Abstract
Introduction
Despite improvements in overall survival, biochemical recurrence of prostate cancer, characterized by rising prostate-specific antigen (PSA) levels after curative intent primary therapy, remains common. With the advent of highly sensitive molecular imaging, men with limited metastatic disease burden, or oligometastatic prostate cancer, are increasingly being identified.
The LOCATE trial (NCT02680041) assessed the impact of positron emission tomography (PET) with 18F-fluciclovine on management of men with prostate cancer recurrence after curative intent primary therapy and negative/equivocal conventional imaging.
Here, we use LOCATE data to characterize the sites of disease recurrence and explore the potential for 18F-fluciclovine-PET/CT to evaluate oligometastatic disease.
Methods
Eligible men (≥18 years; prior curative intent treatment of prostate cancer; recurrence based on rising PSA; negative/equivocal conventional imaging) underwent 18F-fluciclovine-PET/CT according to standard protocols. The primary outcome measure of the LOCATE trial was a revised management plan post-scan. We performed a secondary analysis of the LOCATE imaging data to characterize anatomical sites of disease recurrence and to explore the potential for 18F-fluciclovine-PET/CT to evaluate oligometastatic disease. Imaging results were stratified by baseline PSA levels and prior treatment(s) and the Fisher exact test used to analyze differences between groups. Oligometastatic disease was defined as 1–5 extraprostatic lesions (≤3 lesions in any single organ system) plus negative prostate/bed imaging (as a surrogate for primary tumor control).
Results
Of 213 enrolled patients, 164 (77%) had undergone prostatectomy as their initial treatment; their median PSA was 0.57ng/mL. For the 49 patients with an intact prostate, the median PSA was 5.5ng/mL.
The overall 18F-fluciclovine-PET/CT detection rate was 57%. Detection rates were 84% in men with intact prostates and 49% in those who had undergone prostatectomy, with the difference being attributable to prostate/bed findings (71% vs 18%, respectively). The detection rate in lymph nodes was 29% and in bone was 11%.
In total, 53/213 (25%) had oligometastatic disease. Twenty (38%) oligometastatic patients had PSA ≤1.0ng/mL. Forty-two (79%) experienced a change to their management plan following the scan, commonly to target a lesion identified by 18F-fluciclovine-PET/CT. The majority of management changes (74%) involved a new treatment modality; however, 10 patients (24%) experienced a modification of the existing plan for radiotherapy to incorporate a boost to an area guided by the 18F-fluciclovine-PET/CT results.
Conclusion
Even at low PSA levels, 18F-fluciclovine-PET/CT identified a diverse pattern of recurrence missed with conventional imaging. One-quarter of men had oligometastatic disease, raising the potential for 18F-fluciclovine-PET/CT to guide targeted treatment of oligometastases.
Keywords: Biochemical recurrence, 18F-fluciclovine, oligometastases, positron emission tomography, prostatic neoplasms
1. Introduction
The overall survival of patients with prostate cancer is improving because of advances in primary and salvage treatments [1]. However, as many as 53% of patients undergoing curative intent primary therapy with radical prostatectomy (RP) or radiotherapy will experience biochemical recurrence (BCR), characterized by rising prostate-specific antigen (PSA) levels [2].
Metastases of prostate cancer are either lymphatic – typically spreading first to regional and then to distant lymph nodes (LNs), or hematogenous, most often to bone, which represents the most common metastatic site beyond LNs [3–5]. With the advent of highly sensitive molecular imaging, men with limited metastatic disease burden, or oligometastatic prostate cancer, are increasingly being identified. Oligometastatic prostate cancer represents a transitional state between localized and widespread metastatic disease. While no consensus definition for oligometastatic disease exists, working definitions for recent trials generally define it as the presence of a finite number of metastatic sites on imaging, with a controlled primary tumor [6, 7]. There is increasing evidence to suggest that durable control of oligometastatic disease can be achieved through targeted treatment of oligometastases, potentially avoiding or delaying the morbidity of systemic therapy [8–10]. Given that conventional imaging methods frequently yield negative or equivocal results in men with BCR after definitive treatment of clinically localized disease, especially when PSA levels are low, there is a growing role for molecular imaging for the early and precise localization of any disease recurrence.
18F-Fluciclovine is a positron emission tomography (PET) radiopharmaceutical that is approved for use in the USA and Europe for men with BCR of prostate cancer after prior therapy(ies). LOCATE was a prospective trial to assess the impact of 18F-fluciclovine-PET/computed tomography (CT) findings on management of men with prostate cancer recurrence after curative intent primary therapy, and who had negative/equivocal conventional imaging (CT or MRI and bone scintigraphy) [11]. Here, we characterize anatomic sites of recurrence using data from LOCATE and explore the potential for 18F-fluciclovine-PET/CT in oligometastatic disease.
2. Methods
Full methods are available elsewhere [11], but briefly LOCATE (NCT02680041) was a prospective multicenter trial that recruited patients with histologically confirmed prostate cancer who met the following eligibility criteria: men ≥18 years; prior curative intent treatment; BCR based on rising PSA; and negative/equivocal findings on conventional imaging. BCR was diagnosed post-RP as detectable or rising PSA ≥0.2ng/mL, with a second confirmatory level ≥0.2ng/mL. In patients who had undergone radiotherapy or brachytherapy, BCR was diagnosed by a PSA increase ≥2.0ng/mL above nadir [12]. Patients underwent 18F-fluciclovine-PET/CT according to standard protocols [13]. The physician completed a questionnaire regarding the patient’s treatment plan both before and after the scan to document any changes to management plans resulting from the scan.
