutralization potency (PRNT50) of live SARS-CoV-2 viral infection of live VERO-E6 cells (i.e. the reciprocal of the highest dilution at which 50% plaque reduction neutralization was achieved). Group means were compared using a parametric mixed model ANOVA of the log transformed data conducted in SAS software (model effects: adjuvant, dose, day and random animal); * p < 0.05 relative to the analogous dose level group without adjuvant. ** p < 0.01 relative to the analogous dose level group without adjuvant. † p < 0.05 relative to the analogous day 14 value. ††p < 0.01 relative to the analogous day 14 value. †††p < 0.001 relative to the analogous day 14 value. ††††p < 0.0001 relative to the analogous day 14 value.
Fig. 4.
AKS-452-Induced mouse IgG Isotypes analysis. BALB/c mice 6 to 8 weeks of age were immunized with two doses of AKS-452 (human IgG1 Fc-SP/RBD fusion protein) at a 10 µg dose level formulated with or without Montanide ISA 720 adjuvant. Serum was collected on Days 14 and 35 after the first (administered on Day 0) and second (administered on Day 21) doses, respectively, and evaluated for anti-SP/RBD IgG titer isotypes, (A) IgG1 (Th2 type), (B) IgG2a (Th1 type), and (C) IgG2b (Th1 type) via isotype-specific ELISAs (using a mouse serum reference curve). Group means were compared using a parametric mixed model ANOVA of the log transformed data conducted in SAS software (model effects: adjuvant, day and random animal); * p < 0.05, ** p < 0.01, *** p < 0.001 relative to the respective dose level without adjuvant at each timepoint.
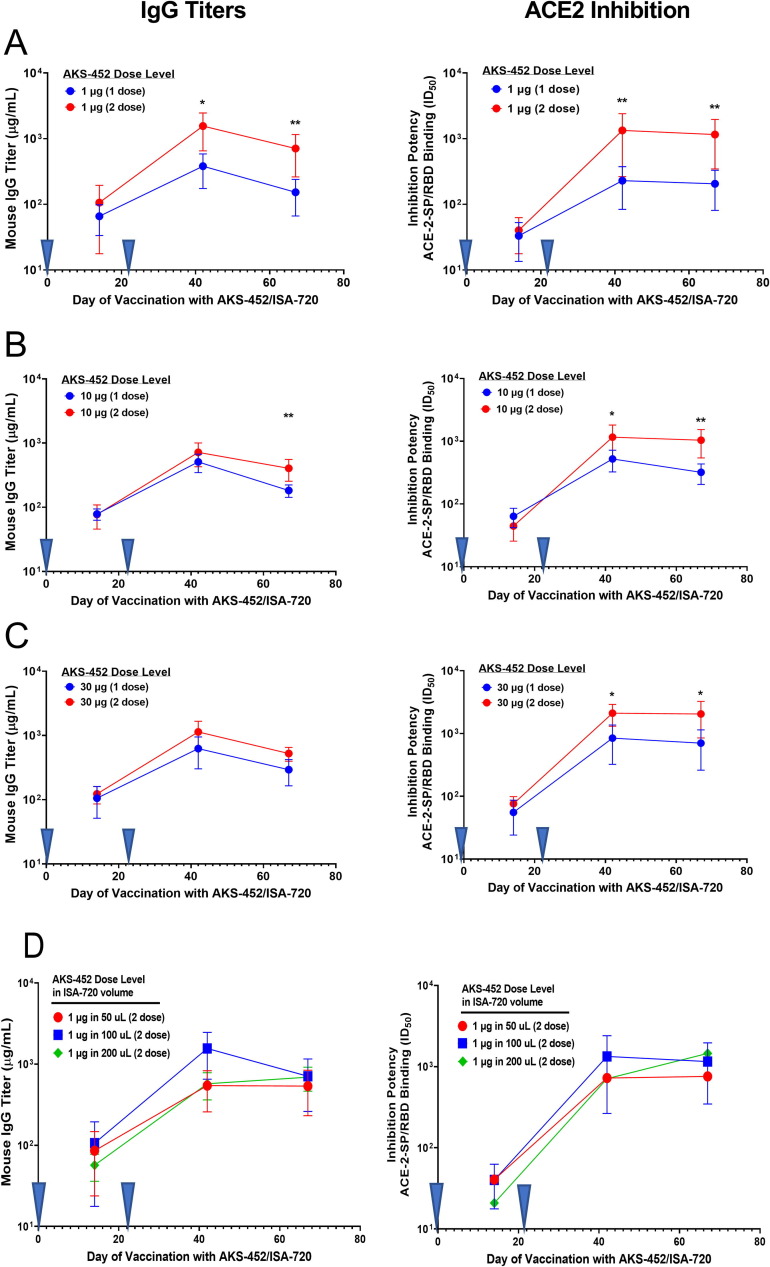
To further understand the immunogenic potential of AKS-452 formulated with ISA 720, a one- versus two-dose regimen study was performed (Fig. 5 ). The two-dose regimen showed a slightly greater enhancement of IgG and ACE2-inhibition potency titers relative to that of a single-dose regimen by 1.4- to 3-fold with the 10 µg (Fig. 5 B, left and right) and 30 µg (Fig. 5 C, left and right) AKS-452 dose levels, and by 5-fold in the presence of the lowest dose level of 1 µg (Fig. 5 A, left and right) on days 42 and 67. These results support that effective immunity of a single dose of AKS-452 in ISA 720 is possible at the higher dose levels and should be evaluated in clinical settings. In further support of possible variables for clinical design, the volume of injection with ISA 720 had no impact on the immunogenic capacity of AKS-452 dose level to induce IgG titers and ACE2-inhibitory potency (Fig. 5 D, left and right) after the first and second doses, demonstrating that a single vaccine formulation can be manufactured in which different dose levels of AKS-452 can be achieved by simply varying the dose volume.
