Abstract
We report the case of a patient with symptoms of myelopathy following acute SARS-CoV-2 infection. MRI documented a longitudinally extensive transverse myelitis and further investigation was unremarkable with the exception of positivity for MOG-IgG in serum. This report extends the spectrum of post-COVID-19 neurological syndromes, and documents a very significant improvement to long-term oral corticosteroid therapy in this setting. Further prospective studies are needed to establish the risk of recurrence in this subset of patients.
Keywords: Myelitis, Post-infectious myelitis, MOG-IgG, SARS-CoV-2, COVID-19
Graphical abstract
1. Introduction
Central nervous system (CNS) disorders related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are being increasingly recognized. Neuroinflammatory syndromes, including acute demyelinating encephalomyelitis-like syndromes and myelitis, are rare and thought to be predominantly para- or post-infectious, rather than a result of direct viral invasion of the CNS (Paterson et al., 2020). The clinical overlap between post-infectious inflammatory syndromes and primary inflammatory or demyelinating diseases is significant, and careful analysis of each patient will determine the best treatment plan.
2. Case report
We report a 31-year-old man with medical history of asthma, obesity and obstructive sleep apnea who was admitted complaining of progressive onset of dysesthesia in the periumbilical region and second and third fingers of both hands over 5 days, followed by progressive ascending lower limb and perineal hyperesthesia. In addition, he referred progressive supra-pubic discomfort with urinary retention requiring urgent urinary catheterization after a previous emergency department evaluation. He had difficulty walking due to apparent lower limb pain, and denied other symptoms including incoordination, gait instability or visual disturbances. Twenty-one days prior, the patient had had a SARS-CoV-2 infection confirmed by antigen detection on a nasal swab with odynophagia, anosmia, ageusia, fever (39 °C) and general malaise. Initial evaluation showed no abnormalities in the chest X-ray nor hypoxia, and symptoms resolved within 10 days without directed therapy.
Neurologic examination identified sensory abnormalities and pyramidal tract involvement. The patient had a lower body hyperalgesia and tactile dysesthesia with a T10 dermatome level, pathologic patellar and achilles deep tendon reflexes (grade 3), and bilateral extensor plantar response. There were no cranial nerve abnormalities, and all upper and lower limb segments had a normal strength (Medical Research Council Scale of Muscle Strength grade 5). Position and vibratory senses and cerebellar function were normal. The patient had a significant limitation of ambulatory ability due to pain, without gait or postural instability, and he maintained the need for urinary catheterization due to urinary retention with overflow incontinency (expanded disability status scale (EDSS) 5.5). General examination was unremarkable.
Topographically, an incomplete thoracic medullary syndrome was assumed with possible conus involvement due to the early and severe sphincter complaints. A thoracic and lumbosacral spinal cord magnetic resonance imaging (MRI) identified a longitudinally extensive (T2 to T10) T2-hyperintense lesion located to the central spinal cord with mild gadolinium enhancement (Fig. 1 A-C). A presumptive diagnosis of longitudinal extensive transverse myelitis (LETM) was made.
Fig. 1.
