Abstract
Treatments for melanoma have significantly advanced with the approval of targeted treatments against the BRAF/MEK pathway and immunotherapy in the form of checkpoint inhibitors. Studies have shown the effectiveness of these treatments against brain metastases. However, the optimum treatment strategy utilising CNS-directed treatments such as stereotactic radiosurgery (SRS) and neurosurgical resection is less clear.
Over six years, 70 patients with metastatic melanoma were treated for brain metastases at a tertiary treatment centre. The median overall survival (OS) for all patients was 10.2 months. 51 patients received localised treatment; 7 resection (median OS 10 months), 11 resection and SRS (median OS 17.3 months) and 33 SRS alone (median OS 17.4 months). For patients treated with SRS those who had <2 cm3 treated had a better median OS (20.5 months) compared to those who had >2 cm3 treated (12 months).
69 Patients received systemic treatment. The median OS of patients who did not have CNS-directed treatment was poor (median OS 1.2 months). Patients treated with first line dual immunotherapy had the best median OS (26.7 months), compared to anti-PD-1 (14.1 months), ipilimumab (14.3 months) and kinase inhibitors (10.9 months).
Despite advancements in treatment, the development of brain metastases in melanoma is associated with worse outcomes. A combination of CNS-directed and systemic treatment is important to improve survival. Dual immunotherapy appears to be the most effective systemic treatment and the use of SRS improved outcomes. As metastatic melanoma treatments evolve there need to be an ongoing focus to ensure these strategies adequately treat intracranial disease.
Keywords: Stereotactic radiosurgery, melanoma, systemic treatment, CNS-directed treatment, immunotherapy
INTRODUCTION
Amongst solid tumours, melanoma has one of the highest propensities to cause brain metastases with over 25% of patients affected at the point of diagnosis with metastatic disease [1]. Autopsy reports show that 75% of melanoma patients die with brain metastases [2].
Both targeted kinase inhibitors and immunotherapy have been shown to have intracranial activity. In asymptomatic patients dual immunotherapy with a combination of ipilimumab, a monoclonal antibody which blocks cytotoxic T-lymphocyte antigen 4 (CTLA-4) and nivolumab, an anti-programme death (PD-1) antibody showed an intracranial clinical benefit rate of 57% [3]. An intracranial response rate of 58.4% was seen in patients treated with dual immunotherapy treatment for asymptomatic brain metastases, however, this response rate dropped to 16.7% in symptomatic patients [4].
The use of stereotactic radiosurgery (SRS) and resection have both demonstrated improvement in outcomes for brain metastases [2, 5-8]. In melanoma, SRS is an established treatment option for the local control of brain metastases. A study of 80 patients treated with immunotherapy after SRS showed 78% of patients were alive at 12 months if treated with nivolumab compared to 68% in patients treated with ipilimumab [9]. This was supported by another study of patients treated with either immunotherapy or targeted therapy after SRS, BRAF wild-type patients treated with immunotherapy had a median overall survival of 12.3 months and BRAF mutated patients treated with either immunotherapy or targeted kinase inhibitors had a median overall survival of 14.8 months [10].
Despite previous studies there remain key questions over the most effective strategy of treating brain metastases. This article addresses the role of CNS-directed treatments and their requirement given the increasing effectiveness of systemic treatments.
METHOD
Retrospective data was collected on consecutive metastatic melanoma patients with radiologically proven brain metastases who were treated between January 2014 and January 2020 in a single tertiary NHS oncology centre in Bristol, UK. Intracranial and systemic treatments received by patients were analysed to see the effect on survival.
For each patient the following demographics was collected; age at time of treatment, gender, BRAF V600 mutational status, date of the development of metastatic disease and brain metastases. All cases were discussed at a neuro-oncology multi-disciplinary meeting and the decision of whether to use CNS-directed treatments for brain metastases were made by the combination of neurosurgeons and stereotactic trained clinical oncologists. Multi-disciplinary meeting outcomes, radiotherapy treatment plans and clinic letters were analysed for details of localised treatments. Systemic treatments were confirmed from electronic patient records. The InSight PACS Medical Imaging system (Insignia medical systems, Basingstoke, UK) was used to assess treatment response by RECIST 1.1 criteria.