Fig. 5.
AKS-452: One vs. two Doses and varying ISA 720 volume BALB/c mice 6 to 8 weeks of age were immunized with either one or two doses (administered at Days 0 and 21, inverted blue triangles) of AKS-452 at (A) 1 µg, (B) 10 µg, or (C) 30 µg dose levels formulated with Montanide ISA 720. In addition, AKS-452 was administered as a two-dose regimen at a (D) 1 µg dose level that was formulated in different volumes of ISA 720. Serum was collected on designated days and evaluated for anti-SP/RBD IgG titers (left panels) via ELISA (using a mouse serum reference curve) and functional potency (right panels) to inhibit huACE2 from binding to SP/RBD (ID50 values that represent the reciprocal of the dilution at which 50% of the ACE2 binding was achieved). Group means were compared using a parametric mixed model ANOVA for each AKS-452 dose level of the log transformed data conducted in SAS software (model effects: regimen, day and random animal); * p < 0.05, ** p < 0.01, relative to the analogous 1-dose timepoint.
In addition, three doses of AKS-452 in ISA 720 (30 µg and 100 µg dose levels) administered 14 days apart demonstrated no difference in immunogenicity between s.c. and i.m. routes of administration in New Zealand White rabbits (Supplemental Methods and Supplemental Fig. 1), in which no significant toxicities were observed in two different studies (a GLP and a non-GLP), confirming that AKS-452 has no direct toxic or pathological effects on lung or other relevant tissues (data not shown).
3.2. Immunogenicity and protective efficacy in NHPs (Cynomolgus monkeys)
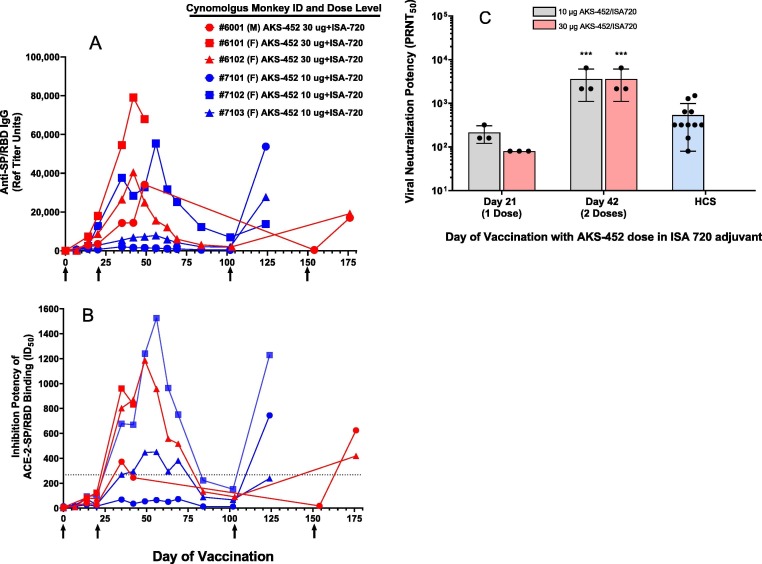
Immunization (s.c.) of the more genetically-relevant NHP species, cynomolgus monkeys, with 10 µg (N = 3) or 30 µg (N = 3) AKS-452 dose levels formulated in ISA 720 induced strong IgG (Fig. 6 A) and ACE2-inhibitory (Fig. 6 B) titers after one, two, and three doses, and all doses achieved ID50 potencies greater than those of all HCS samples tested (dotted line in Fig. 6 B). Moreover, when titers were allowed to resolve after the second dose, such titers were readily restored after the third “booster” dose on day 100 or 150, demonstrating that a durable memory immunity is present even when serum titers wane (Fig. 6 A and B). Also, serum neutralization potency of live SARS-CoV-2 virus infectivity similar to or greater than that of HCS was apparent after the first two doses (Fig. 6 C).
Fig. 6.
