Abstract
Purpose/objectives:
Outcomes of T2N0 lung cancer patients treated with stereotactic radiotherapy are not well known.
Methods and materials:
We conducted a single institution retrospective review of patients with T2N0 NSCLC who were treated with SBRT. The local, regional, distant control rates were calculated from available clinical data. Survival outcomes were determined using the Kaplan Meier method.
Results:
Fifty-six patients met our selection criteria. The two-year local control rate was 84.2%. The two and 5-year disease-free survival (DFS) and OS were 31.9% and 15.3% and 39.9% and 12.1%, respectively. Centroid BED10 > 150Gy was associated with improved DFS, (p = 0.014), and OS on univariable analysis (p=0.0132).
Conclusions:
SBRT provides good local control for T2N0 NSCLC, but systemic failure remains problematic.
Keywords: Radiosurgery, carcinoma, non-small-cell lung cancer, treatment outcomes, retrospective studies
INTRODUCTION
Lung cancer is the second most common form of cancer in the United States with 234,000 cases annually and 154,000 deaths (1). Non-small cell lung cancer (NSCLC) is the predominant histologic subgroup, and is identified in 75-80% patients (1). Lobectomy is the preferred treatment modality for early stage (T1-T2) NSCLC, with a 5-year survival rate of 60-80% (2-4). In the last two decades, high dose conformal stereotactic body radiotherapy (SBRT) has become the preferred choice for medically inoperable patients with early stage NSCLC (5). SBRT utilizes sharp dose gradients to deliver highly conformal radiation dose to the tumors. Early phase I dose escalation trials had shown SBRT to be safe and effective in medically inoperable stage I-II NSCLC patients (6,7). In a multicenter phase II trial, SBRT produced local control rates greater than 80%, and overall survival rate of 55.8% at 3 years (7). Long term follow-up demonstrated primary tumor failure rates of 7%, and a local regional failure rates of 38% (8). The rates of disease free and overall survival at 5 years were 26% and 40% respectively (8). Recent data shows that SBRT of centrally located tumors produces two year local control rates of 87.9%, 72.7%, and progression free survival of 54.5% (9). Other studies showed that SBRT produces three year local control rates of 70 to 90%, and two year survival rates of 50-70% (2,10,11). Based on these and similar studies, SBRT has become the favored treatment modality for early stage (T1-T2) NSCLC patients who are medically inoperable.
Only a few retrospective series specifically looked at use of SBRT in T2 tumors. Surgical series have shown though that larger tumor sizes tend to have worse five year overall survival following lobectomy (12). In addition, larger tumor size have higher distant failure rates (12). Immerman et al found a 11% local failure rate and a 40% distant failure rate in a surgical series of tumors greater than 5 cm in size (13). Additional studies including pT2 and pT3N0 patients showed 5-year OS of only 40 to 50%(14–16).
Larger or T2 tumors are often included with smaller sized tumors in SBRT trials. However, the outcome of only T2 or larger tumors has not been extensively reported.
Only few single institution reports have shown that SBRT treatment of tumors greater than 5 cm is safe, with local control rates of greater than 70-90%, but significantly higher distant failure rates (17). Another retrospective multi-institutional study of SBRT for tumors greater than 5 cm showed two-year local control rates of greater than 70% (18). Given the limited data on the outcome of larger or T2 tumors using SBRT, we undertook a retrospective analysis of our T2N0 NSCLC lung cancer patients treated with SBRT to assess the failure patterns and survival outcome of these patients.
MATERIALS AND METHODS
Patients
Utilizing an Institutional Review Board approved study (University Hospitals ID: CHRV0081), we identified patients with clinically or pathologically staged T2N0 NSCLC tumors who had undergone SBRT at University Hospitals Cleveland Medical Center from 2008-2013. Tumors were classified using clinical and radiographic information using the 7th edition of the American Joint Committee on Cancer staging system.
Histologic information was available for tumors prior to beginning radiation treatment. All cases were presented in our multidisciplinary thoracic tumor board. Only patients with tumors greater than or equal to 3 cm in size were included in this analysis.
Relevant studies included computed tomography (CT) scans of the chest, [18F]-fluorodeoxyglucose positron emission tomography (FDG-PET/CT), and magnetic resonance imaging (MRI) of the brain. All patients were treated using the Cyberknife SBRT system. Patients had fiducial placement for tumor tracking seven to ten days prior to their treatment planning CT scan. Four dimensional CT scans were used to account for tumor motion during treatment planning. Direct tumor motion tracking was performed during treatment using the Cyberknife Synchrony system.
