To the Editor:
We have read with interest the recent cases suggesting the possibility of vaccine-induced immune-mediated hepatitis with Pfizer-BioNTech and Moderna mRNA-1273 vaccines for the SARS-CoV-2 virus.[1], [2], [3], [4], [5], [6], [7] However, as the cohort of vaccinated individuals against COVID-19 increases, the previously reported cases could not exclude a coincidental development of autoimmune hepatitis, which has an incidence of 3/100,000 population per year.8 Our case demonstrates conclusive evidence of vaccine-induced immune-mediated hepatitis with a rapid onset of liver injury after the first Moderna dose, which on re-exposure led to acute severe autoimmune hepatitis.
Case description
A 47-year-old Caucasian man, previously completely well, received his 1st Moderna vaccine dose on the 26 April 2021. He noted malaise and jaundice 3 days after. Investigations on the 30th April showed serum bilirubin 190 μmol/L (normal 0-20), alanine aminotransferase (ALT) 1,048 U/L (normal 10-49), alkaline phosphatase (ALP) 229 U/L (normal 30-130), albumin 41 g/L (normal 35-50). Blood count, renal function and international normalized ratio (INR) were normal. Liver function tests (LFTs) last checked 4 years previously were normal. He denied paracetamol use and reported minimal alcohol intake. Ultrasound scan, CT thorax, abdomen and pelvis and MRI pancreas performed to exclude malignancy, showed no significant findings. Serum IgG was raised at 25.1 g/L (normal 6-16), IgM 2.2 g/L (0.5-2) and serum was positive for anti-nuclear antibody. Serological tests for HAV, HBV, HCV, HEV, EBV and CMV were negative.
His jaundice faded and LFTs improved: bilirubin falling on 25th June to 69 μmol/L and ALT to 332 U/L. The patient received his 2nd Moderna vaccine dose on the 6 July 2021 (despite reporting the jaundice to the vaccination centre) and the jaundice returned a few days after. Blood tests on 20th July found bilirubin 355 μmol/L, ALT 1,084 U/L and a raised prothrombin time (PT) of 18.4 seconds. After liver biopsy on the 21st July 2021, prednisolone 40 mg/day was commenced and he was transferred to our service.
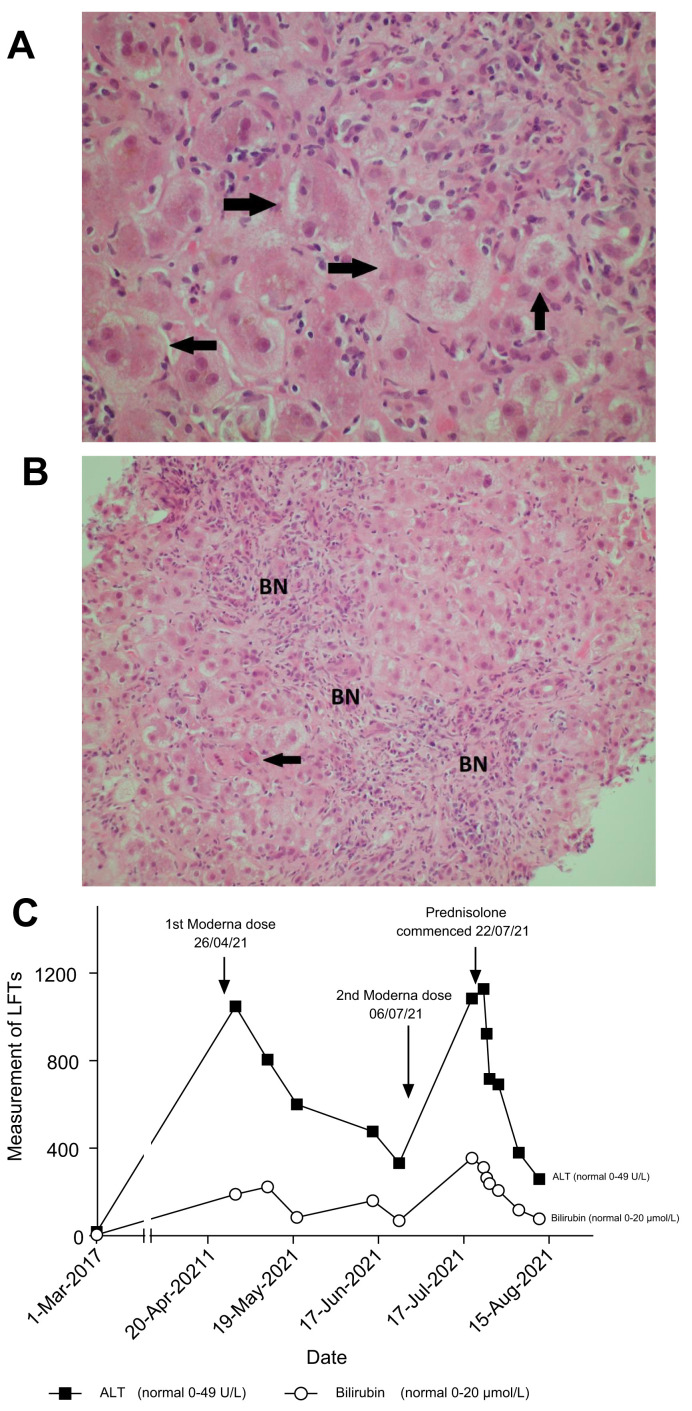
On examination, he was alert, deeply jaundiced, with hepatomegaly but no ascites. Repeat abdominal ultrasound showed a mildly fatty liver, patent portal and hepatic vein flow, with no ascites. Review of the liver biopsy showed acute active hepatitis: widespread areas of bridging necrosis, marked interface hepatitis, lymphoplasmatic inflammation including eosinophils, ballooned hepatocytes, multi-nucleated giant cells, and emperipolesis (Fig.1 ). There was minimal fibrosis, Ishak stage 1. The pattern of injury on histology was consistent with acute hepatitis, with features of autoimmune hepatitis or possible drug-induced liver injury (DILI), triggering an autoimmune-like hepatitis.