AKS-452-induced immunogenicity in NHPs (cynomolgus monkeys) with Montanide ISA 720 adjuvant. N = 3 animals/group received three doses of AKS-452 of either the 10 µg or 30 µg dose level in ISA 720. Serum samples were collected at several days after the first (administered on Day 0), second (administered on Day 21), and third (administered on either Day 100 or 150) doses, respectively, and evaluated for (A) anti-SP/RBD IgG Ab titers (IgG µg/mL via ELISA using an NHP immune IgG standard curve), (B) functional potency to inhibit huACE2 from binding to SP/RBD (ID50 values that represent the reciprocal of the dilution at which 50% of the ACE2 binding was achieved); the dotted line refers to the highest HCS levels from Fig. 3B), and (C) neutralization potency (PRNT50) of live SARS-CoV-2 viral infection of live VERO-E6 cells (i.e. the reciprocal of the highest dilution at which 50% plaque reduction neutralization was achieved). Note that titers were allowed to resolve after the second dose before the third booster dose was given. Human convalescent serum was evaluated for potency to neutralize live SARS-CoV-2 virus from infecting VERO-E6 cells (C). Group means were compared using a parametric mixed model ANOVA of the log transformed data conducted in SAS software (model effects: dose, day and random animal); *** p < 0.001 relative to the respective dose level at Day 21.
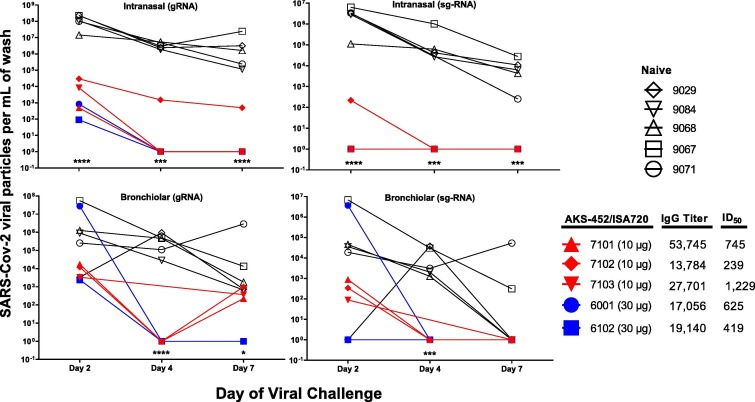
Using these immunized NHPs after the third vaccine dose (N = 5) and a naïve NHP cohort (N = 5), a live SARS-CoV-2 virus challenge study was performed via intranasal and intratracheal delivery of an active SARS-CoV-2 virus (strain WA/1/2020). (One of the six NHPs immunized above, #6101, had a heart condition unrelated to vaccine, i.e., prior to day 0 of immunization, that precluded evaluation in this viral challenge study). The five NHPs immunized with three doses of AKS-452/ISA 720 demonstrated significant anti-SP/RBD IgG titers and ACE2-inhibitory ID50 values assessed 7 days prior to viral challenge compared to the five naïve control NHPs with negligible or undetectable levels (Fig. 7 ). To assess viral infectivity, nasal swabs and bronchiolar lavage (BAL) samples were obtained from all animals on days 2, 4, and 7 after viral inoculation, and analyzed for viral titers via qRT-PCR yielding viral copies per mL of viral gRNA (a measure of both live and dead virus) and sg-RNA (a measure of active viral replication) (Fig. 7). The normal kinetics of viral infection were demonstrated in naïve animals in which the highest viral load in the nasal cavity (gRNA and sg-RNA) was present on day 2 and remained substantial to the end of study on day 7 (Fig. 7). Viral load in the bronchiolar space, while more variable among naïve animals, showed a similar kinetic pattern (although 3 of 5 naïve animals naturally resolved viral levels by day 7 via sg-RNA analysis; Fig. 7). In contrast, all five vaccinated animals showed significantly lower viral levels on day 2 and completely resolved infection by day 4 (Fig. 7), demonstrating a highly protective efficacy of the vaccine. Accordingly, gross pathology and histological evaluation of necropsy lung tissue samples collected at the end of study on day 7 demonstrated significant inflammation scores in naïve animals that were nonexistent or negligible in the vaccinated animals (Table 1 ). Indeed, such results demonstrate the lack of ADE by this vaccine.
Fig. 7.
AKS-452-induced protection from viral challenge in NHPs (cynomolgus monkeys) with Montanide ISA 720 adjuvant. Animals were either untreated (Naïve) or received three doses of AKS-452 of either 10 µg or 30 µg in ISA 720 on Days 0, 21, and 100 (or day 150 for #6001) in which serum was collected on Day 125 (animals #7101, #7102, #7103) or Day 175 (#animals #6001 and #6102) and assessed for anti-SP/RBD-specific IgG titers (NHP serum reference units) and ACE2-inhibition potency (ID50). Seven days after serum collection, animals were challenged via intranasal and intratracheal split dose of live SARS-CoV-2 virus (Washington strain, total of 1.1 × 105 PFU). Intranasal swab and bronchiolar lavage samples were collected on Days 2, 4, and 7 after viral challenge and analysed for levels of virus via quantitative RT-PCR for genomic viral RNA (gRNA; detects live and inactivated virus) and sub-genomic viral RNA (sg-RNA; associated with live virus replication) and reported as viral RNA copies per mL of nasal and bronchiolar wash. * p < 0.05, *** p < 0.001, **** p < 0.0001 for comparison between the “Naïve” and all “AKS-452/ISA720-treated” groups on each day using parametric 2-way ANOVA of log transformed data in GraphPad Prism software (for each panel a model with dose (treatment) and day as effect).