Treatment plans were reviewed to collect prescription dose, prescription isodose line, gross tumor volume (GTV), and planning target volume (PTV). The GTV was isotropically expanded by 5 to 7 millimeters to obtain PTVs. Prescription biological effective dose (BED) was calculated using a tumor alpha-beta ratio of 10 (BED10). Centroid BED10 was defined as the calculated BED of the maximum point dose of the plan using a tumor alpha-beta ratio of 10. Total target doses ranged from 50-60 Gy administered in 3-6 fractions.
Charts were retrospectively reviewed to record age, gender, smoking status, oxygen (O2) use, tumor location, and histology.
Follow-up and endpoints
Patients were followed every three to six months after treatment for the first two years, and annually thereafter. Serial CT scans and PET-CT scans were obtained as indicated. Clinical data from electronic health records, referring physicians, and general practitioners were also used evaluate outcomes. A local failure was defined as a recurrence within the defined PTV for radiation planning. Regional recurrence was defined as a recurrence in the hilar or mediastinal nodes, or in the ipsilateral lung. A distant failure was defined as recurrence in the contralateral lung, pleura, or outside of the thorax.
Local control (LC) was based on clinical examination and surveillance imaging. Pathologic diagnosis was used to confirm the presence of local or distant failure in most cases. Several patients had convincing CT and PET imaging data showing disease progression, and thus pathologic confirmation was not needed.
Overall survival (OS) was defined as the difference in time (months) from the date of diagnosis on imaging or pathology to date of death or date of last follow-up (which ever came sooner). If imaging or pathology was not available, then the date of initial consultation served as the date of diagnosis. Disease free survival (DFS) was defined as the date of diagnosis until the date of any recurrence including loco-regional, systemic, or death.
Statistical analysis
Statistical analysis was performed using Medcalc (Medcalc Software, USA), version 14.8. Univariable analysis (UVA) was performed using a linear regression model to assess factors predictive of OS and DFS. Kaplan Meier survival analysis was performed on endpoints of OS and DFS, and was compared by log rank analysis. P-values less than 0.05 were considered statistically significant.
RESULTS
Fifty-six patients were identified as having peripheral stage T2N0 tumors that were greater than 3 cm in size. The median tumor size was 3.5 cm (range 2.8-6.8 cm). The median follow-up was 19.3 months (range 1.2-74.1 months). The median OS was 19.9 months (range 1.3-74.1 months). Patient baseline demographics and clinical characteristics are summarized in Table 1. Men (55.3%) and women (44.7%) were equally represented, and most of our patients were former smokers (69.6%) who did not use oxygen at home (73.2%). Squamous cell carcinoma was the most common histologic diagnosis (50%), Thirty two percent of tumors were in the right upper lobe. Radiation prescription dose is summarized in Table 2 with the most common dose and fractionation pattern of 50 Gy in 5 fractions.
Table 1.
Demographics, tumor histology, anatomy, smoking status, and oxygen use of study patients
| Median Age | 74 (range 53-90) |
|---|---|
| Median Follow-up | 19.3 months |
| Sex | |
| Male | 31 (55.3%) |
| Female | 25 (44.7%) |
| Histology | |
| Adenocarcinoma | 21 (37.5%) |
| Squamous cell carcinoma | 28 (50%) |
| Other/NOS | 7 (12.5%) |
| Tumor Size | Median 3.5cm (range 3.0-6.8cm) |
| Tumor Location | |
| RUL | 18 (32.1%) |
| RML | 5 (8.9%) |
| RLL | 12 (21.4%) |
| LUL | 16 (28.6%) |
| LLL | 5 (8.9%) |
| Smoking Status | |
| Never | 2 (3.6%) |
| Former | 39 (69.6%) |
| Active | 15 (26.8%) |
| Baseline Oxygen Use | |
| Yes | 15 (26.8%) |
| No | 41 (73.2%) |
Table 2.
Summary of SBRT radiation doses and fractionation schedules
| Radiation Dose (Gy) | n (%) |
|---|---|
| 50 in 5 fractions | 31 (55.3) |
| 50 in 4 fractions | 12 (21.4) |
| 54 in 3 fractions | 9 (16.1) |
| 60 in 3 fractions | 3 (5.4) |
| 60 in 6 fractions | 1 (1.8) |
The one-year and two-year LC rate was 90.3% and 84.2%, respectively (Figure 1). The one, two and five-year DFS was 62.2%, 31.9%, and 15.3%, respectively (Figure 2). The one, two and five-year OS were 75.9%, 39.9% and 12.1%, respectively (Figure 3).