For the current study, the 18F-fluciclovine-PET/CT results were categorized as positive or negative for abnormal tracer uptake (based on the interpretations of local readers), with positivity rates determined on a per patient and regional (prostate/prostate bed vs. extraprostatic (LN, bone or soft tissue)) basis. LNs were categorized as “pelvic”, “retroperitoneal” or “other”; pelvic LNs comprised internal, external and common iliac nodes, pre-sacral nodes, obturator nodes and perirectal nodes. Retroperitoneal LNs comprised paraaortic, retroaortic, retrocaval, aortocaval, paracaval and retrocrural nodes and ‘other’ LNs comprised intraperitoneal and inguinal nodes. Imaging results were stratified by baseline PSA and prior treatment(s) and the Fisher exact test used to analyze differences between groups.
The sites of disease recurrence and the impact of 18F-fluciclovine-PET/CT on management plans were explored in patients with oligometastatic disease. In the absence of consensus criteria for defining oligometastatic disease, the definitions used in recently conducted trials, such as CHAARTED [14] and stereotactic ablative radiotherapy (SABR)-COMET trial [6], provide useful working options. CHAARTED defined low- and high-volume metastatic disease predominantly based on bone metastases. We applied criteria based on those used in SABR-COMET to account for patients who might have potentially salvageable nodal disease. Thus, we defined oligometastatic disease for the purpose of this analysis as 1–5 extraprostatic lesions (≤3 lesions in any single organ system) with negative prostate/bed imaging as a surrogate for primary tumor control.
3. Results
Between June 2016 and May 2017, 213 patients were scanned as part of the LOCATE trial. Patients had a median pre-scan PSA of 1.0ng/mL and were a median 177 days post-diagnosis of BCR. Of the 213 patients, 164 (77%) had undergone RP as their initial treatment; their median PSA was 0.57ng/mL. The median PSA of the 49 patients with an intact prostate was 5.5ng/mL.
Overall, 57% of the 213 patients had a positive 18F-fluciclovine-PET/CT [11], with detection rates of 30% in the prostate/bed, 23% in pelvic LNs, 12% in retroperitoneal LNs, 2.3% in other LNs, and 11% in bone (Figure 1).
Figure 1.
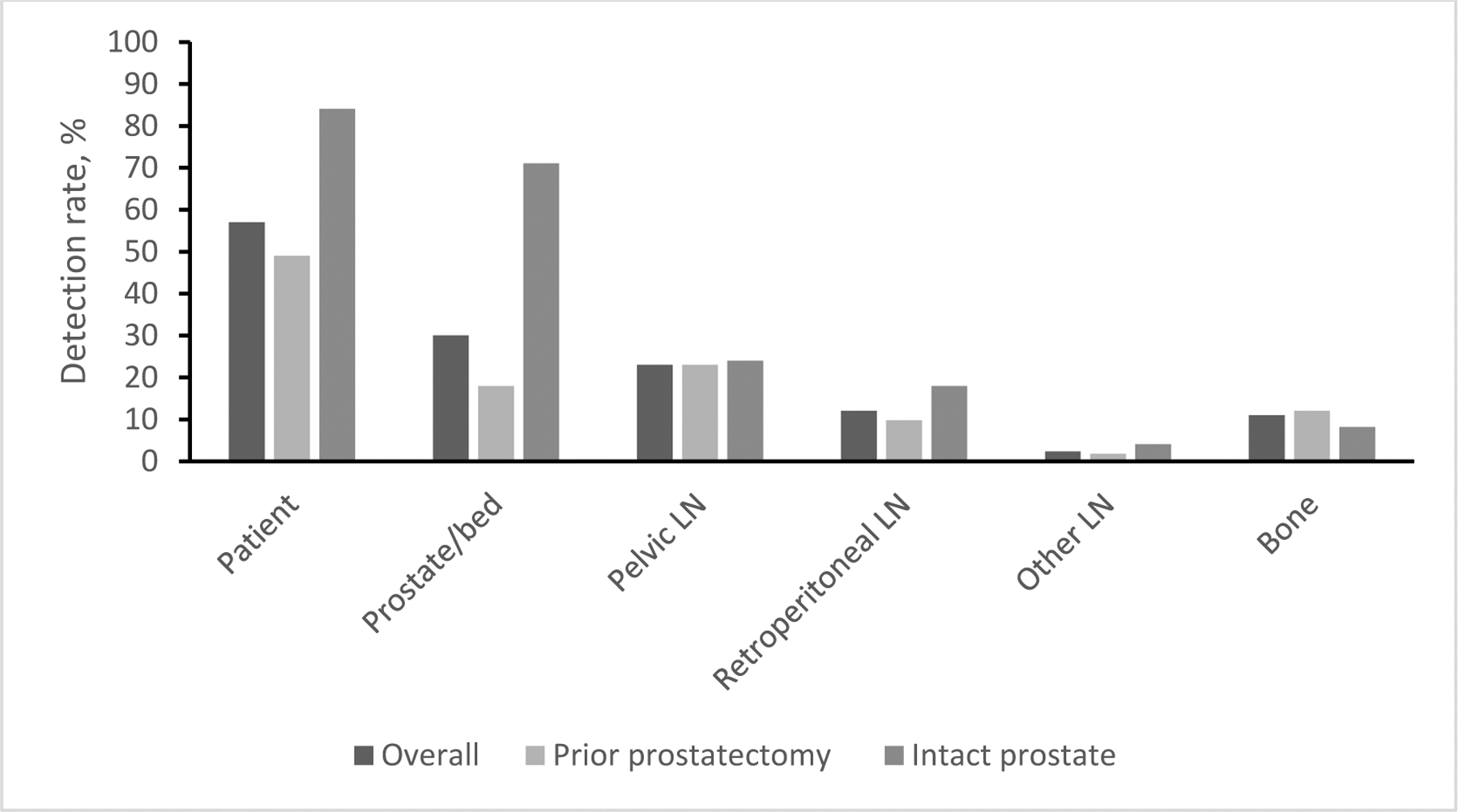
Regional detection rates in the whole population and stratified according to prior prostatectomy
As shown in Figure 1, detection rates in extraprostatic regions were remarkably similar in patients who had undergone RP as in those with intact prostates (38% vs. 37%, p=1.00). Overall, per patient detection rates were higher in men with intact prostates (41/49, 84%) than in those who were post-RP (81/164, 49%) with the difference being attributable to prostate/bed findings (71% vs. 18%, respectively).