Table 1.
Lung Histopathology of NHPs after viral challenge and protection with AKS-452 in Montanide ISA 720 adjuvant.
| Vehicle | AKS-452 |
||
|---|---|---|---|
| (10 µg) | (30 µg) | ||
| Number of Animals | 5 | 3 | 2 |
| Number of animals with minimal (Grade 1) and mild (Grade 2) events | |||
| ACCESSORY LOBE | |||
| Bronchoalveolar lymphoid tissue hyperplasia | 1 | 0 | 0 |
| Increased alveolar macrophages | 0 | 2 | 2 |
| Alveolar inflammation, mononuclear | 5 | 0 | 0 |
| Interstitial inflammation, mononuclear | 3 | 0 | 0 |
| Perivascular inflammation, mixed or mononuclear | 3 | 1 | 1 |
| Fibrosis, interstitial | 0 | 0 | 0 |
| Pneumocyte hyperplasia (non-papillary) | 1 | 0 | 0 |
| LEFT LOBE | |||
| Increased alveolar macrophages | 1 | 1 | 2 |
| Pneumocyte hyperplasia (non-papillary) | 3 | 0 | 0 |
| Fibrosis, interstitial | 2 | 0 | 0 |
| Interstitial inflammation, mononuclear | 2 | 0 | 0 |
| Perivascular inflammation, mononuclear | 4 | 0 | 1 |
| Alveolar inflammation, mononuclear | 5 | 0 | 1 |
| RIGHT LOBE | |||
| Increased alveolar macrophages | 0 | 1 | 2 |
| Bronchoalveolar lymphoid tissue hyperplasia | 1 | 0 | 0 |
| Pneumocyte hyperplasia (non-papillary) | 1 | 0 | 0 |
| Interstitial inflammation, mononuclear | 1 | 0 | 1 |
| Perivascular inflammation, mononuclear or mixed | 5 | 2 | 2 |
| Alveolar inflammation, mononuclear | 5 | 0 | 0 |
| Bronchial inflammation, mixed | 0 | 1 | 0 |
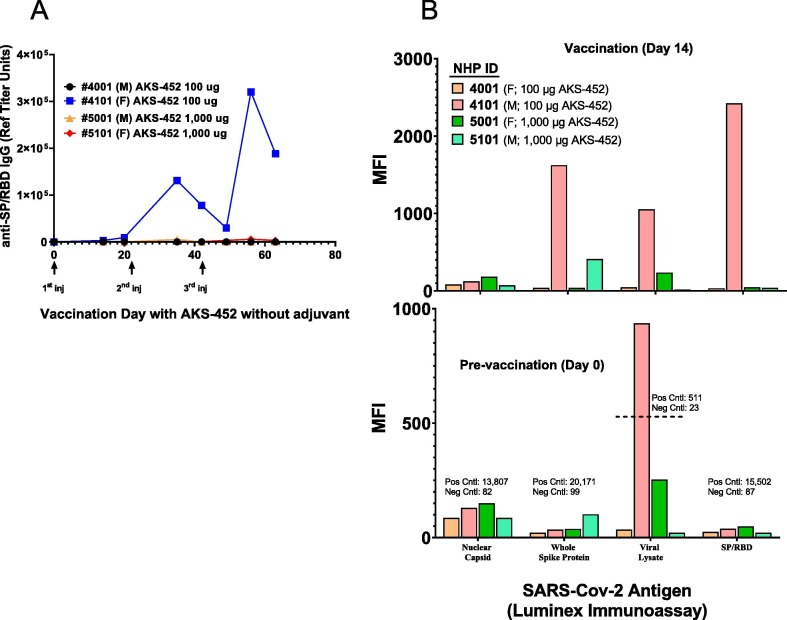
Interestingly, in a separate study with four NHPs that received three doses of AKS-452 in the absence of adjuvant, one animal, #4101, unexpectedly showed a significant anti-SP/RBD IgG titer response after the first dose and a profound response after the second and third doses (Fig. 8 A; ACE2-inhibition potency titers were also observed, data not shown). Note that this animal was dosed with the lower dose level of 100 µg, so dose level was not a factor in its response. We hypothesized that this animal’s immune system had been primed to SP/RBD, and therefore explored the possibility that it had been exposed to SARS-CoV-2 infection during breeding and nurturing prior to this study that was initiated in May 2020, at which time the animal was approximately 2 years of age. While baseline serum samples obtained prior to immunization showed no detectable levels of anti-SP/RBD IgG titers (Fig. 8 A), a more specific titer analysis was performed with a panel of SARS-CoV-2 Ags, including nuclear capsid protein, whole SP, and those undefined Ags in a viral lysate (Fig. 8 B; bottom panel). Surprisingly, significant and substantial titers were detected against the viral lysate antigen only in the samples from animal #4101, but not in those from the other animals, suggesting that #4101 had a prior infection with SARS-CoV-2 or a serologically cross-reactive agent that went undetected that primed its immune system to vaccination with AKS-452. Indeed, these observations support the potential use of AKS-452 in the absence of adjuvant as a booster vaccination in individuals who have contracted SARS-CoV-2 infection or received a primary vaccination and require a safe boosting approach to augment protective titers.
