Abstract
For patients treated with SBRT for spinal metastases in the cervical area, a thermoplastic mask is the usual immobilization technique. This project investigates the impact of shoulder position variability on target coverage for such cases. Eight HN patients treated in a suite equipped with a CT-on-rails system (CTOR) were randomly chosen. Of these, three were treated with shoulder depressors. For each patient, their planning CT was used to contour spine targets at the C5, C6 and C7 levels for which two VMAT plans were developed to deliver 18 Gy to each target per the RTOG 0631 protocol. One plan used full arcs while the other used avoidance sectors around the lateral positions. For each patient, IGRT CTOR images were used to recalculate doses that would have been delivered from these plans. Target coverage and dose to the spinal cord were compared for four scenarios: full and partial arcs, with or without depressors. A Dunn test showed significant differences between groups with and without shoulder depressors, but not between those with full versus partial arcs. For most of the investigated cases, the coverage ended up being higher than planned due to the shoulder position being inferior at treatment compared to simulation. In some cases, this led to higher spinal cord doses than allowed per protocol. The results of this study confirm that, when treating lower cervical spine lesions with SBRT, special care should be taken to ensure that the shoulders are positioned as they were during planning CT acquisition.
Keywords: Spine SBRT, patient positioning, treatment planning
INTRODUCTION
Stereotactic body radiation therapy (SBRT) to the spine is now becoming widely accepted as a method to treat spinal metastases[1,2]. With the close proximity of the spinal cord to the target volume, the geometry of this technique can be particularly challenging, requiring very steep dose gradients to ensure the target receives adequate dose, while simultaneously keeping the dose to the spinal cord below tolerance. Given this demanding geometry, volumetric modulated arc therapy (VMAT) is often employed when planning spinal SBRT[3] due to its ability to create the required sharp gradients and intricate dose distribution that is required.
Another important requirement for this type of treatment is the use of image guided radiation therapy (IGRT) to ensure that this highly conformal dose cloud is delivered precisely and consistently to the target. In our experience, due to the high doses involved with spine SBRT, along with the high visibility of the vertebral body with X-ray imaging, the IGRT registration used to fuse the image of the day with the planning dataset often tends to be highly focused on the target itself, sometimes with less attention paid to the global alignment of the patient as a whole.
For SBRT treatment of targets in the lower-cervical and upper-thoracic area of the spine, the position of the neck and shoulders can have large effects on the dose distribution if they are not well matched to their position during treatment planning image acquisition. This is due to varying thicknesses of tissue being intersected by the beam as it transits toward the target.
At our center, a large proportion of our head-and-neck (HN) patients are treated in a suite equipped with a CT-on-rails system (CTOR) as the primary IGRT solution. Thus, we have a large volume of high-quality fan-beam CT data from these HN patients acquired over their course of treatment. With these HN patients using the same immobilization as we would use in a lower-cervical/upper-thoracic spine SBRT treatment, these IGRT datasets provide an excellent source of information on the small changes in neck and shoulder position that are also observed when treating patients with spine metastases in that region of the body. The aim of this project was to use these CT datasets to precisely characterize the effects of small changes in neck and shoulder positions on the dose distributions to spine targets in the upper-cervical area.
MATERIALS AND METHODS
Patient Selection, Immobilization, and Contouring
Eight patients treated for HN cancer at our center were randomly selected from our database. All patients were immobilized with a 3-point ‘head only’ thermoplastic mask (QFix, Avondale, PA, USA) on a Head and Neck Board (AccuFix, QFix) . Within this cohort, three patients (Cases 1, 2, and 4) were treated using a shoulder suppressor (Shoulder-Loc, QFix) while the other five were treated without the shoulder suppressor. In each case, the planning CT was anonymized and transferred to an image management and contouring software, MIM (version 6.9.7, MIM Software Inc, Cleveland, OH), and a single attending physician, experienced with spine SBRT treatment, contoured three hypothetical targets encompassing the vertebral bodies of the C5, C6 and C7 levels, respectively. The RTOG 0631 protocol [4] was used to provide contouring guidelines for the targets and organs-at-risk that were relevant to those treatment sites.