Overall, per patient detection ranged from 31% at PSA ≤0.5ng/mL to 95% at PSA >10ng/mL. In patients who had undergone RP, this ranged from 31% at PSA ≤0.5ng/mL to 100% at PSA >10ng/mL. Only one patient with an intact prostate had a PSA <1.0ng/mL – he had a positive 18F-fluciclovine-PET/CT. Detection among the patients with an intact prostate and PSA >10ng/mL was 91%.
In the prostate/bed, detection rates ranged from 16% at PSA ≤0.5ng/mL to 53% at PSA >10ng/mL and in pelvic LNs, rates ranged from 5% at PSA ≤0.5ng/mL to 53% at PSA >10ng/mL. Figure 2 presents the regional detection rates stratified by baseline PSA in patients with and without an intact prostate.
Figure 2.
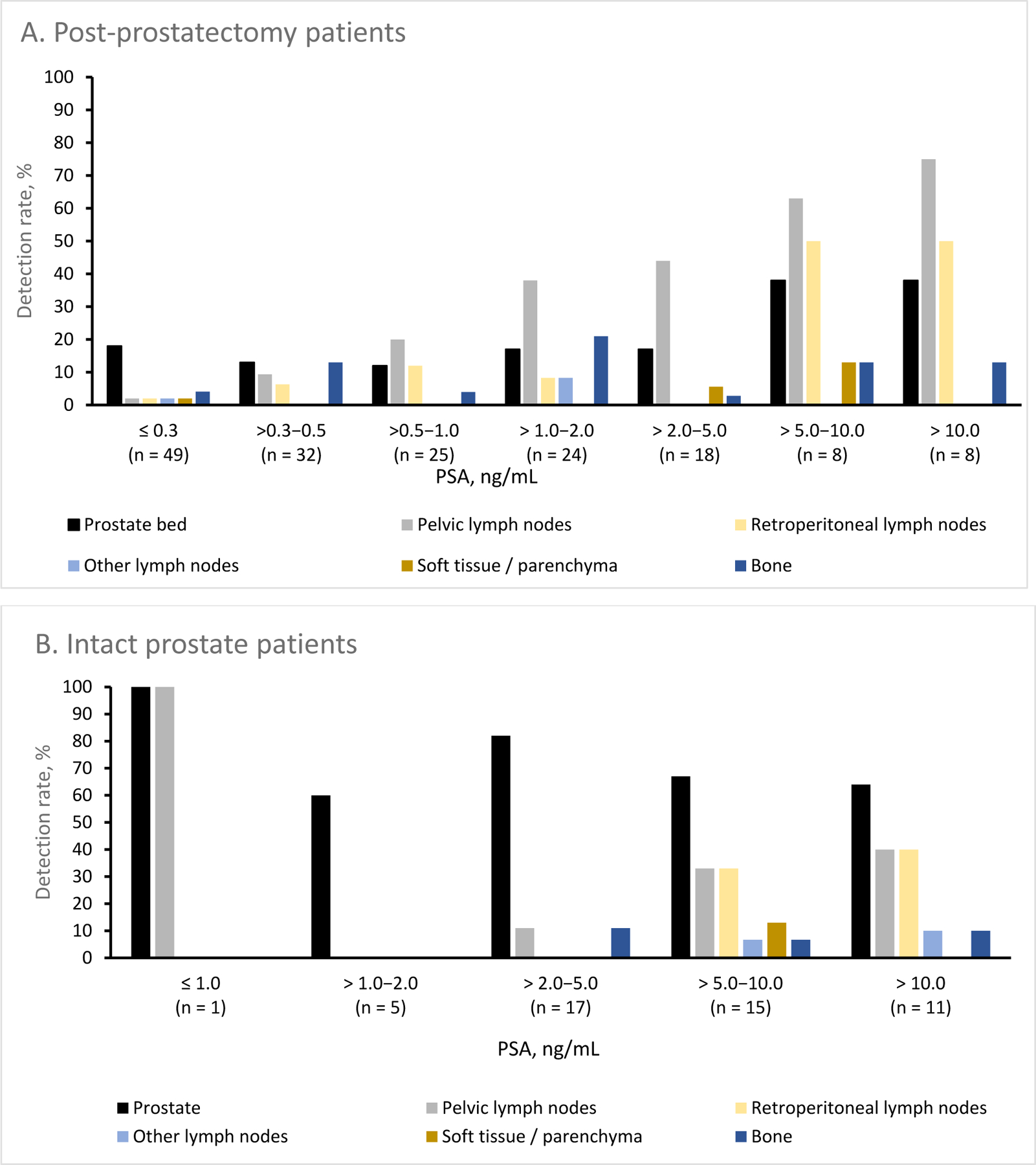
Regional detection rates stratified by PSA in (A) patients who had undergone prostatectomy and (B) with an intact prostate
3.1. Recurrence sites
3.1.1. Lymph nodes
In total, 18F-fluciclovine-avid lesions were found in LNs in 61/213 (29%) patients. Of the patients with positive LNs, 47 (77%) had undergone RP. The median PSA of patients with positive LNs was 2.8ng/mL. For those with lesions in pelvic LNs, the median PSA was 2.0ng/mL and for those with lesions in extrapelvic LNs it was 6.7ng/mL.
We observed overall detection rates of 23% for pelvic LNs, 12% for retroperitoneal LNs and 2.3% for ‘other’ LNs. Even at PSA levels of ≤1.0ng/mL, detection rates of 9.3% in pelvic LNs and 5.6% in retroperitoneal LNs were achieved.
Twenty-eight patients (13%) had positive pelvic LNs (± prostate/bed) but no other involvement. A further 33 (15%) patients had positive LNs that were outside the pelvis (± prostate/bed and/or pelvic LNs).
