Abstract
AIMS:
Although rare, malignant sarcomatoid breast tumours without evidence of epithelial differentiation comprise a diagnostic challenge with management implications. Earlier studies have generally considered these to be primary breast sarcomas; however, supporting evidence is lacking and management remains variable. This study aimed to provide an evidence-based approach to improve consistency of diagnosis and management for such cases.
METHODS AND RESULTS:
A large series (n=140) of metaplastic breast carcinoma (MBC) diagnosed in Nottingham over 18 years was analysed. Only cases with available data on immunohistochemical expression of cytokeratins (CKs) were included. The prevalence and pattern of expression for various CKs were assessed and details of tumours negative for CKs were collected. A diagnostic approach based on our experience is provided. 47 cases (34%) showed foci of conventional type invasive breast carcinoma or DCIS, whereas 93 cases (66%) were diagnosed as MBC based on morphology and/or CK expression. 97 cases (69%) were negative for one or more CKs, with 18 cases (13%) negative for 5 or more CKs. 8 cases (6%) lacked expression of all CKs tested. Further examination showed evidence of carcinomatous nature in 5 cases, whereas 3 were diagnosed as MBC following extensive diagnostic workup and on our experience.
CONCLUSION:
This study suggests that MBC represent a spectrum of neoplasms with some lacking CKs expression. Sarcomatoid neoplasms of the breast lacking evidence carcinomatous morphology and CK expression may represent an extreme end of differentiation that can be considered as carcinomas rather than sarcomas for management purposes (following extensive workup).
Keywords: Breast metaplastic carcinoma, CKs negative, diagnosis
INTRODUCTION
Metaplastic carcinoma of the breast (MBC) is a rare type of BC accounting for 0.5–3% of cases1,2. This comprises a heterogeneous group of tumours4,5 characterized by differentiation of the neoplastic epithelium towards squamous or mesenchymal elements6–14. The diagnosis of adenosquamous carcinoma and mixed squamous and spindle cell carcinomas as MBC is typically straightforward; in particular, when in situ carcinoma is present or when positivity of breast related immunohistochemical markers is seen. MBC with mesenchymal differentiation comprises tumours mostly with spindle cells although more rarely they demonstrate osseous, chondroid, rhabdoid or even neuroglial differentiation15. Morphologically these sarcomatoid MBCs overlap with a variety of lesions including high-grade mesenchymal-looking tumours such as primary16 and metastatic sarcomas, stromal-rich phyllodes tumour, lymphomas and melanomas. Distinguishing MBC from these mimics may be challenging and is based on a constellation of features including clinical context, morphological appearances and immunohistochemical (IHC) / molecular features2,12,14,17–19. Morphologically these tumours are diagnosed as MBC if there is an associated conventional mammary carcinomatous element (either in situ lesions or conventional type invasive breast carcinomas) or by IHC demonstration of epithelial differentiation (i.e. the presence of cytokeratin (CK) positivity). Pathologists typically rely on a panel of IHC markers to demonstrate the epithelial nature of sarcomatoid breast neoplasms lacking definite morphological evidence of a breast epithelial origin, and to exclude mimicking lesions.
Apart from the well-established subtypes of sarcoma in the breast, such as angiosarcoma and malignant phyllodes tumour, several studies have reported a large number of undifferentiated primary breast sarcomas, for example designated as breast malignant fibrous histiocytoma/pleomorphic sarcoma/sarcoma not otherwise specified, fibrosarcoma, myxofibrosarcoma – all of which are diagnoses of exclusion20–22.
Primary breast NOS sarcomas are extremely rare malignant tumours that are purported to arise from the mesenchymal tissue of the breast and are considered as a diagnosis of exclusion16,23–27. Most of the published studies of primary breast NOS sarcomas have not elaborated on how the specific diagnosis was established26,28,29, or used the lack of morphological evidence of carcinomatous differentiation and CKs negativity as criteria for their diagnosis16 and most of the studies have included relatively small numbers of cases with variable panels of IHC markers10,30–35,6,14. To highlight the inconsistency in diagnosis and the challenge associated with relying on CKs positivity in such situations some of these sarcomas were reclassified on review and using different sets of CKs. In a previous study of breast sarcomas27, 11 out of 36 cases (30%) were reclassified as MBC following additional CKs staining. In a different series, 6 out of 27 cases (22%) initially categorised as primary malignant fibrous histiocytoma of the breast were reclassified as MBC after testing for a range of CKs21.
Categorisation of such CK negative sarcomatoid tumours for management purposes often poses great challenges with lack of consensus diagnostic assignments of these patients into specific categories resulting in different treatment strategies in different centres. In this study we evaluated a large number of sarcomatoid breast neoplasms with available data on CKs expression, to determine the nature of those tumours that lacked evidence of epithelial differentiation, including a CKs negative phenotype. We also provide an insight into the diagnostic approach of sarcomatoid MBC.
MATERIALS AND METHODS
Cohort
This study included cases diagnosed at the Nottingham City Hospital, Nottingham between 2000 and 2018. Cases included breast lesions from patients managed locally in Nottingham and others referred for a second opinion for diagnosis. Criteria for inclusion were all cases diagnosed as MBC, including malignant spindle cell and pleomorphic cell lesions, matrix producing MBC and MBC not otherwise specified, all of which were diagnosed using the previously published criteria6,36, summarised in the algorithm described in Figure 6.
Figure 6 –

Diagnostic algorithm used in our department.
MBC – metaplastic breast carcinoma; CK – cytokeratin; PT- phyllodes tumour.