Gamma knife radiosurgery was delivered using the Leksell Gamma Knife Icon with inbuilt high-definition motion management system. Patients were immobilised with fixed frames. SRS treatment was planned from gadolinium contrasted T1 and T2 weighted MRI imaging, performed from the base ring to the vertex at 1-1.5 mm slice thickness. Any discrepancy or distortion on the MRI images was checked via CT imaging for co-registration. The planned treatment volume was defined as the gross tumour volume on MRI imaging. Dose was prescribed as a single fraction according to tumour diameter: <2 cm, 20-22 Gy; 2-3 cm, 16-20 Gy; 3-4 cm, 16-18 Gy. In single metastases doses were increased by 2 Gy. 192 cobalt-60 sources delivered multiple narrow low energy beams of radiation. Patient motion was detected on any movement of >0.5 mm and an automatic stop was inserted at 1.5 mm and with consistent movements of >0.7 mm a second stereotactic cone beam CT was used to reset the treatment plan [11].
Univariate analysis was undertaken on survival data, Cox-Mantel log-rank tests and the Bonferroni correction was used to set the p value for significance on Microsoft Excel. Kaplan-Meier survival curves were generated to compare survival of different groups.
RESULTS
70 metastatic melanoma patients were treated for brain metastases over the five year period. The demographics for patients including the treatment summaries are detailed in Table 1. The mean age at diagnosis was 63 (range 32-87). 51 patients received CNS-directed therapy, 7 had surgery alone, 11 had surgery then adjuvant SRS and 33 had SRS alone. At the date of censorship, 18 (25.7%) of patients were alive and 52 (74.3%) of patients had died.
Table 1.
Patient demographics including details of localised and systemic treatment received
| Number (%) | Mean | Range | Interquartile range | ||
|---|---|---|---|---|---|
| Male | 51 (72.9) | ||||
| Female | 19 (27.1) | ||||
| Age | 63 | 32–87 | 54–73 | ||
| BRAF V600 status | Mutant | 46 (65.7) | |||
| Wild-type | 24 (34.3) | ||||
| At point of Diagnosis | Localised | 49 (70.0) | |||
| Metastatic disease | 21 (30.0) | ||||
| Brain metastases | 11 (15.7) | ||||
| Time from diagnosis to development of brain metastases (months) | 29.6 | 0–148 | 4.2–39.2 | ||
| At point of diagnosis with metastatic melanoma | Brain metastases | 37 (52.9) | |||
| No brain metastases | 33 (47.1) | ||||
| Time from metastatic diagnosis to development of brain metastases (months) | 3.5 | 0–22.7 | 0–4.5 | ||
| Localised treatment | Total | 51 (72.9) | |||
| Surgery only | 7 (10.0) | ||||
| SRS only | 33 (47.1) | ||||
| Surgery and SRS | 11 (15.7) | ||||
| First line systemic treatment | Total | 69 (98.6) | |||
| BRAF inhibitor | 15 (21.4) | ||||
| BRAF + MEK inhibitors | 19 (27.1) | ||||
| Ipilimumab | 7 (10.0) | ||||
| Anti-PD-1 | 21 (30.0) | ||||
| Dual immunotherapy | 5 (7.1) | ||||
| No localised treatment | 19 (27.1) | ||||
The median overall survival (OS) for all patients was 10.1 months with a 3 year survival of 21.1%. For patients who received surgery the median OS was 10.0 months (95% CI ± 25.7) (3 year survival: 28.6%), for patients who received SRS the median OS was 17.4 months (95% CI ± 6.2) (3 year survival: 19.0%), for patients who received surgery and SRS the median OS was 17.3 months (95% CI ± 10.4) (3 year survival: 45.5%) and for patients who received only systemic treatment the median OS was 1.2 months (95% CI ± 1.7) (3 year survival: 0%). Both SRS and the combination of surgery and SRS had statistically significant better median OS than patients treated with systemic treatment only, p ≤ 0.001 HR 0.25 and p ≤ 0.001 HR 0.22 respectively (see Figure 1).