3.1.2. Bone
In total, 18F-fluciclovine-avid bone lesions were found in 23/213 (11%) patients. Of the patients with PSA ≤1.0ng/mL, 6.5% had a 18F-fluciclovine-avid bone lesion. The 23 patients with bone metastases had a median (range) baseline PSA of 1.5 (0.2–70)ng/mL and 19 (83%) had undergone RP.
Of the 23 patients with 18F-fluciclovine-avid bone lesion(s), 21 (91%) had undergone 99mTc-MDP bone scintigraphy prior to 18F-fluciclovine-PET/CT − 20 with a negative result and 1 with an equivocal result. One patient (4%) had an unspecified bone scan, which was negative, and the remaining patient (4%) did not undergo a bone-specific scan.
Bone lesions were located in 30 anatomical sites, most commonly the ilium (n=10), the lumbar vertebrae (n=6) and the sacrum (n=4). Eighteen patients had bone lesions at one site, three patients at two sites and two patients at three sites. An evaluation of all recurrence sites in the 23 patients with bone lesions showed that 13 (57%) patients had no other 18F-fluciclovine-avid lesions. Three patients (13%) also had recurrence in the prostate/bed, four (17%) had bone and pelvic LN involvement (± prostate/bed), two (8.7%) had findings in bone, pelvic LNs and retroperitoneal LNs and one (4.3%) had positivity in bone and soft tissue only.
3.2. Oligometastatic disease
Fifty-three (25%) of the enrolled patients met our criteria for oligometastatic disease; 52 patients with negative prostate/bed results had 1–3 extraprostatic metastases and 1 patient had 5 metastases. In total, 91% of patients with oligometastatic disease had metastases in one tissue type only, with LNs being the most common site (n=35), approximately 3 times more common than bone (n=13; Table 1). Five patients had metastases in more than one tissue type.
Table 1.
Location of metastases in patients with oligometastatic disease and corresponding baseline PSA levels.
| Recurrence sites: | Bone only | Lymph nodes only | Bone plus lymph nodes | Bone plus soft tissue | Lymph nodes plus soft tissue |
|---|---|---|---|---|---|
| n (N=53) | 13 | 35 | 3 | 1 | 1 |
| % | 25 | 66 | 5.7 | 1.9 | 1.9 |
| Median (range) PSA, ng/mL | 1.10 (0.2–4.2) | 1.69 (0.2–17) | 2.20 (1.5–5.3) | 3.5* | 8.9* |
PSA value of single patient.
We explored baseline PSA levels in patients with oligometastatic disease (Figure 3). Twenty (38%) patients had PSA ≤1.0ng/mL. All 5 patients with metastases in more than one tissue type had PSA >1.0ng/mL. All instances of oligometastatic bone metastases occurred in patients with a PSA <10ng/mL. All instances of soft tissue metastases occurred in patients with a PSA level between 2 and 10ng/mL.
Figure 3.
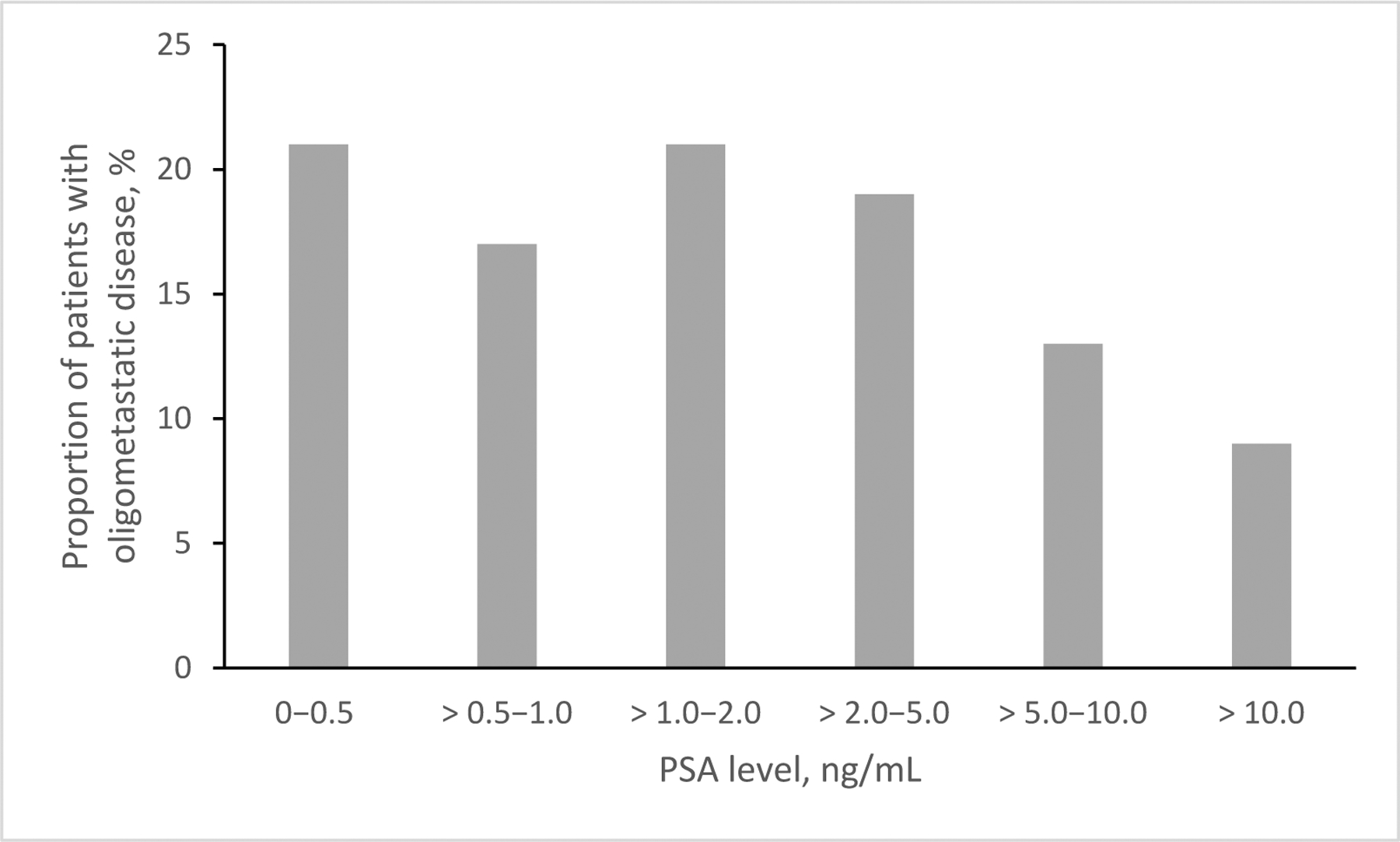
Patients with oligometastatic disease stratified by baseline PSA level.