Fig. 8.
Substantial AKS-452-induced immunogenicity in an NHP (cynomolgus monkeys) in the absence of adjuvant. Animals received three doses of either 100 µg or 1,000 µg AKS-452 without adjuvant at 21 days apart. Serum was collected at designated times and evaluated for (A) anti-SP/RBD IgG Ab titers by ELISA (using an NHP serum reference curve reported as Reference Unit Titer values) and (B) SARS-CoV-2 anti-nucleocapsid, anti-whole SP Ag, anti-viral lysate, and anti-SP/RBD via Luminex immunoassay (reported as raw MFI values).
In further support of the boosting potency in the absence of adjuvant, a single dose of 10 µg AKS-452 without adjuvant was able to substantially re-stimulate IgG titers in previously-immunized BALB/c mice (Supplemental Fig. 2) after their previous adjuvanted AKS-452-induced titers were allowed to subside below 500 µg/mL prior to the booster dose.
3.3. Immunogenicity and protective efficacy against the B.1.351 variant in huACE2-Tg mice
It is clear that different variants of SARS-CoV-2 containing unique mutations are associated with increased infection rates in different parts of the world including the B.1.1.7 (N501Y), B.1.351 (K417N, E484K, N501Y), and B.1.617.2 (L452R, T478K) variants [30], [31], [32]. Therefore, protective immunity against the more evasive B.1.351 variant of SARS-CoV-2 by vaccination with AKS-452 formulated in ISA 720 live virus was confirmed in the murine model of SARS-CoV-2 infection using the K18-huACE2-Tg mouse model [33], [34] containing the human ACE2 receptor which confers SARS-CoV-2 infection susceptibility versus wild-type mice [35], [36]. K18-huACE2-Tg mice were immunized with 1, 10, or 30 µg of AKS-452 formulated in ISA 720 in which 10 µg and 30 µg dose levels were evaluated at a single dose, and 1 µg and 10 µg dose levels were evaluated at two doses administered 21 days apart prior to viral challenge on day 35 (Fig. 9). Four days prior to viral challenge, IgG titers were similar among all four groups of mice (Fig. 9 A). Upon viral challenge, unvaccinated (vehicle; naïve) control animals showed an aggressive decline in body weight from days 5 to 10 (Fig. 9 B) that correlated with a decrease in survival incidence to 10% by day 9 (Fig. 9 C) and a maximum mean disease score of approximately 4.5 by day 9 (Fig. 9 D). Clear dose responses in body weight, survival incidence, and disease score were observed between the 10 and 30 µg one-dose groups, and between the 1 and 30 µg two-dose groups. Interestingly, one dose of the highest dose level of 30 µg and 2 doses of the lowest dose level of 1 µg showed similar efficacies that were greater than those of both one- and two-doses of 10 µg such that animal weight was preserved and survival was enhanced to 60% to 70% with associated mean disease scores of 1.6 to 2.0. While an expected dose response in efficacy was observed between the 10 µg and 30 µg one-dose groups, there was an inconsistency with the two-dose 10 µg response relative to that of the two-dose 1 µg response. While both of these two-dose groups induced similar IgG titers (Fig. 9 A) and were able to induce protection (Fig. 9 B, C, D), it remains unclear why this dose response was reversed. Nevertheless, these results support the possibility that a single dose of a higher dose level could be used to achieve protective immunity even against the more infectious B.1.351 variant.
4. Discussion
This study describes the development of a unique protein subunit COVID-19 vaccine, AKS-452, based on the concept of fusing the immune-enhancing human IgG Fc moiety with an immunogenically-focused antigenic component of the SARS-CoV-2 virus, SP/RBD. Consistent with our results here, fusion of IgG Fc with the RBD fragment of SP from the original SARS virus demonstrated significant immunogenicity in mice relative to the SARS-RBD fragment alone [37], [38]. In fact, this human IgG Fc-fusion enhancing approach has been demonstrated with the development of a MERS coronavirus vaccine containing recombinant protein of a truncated MERS SP/RBD fragment (residues 377–588) fused to human IgG Fc that increased immunogenicity via FcγR-binding on APCs, in addition to increasing in vivo half-life and stability [17], [39].