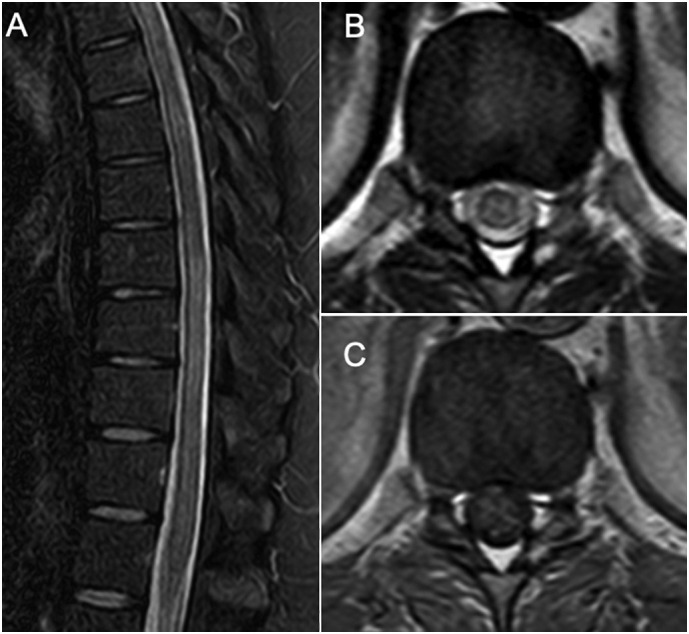
Sagittal MRI shows a longitudinally extensive hyperintense lesion from T2 to T10 on T2/STIR sequences (A), located to the centromedular gray matter on the horizontal plane as seen on the T2-weighted DRIVE sequences (“H sign”) at the T11-T12 level (B), with mild enhancement following gadolinium injection (postcontrast axial T1-weighted fast spin-echo) (C). Brain MRI did not show additional lesions. This pattern of a longitudinally extensive transverse myelitis with gray matter involvement and no or mild gadolinium enhancement is suggestive of a MOG-IgG myelitis.
Initial inpatient investigation focused on ancillary studies directed to CNS inflammatory and demyelinating disorders, namely multiple sclerosis and neuromyelitis optica spectrum disorders (NMOSD). Brain and cervical spine MRI were unremarkable, and visual evoked potentials were normal, excluding sub-clinical multifocal CNS involvement. Cerebrospinal fluid (CSF) analysis revealed a marked pleocytosis with 168 cells/mm3, with mononuclear predominance, high protein count (85 mg/dL), normal glucose, and two oligoclonal bands (OCB) in a mirror pattern to serum. All systemic inflammatory, autoimmune, and infectious markers were unremarkable or non-specific (see Table 1 for the summary of laboratory findings). Serum analysis revealed a low-titer positive myelin oligodendrocyte glycoprotein antibody (MOG-IgG), detected by fixed cell-based immunofluorescence assay (CBA), anti-aquaporin 4 antibodies (AQP4-IgG) were not present. Due to the temporal relation with a recent SARS-CoV infection, PCR and serologies for SARS-CoV-2 were investigated both in the serum and the CSF; SARS-CoV-2 PCR was negative in CSF, with positive antibody markers of recent SARS-CoV-2 infection in both CSF and serum. No clear evidence of current blood-brain barrier dysfunction was found.
Table 1.
Summary of laboratory findings.
| Blood and serum | |
|---|---|
| Autoimmunity and inflammation | |
| Anti-ds-DNA | negative |
| ANA screening | negative |
| Anti-MPO and anti-PR3 | negative |
| Anti-phospholipid antibodies | negative |
| Angiotensin converting enzyme | 29 U/L (N: 8–52 U/L) |
| ESR | 29 mm (N: <10 mm) |
| Anti-MOG | positive (titer 1:20), CBA |
| Anti-AQP4 | negative |
| Infections | |
| Anti-SARS-CoV-2 (S1-RBD) | IgG and IgM positive |
| Anti-SARS-CoV-2 (S1-RBD Ig) | >250 U/mL |
| HIV 1/2 | negative |
| HSV 1 and 2 | IgG and IgM negative |
| HTLV I/II | negative |
| CMV | IgG and IgM negative |
| EBV | IgG, IgM, EBNA negative |
| Chlamydia pneumoniae | IgG positive, IgA negative |
| Mycoplasma pneumoniae | IgG and IgM negative |
| Borrelia burgdorferi. | IgG and IgM negative |
| Brucella spp. | negative |
| Treponema pallidum | non-reactive |
| Cerebrospinal fluid | |
|---|---|
| Cells | 168/mm3 (mononuclear) |
| Proteins | 85 mg/dL |
| Glucose | 56 mg/dL |
| Oligoclonal bands | present in mirror pattern |
| PCR SARS-CoV-2 | negative |
| Anti-SARS-CoV-2 (S1-RBD) | IgG positive (1.68 U/mL), IgM negative |
| PCR multiple array* | negative |
| Cultures (bacterial, fungal, mycobacterial) | negative |
Notes: * Array including PCR for E. coli K1, H. influenzae, L. monocytogenes, N. meningitidis, S. agalactiae, S. pneumoniae, CMV, enterovirus, HSV 1, HSV 2, HHV6, VZV, human parechovirus, and cryptococcus neoformans. ANA – antinuclear antibodies; AQP4 - Aquaporin-4; CBA – cell-based assay; CMV – cytomegalovirus; EBV – Epstein-Barr vírus; ESR – erythrocyte sedimentation rate; HIV - human immunodeficiency virus; HSV – herpes simplex virus; HTLV - human T-lymphotropic vírus; MOG - myelin oligodendrocyte glycoprotein; N – normal range/value; PCR – polymerase chain reaction; SARS-CoV-2 - severe acute respiratory syndrome coronavirus 2.