Forty-two (79%) of the oligometastatic patients experienced changes to their management plans following the 18F-fluciclovine-PET/CT. As shown in Figure 4, the majority of changes (n=31, 74%) involved a change to the treatment modality; however, 11 patients (26%) experienced a modification of the existing plan, most commonly a change to radiotherapy fields to incorporate a boost to an area guided by the 18F-fluciclovine results.
Figure 4.
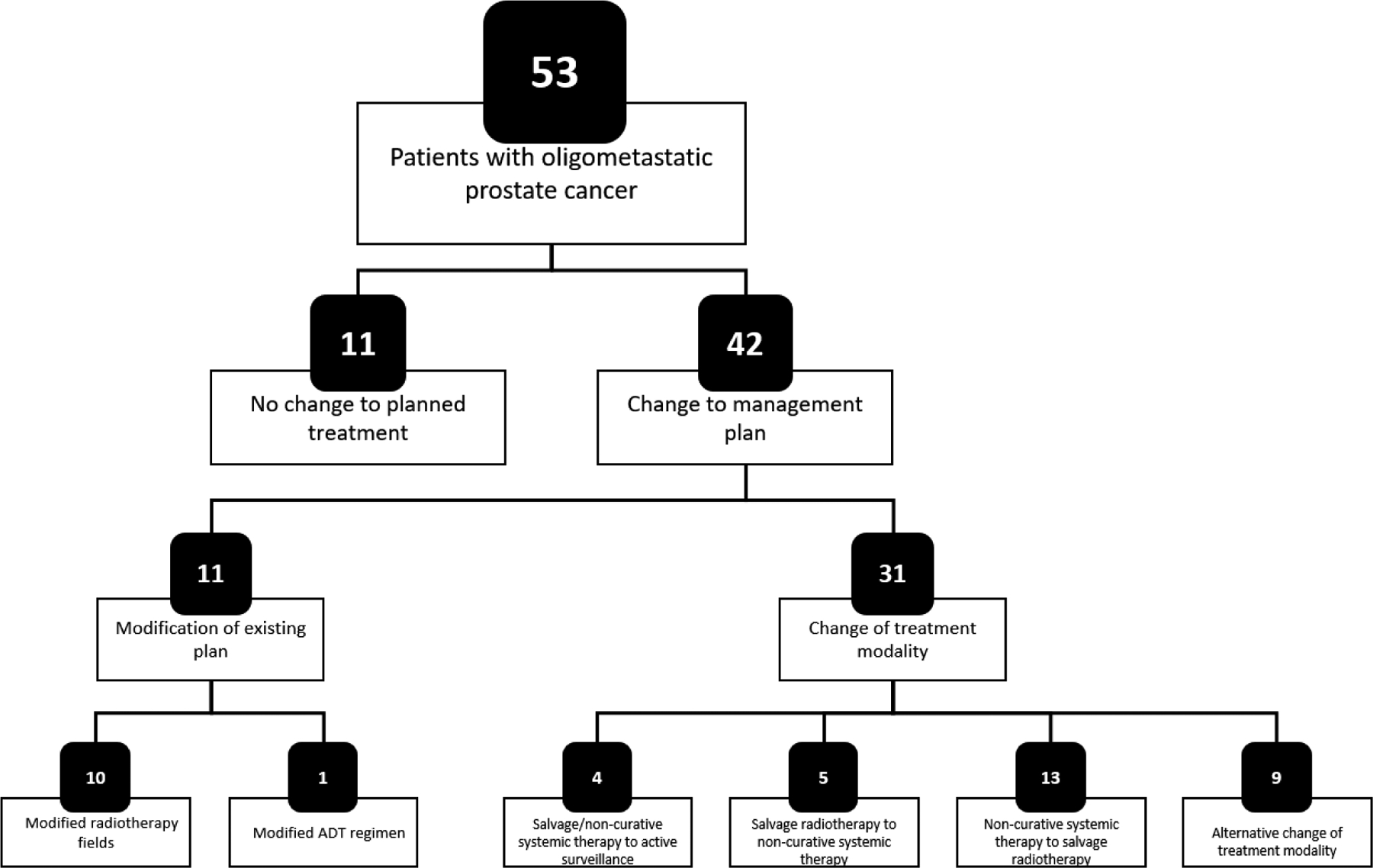
Changes to management plans in patients with oligometastatic disease
Figure 5 presents images from a patient with oligometastatic prostate cancer.
Figure 5.