Figure 1.

Kaplan Meier survival curve assessing the overall survival after localised treatment for brain metastases
67 Patients received systemic treatment as part of their management, they had a median OS of 13.1 months (3 year survival: 15.4%). As first line treatment 34 patients were treated with targeted kinase inhibitors against BRAF alone (15 patients) or in combination with MEK inhibitors (19 patients) and had a median OS of 10.9 months (95% CI ± 4.5) (3 year survival: 6.7%), 7 patients were treated with ipilimumab and had a median OS of 14.3 months (95% CI ± 7.0) (3 year survival: 28.6%), 21 patients were treated with anti-PD-1 therapy, either nivolumab or pembrolizumab and had a median OS of 14.1 months (95% CI ± 6.4) (3 year survival: 34.1%) and 5 patients were treated with dual immunotherapy, the combination of ipilimumab and nivolumab and had a median OS on 26.7 months (95% CI ± 8.6) (3 year survival: 0%). There were no statistically significant differences between treatments (see Figure 2a).
Figure 2.
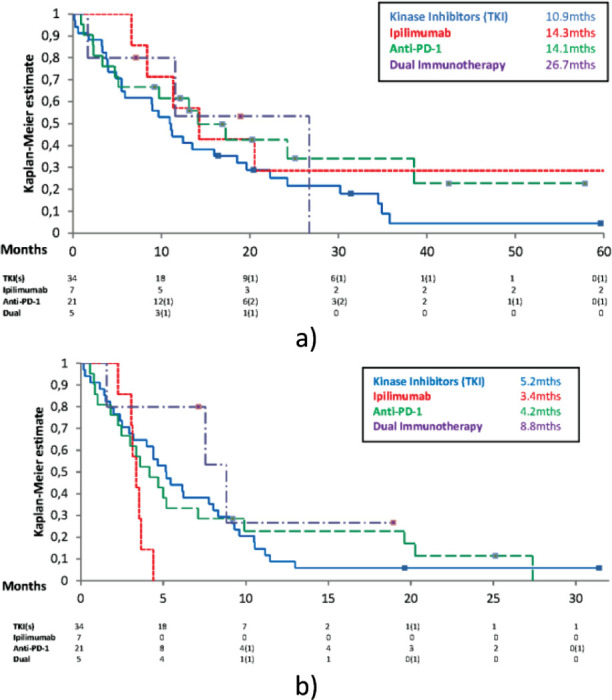
a) Kaplan Meier survival curve assessing the overall survival for systemic treatment received to treat brain metastases; b) Kaplan Meier survival curve assessing progression free survival for systemic treatment received to treat brain metastases
The first line progression free survival (PFS) was also assessed; targeted kinase inhibitors - median PFS 5.16 months (95% CI ± 2.1) (12 month PFS: 8.8%), ipilimumab - median PFS 3.4 months (95% CI ± 0.5) (12 month PFS: 0%), anti-PD-1 therapy - median PFS 4.2 months (95% CI ± 3.5) (12 month PFS: 22.9%) and dual immunotherapy - median PFS 8.8 months (95% CI ± 5.5) (12 month PFS: 26.7%). There were no statistically significant differences between treatments, although there were trends to suggest both targeted kinase inhibitors and dual immunotherapy were better than ipilimumab. Kinase inhibitors versus ipilimumab p = 0.029 with HR 0.43 and dual immunotherapy versus ipilimumab p = 0.02 with HR 0.27 (see Figure 2b).
44 Patients received SRS, these patients had a median OS of 17.3 months (3 year survival 26.7%). 22 Patients had a total volume treated of less than 2 cm3, median OS of 20.5 months (95% CI ± 8.5) (3 year survival 28.0%) compared to 22 patients who had a total volume treated of greater than 2 cm3, median OS 12.0 months (95% CI ± 6.1) (3 year survival 25.5%). There were no statistically significant differences between groups (see Figure 3). 20 Patients had one lesion treated, median OS of 12.5 months (95% CI ± 8.0) (3 year survival 18.8%), 15 patients had either two or three lesions treated, median OS of 18.6 months (95% CI ± 7.2) (3 year survival 17.5%) and 9 patients had four or more lesions treated, median OS 40.7 months (95% CI ± 14.7) (3 year survival 55.6%). There was no statistical difference between these groups.