A diagnosis of probable post-infectious inflammatory LETM was made. Because of the clinical severity, and a dubious MOG-IgG serology, intravenous methylprednisolone 1 g/day for 5 days was administered. There was no progression of deficits or other complications during in-hospital stay. There was a slight improvement of sensitive abnormalities after corticoid therapy and introduction of pregabalin 75 mg bid, with improved ambulatory ability; and mild improvement of urinary symptoms with need for intermittent self-catheterization. At the time of hospital discharge, there was an uncertain risk of recurrence and after careful consideration and patient discussion oral prednisolone (60 mg/day) was started in addition to gastroprotection with omeprazole 20 mg/day and osteoporosis prophylaxis with calcium and vitamin D supplementation. At the 3 and 6 months follow-up appointment, the patient showed an almost complete resolution of the sensitive abnormalities despite a protracted recovery of urinary retention - mild urinary retention symptoms only, without need for urinary catheterization, accounting for a EDSS 2.0 at 3 months and EDSS 1.0 at 6 months. After 6 months of 60 mg/day, oral prednisolone was kept until further re-evaluation, with a slow tapering plan.
3. Discussion
LETM is the most common myelitis pattern associated with SARS-CoV-2 infection. A recent review characterized myelitis in presumed relation to a SARS-CoV-2 infection (de Antonio et al., 2021). Similar to our case, 77.7% of patients presented with sensitive symptoms and 88.8% with urinary dysfunction. Clinical severity ranged from mild to severe myelitis. MRIs identified LETM in 64.7% of patients. In most, there was mild pleocytosis in CSF analysis (mean 40.9 ± 49.7/μL), moderate elevation of proteins, and oligoclonal bands in a mirror pattern were found in 2/9 patients. Another recent report documented a LETM following asymptomatic SARS-CoV-2 infection with improvement after corticosteroid therapy, determining the need for considering neurological presentations even in the absence of classical respiratory signs of COVID-19 (Lee, 2021). Other patterns including LETM associated with mild encephalitis/encephalopathy with a reversible splenial lesion have been reported although, in most cases, establishment of possible causation in lieu of association is challenging (Kim et al., 2021). Therefore, there is still a need for establishing SARS-CoV-2 specific neurologic manifestations, their long-term prognosis, and to define probable or definite causation (Ellul et al., 2020; Samudralwar, 2021).
Our patient presented with a classical myelitis syndrome associated with MOG-IgG, with severe urinary retention and LETM. Accordingly, we found a positive low-titer MOG-IgG as determined by CBA (the gold-standard), which may nevertheless be non-specific or of uncertain significance, and is insufficient to propose a diagnosis of anti-MOG associated disease (MOGAD) or NMOSD at this stage (Jarius et al., 2020; Reindl and Waters, 2019). Radiologically, the MRI pattern was also suggestive of MOG-IgG myelitis, including a predominantly gray-matter medullary involvement (H-sign) with mild enhancement after gadolinium (Fig. 1) (Chiriboga and Flanagan, 2021; Jarius et al., 2020). As such, despite not having chronic/recurrent neurological events at this point of follow-up, all these factors led to the decision of a prolonged steroid course of treatment.