While the Fc moiety is known to enhance Ag presentation via FcγR binding to induce activation signals in APCs, such signals are typically not strong enough by a vaccine to achieve the activation threshold of naïve lymphocytes, in addition to not ensuring commitment to Th1 development. However, as we have demonstrated here with a cynomolgus monkey that possibly had a prior infection with SARS-CoV-2 or a cross reactive agent, Fc-mediated signals may be strong enough to re-activate memory T and B cells of primed individuals that have a low-threshold of activation relative to that of naïve T cells. Nevertheless, these limitations in the naïve immune system were overcome by fortifying vaccine potency with a formulation of the SP/RBD-Fc Ag in the adjuvant, ISA 720, a water-in-oil emulsification agent containing the Th1-promoting and human-safe squalene oil [19], [26], [27], [28], [29], [40], [41], [42], developed for its low reactogenicity in humans. Collectively, the Fc moiety of AKS-452 was designed to act as a mild adjuvant via activation of APCs and to work in concert with a strong classical adjuvant, ISA 720, to enhance the duration of Ag exposure to APCs and possibly direct Ag entry into lymph nodes locally and systemically where additional APCs reside. Indeed, our demonstration that a single dose of AKS-452 in ISA 720 showed immunogenic potency and protection of viral infection similar to that of a two-dose regimen in K18-huACE2-Tg mice supports a dose-sparing potential.
Several outcomes of this study support the potential use of AKS-452 in the absence or presence of ISA 720 as a booster vaccine for subjects that had prior COVID-19 or after vaccination with other vaccines. First, the response to AKS-452 without adjuvant by one of the four treated NHPs appeared to be associated with a primed immune status, perhaps induced via a prior viral infection. While such responses in the absence of adjuvant appear to be more readily induced in naïve mice (as our results demonstrate), they are not expected in naïve animals of NHP species that are genetically-proximal to humans where reaction to a human protein is less likely after one or two doses without adjuvant (i.e., as demonstrated in three of four animals in our study). Second, NHPs immunized with AKS-452 in ISA 720 that were allowed a rest from vaccination for several weeks during which IgG titers resolved could be readily re-stimulated with a single booster injection that achieved maximum neutralizing titers within 21 days. This is consistent with another report demonstrating a strong booster effect with a subunit SP vaccine in combination with a novel DNA priming vaccine encoding the full-length SP of SARS-CoV-2 [43]. Indeed, additional studies are required to definitively demonstrate the potential of AKS-452 with or without adjuvant to safely boost the immune response induced by other previously administered vaccines or prior COVID-19 infection.
Vaccines based on the single SP Ag are likely to protect against the known SARS-CoV-2 SP mutant strains as has been demonstrated in humans with EUA-allowed vaccines (reviewed in [44]). It is worth noting that a recent study evaluating HCS binding to SP/RBD mutant proteins [45] demonstrated that the vast majority of SP binding was focused to SP/RBD epitopes (70% to 99% attributed to SP/RBD binding). This observation supports that the focused SP/RBD-specific nAb response induced by AKS-452 is expected to yield a strong protective efficacy and that the risk of variant escape from SP/RBD-specific vaccinations should not be any greater than the risk with whole SP Ag vaccinations. Indeed, immunization with the SP/RBD-specific AKS-452 in ISA 720 effectively protected NHPs from viral infection with the original USA-WA1/2020 virus resulting in significantly lower viral levels and nonexistent or negligible inflammation scores in lung tissue samples and effectively protected K18-huACE2-Tg mice from viral infection with the B.1.351 variant resulting in preserved body weight and increased survival. Thus, the SP/RBD-focused immunogenicity of AKS-452 is likely to provide effective protection from viral variants with SP/RBD mutations as was demonstrated with the B.1.351 variant.
Our current experience with manufacturing AKS-452 has demonstrated that a single 2,000L bioreactor production train run yields enough material for an expected 45 µg dose of drug substance to treat approximately 100 million people receiving a single dose. This manufacturing capacity is extremely significant and far superior to the production throughput and costs of the other viral-based, nucleic acid-based, and full-length recombinant SP subunit-based vaccines. Furthermore, ongoing stability studies indicate preserved physicochemical characteristics and potency at 25 °C for over six months and 37 °C for up to one month (Supplemental Methods, Supplemental Table 3) as might be expected for a protein subunit Ag built on the stable backbone of a monoclonal antibody structure. The potency, manufacturability, stability, and mechanism-of-action of the AKS-452 Fc-fusion protein formulated with adjuvant, therefore, offer an opportunity to immunize billions of people globally as frequently as necessary to maintain high levels of neutralizing anti-SP/RBD Ab titers throughout the population. It is expected that single dosing, high expression yields, and mild storage temperatures of AKS-452 will help address the world’s large-scale manufacturing and distribution needs of an effective COVID-19 vaccine.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The following authors declare the following financial interests/personal relationships which may be considered as potential competing interests: the following authors were employed by and received monetary compensation from Akston Biosciences, Inc.: DGA, ARD, MMS, SM, RR, EKG, TS, JRH, NJS, TML, TZ. No other authors were personally compensated by Akston Biosciences.