Fig. 1.
Histological findings and biochemical findings.
H&E-stained section of liver biopsy indicates acute hepatitis. (A) The parenchymal hepatocytes are arranged into rosette forms (marked with arrows) with cholestasis. (B) BN from hepatocyte loss, some by apoptosis (arrow). (C) Diagram showing trend of bilirubin and ALT following Moderna vaccine dose 1 and 2 with response to prednisolone. ALT, alanine aminotransferase; BN, bridging necrosis. (This figure appears in color on the web.)
Prednisolone 40 mg/day was continued and LFTs improved (Fig. 1). He was discharged on prednisolone and on follow-up, blood tests continue to improve, and PT normalised within 2 weeks.
Discussion
This case illustrates immune-mediated hepatitis secondary to the Moderna vaccine, which on inadvertent re-exposure led to worsening liver injury with deranged synthetic function. This occurred in a well man with no other medical problems. The onset of jaundice associated with the mRNA vaccine was unusually rapid. This was also illustrated in the other cases where symptoms developed over a median of 7 days (range 4-35). Latency is usually longer in other causes of DILI, but can vary depending on mode of injury.
The mRNA vaccine pathway triggers pro-inflammatory cytokines including interferon and cross-reactivity has been illustrated between the antibodies against the spike protein and self-antigens.9 , 10
Seven cases of suspected immune-mediated hepatitis have been reported with SARS-2-COV mRNA vaccines (3 with Pfizer and 4 with Moderna).[1], [2], [3], [4], [5], [6], [7] Liver histology was assessed in every case and findings were similar to ours, indicating acute hepatitis with interface hepatitis, lymphoplasmacytic infiltrate and absence of fibrosis. Eosinophils as part of the infiltrate, which can be noted in DILI were present in 3 cases. All 7 patients responded well to steroids (n = 5 prednisolone, n = 1 budesonide and n = 1 methylprednisolone). In 3 cases there were features suggesting coincidental autoimmune hepatitis: a 35-year-old lady in her third trimester of pregnancy with positive double-stranded DNA, an 80-year-old lady with a history of autoimmune conditions and a 41-year-old lady with strongly positive auto-antibody panel after both doses of vaccination. In the other 4 cases, a raised IgG, with at least 1 positive antibody was noted in 3 cases.[4], [5], [6], [7]
This case has confirmed immune-mediated hepatitis secondary to the Moderna vaccine, which on inadvertent re-exposure led to acute severe hepatitis. Treatment with corticosteroid therapy appears to be favourable. We wish to highlight that immune-mediated reactions from the SARS-CoV-2 mRNA vaccines are very rare and during the COVID pandemic, the vaccination programme continues to be crucial. We report this case to encourage vigilance for drug-induced reactions and to raise awareness to vaccination centres to incorporate it into their routine checks before administering second doses. Long-term follow up of identified individuals will be essential in determining the prognosis of this immune-mediated liver injury.
Financial support
The authors received no financial support to produce this manuscript.
Authors’ contributions
DG and AAJ conceptualised the work. GT wrote the initial draft and all authors contributed to and approved the final manuscript.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2021.09.031.
Supplementary data
The following is the supplementary data to this article:
References
- 1.Bril F., Al Diffalha S., Dean M., Fettig D.M., et al. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J Hepatol. 2021;75(1):222–224. doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rocco A., Sgamato C., Compare D., Nardone G., et al. Autoimmune hepatitis following SARS-CoV-2 vaccine: may not be a casualty. J Hepatol. 2021;75(3):728–729. doi: 10.1016/j.jhep.2021.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Londono M.C., Gratacos-Gines J., Saez-Penataro J. Another case of autoimmune hepatitis after SARS-CoV-2 vaccination. Still casualty? J Hepatol. 2021 doi: 10.1016/j.jhep.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghielmetti M., Schaufelberger H.D., Mieli-Vergani G., Cerny A., et al. Acute autoimmune-like hepatitis with atypical anti-mitochondrial antibody after mRNA COVID-19 vaccination: a novel clinical entity? J Autoimmun. 2021;123:102706. doi: 10.1016/j.jaut.2021.102706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McShane C., Kiat C., Rigby J., Crosbie O., et al. The mRNA COVID-19 vaccine - a rare trigger of Autoimmune Hepatitis? J Hepatol. 2021 doi: 10.1016/j.jhep.2021.06.044. S0168-8278(21)01896-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan C.K., Wong Y.J., Wang L.M., Ang T.L., et al. Autoimmune hepatitis following COVID-19 Vaccination: true causality or mere association? J Hepatol. 2021;18 doi: 10.1016/j.jhep.2021.06.009. S0168-8278(21)00424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lodato F., Larocca A., D’Errico A., et al. An unusual case of cholestatic hepatitis after m-RNABNT162b2 (Comirnaty) SARS-CoV-2 vaccine: coincidence, autoimmunity or drug related liver injury? J Hepatol. 2021 doi: 10.1016/j.jhep.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grønbaek L., Otete H., Ban L., Crooks C., et al. Incidence, prevalence and mortality of autoimmune hepatitis in England 1997-2015. A population-based cohort study. Liver Int. 2020;40(7):1634–1644. doi: 10.1111/liv.14480. [DOI] [PubMed] [Google Scholar]
- 9.Vojdani A., Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teijaro J.R., Farber D.L. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21:195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.