Cases that were not assessed for immunohistochemical markers of epithelial differentiation, namely CKs (Table 1), were excluded. Cases diagnosed as metastatic sarcoma or carcinoma, angiosarcoma or phyllodes tumours were also excluded. As a result, of the 160 MBCs identified in our database, 20 cases were excluded. The CKs which were used are (not all of them tested in each case): AE1/AE3, CK5, CK5/6, CK7, CK8, CK18, CK8/18, CK19, CAM5.2, MNF116, 34ßE12, CK20, CK17, CK14. Details related to p63 staining were not purposefully recorded except in individual cases.
Table 1a:
Cytokeratin (CKs) antibodies used in this study with frequency of positivity.
| CK antibody used* | No of positive cases | No. of negative cases | Percent of positivity (%) |
|---|---|---|---|
| CK5/6 | 55 | 33 | 63 |
| CK14 | 53 | 26 | 67 |
| AE1/AE3** | 40 | 25 | 62 |
| CAM5.2 | 54 | 30 | 64 |
| MNF116 | 44 | 24 | 67 |
| 34βE12 | 15 | 22 | 41 |
| CK7 | 33 | 36 | 49 |
| CK8 | 17 | 10 | 63 |
| CK18 | 18 | 12 | 60 |
| CK17 | 9 | 1 | 80 |
| CK19 | 18 | 26 | 41 |
The CKs used in this study were not uniformly tested in all cases; different combinations of CKs were used for each case;
Each pan-cytokeratin is considered as one CK antibody in this study. Other CKs including CK5, CK20 and panCK were stained in few cases only, so they were not included in this table.
Variables collected included histological subtype, presence of overt mammary carcinomatous elements and of associated ductal carcinoma in situ (DCIS), along with data on all CKs tested. Clinical history and details of the microscopic description of CKs negative tumours were reviewed. The available slides from all cases in this study were reviewed by a single observer (MM) and doubtful cases were discussed with a second observer (RM) to confirm the pattern of expression for CKs and the presence of any associated carcinomatous component. The slides of a subset of this cohort were reviewed as part of a previous study12 and the data was also available. Slides of other markers were not reviewed. Details related to p63 staining were not purposefully recorded except in individual cases. The clinicopathological variables were compared using contingency tables and chi-squared and Pearson’s correlation tests. All comparisons were two-sided and a p-value of <0.05 was considered significant.
This study was approved by the Nottingham Research Biorepository (NRB) Access Committee under the biobank ethical approval REC reference: 10/H1008/72 (NRES Committee North West - Greater Manchester Central)
RESULTS
This study included 140 MBCs with available CKs expression data. 25 cases (18%) showed foci of associated conventional invasive breast carcinoma, NST; 30 (21%) cases showed associated DCIS, and 8 cases (6%) showed both in situ and invasive components. The number of CKs examined with IHC per case ranged from 1 to 9 (median 5) (Table 1). Most cases had 4–5 CKs (46%) performed; the number of cases positive for any one specific CK matched the number of cases with negative staining, suggesting that CKs sensitivity is variable. 88% of cases (118/140) showed expression of at least one CKs (range 1–8, median 3) but the expression was variable from diffuse strong to focal and weak (Figure 1). The remaining 22 cases were negative for all CKs tested (Table 2). Of all the CKs examined per case, 69% (n=97) of tumours were negative in the sarcomatous component for at least one (Table 2a), including cases with or without an associated carcinomatous component (Table 2b).
Figure 1A:

This case with spindle cell morphology showed very focal CK expression: H&E (A), CK AE1/AE3 (B), CK5/6 (C), and p63 (D). In this case, p63 positivity was used in conjunction with focal CK expression to support a diagnosis of MBC.
Table 2a:
Correlation between the number of negative cytokeratins (CKs) and the total number of CKs requested – first half of the table - and the number of positive CKs – second half of the table (p<0.001).
| Number of cases distributed by number of negative CK immunostains |
Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0~ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| Total no. of CK immunostains | |||||||||||
|
| |||||||||||
| 1 | 6* | 0 | 6 | ||||||||
| 2 | 6 | 4 | 0 | 10 | |||||||
| 3 | 6 | 4 | 0 | 2 | 12 | ||||||
| 4 | 12 | 13 | 5 | 4 | 2 | 36 | |||||
| 5 | 7 | 5 | 6 | 2 | 2 | 6 | 28 | ||||
| 6 | 3 | 2 | 2 | 3 | 3 | 2 | 3 | 18 | |||
| 7 | 2 | 1 | 0 | 1 | 0 | 0 | 1 | 4 | 9 | ||
| 8 | 1 | 1 | 3 | 2 | 0 | 1 | 1 | 0 | 2 | 11 | |
| 9** | 0 | 0 | 1 | 3 | 1 | 1 | 0 | 1 | 0 | 3 | 10 |
|
| |||||||||||
| No. of positive CK immunostains | |||||||||||
|
| |||||||||||
| 0 | 0 | 0 | 0 | 2 | 2 | 6 | 3 | 4 | 2 | 3 | 22 |
| 1 | 6* | 4 | 0 | 4 | 2 | 2 | 1 | 0 | 0 | 19 | |
| 2 | 6 | 4 | 5 | 2 | 3 | 0 | 1 | 1 | 22 | ||
| 3 | 6 | 13 | 6 | 3 | 0 | 1 | 0 | 29 | |||
| 4 | 12 | 5 | 2 | 1 | 0 | 1 | 21 | ||||
| 5 | 7 | 2 | 0 | 2 | 1 | 12 | |||||
| 6 | 3 | 1 | 3 | 3 | 10 | ||||||
| 7 | 2 | 1 | 1 | 4 | |||||||
| 8 | 1 | 0 | 1 | ||||||||
|
| |||||||||||
| Total | 43 | 29 | 17 | 17 | 9 | 10 | 5 | 5 | 2 | 3 | 140 |
column lists all cases which were positive with all CKs testes (none were negative)
6 cases were stained with only 1 CK and they were all positive.