Figure 3.
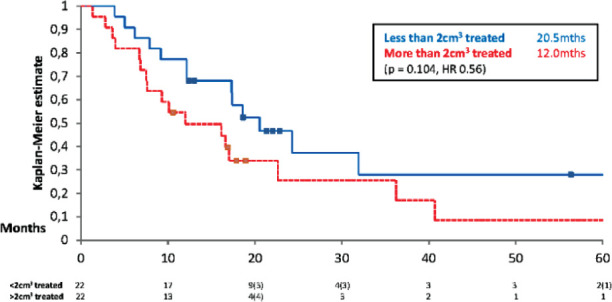
Kaplan Meier survival curve assessing overall survival from the volume of metastases treated with SRS
BRAF V600 mutant patients had a median OS of 12.1 months (95% CI ± 6.2) (3 year survival: 20.0%), BRAF V600 wild type patients had a median OS of 7.6 months (95% CI ± 5.3) (3 year survival: 24.3%). There was no statistically significant difference between the two groups (see Figure 4).
Figure 4.
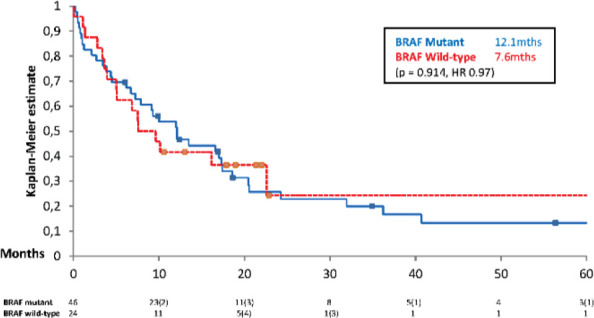
Kaplan Meier survival curve assessing overall survival of patients from brain metastases based on BRAF V600 status
DISCUSSION
This study is in keeping with previous published data, showing a significant proportion of melanoma patients develop brain metastases and in the majority of cases this occurred at the point of first developing metastatic disease (52.9%). In the Checkmate 067 study, the median OS for dual immunotherapy has not been reached at 60 months and was 36.9 months for nivolumab alone [12]. The survival of melanoma patients with brain metastases is much worse. One study reported median survival of 12.3 months for BRAF wild-type patients and 14.8 months for BRAF mutant patients treated with immunotherapy [10]. This was supported by a further study where the median survival after the development of brain metastases was just 12.8 months [13]. Given the worse prognosis seen in brain metastases, improvements in management for this group of patients should be a significant focus.
The median OS for this study was 10.1 months, which is in keeping with previous studies especially considering patients who did not receive systemic treatment were included. The survival for patients who did not receive CNS-directed treatment was poor at just 1.2 months, it is possible this is because their intracerebral disease was so extensive that CNS-directed treatment was not an option but this study gives weight to the argument that if technically feasible patients should receive CNS-directed treatment upfront. There was no significant difference between the median OS for patients who received surgery, SRS or a combination of the two although there was a trend to suggest SRS was at least equally as effective as surgery (median OS 17.4 months v 10.0 months). The 3 year survival in patients who received surgery and SRS was 45.5% (surgery alone 28.6%, SRS alone 19%) which suggests in appropriately selected patients this option may give the greatest chance of long-term disease control.
The use of systemic treatment improved median OS in this study (13.1 v 10.1 months). There were no significant differences in the median OS based on the first line treatment received. Patients receiving dual immunotherapy had the longest median OS (26.7 months) and PFS (8.8 months). This supports the data from Checkpoint 204 where melanoma patients with brain metastases treated with dual immunotherapy had response rates of 57% [3]. Although there were minimal differences, the slightly better median OS, 3 year survival percentage and median PFS for anti-PD-1 treatments compared to ipilimumab support the usage of anti-PD-1 treatments over ipilimumab as first line treatments in patients with brain metastases.