Plan Generation
Once the contours were generated, the CT dataset and structure set were exported via DICOM to the RayStation treatment planning system (version 9A, RaySearch Inc, Stockholm, Sweden). Consistent with our typical clinical approach, treatment plans were generated using the flattening filter free (FFF) 6MV beam model for a TrueBeam (Varian Medical Systems, Palo Alto, CA, USA) outfitted with a 120 leaf HD MLC. Each of the three targets per patient was planned for treatment independently of the other two targets. For each target, two VMAT plans were generated using the guidelines from RTOG 0631. In the first plan, two full arcs were utilized, with collimator rotation being the only difference between the arcs. In the second plan, the same two arc geometry was used, but avoidance areas were created between gantry angles of 60 degrees and 120 degrees and 240 degrees and 300 degrees (angles defined according to IEC 1217[5]). The rationale for these avoidance sectors was to attempt to limit the dose entering laterally that could be most impacted by shoulder position, thereby reducing the importance of consistent shoulder position. For each patient and target, both plans were normalized to give a dose of 18 Gy to 90% of the target while ensuring that all other constraints mandated by RTOG 0631 were met.
IGRT CT Dataset Selection
For the eight patients selected for this study, there were between 7 and 37 daily IGRT datasets acquired, with an average of 24 per patient. Since these were HN cancer patients, who generally lose weight throughout their treatment[6], it was decided to only include IGRT datasets acquired when the patient’s weight was within 5 pounds (2.5%-4.5%) of their weight at the time of the planning CT acquisition. Once this criterion was enforced, the number of CT datasets per patient ranged between 5 and 19, with an average of 12 per patient, all of which were used to generate the results presented in this report.
Dose Computation on Additional CT datasets
The daily CT datasets identified for each case were imported into RayStation and the Raystation scripting language was used to create a custom script to rigidly register each of these datasets to the planning CT, while specifically focusing on the target and the partial cord for that target (defined as the cord contoured sup/inf through the target, plus 6 mm superior and inferior of the target). The script then followed with a deformable registration that also focused on the target and partial cord for that target. This deformable registration was used as the basis for mapping the target, partial cord and spinal cord onto the additional CT. Once the structures were mapped, the deformable registration was removed.
After the registration and contour mapping, the quality of the registration was reviewed by a medical physicist highly experienced in spine SBRT, and also to ensure that the pitch and roll were within 3 degrees, the maximum rotations achievable by our 6 degree of freedom couch (PerfectPitch, Varian Medical Systems, Palo Alto, CA, USA). The mapped structures were visually checked to ensure they matched the target by the same medical physicist. As an additional quality assurance step, the volume of each mapped target was extracted and compared to the volume of the target on the planning CT.
A second script was then executed to map the two plans for that target onto each additional CT and calculate the dose on that CT. The script was then used to automatically extract the relative target volume receiving the prescription dose of 18 Gy, the relative volume of the partial cord receiving 10 Gy, the dose received by 10% of the partial cord, and the volume of spinal cord receiving doses of 7, 10 and 14 Gy. From the RTOG protocol, the maximum volumes of spinal cord allowed to receive these dose values were 1.2 cc, 0.35 cc and 0.035 cc respectively. These dose metrics, as calculated on each treatment CT dataset, were compared for each target and each treatment plan (with or without the avoidance sectors).
With three targets per patient, two plans per target and an average of 12 daily image sets per patient a total of 570 different dose distributions were evaluated for this work. Of these, 234 included the use of shoulder depressors, while the other 336 did not.