9 CK antibodies were used in 10 cases: 3 were negative for all of the 9 CKs, 1 was negative for 7 CKs and positive for 2 CKs, 1 case was positive for 5 CKs and negative for 4, whereas 3 cases were positive for 3 CKs and negative for the remaining 6 CKs.
Distribution of the number of negative CKs in the 22 cases that did not show any positivity for any of the tested CKs is highlighted in bold. The table shows that all CK negative MBC were tested for at least 3 CKs, with 18 of them being stained with more than 5 CKs.
Table 2b:
Correlation between the number of negative cytokeratins (CKs) and the presence of associated adenocarcinomatous component and/or DCIS.
| Number of total CKs tested | Number of cases distributed by number of negative CKs |
Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| No carcinomatous component | 29 | 21 | 12 | 11 | 8 | 5 | 4 | 1 | 2 | 1 | 94 |
| Associated carcinomatous component | 14 | 9 | 5 | 6 | 0 | 5 | 1 | 4 | 0 | 2 | 46 |
There was a strong positive correlation between the number of negative CKs and the total number of CKs requested (r = 0.6, p<0.001), but a negative correlation with the number of positive CKs (r = −0.6, p<0.001), representative of the fact that the more puzzling cases were more thoroughly investigated. A significant correlation was also identified between the presence of an invasive breast, NST component or DCIS and the number or pattern of CKs expression (p<0.001), highlighting that cases without these elements required more comprehensive immunohistochemical analysis for a diagnosis to be rendered. It should also be noted, however, that cases with those components that were not subjected to IHC for CKs were excluded from the analysis.
The most commonly used CKs were as follows: CK14: 72% (72 positive of 100 cases stained), CK5/6: 64% (65/102), AE1/AE3: 60% (43/68), MNF116: 60% (46/77) and CAM5.2: 59% (51/86). Although CK17 was rarely used, it was positive in 75% of informative cases (9/12). CK8/18 was the least used and the least frequently positive CK (2/6; 33%).
CK-negative tumours:
22 cases (16%) were negative for all CKs tested of the 3–9 CKs per case (median=6; Table 1) with 18 tumours (13%) negative for all CKs when 5 or more CKs were used (Table 2). These 22 CKs negative tumours were spindle cell neoplasms, mainly pleomorphic sarcoma-like tumours, together with 3 cases of osteosarcoma-like, 1 chondrosarcoma-like and 1 rhabdomyosarcoma-like malignancy. None were squamous cell carcinoma or adenosquamous carcinoma. These tumours uniformly displayed a triple-negative phenotype (oestrogen receptor (ER), progesterone receptor (PR) and HER2 negative).
Of these 22 CKs negative tumours, 12 had an associated carcinomatous component and were diagnosed as MBC based on this association. 10 had no identifiable carcinomatous elements in the primary tumours during initial examination, and these were diagnosed MBC following more extensive workup. Of these 10, one high grade sarcomatoid tumour was sent for a second opinion and on preparation of the case, deeper levels were carried out which showed scanty foci of DCIS; therefore, a final diagnosis of MBC was rendered. In another case, which was a low to intermediate grade spindled cell tumour, a focus of DCIS was seen on extra tissue sampled at a later date following discussion with an expert, after an initial diagnosis of malignant spindle cell lesion of uncertain nature.
Eight cases remained with no evidence of DCIS, conventional type breast carcinoma components or CK expression and no positivity for other markers characteristic of breast carcinoma, such as hormone receptors, GATA3, GCDFP-15 or HER2. The details of the 8 cases that were diagnosed as MBC after our routine workup are as follows:
* Four patients had a history of previous breast carcinoma in the same breast and review of the previous slides showed similarity with the current malignant neoplasm. In one patient the primary tumour was MBC diagnosed 6 years previously with spindle cell and squamous components. The spindle cell component morphologically matched the current tumour (Figure 2). The second patient had a prior invasive breast carcinoma (NST) with, on review, focal areas of spindle cell differentiation that were not recorded in the original report. The third patient presented with a mass following neoadjuvant chemotherapy for MBC. Although this excised lesion did not show evidence of epithelial differentiation or CKs expression, it was diagnosed as MBC because of its similarity to the original tumour seen on the core biopsy which was CKs positive. The fourth patient presented with axillary and disseminated metastases with a spindle cell morphology and no CKs expression; a previous fibromatosis-like MBC was identified with convincing CK expression for 4 markers. The axillary metastasis presented 3 years after the initial lesion, while the disseminated gastro-intestinal and paraspinal metastases developed 5 years after the initial event (Figure 3).
Figure 2:

This case of malignant spindle cell lesion showed no evidence of carcinomatous differentiation and it was negative for all CKs tested (A, B). Reviewing the previous carcinoma in the same breast (C, D) revealed MBC with both squamous areas and spindle cell areas which were similar to those seen in the recurrences. The primary tumour was not tested for CKs due to the presence of squamous areas. In the recurrence, no such squamous differentiation was seen.
Figure 3:

This case showed an initial fibromatosis-like MBC (A) with CK5/6 positivity (B). Three years later the patient developed an axillary metastasis (C), which tested negative for CK5/6, CK14 (D), AE1/3, CAM5.2 and p63. Multiple metastatic deposits (digestive and paraspinal) developed after another 2 years and all showed similar spindle cell morphology with no CK positivity.