Figure 1.
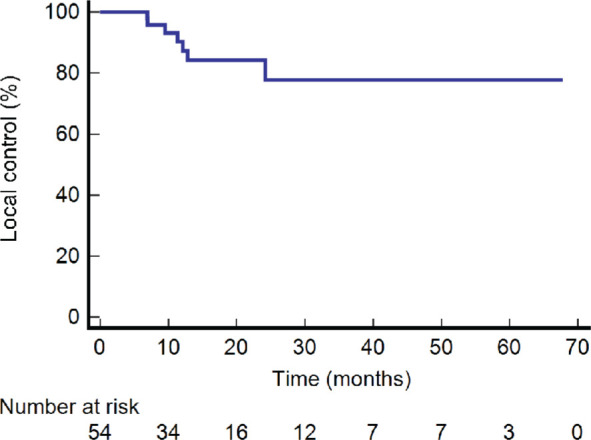
Kaplan-Meier graph of local control.
Figure 2.
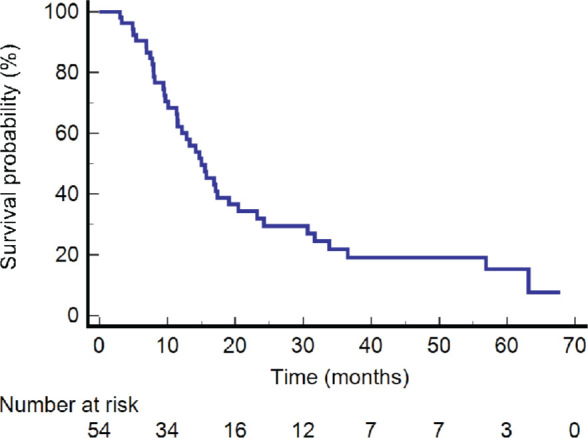
Kaplan-Meier graph of disease-free survival.
Figure 3.
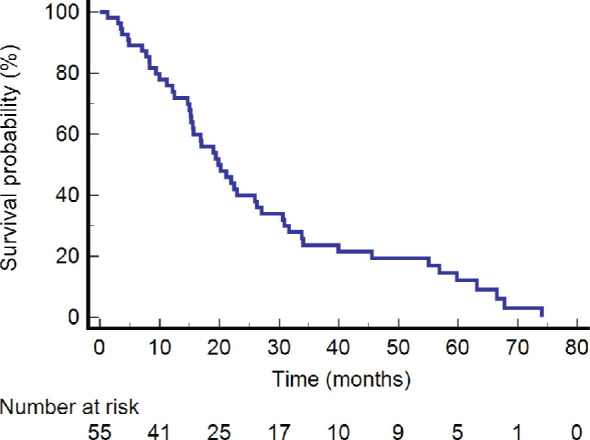
Kaplan-Meier graph of overall survival.
Patterns of failure are summarized in Table 3. We found isolated local failure rate of 1.8%, a loco-regional failure rate of 8.9%, and distant failure rate of 19.6%. Distant failure along with regional and loco-regional failure was observed in 30.3% of our patients.
Table 3.
Summary of sites of first failure
| Sites of Failure | n (%) |
|---|---|
| Local only | 1 (1.8) |
| Regional only | 7 (12.5) |
| Loco-regional only | 5 (8.9) |
| Regional and distant | 5 (8.9) |
| Loco-regional and distant | 1 (1.8) |
| Distant only | 11 (19.6) |
| No failure | 26 (46.4) |
Centroid BED10 greater than 150 Gy was associated with improved DFS (log-rank p=0.014) (Figure 4). Univariable logistic regression showed that BED10 greater than 150 Gy predicted improved survival (p = 0.0132).As a continuous variable, centroid BED10 (p=0.0036) as well as prescription BED10 (p=0.035) were also associated with improved OS. Adenocarcinoma was associated with inferior OS (p=0.026). There was no association seen between PTV size, smoking, or oxygen use, and OS. Multivariable analysis was limited due to sample size.
Figure 4.