There are only two other cases of myelitis following SARS-CoV-2 infection associated with MOG-IgG: one case of bilateral optic neuritis and LETM, and a multifocal mid-thoracic spinal cord myelitis and HHV6 coinfection/reactivation which confounds interpretation of the clinical findings (Jumah et al., 2020; Zhou et al., 2020). Furthermore, MOG-IgG was not determined by cell based assay in these cases, and thus laboratory significance of the finding is unknown (Reindl et al., 2020; Reindl and Waters, 2019).
The analysis of this case raises several questions. Firstly, we cannot rule out simple association of SARS-CoV-2 infection and myelitis instead of causation; nevertheless, previous reports support the strength and consistency of the association, and our report adds to this body of evidence with a clear temporal association and biological plausibility (Ellul et al., 2020). In fact, the patient had serum biomarkers of a recent SARS-CoV-2 infection with total immunoglobulin, IgM and IgG positivity indicating a recent infection. The presence of these markers may reinforce the temporal association between the two conditions (Cheng et al., 2020; Qu et al., 2020). Secondly, we do not know if SARS-CoV-2 played a role as a trigger of another inflammatory disorder or as a etiological pathogen, as we know this virus is possibly neurotropic (Pezzini and Padovani, 2020). Myelitis after SARS-CoV-2 infection is mostly thought to be part of a para/post-infectious mechanism, and a cytokine storm has been postulated as a possible cause for neural injury in SARS-CoV-2 patients, but this theory does not explain myelitis in mild COVID, as in our case (de Antonio et al., 2021; Paterson et al., 2020). Other proposed mechanisms include an hypercoagulable state, direct invasion of the CNS and multiorgan failure, also in severe disease (Pezzini and Padovani, 2020). Finally, if SARS-CoV-2 is considered as a possible trigger to an inflammatory disorder, whether this represents a monophasic – acute/subacute - response or rather a first manifestation of a chronic recurrent debilitating disorder such as NMOSD is also undetermined. Therefore, ideal treatment for these patients must still be decided in a case by case analysis, and prognosis uncertainty must be discussed with the patient.
4. Conclusion
LETM and low MOG-IgG may be part of a secondary and monophasic immunological response to SARS-CoV-2, or alternatively SARS-CoV-2 infection triggered a chronic MOG-IgG disease. Systematic assessment of neurological syndromes following COVID-19 is needed to advance knowledge of para/post-infectious neuroimmune syndromes in this setting, and to determine both treatment and potential risk of recurrence.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical standards
The authors declare that they complied with ethical standards and the patient gave written informed consent for the publication of this report.
Declaration of Competing Interest
None.