* One patient presented with a well-defined ossifying lesion of the breast formed by osteoid trabeculae and focally loose stroma featuring a proliferation of bland fibroblast-like cells that lacked expression for a wide range of CKs. Foci of chondroid matrix at the centre of the lesion were seen. The lesion was originally thought to be a florid reactive process with bone formation and was classified as ossifying fasciitis, which is regarded as a form of nodular fasciitis. Four years later, however, the patient developed a bone metastasis and the histology of the metastasis revealed a malignant neoplasm with chondroid and osseous differentiation. This was similar to the original tumour, but also showed additional atypical features with focal epithelial differentiation (carcinomatous morphology) in keeping with metastatic matrix producing MBC (Figure 4). The original diagnosis of the tumour was retrospectively reviewed and regarded as MBC, given the new evidence brought by the metastatic focus.
Figure 4:
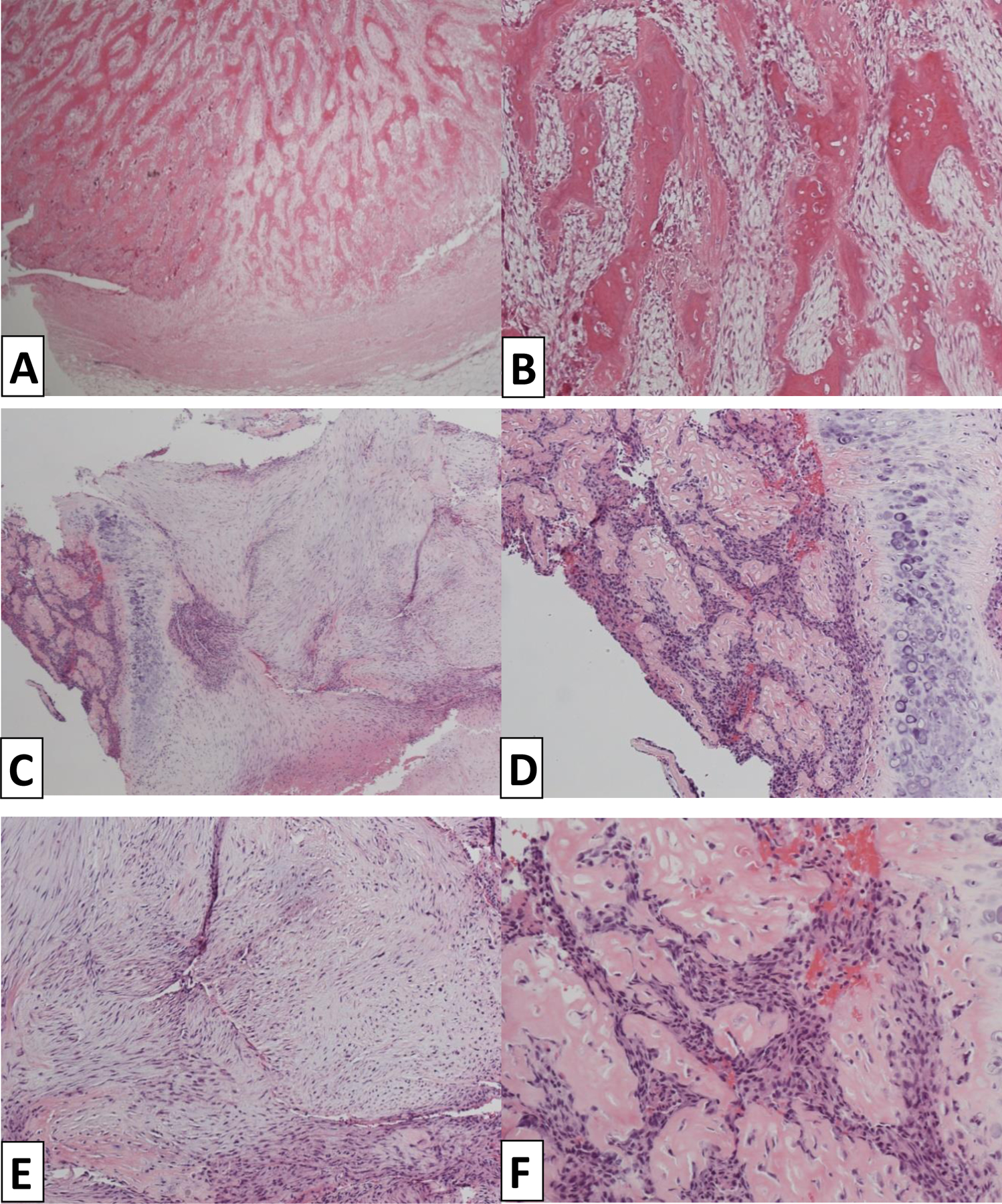
This case presented as an ossifying lesion of the breast initially catalogued as ossifying fasciitis; morphologically it presented with woven bone and osteoid trabeculae lined by florid osteoblastic proliferation with scattered osteoclasts and focally loose stroma featuring a proliferation of non-atypical fibroblast-like cells – no CK expression was identified (A, B).
4 years later a bone metastasis was identified with similar features, however this time the lesion showed epithelial differentiation as well as spindle elements. (C,D,E,F).
*In three cases, the final diagnosis of MBC was based on the balance of probability and after the exclusion of other entities. One presented with spindle cell areas admixed with chondroid and osteoid formation. The presence of all these elements transitioning from one to another is the very definition of MBC and the diagnosis was based on the typical morphology, despite the lack of CKs. One was a spindle cell malignant neoplasm, not otherwise specified (Figure 5), while the other case displayed highly pleomorphic cells in a variable collagenous to myxoid background. In these three cases, the diagnosis of MBC carcinoma was made in routine practice based on the balance of probability after exclusion of the entities included in the differential diagnosis, thorough sampling and immunoprofiling (Figure 6). As this was a diagnosis of exclusion, details of the diagnostic workup and why the diagnosis of MBC was favoured for management purpose were provided in the report.