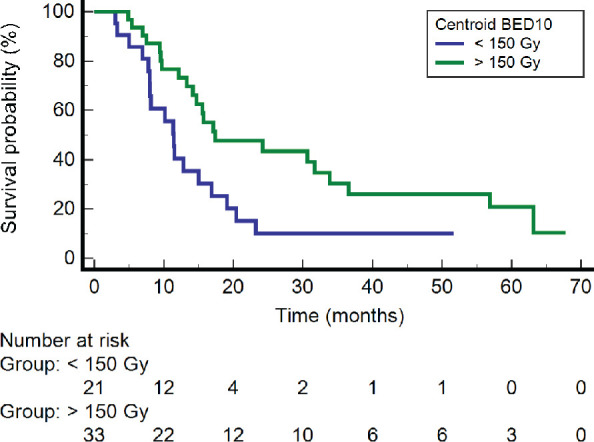
Kaplan-Meier graph of disease-free survival as a function of centroid BED10.
DISCUSSION
SBRT has become the standard of care for medically inoperable patients with early-stage NSCLC. While for smaller T1 tumors, the outcome of SBRT is excellent, for larger sized tumors, distant recurrence appears quite significant. In a single institution retrospective study, Woody et al reported local control rates of 91.2%. However, distant failure rates was significantly higher at 32.5% at 18 months for tumors greater than 5 cm treated with SBRT (17). Peterson et al found a local failure rate of only 4.8%, and a disproportionately high distant failure rate of 31% at a median follow-up of 16 months for tumors greater than 5 cm (19). They also noted an OS of 65% and 34% at 1 and 2 years (19). In a large multi-institutional study, Verma et al reported excellent LC rates of 95.7% at 1 year and relatively lower than expected LC of 73.2% at two years for larger tumors treated with SBRT(18). Overall survival was 76.2% and 46.4% at one and two years respectively(18). Distant failure rates again was higher at 33%, with a local failure rate of 26%, and local regional failure rate of 23% (18). Similarly, Tekatli et al observed local, regional, and distant control rates of 95.8%, 93.7%, and 83.6% respectiveley in tumors greater than 5 cm at 2 years (20). Jumeau et al found one-year and three-year survival rates of 88% and 70%, respectively, but excellent local control rates of 91% in patients with T2 disease (21). Twenty-one percent of their patients experienced a recurrence, and 84% of the relapses were nodal or distant (21). Our single institutional series showed similar findings for patients with T2 disease with tumors larger than 3 cm treated with SBRT. We noted high distant failure rates with an isolated distant failure rate of 19.6% and combined distant and loco-regional failure rate of 30.3%. All these studies have shown that the common failure patterns for SBRT for larger tumors are often isolated distant recurrence, which was seen in our data set (22).
Several studies have looked at factors predictive of local or distant failures, and increasing tumor size consistently was associated with regional and distant failure [24, 25]. Allibhai et al reported GTV size greater than 11.79 cubic cm was associated with poorer non-local recurrence free survival, disease free survival, and cause specific survival (23). Parker et al noted worse local control rates in tumors with diameter greater than 5 cm when compared to tumors with diameter less than 5 cm (79.8% vs 98.2%) (24). These studies and the current report support that SBRT alone for these larger tumors is associated with inferior outcomes because of distant and regional failures.
Many strategies were employed by various groups to try to improve outcomes in larger tumors, including radiation dose escalation. The Japanese Clinical Oncology Group study 0702 investigated dose escalation in T2N0 tumors with PTVs greater than 100 cubic centimeters (25). They found an OS of 83.3% and PFS of 76.2% at 3-years (25). This led them to recommend a dose of 50 Gy in 4 fractions for patients with T2N0 tumors (25). However, their findings are difficult to translate into standard practice due to poor accrual of only 13 patients (25). Miyakawa et al did not show any difference in OS, PFS, and local recurrence between radiation dose of 48 Gy, 50 Gy, and 52 Gy in patients with T1-T2a tumors (≤ 5 cm) (26). Mitsuyoshi et al observed local control rates of 95.7% and OS of 85.2% at 2 years in patients with T1-T2a (≤ 5 cm) tumors treated with 70 Gy in 4 fractions (27). Our study uniquely demonstrates that a centroid BED10 greater than 150 Gy led to significant improvement in disease free survival and overall survival. However, dose escalation strategies can have limitations due to concern for treatment related toxicities. Earlier reports by Fakiris et al and Timmerman et al showed significantly increased toxicity for larger tumors treated with SBRT with higher doses (28–30).