Although not reaching significance, there was a trend to suggest patients with lower total volume treated by SRS had improved survival compared to those with larger volumes treated (<2 cm3 median OS 20.5 cm compared to >2 cm3 median OS 12.0 months). The number of lesions treated did not appear to have any effect on survival. This is in keeping with our previous published study in breast cancer where volume and not number of lesions treated predicted survival post-SRS [5].
One weakness of this study is the number of patients investigated have made multi-variant analysis unfeasible and therefore care needs to be taken in drawing conclusions over the significance of findings. However, our principle findings are in-keeping with other published studies and this study increases the weight of these arguments. Further studies with increased patient numbers would strengthen confidence in the conclusions drawn from this study.
The emergence of different checkpoint inhibitors, notably anti-LAG-3 will hopefully lead to new first line treatment options in metastatic melanoma. The early results from the Relativity-047 clinical trial are promising, with an improved PFS seen an anti-LAG-3 agent in combination with nivolumab versus nivolumab alone (10.1 months vs 4.6 months) and grade 3/4 toxicity levels of 18.9% from the combination treatment [14], as opposed to 59% seen in Checkmate-067 with ipilimumab and nivolumab [12]. Further evaluation of anti-LAG-3 agents combined with anti-PD-1 agents in the treatment of melanoma patients with brain metastases.
Tumour-infiltrating lymphocytes (TILs) have also emerged as an effective management option in metastatic melanoma. There is emerging evidence that TILs have efficacy in brain metastases, although a study of 33 patients suggested that these patients still require CNS-directed therapy to achieve durable response [15]. The widespread use of TILs are currently limited by the expense and expertise required for creation and in patient management after infusion. Hopefully in the future their use will become more widespread as costs reduced and experience increases.
CONCLUSION
The development of brain metastases leads to worse outcomes for metastatic melanoma patients.
To improve outcomes in these patients the combination of localised treatment and systemic treatment appears important.
The best survival was seen in patients who had a combination of upfront neurosurgery and SRS as CNS-directed treatment and dual immunotherapy as systemic treatment.
Reassuringly, all systemic treatments appeared effective.
As in other cancers, it is likely the volume of brain metastases treated with SRS can act as a predictive biomarker.
Further studies are required to prove the optimum sequencing of current treatments in melanoma patients with brain metastases. In combination, the emergence of new treatment options, such as the promising early results of anti-LAG-3 antibodies in combination with anti-PD-1 agents and the wider use of TILs will hopefully lead to more treatment options for melanoma patients with brain metastases.
ABBREVIATIONS
SRS: Stereotactic radiosurgery, OS: Overall survival, PFS: Progression free survival, anti-PD-1: anti-programmed cell death protein- 1, CTLA-4: cytotoxic T-lymphocyte antigen 4
ACKNOWLEDGMENTS
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
The authors would like to thank all the patients who underwent treatment and whose data allowed us to undertake this study. We would also like to thank all the staff at the Bristol Haematology and Oncology centre.
Authors’ disclosure of potential conflicts of interest
The authors have nothing to disclose.