Figure 5:

This case was a malignant spindle cell tumour of the breast infiltrating the deep skeletal muscle; although no CK expression, DCIS or conventional invasive carcinoma were identified, it was defaulted to CK-MBC, after other lesions were excluded (DFSP, malignant melanoma, lymphoma, malignant phyllodes tumour). The lesion was negative for CD34 and p63 but shows some epithelioid morphology.
DISCUSSION
The concept of CK negative MBC is typically difficult to prove for several reasons; firstly, there is a wide range of CKs immunostains available but not all are used consistently across histopathology departments; secondly, these cases are rare and, thirdly, CKs are not routinely employed (or necessary) to diagnose MBC when there is morphological evidence of epithelial mammary gland origin. The number of CKs IHC assays a pathology laboratory has available can be limited and may not cover the range expressed by such poorly differentiated tumours. There is accumulating evidence that breast carcinomas may variably lack expression of one or more CKs, and even when positive may show only focal expression, supporting the existence of MBCs that lose expression of a range of CKs. 16,18,31,34,37–39 Even in conventional no special type/ductal carcinomas of the breast, approximately 5% show no expression of low molecular weight CKs and more than 70% are typically negative for high molecular weight CKs40–42. Leibl et al.34 studied 20 sarcomatoid MBC and found that 2 (10%) lacked expression of all CKs whereas 6 (30%) showed weak and/or focal CKs expression. Interestingly, these authors found that myoepithelial marker expression was frequent in these tumours and they concluded that CKs negative sarcomatous breast lesions should be designated as MBC provided that they express myoepithelial cell markers such as p63, CD10 or SMA34. In a subsequent study16 the same authors investigated 7 cases that had previously been diagnosed as primary mammary sarcomas using the same criteria and suggested that these mammary NOS-type sarcomas could represent the extreme sarcomatous end of MBCs, which is in line with our experience. In a similar study of 36 MBC, 4 (10%) lacked all CKs expression43. In a large study of MBC by McCart Reed 39, 12 of 166 MBC (7%) were negative for AE1/AE3 whereas 22% (28/126) and 26% (36/140) of the cases were negative for CK14 and CK5/6 respectively. Our study, in line with those above, provides further evidence that a significant proportion of MBCs (69%) fails to express one or more CKs in the sarcomatous component of the tumours and that a proportion of cases (16%) completely lack expression of the CKs that were utilised in the current study. Of those 22 that lacked CK expression in the current study, 14 cases showed morphological evidence of carcinomatous differentiation in the primary tumours whereas 5 cases showed indirect evidence of the carcinomatous nature as demonstrated by comparison with previous lesions or recurrences.
Even when CK expression was seen in the MBCs in this series, variable reactivity was identified, ranging from cases with diffuse CKs expression to heterogeneous CKs expression. Patterns included a CK positive conventional carcinomatous component admixed with CK negative MBC elements and MBC with both focal positive and negative mesenchymal-looking areas, as well as cases with total negativity for CKs. The carcinomatous nature of these CK negative malignant mesenchymal-looking elements was confirmed by association with conventional carcinomatous components in some cases. Most of these mixed cases were referred to us for a second opinion because the reporting pathologist had concerns about the nature of the CK negative components. In our practice, we consider these CK negative components to be part of MBC based on their co-existence with areas of carcinomatous nature, regardless of their lack of CKs expression. This approach has been recently endorsed by the WHO working group14. We also highlight in this study that MBCs that are initially CK positive may lose CK expression in recurrences and/or metastases, as well as cases that were defaulted to CK negative MBCs after extensive work-up. This is based on the findings that:
1 - a proportion of conventional type invasive breast carcinomas can be negative for CKs40–42, and this proportion is higher in MBC2,18,23,26,29,30,39–41;
2 - the biological behaviour of some of these tumours with available outcome data supports their breast carcinomatous nature with change of morphology or immunoprofile between primary and metastatic or recurrent tumours2;
3 - the statistical probability of BC with one or more CKs negative phenotype versus primary breast NOS sarcomas favours MBC24,36;
4 - a history of previous malignancy should be excluded or, if a previous malignancy is documented, review of the previous slides should be performed to compare and exclude the possibility of the lesion representing recurrent or metastatic tumour;
4 - lack of definite morphological and/or molecular evidence of other histogenetically defined sarcomas.
It is important to note that some sarcomas express CKs44 and their diagnosis in such circumstances is based on other morphological and/or molecular features. Thus, CK expression (positive or negative) is not irrefutable evidence to support either a carcinomatous or sarcomatous nature and in essence, at this end of the differentiation spectrum, we are often left with a diagnosis of exclusion. The diagnostic assays used in routine practice to investigate both soft tissue sarcomas and other tumours included in the differential diagnosis of these poorly differentiated breast lesions are not highly specific or sensitive; additional studies are required to investigate their presence in some breast cancers, and in particular the sarcomatoid variant of MBC. Next generation sequence (NGS) testing is performed in occasional cases, but the interpretation of these results remains of limited value, as further evidence that the various genetic alterations identified can distinguish specific tumours types remains to be confirmed 45.
Beyond the academic interest in histogenesis, these cases comprise a diagnostic challenge with significant management implications. In a previous study we concluded that a range of malignant matrix-producing breast tumours were all variants of MBC.16 In the present study, we have highlighted the existence of additional CK-negative MBC and provided evidence that sarcomatous-looking neoplasms in the breast, whether matrix-producing or spindle/pleomorphic cell neoplasms, may be diagnosed as MBC even in the absence of conventional evidence of mammary epithelial differentiation. Such a diagnosis of CKs negative MBC, however, must be rendered with caution and only after the careful exclusion of other specific entities. Although this is essentially a diagnosis of exclusion, the outcome of this diagnostic algorithm is establishing a final diagnosis of MBC rather than sarcoma, with its subsequent management implications. In such workup, the diagnosis of MBC must be considered in the clinical context, morphology, immunoprofile and, whenever possible, molecular profile of the lesion. Fibromatosis-like MBC is diagnosed with confidence when it looks like fibromatosis but shows positivity for CKs and p63. Other low to intermediate grade spindle cell MBCs are diagnosed when arising in a background of other mammary lesions such as papillomas or complex sclerosing lesion, (or it is associated with DCIS or conventional type mammary carcinoma) and is CK positive. Melanoma and lymphoma are excluded based on morphology, history and IHC. The subtype of sarcomas included in the differential diagnosis is based on morphological features of the lesions and those included in the differential diagnosis will be excluded based on IHC and specific molecular alteration testing whenever available.