Statistical Analysis
The various dose metrics investigated here were evaluated for four groups: 1. full arc geometry with shoulder depressor, 2. full arc geometry without shoulder depressor, 3. partial arc geometry (with avoidance sectors) with shoulder depressor and 4. partial arc geometry without shoulder depressor. To compare the achieved target coverage, the Kruskal-Wallis test[7] was used as implemented in the SciPy[8] statistics package. If the Kruskal-Wallis test showed significant differences existed between the groups, Dunn’s test[9,10] was used post-hoc for pairwise comparisons to determine which groups were significantly different from each other.
RESULTS
Verification of mapped target volumes
A comparison of the volumes of the mapped targets to the original volumes as drawn on the planning CT for each patient showed that the mapped regions of interest (ROI) volumes were all within 0.5% of the original volume.
Dose Metric Comparison and Statistical Analysis
Figure 1 shows the distribution of the target volume receiving the prescription dose for each of four different types of plan for each target. Recall that the planning goal was for 90% of each target to receive the prescription dose. The Kruskal-Wallis test on the data showed the average coverage from dose calculations on all treatment CT datasets was significantly different for all targets (C5 p = 6.59E-7), the C6 target (p = 2.82E-15) and C7 target (p = 1.06E-19). In all cases, Dunn’s post-hoc test showed the target coverage for patients treated with shoulder depressors was significantly different compared to patients treated without the depressors. Within each of these groups, there were no significant differences between treatments with and without avoidance sectors.
Figure 1.
Comparison of target coverage for the a) C5, b) C6 and c) C7 target for the four categories of plans run.
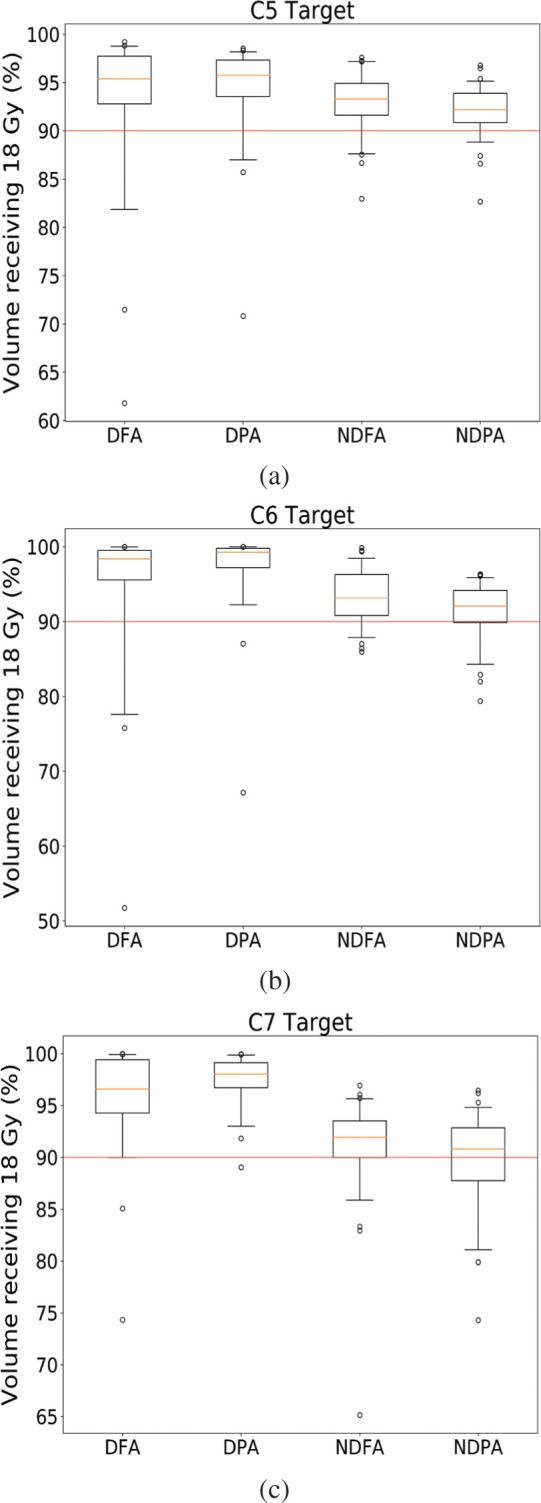
DFA: with depressor, full arc
DPA: with depressor, partial arc
NDFA: no depressor, full arc
NDPA: no depressor, partial arcs
Red lines show the protocol requirement of 90% coverage.