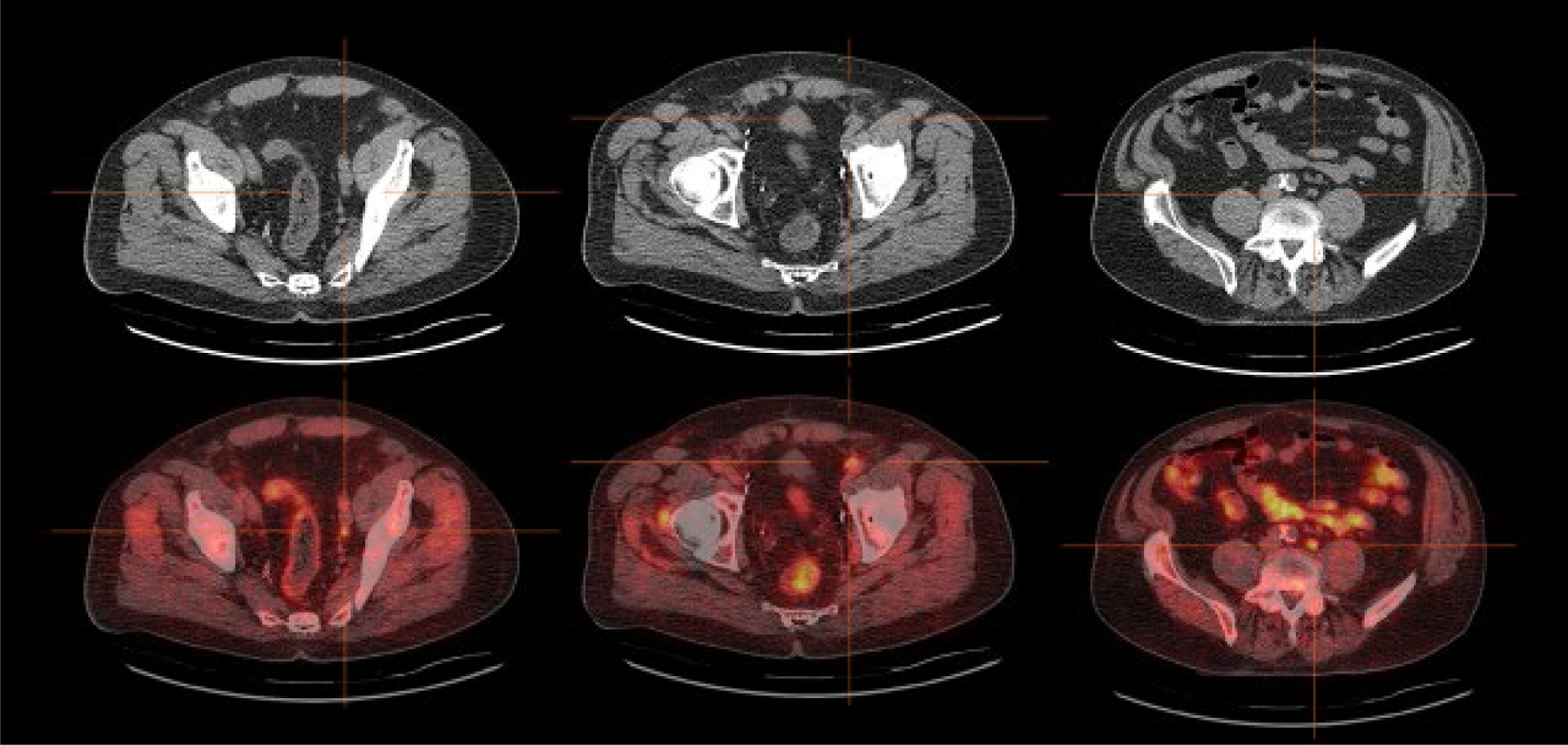
Example of impact of 18F-fluciclovine-PET/CT on patient management. This 67-year-old man underwent radical prostatectomy for pT2cN0, Gleason 3+4 disease after which his PSA remained undetectable for 2.5 years. He was then lost to follow-up, but was found to have biochemical recurrence 14 years after prostatectomy (PSA 11.6ng/mL increasing over 3 months to 16.3ng/mL). Bone scintigraphy and CT of the abdomen and pelvis showed no evidence of recurrent disease. 18F-Fluciclovine-PET/CT showed focally increased uptake in three normal-sized lymph nodes: left obturator (left panel), left external iliac (middle panel) and paraaortic (right panel). The patient’s treatment plan was adjusted to include boost radiotherapy to these oligometastatic sites, as well as conventional salvage prostate bed/pelvic radiotherapy and androgen-deprivation therapy for 1.5 years. The patient’s PSA remained undetectable for three years, but had increased to 0.17ng/mL at last follow-up.
4. Discussion
Here, we describe anatomical sites of recurrence based on 18F-fluciclovine-PET/CT in patients with BCR of prostate cancer. These patients underwent 18F-fluciclovine-PET/CT following negative or equivocal conventional imaging results. 18F-Fluciclovine-PET/CT has been established as an effective imaging technique in this setting [15, 16], with a detection rate that increases with increasing PSA. Here we also show that detection in extraprostatic regions is consistent, irrespective of whether or not the patient has an intact prostate.
Almost one-third of patients showed recurrence in the prostate region, while 38% of patients had extraprostatic involvement [11]. Accurately determining the extent of disease is an important clinical consideration owing to the distinct management approaches used in these disease states. LNs were the most common recurrence site outside the prostate/bed, occurring in almost one-third of patients. In just over half of the patients with positive LNs, the lesions were outside the pelvis. In line with the expected spread of prostate cancer [3–5], we found the most common metastatic site beyond LNs was bone, occurring in 11% of all patients. Current National Comprehensive Cancer Network (NCCN) guidelines suggest that conventional bone scintigraphy should be considered after RP if PSA does not fall to undetectable levels, or following two or more consecutive PSA rises. After radiotherapy, bone scintigraphy is recommended for those with rising PSA who would be candidates for local or systemic therapy [17] with further guidance that 18F-fluciclovine-PET/CT be considered after bone scintigraphy for further evaluation when clinical suspicion of bone metastases is high. All but one of our patients with 18F-fluciclovine-avid osseous disease had negative/equivocal conventional bone scintigraphy prior to enrollment. Accordingly, our findings indicate a potential application of 18F-fluciclovine in the early determination of bone metastases, sooner than suggested by current guidelines, and at lower PSA levels. One likely reason is that 18F-fluciclovine is identifying metastases while in the marrow, prior to changes in bone remodeling that can be visualized with bone scintigraphy [18]. Bone metastases were identified in our patients with PSA levels as low as 0.2ng/mL (median 1.5ng/mL); generally, early identification of metastases at low PSA levels is associated with a higher chance of salvage treatment success [19] and in the case of bone metastases can avoid the associated morbidity of more advanced lesions, such as pain and fractures.