There is some clinical uncertainty whether MBC lacking evidence of epithelial differentiation should be treated as sarcoma and managed by specialised sarcoma teams31,46,47 or treated using the same principles as conventional invasive breast carcinoma, a dilemma that prompted this study. Most studies on what has been labelled “breast sarcoma” have included a mix of tumours, so a conclusion on the outcome and response to chemotherapy of specific subtypes is not possible (with the exception of the well-established phyllodes tumours and mammary angiosarcomas).25,28,48,50 For example, one previous study which set out to compare the outcome of “primary breast sarcomas” versus phyllodes tumours included 12 cases of carcinosarcoma (34%), currently considered as variants of MBC. In that series, lymph node metastasis was reported in 11% of patients and adjuvant chemotherapy was offered to 63% 25. In the McCart Reed study, MBC that was negative for epithelial markers (AE1/AE3) showed an association with poorer outcome39. Similar to other types of breast carcinomas, poorly differentiated MBC that has only focal evidence of epithelial differentiation are associated with a worse prognosis than well differentiated MBC with bona fide evidence of mammary epithelial differentiation47. This supports the view that CKs negative MBC is not much different from MBC with focal evidence of epithelial differentiation - which is currently catalogued as MBC, regardless of its outcome pattern.
Given the rarity of what is called primary breast sarcomas NOS, there are no prospective randomized trials to guide therapy; treatment principles have been derived from small retrospective case reviews and inferred from studies of soft tissue sarcomas in other locations. Some reports indicate that breast sarcomas may benefit from radiotherapy and chemotherapy.49 Although a poorer outcome has been reported in patients defaulted to a diagnosis of sarcoma, despite being offered chemotherapy, when compared to conventional type carcinomas, this may be the result of chemotherapy being given to patients with other unfavourable tumour features49,50. Conversely, at present MBC, regardless of its morphology or CK expression, is treated similarly to other triple-negative BC of similar grade and stage. We believe that MBC comprises a spectrum of lesions and the threshold to recommend chemotherapy should not be determined based on the presence of CK expression alone (e.g. MBC with focal CK positivity vs CK negative MBC). Evidence to demonstrate that tumours diagnosed as primary breast sarcoma NOS behave differently from poorly differentiated MBC, and their likelihood of response (or not) to systemic chemotherapy, is lacking. Further studies are needed but these should be based on lesions diagnosed and classified using clearer definitions.
Based on the findings of this study, and on our experience, we recommend classifying these tumours as MBC in surgical excision specimens following an extensive work-up protocol as described above. We usually recommend lymph node examination, for staging purposes, and that radiotherapy and chemotherapy should be considered akin to other grade-, stage- and receptor-matched MBC. These tumours are likely to be clinically aggressive and depriving patients of the potential benefits of available regimens for treatment may not be justified, regardless of their histogenesis, as this is mainly of academic interest. When attempting to diagnose these malignant tumours in a pre-operative core biopsy, we recommend that the clinicians be alerted to the possible differential diagnoses. At present, we believe neoadjuvant chemotherapy should not be recommended in such CK negative malignant mesenchymal tumours diagnosed on core biopsies, until a definite diagnosis can be made on the surgical excision specimen. Granted, this is mostly a pragmatic approach guided by our experience with these lesions and further studies are required to produce the evidence needed for changing protocols.
The current study has some limitations. No outcome data is available in the cases included in the study. The use of historical data might introduce some bias as immunohistochemistry protocols have evolved over time and the cytokeratin stains have been used with variable frequency. Ideally, a panel of cytokeratin stains should have been performed uniformly on this large series to overcome any potential bias, but the availability of tissue blocks precluded such an option. In addition, other markers to confirm the diagnosis such as GCDFP-15, mammaglobin, SOX10, SATB2 and S100 were not routinely performed all cases. p63 stains were available in a subset of cases during the histological review process.
In conclusion, this study provides evidence that BC can show extensive mesenchymal differentiation and lose evidence of epithelial differentiation including the expression of a range of CKs. Following exclusion of all other possibilities, these tumours can be diagnosed and managed as poorly differentiated MBC.
Figure 1B:

This MBC showed a heterogeneous appearance (A) with squamous cell differentiation positive for CK AE1/AE3 (B), while the spindle cell component was negative.
Table 1b:
Number of cytokeratin antibody combinations per case in the studied cohort (n=140).
| No. of CKs used per case | No and (%) of cases |
|---|---|
| 1 | 6 (4)* |
| 2 | 10 (7) |
| 3 | 13 (9) |
| 4 | 36 (26) |
| 5 | 28 (20) |
| 6 | 18 (13) |
| 7 | 9 (7) |
| 8 | 10 (7) |
| 9 | 10 (7)** |
6 cases (out of 140) were tested for only one CK;
10 cases (out of 140) were tested for a total of 9 CKs;
In this study, 22 cases were negative with all the CKs they were tested for, whereas 43 cases were positive for all the CKs used in their investigation.