For each box plot, small circles show data points <5th or >95th percentile
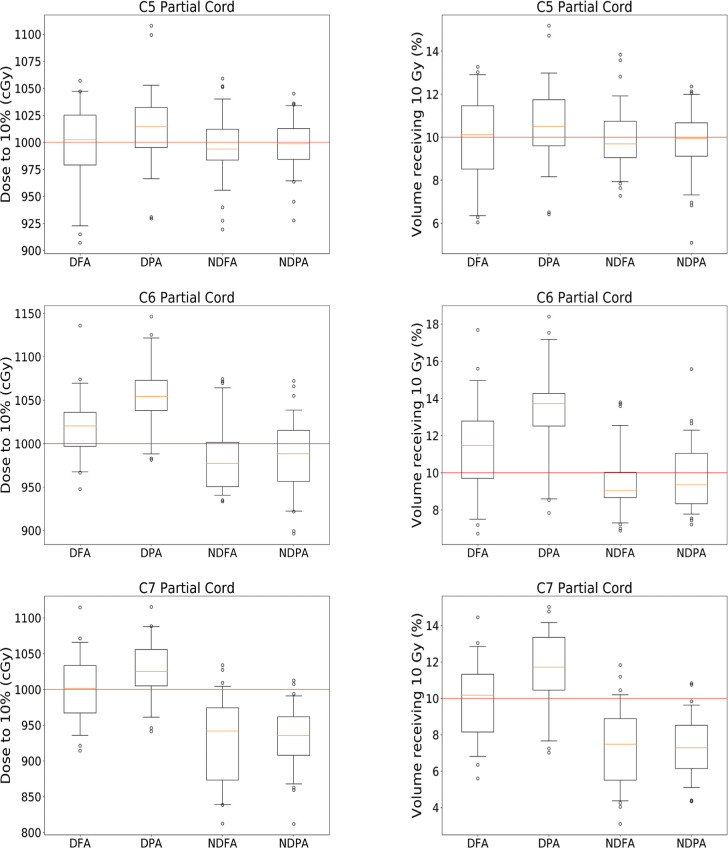
Figure 2 shows the distribution of doses to the partial cord for each of the targets.
Figure 2.
Comparison of doses received by the partial cord for each of the three targets for the four different categories of cases investigated.
DFA: with depressor, full arc
DPA: with depressor, partial arc
NDFA: no depressor, full arc
NDPA: no depressor, partial arcs
Finally, Tables 1, 2 and 3 shows the distribution of the volume of spinal cord receiving 7, 10 and 14 Gy respectively for each of the four categories of plans investigated. Note that the maximum values show that plans exceed the volume of cord allowed to get these dose levels per protocol for each plan category.
Table 1.
Volume (cc) of spinal cord receiving 7 Gy for each plan category
| Target Location | DFA Plans | DPA Plans | NDFA Plans | NDPA Plans | |
|---|---|---|---|---|---|
| C5 | Mean | 0.9952 | 1.0326 | 1.1736 | 1.1236 |
| Standard Deviation | 0.0848 | 0.0967 | 0.1245 | 0.0967 | |
| Minimum | 0.8194 | 0.8351 | 0.9561 | 0.8959 | |
| Maximum | 1.1847 | 1.1830 | 1.4102 | 1.3296 | |
| C6 | Mean | 1.0451 | 1.0188 | 1.2528 | 1.1140 |
| Standard Deviation | 0.1473 | 0.1643 | 0.1202 | 0.0786 | |
| Minimum | 0.7269 | 0.6899 | 1.0095 | 0.9230 | |
| Maximum | 1.2425 | 1.2961 | 1.5076 | 1.3440 | |
| C7 | Mean | 1.1415 | 1.0797 | 0.9903 | 0.9444 |
| Standard Deviation | 0.1926 | 0.1137 | 0.1643 | 0.0140 | |
| Minimum | 0.7950 | 0.8552 | 0.6090 | 0.6713 | |
| Maximum | 1.4195 | 1.2926 | 1.5573 | 1.3050 |
DFA: with depressor, full arc; DPA: with depressor, partial arc; NDFA: no depressor, full arc; NDPA: no depressor, partial arcs
Table 2.