References
- Cheng M.P., Papenburg J., Desjardins M., Kanjilal S., Quach C., Libman M., Dittrich S., Yansouni C.P. Diagnostic testing for severe acute respiratory syndrome–related Coronavirus-2. Ann. Intern. Med. 2020;172:726–734. doi: 10.7326/M20-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiriboga S.L., Flanagan E.P. Myelitis and other autoimmune myelopathies. Contin. Lifelong Learn. Neurol. 2021;27:62–92. doi: 10.1212/con.0000000000000900. [DOI] [PubMed] [Google Scholar]
- de Antonio L.A.R., González-Suárez I., Fernández-Barriuso I., Pérez M.R. Para-infectious anti-GD2/GD3 IgM myelitis during the Covid-19 pandemic: case report and literature review. Mult. Scler. Relat. Disord. 2021;49 doi: 10.1016/j.msard.2021.102783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellul M., Varatharaj A., Nicholson T.R., Pollak T.A., Thomas N., Easton A., Zandi M.S., Manji H., Solomon T., Carson A., Turner M.R., Kneen R., Galea I., Pett S., Thomas R.H., Michael B.D. Defining causality in COVID-19 and neurological disorders. J. Neurol. Neurosurg. Psychiatry. 2020;91:811–812. doi: 10.1136/jnnp-2020-323667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarius S., Paul F., Weinshenker B.G., Levy M., Kim H.J., Wildemann B. Neuromyelitis optica. Nat. Rev. Dis. Prim. 2020;6 doi: 10.1038/s41572-020-0214-9. [DOI] [PubMed] [Google Scholar]
- Jumah M., Rahman F., Figgie M., Prasad A., Zampino A., Fadhil A., Palmer K., Arthur R., Gunzler S., Gundelly P., Abboud H. COVID-19, HHV6 and MOG antibody: a perfect storm. J. Neuroimmunol. 2020:353. doi: 10.1016/j.jneuroim.2021.577521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Abdullayev N., Neuneier J., Fink G.R., Lehmann H.C. Post-COVID-19 encephalomyelitis. Neurol. Res. Pract. 2021;4:4–6. doi: 10.1186/s42466-021-00113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. Acute longitudinal extensive transverse myelitis secondary to asymptomatic SARS-CoV-2 infection. BMJ Case Rep. 2021;14(e244687):1–3. doi: 10.1136/bcr-2021-244687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R.W., Brown R.L., Benjamin L., Nortley R., Wiethoff S., Bharucha T., Jayaseelan D.L., Kumar G., Raftopoulos R.E., Zambreanu L., Vivekanandam V., Khoo A., Geraldes R., Chinthapalli K., Boyd E., Tuzlali H., Price G., Christofi G., Morrow J., McNamara P., McLoughlin B., Lim S.T., Mehta P.R., Levee V., Keddie S., Yong W., Trip S.A., Foulkes A.J.M., Hotton G., Miller T.D., Everitt A.D., Carswell C., Davies N.W.S., Yoong M., Attwell D., Sreedharan J., Silber E., Schott J.M., Chandratheva A., Perry R.J., Simister R., Checkley A., Longley N., Farmer S.F., Carletti F., Houlihan C., Thom M., Lunn M.P., Spillane J., Howard R., Vincent A., Werring D.J., Hoskote C., Jäger H.R., Manji H., Zandi M.S. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzini A., Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat. Rev. Neurol. 2020;16:636–644. doi: 10.1038/s41582-020-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J., Wu C., Li Xiaoyong, Zhang G., Jiang Z., Li Xiaohe, Liu L. Profile of IgG and IgM antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;71:2255–2258. doi: 10.1093/cid/ciaa489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reindl M., Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat. Rev. Neurol. 2019;15:89–102. doi: 10.1038/s41582-018-0112-x. [DOI] [PubMed] [Google Scholar]
- Reindl M., Schanda K., Woodhall M., Tea F., Ramanathan S., Sagen J., Fryer J.P., Mills J., Teegen B., Mindorf S., Ritter N., Krummrei U., Stöcker W., Eggert J., Flanagan E.P., Ramberger M., Hegen H., Rostasy K., Berger T., Leite M.I., Palace J., Irani S.R., Dale R.C., Probst C., Probst M., Brilot F., Pittock S.J., Waters P. International multicenter examination of MOG antibody assays. Neurol. Neuroimmunol. Neuroinflam. 2020;7:1–12. doi: 10.1212/NXI.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samudralwar R.D. The spectrum of neurological manifestations related to COVID-19 and vaccinations. J. Neuroimmunol. 2021;358:577660. doi: 10.1016/j.jneuroim.2021.577660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Jones-lopez E.C., Soneji D.J., Azevedo C.J., Patel V.R. Myelin Oligodendrocyte glycoprotein antibody – associated optic neuritis and myelitis in COVID-19. J. Neuro-Ophtalmol. 2020;40:398–402. doi: 10.1097/WNO.0000000000001049. [DOI] [PMC free article] [PubMed] [Google Scholar]