Evidence for a potential role of PET in detection of oligometastatic prostate cancer is growing [20]. Although NCCN guidelines already recommend 18F-fluciclovine-PET/CT in the clinical workup of patients with BCR of prostate cancer [17], we explored its potential for identifying patients with oligometastatic disease. Approximately one-quarter of LOCATE patients were found to have oligometastatic disease, defined as negative prostate/bed imaging and 1–5 extraprostatic lesions (≤3 lesions in any single organ system) on 18F-fluciclovine-PET/CT. These subjects had a median PSA level of 1.4ng/mL and all had negative/equivocal conventional imaging, highlighting the potential for 18F-fluciclovine-PET/CT in the early identification of patients who may benefit from metastasis-directed therapy. Indeed, our data reveal that, of patients whose plan for salvage radiotherapy was modified post-scan, 100% of the changes were to encompass a boost targeted at an 18F-fluciclovine-avid lesion. Moreover, almost one-quarter of management changes in patients with oligometastatic disease were from non-curative ADT to targeted salvage radiotherapy. Avoiding the significant morbidity associated with ADT [21, 22] is an important factor in treatment decisions for such patients. The results of a phase II randomized trial showed that patients who underwent metastasis-directed therapy for oligometastatic recurrent prostate cancer, identified by 11C-choline-PET/CT, had a longer ADT-free survival than those undergoing active surveillance [20]. The randomized, open-label, phase II SABR-COMET trial compared palliative standard of care treatment with standard-of-care plus SABR to all metastatic lesions in 99 patients with a variety of oligometastatic cancers (16% with prostate cancer). The results, which offer further support to the benefits of targeted therapy in patients with oligometastatic disease, demonstrated a 13-month increase in overall survival and a doubling of progression-free survival in the SABR arm [6].
A recent study suggests that in patients with nodal-only recurrence, metastasis-directed therapy via salvage LN dissection should be considered as part of a multimodal approach rather than as an exclusive treatment strategy, after showing a significant survival benefit associated with the administration of ADT following salvage LN dissection [23]. An upcoming Phase III trial [24] may help further delineate the role that molecular imaging can play in metastases-directed therapy (either alone or as part of a multimodal approach). The trial aims to determine the effect of apalutamide with or without targeted radiation therapy by comparison with standard-of-care treatment on outcomes for patients with BCR of prostate cancer. The investigators will use 18F-fluciclovine-PET/CT to determine the target fields for radiation therapy.
The present study is not without limitations. While we did not routinely confirm imaging results with histological findings, FDA approval of 18F-fluciclovine was based on data that included correlation with histological findings [15]. Although those data indicated a false-positive rate of 14.7%, 18F-fluciclovine reportedly has fewer false-positives than other PET tracers because of its comparatively lower uptake by inflammatory cells [15, 25–27]. Uptake of 18F-fluciclovine is not specific for prostate cancer and may occur with other types of cancer, prostatitis and benign prostatic hyperplasia and false-positives have been described in association with an inflammatory response after cryotherapy and radiotherapy [28]. However, the present study design included measures to limit such occurrences. Our exclusion criteria ensured that any non-surgical local treatment must have occurred at least 1 year before enrollment, with brachytherapy occurring at least 2 years previously. Moreover, the focus of the present study was primarily on extraprostatic sites of oligometastatic disease, and as shown by prior histologically confirmed data, the PPV for 18F-fluciclovine-PET/CT in extraprostatic regions is excellent at 92% [15].
A further limitation of these data is the lack of long-term follow up. A change in management does not necessarily confer improved outcomes and thus the results of ongoing trials, such as those discussed above, are eagerly awaited to confirm how incorporation of 18F-fluciclovine-PET/CT into therapy planning will affect outcomes for patients [24].
5. Conclusions
Even at low PSA levels, 18F-fluciclovine-PET/CT identified a diverse pattern of recurrence missed with conventional imaging. One-quarter of men had oligometastatic disease raising the potential for 18F-fluciclovine-PET/CT to guide targeted treatment of oligometastases.
18F-Fluciclovine-PET/CT is widely used for the detection and localization of disease in men with biochemical recurrence (BCR) prostate cancer.
It detects both locoregional and distant disease, including osseous metastases, not found by conventional imaging, even at PSA levels as low as 0.2 ng/mL.
18F-Fluciclovine-PET/CT allows for identification of men with oligometastatic disease (in about 25% of BCR patients) and often leads to changes in patient management to include targeted therapy to these metastatic lesions.
Acknowledgments
The LOCATE study was funded by Blue Earth Diagnostics Ltd, Oxford, UK. Medical writing support was provided by Dr Catriona Turnbull, Blue Earth Diagnostics Ltd, Oxford, UK.
References
- [1].Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol. 2020;77:38–52. [DOI] [PubMed] [Google Scholar]
- [2].Mottet N, Bellmunt J, Briers E, Bolla M, Bourke L, Cornford P, et al. The EAU prostate cancer guidelines. http://uroweb.org/guideline/prostate-cancer/.2020. (Accessed, August 2020).