Volume (cc) of spinal cord receiving 10 Gy for each plan category
| Target Location | DFA Plans | DPA Plans | NDFA Plans | NDPA Plans | |
|---|---|---|---|---|---|
| C5 | Mean | 0.3011 | 0.3243 | 0.3816 | 0.3824 |
| Standard Deviation | 0.0545 | 0.0646 | 0.1245 | 0.0967 | |
| Minimum | 0.1938 | 0.2115 | 0.2890 | 0.1668 | |
| Maximum | 0.4084 | 0.5135 | 0.5175 | 0.5242 | |
| C6 | Mean | 0.2872 | 0.3416 | 0.3500 | 0.3587 |
| Standard Deviation | 0.0686 | 0.0769 | 0.0707 | 0.0504 | |
| Minimum | 0.1406 | 0.1638 | 0.2210 | 0.2348 | |
| Maximum | 0.4289 | 0.5278 | 0.5331 | 0.5624 | |
| C7 | Mean | 0.3251 | 0.3773 | 0.2914 | 0.2977 |
| Standard Deviation | 0.0775 | 0.0719 | 0.0665 | 0.0608 | |
| Minimum | 0.1682 | 0.2177 | 0.1511 | 0.1964 | |
| Maximum | 0.4835 | 0.4967 | 0.5320 | 0.4320 |
DFA: with depressor, full arc; DPA: with depressor, partial arc; NDFA: no depressor, full arc; NDPA: no depressor, partial arcs
Table 3.
Volume (cc) of spinal cord receiving 14 Gy for each plan category
| Target Location | Cord volume receiving 14 Gy (cc) | DFA Plans | DPA Plans | NDFA Plans | NDPA Plans |
|---|---|---|---|---|---|
| C5 | Mean | 0.0227 | 0.0258 | 0.0231 | 0.0131 |
| Standard Deviation | 0.0147 | 0.0205 | 0.0189 | 0.0089 | |
| Minimum | 0.0010 | 0.0006 | 0.0033 | 0.0001 | |
| Maximum | 0.0479 | 0.1008 | 0.0747 | 0.0330 | |
| C6 | Mean | 0.0342 | 0.0410 | 0.0181 | 0.0120 |
| Standard Deviation | 0.0252 | 0.0268 | 0.0161 | 0.0109 | |
| Minimum | 0.0000 | 0.0002 | 0.0003 | 0.0007 | |
| Maximum | 0.1187 | 0.1189 | 0.0819 | 0.0724 | |
| C7 | Mean | 0.0264 | 0.0303 | 0.0113 | 0.0080 |
| Standard Deviation | 0.0215 | 0.0185 | 0.0103 | 0.0083 | |
| Minimum | 0.0002 | 0.0012 | 0.0000 | 0.0000 | |
| Maximum | 0.0779 | 0.0688 | 0.0492 | 0.0365 |
DFA: with depressor, full arc; DPA: with depressor, partial arc; NDFA: no depressor, full arc; NDPA: no depressor, partial arcs
DISCUSSION
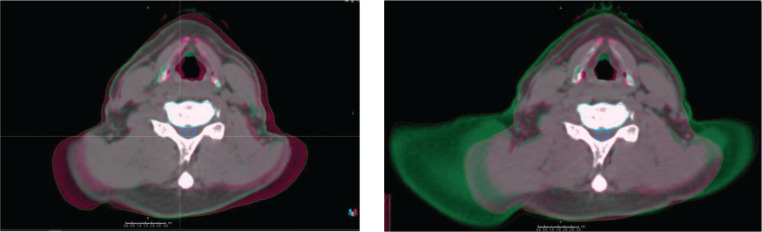
Figure 1 confirms that, when doses are recalculated on the daily CT images, the volume of target receiving the prescription dose varies significantly. As depicted in Figure 3, a large part of the observed change in target coverage can be attributed to either missing tissue at treatment time compared to planning CT (left panel) or extra tissue being present in the treatment field as depicted in the right panel of Figure 3 for the same patient.