Acknowledgments
Acknowledgements
The following reagents were deposited by the Centers for Disease Control and Prevention and obtained through BEI Resources, NIAID, NIH: SARS-Related Coronavirus 2, Isolate USA-WA1/2020, Gamma-Irradiated, NR-52287 and the SARS-Related Coronavirus 2, Isolate hCoV-19/South Africa/KRISP-K005325/2020, NR-54009, and the authors thank Alex Sigal and Tulio de Oliveira at BEI Resources.
Funding statement
This research was not funded by any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.09.077.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.University, J.H., COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University; https://www.covidtracker.com/.
- 2.Heaton P.M. The Covid-19 Vaccine-Development Multiverse. N Engl J Med. 2020;383(20):1986–1988. doi: 10.1056/NEJMe2025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karnik M., et al. A Review on SARS-CoV-2-Induced Neuroinflammation, Neurodevelopmental Complications, and Recent Updates on the Vaccine Development. Mol Neurobiol. 2021 doi: 10.1007/s12035-021-02399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu F.-C., Guan X.-H., Li Y.-H., Huang J.-Y., Jiang T., Hou L.-H., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bos R., et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. npj Vaccines. 2020;5:91. doi: 10.1038/s41541-020-00243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson MG et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers - Eight U.S. Locations, December 2020-March 2021. MMWR Morb Mortal Wkly Rep 2021;70(13):495–500. [DOI] [PMC free article] [PubMed]
- 8.Verbeke R., Lentacker I., De Smedt S.C., Dewitte H. The dawn of mRNA vaccines: The COVID-19 case. J Control Release. 2021;333:511–520. doi: 10.1016/j.jconrel.2021.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Organization, W.H., Status of COVID-19 Vaccines within WHO EUL/PQ evaluation process. WHO Guidance Document, 2021; May 18th, 2021.
- 13.Kounis N.G., Koniari I., de Gregorio C., Velissaris D., Petalas K., Brinia A., et al. Allergic Reactions to Current Available COVID-19 Vaccinations: Pathophysiology, Causality, and Therapeutic Considerations. Vaccines (Basel) 2021;9(3):221. doi: 10.3390/vaccines9030221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ewer K., Sebastian S., Spencer A.J., Gilbert S., Hill A.V.S., Lambe T. Chimpanzee adenoviral vectors as vaccines for outbreak pathogens. Hum Vaccin Immunother. 2017;13(12):3020–3032. doi: 10.1080/21645515.2017.1383575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park K.S., Sun X., Aikins M.E., Moon J.J. Non-viral COVID-19 vaccine delivery systems. Adv Drug Deliv Rev. 2021;169:137–151. doi: 10.1016/j.addr.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong S.K., Li W., Moore M.J., Choe H., Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J Biol Chem. 2004;279(5):3197–3201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 18.Loureiro S., Robinson E., Chen H., Phapugrangkul P., Colaco C., Jones I.M. Innovation in Vaccinology: from design, through to delivery and testing. Springer Netherlands; Dordrecht: 2012. pp. 45–63. [DOI] [Google Scholar]
- 19.Aucouturier J., Dupuis L., Deville S., Ascarateil S., Ganne V. Montanide ISA 720 and 51: a new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev Vaccines. 2002;1(1):111–118. doi: 10.1586/14760584.1.1.111. [DOI] [PubMed] [Google Scholar]
- 20.Elizaldi SR et al. SARS-CoV-2 infection induces germinal center responses with robust stimulation of CD4 T follicular helper cells in rhesus macaques. bioRxiv 2020.