Table 1c:
Number and percent (%) of cases positive or negative for the same number of CKs.
| No. of CKs | No. and percent (%) of cases positive | No. and percent (%) of cases negative |
|---|---|---|
| 1 | 19 (14)* | 30 (21)** |
| 2 | 22 (16) | 17 (12) |
| 3 | 29 (21) | 17 (12) |
| 4 | 21 (15) | 8 (6) |
| 5 | 12 (8) | 10 (7) |
| 6 | 10 (7) | 5 (4) |
| 7 | 4 (3) | 5 (4) |
| 8 | 1 (1) | 2 (1) |
| 9 | 0(0) | 3 (2) |
19 cases (out of 140) were positive for 1 CK only regardless of the number of CKs tested per case
30 cases (out of 140) were negative for 1 CK regardless of the number of CKs tested per case
Acknowledgement:
JSR-F is funded in part by the Breast Cancer Research Foundation. Research reported in this publication was funded in part by a Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute (grant No P30CA008748). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest:
JSR-F is a consultant of Goldman Sachs, Paige and REPARE Therapeutics, a member of the scientific advisory board of Volition RX, Paige and REPARE Therapeutics, and an ad hoc member of the scientific advisory board of Ventana Medical Systems/ Roche Tissue Diagnostics, Roche, Genentech, Novartis, InVicro and GRAIL. There are no conflicts of interest to disclose.
REFERENCES
- 1.Rosen PP: Rosen’s Breast Pathology (ed 3rd). Philadelphia, Lippincott Williams & Wilkins, 2009 [Google Scholar]
- 2.van Deurzen CH, Lee AH, Gill MS, et al. : Metaplastic breast carcinoma: tumour histogenesis or dedifferentiation? J Pathol 224:434–7, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Mori I, Tang W, et al. : Metaplastic carcinoma of the breast: p53 analysis identified the same point mutation in the three histologic components. Mod Pathol 14:1183–6, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Smith BH, Taylor HB: The occurrence of bone and cartilage in mammary tumors. Am J Clin Pathol 51:610–8, 1969 [DOI] [PubMed] [Google Scholar]
- 5.Spagnolo DV, Shilkin KB: Breast neoplasms containing bone and cartilage. Virchows Arch A Pathol Anat Histopathol 400:287–95, 1983 [DOI] [PubMed] [Google Scholar]
- 6.Reis-Filho JS, Lakhani SR, Gobbi H, et al. : Metaplastic carcinoma, in Lakhani SR, I.O. E, Schnitt SJ, et al. (eds): WHO Classification of Tumours of the Breast (ed Fourth). IARC press, Lyon: 2012, pp 48–52 [Google Scholar]
- 7.Pezzi CM, Patel-Parekh L, Cole K, et al. : Characteristics and treatment of metaplastic breast cancer: analysis of 892 cases from the National Cancer Data Base. Ann Surg Oncol 14:166–73, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Tseng WH, Martinez SR: Metaplastic breast cancer: to radiate or not to radiate? Ann Surg Oncol 18:94–103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaguchi R, Horii R, Maeda I, et al. : Clinicopathologic study of 53 metaplastic breast carcinomas: their elements and prognostic implications. Hum Pathol 41:679–85, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Tse GM, Tan PH, Putti TC, et al. : Metaplastic carcinoma of the breast: a clinicopathological review. J Clin Pathol 59:1079–83, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hennessy BT, Giordano S, Broglio K, et al. : Biphasic metaplastic sarcomatoid carcinoma of the breast. Ann Oncol 17:605–13, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Rakha EA, Tan PH, Varga Z, et al. : Prognostic factors in metaplastic carcinoma of the breast: a multi-institutional study. Br J Cancer 112:283–9, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakhani SR IOE, Schnitt SJ, et al. : WHO Classification of Tumours of the Breast, (ed Fourth). IARC press, Lyon: 2012 [Google Scholar]
- 14.Reis Filho JS, Gobbi H, McCart Reed A, E, et al. : Metaplastic Carcinoma in Board TWCoTE (ed): WHO Classification of Tumours: Breast Tumours (ed Fifth). IARC press, Lyon: 2019, pp 134–138 [Google Scholar]
- 15.Golshan M, Kuten A, William J, et al. : Metaplastic carcinoma of the breast with neuroglial differentiation. Breast 15:545–9, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Leibl S, Moinfar F: Mammary NOS-type sarcoma with CD10 expression: a rare entity with features of myoepithelial differentiation. Am J Surg Pathol 30:450–6, 2006 [DOI] [PubMed] [Google Scholar]
- 17.The WHO Classification of Tumours: Breast Tumours (ed Fifth). IARC press, Lyon: 2019 [Google Scholar]
- 18.Rakha EA, Coimbra ND, Hodi Z, et al. : Immunoprofile of metaplastic carcinomas of the breast. Histopathology 70:975–985, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Lee H, Jung SY, Ro JY, et al. : Metaplastic breast cancer: clinicopathological features and its prognosis. J Clin Pathol 65:441–6, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Zelek L, Llombart-Cussac A, Terrier P, et al. : Prognostic factors in primary breast sarcomas: a series of patients with long-term follow-up. J Clin Oncol 21:2583–8, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Hartel PH, Bratthauer G, Hartel JV, et al. : Primary malignant fibrous histiocytoma (myxofibrosarcoma/pleomorphic sarcoma not otherwise specified) of the breast: clinicopathologic study of 19 cases. Ann Diagn Pathol 15:407–13, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Terrier P, Terrier-Lacombe MJ, Mouriesse H, et al. : Primary breast sarcoma: a review of 33 cases with immunohistochemistry and prognostic factors. Breast Cancer Res Treat 13:39–48, 1989 [DOI] [PubMed] [Google Scholar]
- 23.Ciatto S, Bonardi R, Cataliotti L, et al. : Sarcomas of the breast: a multicenter series of 70 cases. Neoplasma 39:375–9, 1992 [PubMed] [Google Scholar]