Figure 3.
Comparison of planning CT and daily CT for two different days' images for the same patient. The planning CT is magenta while the CT of the day is in green.
While some degree of variation was expected, and was indeed the basis for the present investigation, this effect is especially important when the treatment is being delivered in a single treatment. A surprising observation is that for all three targets investigated, the majority of the cases studied show higher coverage than planned. Indeed, for all three targets and all four scenarios investigated, the median coverage exceeded the 90% of the target coverage stipulated by the protocol. As a consequence, increased dose to nearby organs-at-risk was observed; primarily, the spinal cord. It can be inferred from the mean and standard deviation values in Table 3 that several plans from each plan category would also have the volume of cord receiving a dose of 14 Gy being well above the 0.035 cc allowed by the protocol. This is especially concerning for a single fraction treatment where a fraction-to-fraction dose feathering effect is absent and, therefore, the subsequent reduction in maximum dose that typically accompanies such ‘feathered’ treatments.
While we did not specifically study this, one could speculate that patients may be more nervous at the time of treatment planning CT acquisition and, thus, tense up their shoulders more while, perhaps, being more relaxed at the time of treatment. This could lead to the shoulder positions being more inferior to the target at treatment time, leading to less tissue within the field and therefore higher doses in general, as we observed here.
A second interesting result from this analysis comes from the use of the shoulder depressors. The Dunn test shows that the median coverage seen in patients treated with shoulder depressors is statistically different from the median coverage for patients treated without. While one could assume that shoulder depressors would lead to less variability of coverage, the opposite is seen in Figure 1 for the C5 and C6 targets. While the median coverage seems to be higher for cases using the depressors, which is attributable to the fact that shoulder depressors limit how far superior the shoulders can extend, not how inferior they can go, the lowest coverage seen in this study also belongs to a patient with depressors. In that case, the coverage fell to 61%, and this can be seen in the right panel of Figure 3. This particular scenario can happen when the depressors are not positioned tightly against the shoulders at simulation time, thus enabling the shoulders to creep slightly more superior compared to the simulated position. These observations confirm the fact that the clinical use of new immobilization devices should be carefully investigated to ensure that they are achieving the desired results. In this case, even with the use of rigid shoulder depressors, it is clear that the shoulders were still able to move in both directions, which led to the changes in target coverage reported here.
Another possible system that is commercially available to help with shoulder position the 5-point head and shoulder mask. While thermoplastic masks are less rigid than the shoulder suppressor system used, they do cover the whole shoulder area and may provide more tactile feedback to the patients and help keep the shoulders in a more reproducible position. Unfortunately, none of the patients investigated here used such a mask and we therefore do not have data to provide conclusions on how these masks do immobilize the shoulders.
Regarding the use of avoidance sectors that miss the shoulder, the results of this study do not show a statistically different distribution of coverage between cases using depressors and those not using them. This could be because the avoidance sectors used were not large enough to fully mitigate the effect of shoulder position, but larger sectors would have likely increased the required plan complexity to achieve the required dose gradients. This increased complexity would lead to even higher susceptibility for negative dosimetric impact from small changes in shoulder geometry.