- 21.Weiss S et al. A High Through-put Assay for Circulating Antibodies Directed against the S Protein of Severe Acute Respiratory Syndrome Corona virus 2. medRxiv 2020. [DOI] [PMC free article] [PubMed]
- 22.Yee J.L., Van Rompay K.K.A., Carpenter A.B., Nham P.B., Halley B.M., Iyer S.S., et al. SARS-CoV-2 surveillance for a non-human primate breeding research facility. J Med Primatol. 2020;49(6):322–331. doi: 10.1111/jmp.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park J.G., et al. Rapid in vitro assays for screening neutralizing antibodies and antivirals against SARS-CoV-2. J Virol Methods. 2021;287 doi: 10.1016/j.jviromet.2020.113995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandrashekar A., et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science. 2020 doi: 10.1126/science.abc4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harcourt J., et al. Severe Acute Respiratory Syndrome Coronavirus 2 from Patient with 2019 Novel Coronavirus Disease, United States. Emerg Infect Dis. 2020;26(6) doi: 10.3201/eid2606.200516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herrera S., et al. Phase I safety and immunogenicity trial of Plasmodium vivax CS derived long synthetic peptides adjuvanted with montanide ISA 720 or montanide ISA 51. Am J Trop Med Hyg. 2011;84(2 Suppl):12–20. doi: 10.4269/ajtmh.2011.09-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliveira G.A., Wetzel K., Calvo-Calle J.M., Nussenzweig R., Schmidt A., Birkett A., et al. Safety and enhanced immunogenicity of a hepatitis B core particle Plasmodium falciparum malaria vaccine formulated in adjuvant Montanide ISA 720 in a phase I trial. Infect Immun. 2005;73(6):3587–3597. doi: 10.1128/IAI.73.6.3587-3597.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miles A.P., McClellan H.A., Rausch K.M., Zhu D., Whitmore M.D., Singh S., et al. Montanide ISA 720 vaccines: quality control of emulsions, stability of formulated antigens, and comparative immunogenicity of vaccine formulations. Vaccine. 2005;23(19):2530–2539. doi: 10.1016/j.vaccine.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence G.W., Saul A., Giddy A.J., Kemp R., Pye D. Phase I trial in humans of an oil-based adjuvant SEPPIC MONTANIDE ISA 720. Vaccine. 1997;15(2):176–178. doi: 10.1016/s0264-410x(96)00150-8. [DOI] [PubMed] [Google Scholar]
- 30.Greaney A.J., Loes A.N., Crawford K.H.D., Starr T.N., Malone K.D., Chu H.Y., et al. Comprehensive mapping of mutations in the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human plasma antibodies. Cell Host Microbe. 2021;29(3):463–476.e6. doi: 10.1016/j.chom.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wall E.C., Wu M., Harvey R., Kelly G., Warchal S., Sawyer C., et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397(10292):2331–2333. doi: 10.1016/S0140-6736(21)01290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie X et al. Neutralization of N501Y mutant SARS-CoV-2 by BNT162b2 vaccine-elicited sera. bioRxiv 2021. [DOI] [PubMed]
- 33.Sarkar J., Guha R. Infectivity, virulence, pathogenicity, host-pathogen interactions of SARS and SARS-CoV-2 in experimental animals: a systematic review. Vet Res Commun. 2020;44(3-4):101–110. doi: 10.1007/s11259-020-09778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang R.-D., Liu M.-Q., Chen Y., Shan C., Zhou Y.-W., Shen X.-R., et al. Pathogenesis of SARS-CoV-2 in Transgenic Mice Expressing Human Angiotensin-Converting Enzyme 2. Cell. 2020;182(1):50–58.e8. doi: 10.1016/j.cell.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5(4):562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan R., Zhang Y., Li Y., Xia L.u., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen W.-H., Strych U., Hotez P.J., Bottazzi M.E. The SARS-CoV-2 Vaccine Pipeline: an Overview. Curr Trop Med Rep. 2020;7(2):61–64. doi: 10.1007/s40475-020-00201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lurie N., Saville M., Hatchett R., Halton J. Developing Covid-19 Vaccines at Pandemic Speed. N Engl J Med. 2020;382(21):1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 39.Zhang N., Channappanavar R., Ma C., Wang L., Tang J., Garron T., et al. Identification of an ideal adjuvant for receptor-binding domain-based subunit vaccines against Middle East respiratory syndrome coronavirus. Cell Mol Immunol. 2016;13(2):180–190. doi: 10.1038/cmi.2015.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ko E.-J., Kang S.-M. Immunology and efficacy of MF59-adjuvanted vaccines. Hum Vaccin Immunother. 2018;14(12):3041–3045. doi: 10.1080/21645515.2018.1495301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu Q.i., Wang R.-H., Jiao X., Jin B.o., Sugauchi F., Grandinetti T., et al. Induction of multispecific Th-1 type immune response against HCV in mice by protein immunization using CpG and Montanide ISA 720 as adjuvants. Vaccine. 2008;26(43):5527–5534. doi: 10.1016/j.vaccine.2008.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ascarateil S., Puget A., Koziol M.-E. Safety data of Montanide ISA 51 VG and Montade ISA 720 VG, two adjuvants dedicated to human therapeutic vaccines. J Immunotherapy Cancer. 2015;3(Suppl 2):428. [Google Scholar]
- 43.Li Y., Bi Y., Xiao H., Yao Y., Liu X., Hu Z., et al. A novel DNA and protein combination COVID-19 vaccine formulation provides full protection against SARS-CoV-2 in rhesus macaques. Emerg Microbes Infect. 2021;10(1):342–355. doi: 10.1080/22221751.2021.1887767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Caro A., Cunha F., Petrosillo N., Beeching N.J., Ergonul O., Petersen E., et al. Severe acute respiratory syndrome coronavirus 2 escape mutants and protective immunity from natural infections or immunizations. Clin Microbiol Infect. 2021;27(6):823–826. doi: 10.1016/j.cmi.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greaney AJ et al. Comprehensive mapping of mutations to the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human serum antibodies. bioRxiv 2021;2021. https://doi.org/10.1101/2020.12.31.425021. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.