- 24.McGowan TS, Cummings BJ, O’Sullivan B, et al. : An analysis of 78 breast sarcoma patients without distant metastases at presentation. Int J Radiat Oncol Biol Phys 46:383–90, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Wang F, Jia Y, Tong Z: Comparison of the clinical and prognostic features of primary breast sarcomas and malignant phyllodes tumor. Jpn J Clin Oncol 45:146–52, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Pencavel T, Allan CP, Thomas JM, et al. : Treatment for breast sarcoma: a large, single-centre series. Eur J Surg Oncol 37:703–8, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Adem C, Reynolds C, Ingle JN, et al. : Primary breast sarcoma: clinicopathologic series from the Mayo Clinic and review of the literature. Br J Cancer 91:237–41, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Confavreux C, Lurkin A, Mitton N, et al. : Sarcomas and malignant phyllodes tumours of the breast--a retrospective study. Eur J Cancer 42:2715–21, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Holm M, Aggerholm-Pedersen N, Mele M, et al. : Primary breast sarcoma: A retrospective study over 35 years from a single institution. Acta Oncol 55:584–90, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Adem C, Reynolds C, Adlakha H, et al. : Wide spectrum screening keratin as a marker of metaplastic spindle cell carcinoma of the breast: an immunohistochemical study of 24 patients. Histopathology 40:556–62, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Carter MR, Hornick JL, Lester S, et al. : Spindle cell (sarcomatoid) carcinoma of the breast: a clinicopathologic and immunohistochemical analysis of 29 cases. Am J Surg Pathol 30:300–9, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Reis-Filho JS, Milanezi F, Steele D, et al. : Metaplastic breast carcinomas are basal-like tumours. Histopathology 49:10–21, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Grenier J, Soria JC, Mathieu MC, et al. : Differential immunohistochemical and biological profile of squamous cell carcinoma of the breast. Anticancer Res 27:547–55, 2007 [PubMed] [Google Scholar]
- 34.Leibl S, Gogg-Kammerer M, Sommersacher A, et al. : Metaplastic breast carcinomas: are they of myoepithelial differentiation?: immunohistochemical profile of the sarcomatoid subtype using novel myoepithelial markers. Am J Surg Pathol 29:347–53, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Gilbert JA, Goetz MP, Reynolds CA, et al. : Molecular analysis of metaplastic breast carcinoma: high EGFR copy number via aneusomy. Mol Cancer Ther 7:944–51, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosen PP: Rosen’s Breast Pathology (ed 3rd). Philadelphia, Lippincott Williams & Wilkins, 2009 [Google Scholar]
- 37.Dunne B, Lee AH, Pinder SE, et al. : An immunohistochemical study of metaplastic spindle cell carcinoma, phyllodes tumor and fibromatosis of the breast. Hum Pathol 34:1009–15, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Chhieng C, Cranor M, Lesser ME, et al. : Metaplastic carcinoma of the breast with osteocartilaginous heterologous elements. Am J Surg Pathol 22:188–94, 1998 [DOI] [PubMed] [Google Scholar]
- 39.McCart Reed AE, Kalaw E, Nones K, et al. : Phenotypic and molecular dissection of metaplastic breast cancer and the prognostic implications. J Pathol 247:214–227, 2019 [DOI] [PubMed] [Google Scholar]
- 40.Chu PG, Weiss LM: Keratin expression in human tissues and neoplasms. Histopathology 40:403–39, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Hashmi AA, Naz S, Hashmi SK, et al. : Cytokeratin 5/6 and cytokeratin 8/18 expression in triple negative breast cancers: clinicopathologic significance in South-Asian population. BMC Res Notes 11:372, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abd El-Rehim DM, Pinder SE, Paish CE, et al. : Expression of luminal and basal cytokeratins in human breast carcinoma. J Pathol 203:661–71, 2004 [DOI] [PubMed] [Google Scholar]
- 43.D’Alfonso TM, Ross DS, Liu YF, et al. : Expression of p40 and laminin 332 in metaplastic spindle cell carcinoma of the breast compared with other malignant spindle cell tumours. J Clin Pathol 68:516–21, 2015 [DOI] [PubMed] [Google Scholar]
- 44.Wei S, Henderson-Jackson E, Qian X, et al. : Soft Tissue Tumor Immunohistochemistry Update: Illustrative Examples of Diagnostic Pearls to Avoid Pitfalls. Arch Pathol Lab Med 141:1072–1091, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang HY, Koh VCY, Md Nasir ND, et al. : MED12, TERT and RARA in fibroepithelial tumours of the breast. J Clin Pathol 73:51–56, 2020 [DOI] [PubMed] [Google Scholar]
- 46.Silver SA, Tavassoli FA: Primary osteogenic sarcoma of the breast: a clinicopathologic analysis of 50 cases. Am J Surg Pathol 22:925–33, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Davis WG, Hennessy B, Babiera G, et al. : Metaplastic sarcomatoid carcinoma of the breast with absent or minimal overt invasive carcinomatous component: a misnomer. Am J Surg Pathol 29:1456–63, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Bousquet G, Confavreux C, Magne N, et al. : Outcome and prognostic factors in breast sarcoma: a multicenter study from the rare cancer network. Radiother Oncol 85:355–61, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Al-Benna S, Poggemann K, Steinau HU, et al. : Diagnosis and management of primary breast sarcoma. Breast Cancer Res Treat 122:619–26, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Lahat G, Lev D, Gerstenhaber F, et al. : Sarcomas of the breast. Expert Rev Anticancer Ther 12:1045–51, 2012 [DOI] [PubMed] [Google Scholar]