- [3].Gandaglia G, Abdollah F, Schiffmann J, Trudeau V, Shariat SF, Kim SP, et al. Distribution of metastatic sites in patients with prostate cancer: A population-based analysis. Prostate. 2014;74:210–6. [DOI] [PubMed] [Google Scholar]
- [4].Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106:1624–33. [DOI] [PubMed] [Google Scholar]
- [5].Paño B, Sebastià C, Buñesch L, Mestres J, Salvador R, Macías NG, et al. Pathways of lymphatic spread in male urogenital pelvic malignancies. Radiographics. 2011;31:135–60. [DOI] [PubMed] [Google Scholar]
- [6].Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393:2051–8. [DOI] [PubMed] [Google Scholar]
- [7].Royal Marsden NHS Foundation Trust, Institute of Cancer Research United Kingdom, National Health Service United Kingdom. Conventional care versus radioablation (stereotactic body radiotherapy) for extracranial oligometastases. https://ClinicalTrials.gov/show/NCT02759783; 2016. (AccessedSeptember 2020).
- [8].Reyes DK, Pienta KJ. The biology and treatment of oligometastatic cancer. Oncotarget. 2015;6:8491–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ost P, Jereczek-Fossa BA, As NV, Zilli T, Muacevic A, Olivier K, et al. Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: a multi-institutional analysis. Eur Urol. 2016;69:9–12. [DOI] [PubMed] [Google Scholar]
- [10].Conde Moreno AJ, Ferrer Albiach C, Muelas Soria R, Gonzalez Vidal V, Garcia Gomez R, Albert Antequera M. Oligometastases in prostate cancer: restaging stage IV cancers and new radiotherapy options. Radiat Oncol. 2014;9:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Andriole GL, Kostakoglu L, Chau A, Duan F, Mahmood U, Mankoff DA, et al. The impact of positron emission tomography with 18F-fluciclovine on the management of patients with biochemical recurrence of prostate cancer: results from the LOCATE trial. J Urol. 2019;201:322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Roach M, Hanks G, Thames H, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–74. [DOI] [PubMed] [Google Scholar]
- [13].Miller MP, Kostakoglu L, Pryma D, Yu JQ, Chau A, Perlman E, et al. Reader training for the restaging of biochemically recurrent prostate cancer using 18F-fluciclovine PET/CT. J Nuc Med. 2017;58:1596–602. [DOI] [PubMed] [Google Scholar]
- [14].ECOG-ACRIN Cancer Research Group, National Cancer Institute, Eastern Cooperative Oncology Group. Androgen ablation therapy with or without chemotherapy in treating patients with metastatic prostate cancer. https://ClinicalTrials.gov/show/NCT00309985; 2006. (AccessedSeptember 2020).
- [15].Bach-Gansmo T, Nanni C, Nieh PT, Zanoni L, Bogsrud TV, Sletten H, et al. Multisite experience of the safety, detection rate and diagnostic performance of fluciclovine (18F) positron emission tomography/computerized tomography imaging in the staging of biochemically recurrent prostate cancer. J Urol. 2017;197:676–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Scarsbrook AF, Bottomley D, Teoh EJ, Bradley KM, Payne H, Afaq A, et al. Impact of 18F-fluciclovine positron emission tomography on the management of patients with recurrence of prostate cancer: results from the FALCON trial. Int J Radiat Oncol Biol Phys. 2020;107:316–24. [DOI] [PubMed] [Google Scholar]
- [17].NCCN. NCCN clinical practice guidelines in oncology: prostate cancer. Version 4.2019. https://www2.tri-kobe.org/nccn/guideline/urological/english/prostate.pdf 2019.(AccessedSeptember 2020).
- [18].Ibrahim T, Flamini E, Mercatali L, Sacanna E, Serra P, Amadori D. Pathogenesis of osteoblastic bone metastases from prostate cancer. Cancer. 2010;116:1406–18. [DOI] [PubMed] [Google Scholar]
- [19].Stephenson AJ, Scardino PT, Kattan MW, Pisansky TM, Slawin KM, Klein EA, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36:446–53. [DOI] [PubMed] [Google Scholar]
- [21].Tsai HK, D’Amico AV, Sadetsky N, Chen MH, Carroll PR. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99:1516–24. [DOI] [PubMed] [Google Scholar]
- [22].Saigal CS, Gore JL, Krupski TL, Hanley J, Schonlau M, Litwin MS, et al. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110:1493–500. [DOI] [PubMed] [Google Scholar]
- [23].Bravi CA, Fossati N, Gandaglia G, Suardi N, Mazzone E, Robesti D, et al. Long-term outcomes of salvage lymph node dissection for nodal recurrence of prostate cancer after radical prostatectomy: not as good as previously thought. Eur Urol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].ECOG-ACRIN Cancer Research Group, National Cancer Institute, Eastern Cooperative Oncology Group. Treating prostate cancer that has come back after surgery with apalutamide and targeted radiation using PET/CT imaging. https://ClinicalTrials.gov/show/NCT04423211; 2020. (AccessedSeptember 2020).
- [25].Oka S, Kanagawa M, Doi Y, Schuster DM, Goodman MM, Yoshimura H. PET tracer 18F-fluciclovine can detect histologically proven bone metastatic lesions: a preclinical study in rat osteolytic and osteoblastic bone metastasis models. Theranostics. 2017;7:2048–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Oka S, Okudaira H, Ono M, Schuster DM, Goodman MM, Kawai K, et al. Differences in transport mechanisms of trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid in inflammation, prostate cancer, and glioma cells: comparison with L-[methyl-11C]methionine and 2-deoxy-2-[18F]fluoro-D-glucose. Mol Imaging Biol. 2014;16:322–9. [DOI] [PubMed] [Google Scholar]
- [27].Kanagawa M, Doi Y, Oka S, Kobayashi R, Nakata N, Toyama M, et al. Comparison of trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid (anti-[18F]FACBC) accumulation in lymph node prostate cancer metastasis and lymphadenitis in rats. Nucl Med Biol. 2014;41:545–51. [DOI] [PubMed] [Google Scholar]
- [28].Blue Earth Diagnostics. Axumin prescribing information at: https://www.axumin.com/prescribing-information.pdf (AccessedSeptember 2020).