Author contributions
Conception and design: Christopher Herbert, Hannah Taylor, Thomas G Wilson, Helen Winter
Data collection: Thomas G Wilson
Data analysis and interpretation: Thomas G Wilson
Manuscript writing: Thomas G Wilson
Final approval of manuscript: Christopher Herbert, Hannah Taylor, Thomas G Wilson, Helen Winter
REFERENCES
- 1.Cagney DN, Martin AM, Catalano PJ, Redig AJ, Lin NU, Lee EQ, Wen PY, Dunn IF, Bi WL, Weiss SE, Haas-Kogan DA, Alexander BM, Aizer AA. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: A population-based study. NeuroOncol. 2017;19(11):1511-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sloan AE, Nock CJ, Einstein DB. Diagnosis and treatment of melanoma brain metastasis: A literature review. Cancer Control. 2009;16:248-255. [DOI] [PubMed] [Google Scholar]
- 3.Tawbi H, Forsyth P, Algazi A, Hamid O. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379:722-730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tawbi H, Forsyth P, Hodi S, Lao C. Efficacy and safety of the combination of nivolumab (NIVO) plus ipilimumab (IPI) in patients with symptomatic melanoma brain metastases (CheckMate 204).. J Clin Oncol. 2019;37(15):9501-9501. [Google Scholar]
- 5.Wilson TG, Robinson T, MacFarlane C, Spencer T, Herbert C, Wade L, Reed H, Braybrooke JP. Treating brain metastases from breast cancer: Outcomes after stereotactic radiosurgery. Clin Oncol. 2020;32(6):390-396. [DOI] [PubMed] [Google Scholar]
- 6.Schapira E, Hubbeling H, Yeap B, Mehan Jr W, Shaw A, Oh K, Gainor JF, Shih HA. Improved overall survival and locoregional disease control with concurrent PD-1 pathway inhibitors and stereotactic radiosurgery for lung cancer patients with brain metastases. Int J Radiat Oncol Biol Phys. 2018;101(3):624-629. [DOI] [PubMed] [Google Scholar]
- 7.Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45(2):427-34 [DOI] [PubMed] [Google Scholar]
- 8.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ Jr. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422): 1665-72. [DOI] [PubMed] [Google Scholar]
- 9.Minniti G, Anzellini D, Reverberi C, Cappellini GCA, Marchetti L, Bianciardi F, Bozzao A, Osti M, Gentile PC, Esposito V. Stereotactic radiosurgery combined with nivolumab or ipilimumab for patients with melanoma brain metastases: Evaluation of brain control and toxicity. J Immunother Cancer. 2019;7:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaudy-Marqueste C, Dussouil AS, Carron R, Troin L, Malissen N, Loundou A, Monestier S, Mallet S, Richard MA, Régis JM, Grob JJ. Survival of melanoma patients treated with targeted therapy and immunotherapy after systematic upfront control of brain metastases by radiosurgery. Eur J Cancer. 2017;84:44-54. [DOI] [PubMed] [Google Scholar]
- 11.https://www.elekta.com/dam/jcr:bd34ae75-f0d0-4c8a-ac70-03ed568e2479/LGK-Icon-Bristol-Gamma-Knife-Centre-customer-perspective.pdf (accessed 4/12/20 at 1240).
- 12.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hogg D, Hill A, Márquez-Rodas I, Haanen J, Guidoboni M, Maio M, Schöffski P, Carlino MS, Lebbé C, McArthur G, Ascierto PA, Daniels GA, Long GV, Bastholt L, Rizzo JI, Balogh A, Moshyk A, Hodi FS, Wolchok JD. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019;381:1535-1546. [DOI] [PubMed] [Google Scholar]
- 13.Vosoughi E, Lee JM, Miller JR, Nosrati M, Minor DR, Abendroth R, Lee JW, Andrews BT, Leng LZ, Wu M, Leong SP, Kashani-Sabet M, Kim KB. Survival and clinical outcomes of patients with melanoma brain metastasis in the era of checkpoint inhibitors and targeted therapies. BMC Cancer. 2018;18:490. 10.1186/s12885-018-4374-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipson EJ, Tawbi HA, Schadendorf D, Ascierto PA, Matamala L, Gutiérrez EC, Rutkowski P, Gogas H, Lao CD, de Menezes JJ, Dalle S, AM, Grob JJ, Srivastava S, Abaskharoun M, Simonsen KL, Li B, Long GV, Hodi S. Relatlimab (RELA) plus nivolumab (NIVO) versus NIVO in first-line advanced melanoma: Primary phase III results from RELATIVITY-047 (CA224-047).. J Clin Oncol. 2021;39:15_suppl9503-9503 [Google Scholar]
- 15.Mehta GU, Malekzadeh P, Shelton T, White DE, Butman JA, Yang JC, Kammula US, Goff SL, Rosenberg SA, Sherry RM. Outcomes of adoptive cell transfer with tumor-infiltrating lymphocytes for metastatic melanoma patients with and without brain metastases. J Immunother. 2018;41(5):241-247. doi: 10.1097/CJI.0000000000000223 [DOI] [PMC free article] [PubMed] [Google Scholar]