Finally, it should be noted that the chin position also has the potential to impact the dose distribution when VMAT is used. With our particular cohort of patients, the mask did a good job of immobilizing the chin in a reproducible position. However, this may be specific to the mask system used at our clinic and the mask making methodology. With thermoplastic masks having a range of flexibility, it is not improbable that a mask that does not fit very snugly around the chin area may allow for motion, which would also lead to changes in dose distribution in the cervical area.
CONCLUSIONS
This study confirms that large dosimetric variations can be seen in coverage of lower-cervical spine SBRT targets when shoulder position variability is not fully controlled. The use of avoidance sectors when planning VMAT treatments did not reduce the variation, at least for the avoidance sector strategy used in this investigation. Interestingly, the use of shoulder depressors resulted in more variability in target coverage for the cohort studied. It is our conclusion that one of the ways to minimize the type of variation seen here without changing treatment technique is to ensure the shoulders are also repositioned correctly in addition to accurate target alignment when using image guidance.
ACKNOWLEDGMENTS
Authors’ disclosure of potential conflicts of interest
The authors have nothing to disclose.
Author contributions
Conception and design: All Authors
Data collection: Vikren Sarkar, Shane Lloyd
Data analysis and interpretation: Vikren Sarkar, Shane Lloyd
Manuscript writing: Vikren Sarkar, Shane Lloyd, Bill Salter
Final approval of manuscript: All authors
REFERENCES
- 1.Sahgal A, Larson DA, Chang EL. Stereotactic body radiosurgery for spinal metastases: A critical review. Int J Radiat Oncol Biol Phys. 2008;71(3):652-65. [DOI] [PubMed] [Google Scholar]
- 2.Yamada Y, Bilsky MH, Lovelock DM, Venkatraman ES, Toner S, Johnson J, Zatcky J, Zelefsky MJ, Fuks Z. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71(2):484-90. [DOI] [PubMed] [Google Scholar]
- 3.Wu QJ, Yoo S, Kirkpatrick JP, Thongphiew D, Yin F-F. Volumetric arc intensity-modulated therapy for spine body radiotherapy: comparison with static intensity-modulated treatment. Int J Radiat Oncol Biol Phys. 2009;75(5): 1596-604. [DOI] [PubMed] [Google Scholar]
- 4.Ryu S, Pugh SL, Gerszten PC, Yin FF, Timmerman RD, Hitchcock YJ, Movsas B, Kanner AA, Berk LB, Followill DS, Kachnic LA. RTOG 0631 phase 2/3 study of image-guided radiosurgery/ for localized (1-3) spine metastases: Phase 2 results.. Pract Radiat Oncol. 2014;4:76-81. doi: 10.1016/j.prro.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Standard IEC 61217:2011, Radiotherapy equipment — Coordinates, movements and scales.. Geneva: International Electrochnical Commission. 134 pp. [Google Scholar]
- 6.Larsson M, Hedelin B, Johansson I, Athlin E. Eating problems and weight loss for patients with head and neck cancer: A chart review from diagnosis until one year after treatment. Cancer Nurs. 2005;28(6):425-35. [DOI] [PubMed] [Google Scholar]
- 7.McKight PE, Najab J. Kruskal-Wallis test. In: Weiner IB, Craighead WE. Corsini Encyclopedia of Psychology. Hoboken, NJ: Wiley Online. 2010,1 pp. DOI: 10.1002/9780470479216.corpsy0491 [DOI] [Google Scholar]
- 8.Jones E, Oliphant T, Peterson P. SciPy: Open source scientific tools for Python. scienceopen.com. 2001; Available [viewed 2021-06-17] from: https://www.scienceopen.com/document?vid=ab12905a-8a5b-43d8-a2bb-defc771410b9
- 9.Dunn OJ. Multiple comparisons using rank sums. Technometrics. 1964;6(3):241-52. [Google Scholar]
- 10.Terpilowski M. Scikit-posthocs: Pairwise multiple comparison tests. J Open Source Softw. 2019;4(36):1169. [Google Scholar]