The use of adjuvant systemic chemotherapy in combination with SBRT has been explored to address distant failures in SBRT treatment of large tumors. Ernani et al found that addition of adjuvant chemotherapy improved OS in patients with T2bN0 and T3N0 disease in a NCDB analysis (31). Chen et al found that patients with T1-T3N0 tumors treated with SBRT and adjuvant cisplatin had better overall survival and lower relapse rate than patients treated with SBRT alone (32). Similarly, adjuvant chemotherapy was shown to be beneficial after surgical resection of NSCLC tumors greater than 4 cm in CALGB 9633 (33). While the current practice is to consider adjuvant systemic therapy for larger node negative tumors in patients who are able to tolerate systemic chemotherapy following surgery, it is not used following SBRT due to lack of evidence. Moreover, addition of chemotherapy may not be feasible due to poor performance status and presence of medical co-morbidities in this population. Therefore, future trials incorporating chemotherapy with SBRT for larger primary tumors may be unlikely. Immunotherapy is often better tolerated than standard of care chemotherapy, and provides improved survival in locally advanced NSCLC (34). Combining SBRT with immunotherapy is another promising treatment strategy to improve systemic control. The role of immunotherapy in combination with SBRT in medically inoperable patients is currently being evaluated in the ongoing PACIFIC-4/RTOG 3515 trial (NCT03833154) and in the SWOG/ NRG S1914 trial (NCT03811002). Both these studies will address whether the addition of Durvalumab or Atezolizumab is able to reduce the distal relapse rate in this patient population and improve overall survival.
CONCLUSIONS
Lung SBRT continues to provide adequate local control even for T2N0 tumors. Dose escalation has the potential to improve disease free and overall survival. However, recurrence at distant sites remains the predominant pattern of failure. Future trials are needed to reduce the distant failure rate by incorporating novel systemic agents in these vulnerable patient population who are otherwise not candidates for surgery and adjuvant chemotherapy.
ACKNOWLEDGMENTS
Funding: the study did not require funding.
Human subjects: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (University Hospitals IRB study number: CHRV0081), and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was not needed for this study due to its retrospective nature
Data sharing: De-identified individual data that supports the results will be shared upon reasonable request beginning 9 to 36 months following publication provided the investigators has approval from an Institutional Review Board (IRB), Independent Ethics Committee (IEC), or Research Ethics Board (REB), as applicable, and executes a data use/sharing agreement with University Hospitals Seidman Cancer Center.
Authors’ disclosure of potential conflicts of interest
Mitchell Machtay has received grant funding from Elekta and honoraria and additional support from Elekta and Varian that is unrelated to this work. Stephen J Shamp, Saad Sheikh, Tangel Chang, Nicholas Damico, Phillip Linden, Afshin Dowlati, and Tithi Biswas declare that they have no conflict of interest
Author contributions
Conception and design: Stephen Shamp, Saad Sheikh, Tangel Chang, Nicholas Damico, Mitchell Machtay, Tithi Biswas
Data collection: Stephen Shamp, Tangel Chang, Nicholas Damico,
Data analysis and interpretation: Stephen Shamp, Saad Sheikh, Tangel Chang, Phillip Linden, Afshin Dowlati, Mitchell Machtay, Tithi Biswas
Manuscript writing: Stephen Shamp, Saad Sheikh, Tithi Biswas
Final approval of manuscript: Stephen J Shamp Saad Sheikh, Tangel Chang, Nicholas Damico, Phillip Linden, Afshin Dowlati, Mitchell Machtay, Tithi Biswas
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018January;68(1):7-30. [DOI] [PubMed] [Google Scholar]
- 2.Dunlap NE, Larner JM, Read PW, Kozower BD, Lau CL, Sheng K, Jones DR. Size matters: A comparison of T1 and T2 peripheral non-small-cell lung cancers treated with stereotactic body radiation therapy (SBRT). J Thorac Cardiovasc Surg. 2010September;140(3):583-9. [DOI] [PubMed] [Google Scholar]
- 3.Naruke T, Goya T, Tsuchiya R, Suemasu K. Prognosis and survival in resected lung carcinoma based on the new international staging system. J Thorac Cardiovasc Surg. 1988;96(3):440-7. [PubMed] [Google Scholar]
- 4.Mountain CF. A new international staging system for lung cancer. Chest. 1986April;89(4 Suppl):225S-233S. [DOI] [PubMed] [Google Scholar]
- 5.Potters L, Kavanagh B, Galvin JM, Hevezi JM, Janjan NA, Larson DA, Mehta MP, Ryu S, Steinberg M, Timmerman R, Welsh JS, Rosenthal SA. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2010February;76(2):326-32. [DOI] [PubMed] [Google Scholar]
- 6.Timmerman R, Papiez L, McGarry R, Likes L, DesRosiers C, Frost S, Williams M. Extracranial Stereotactic Radioablation: Results of a Phase I Study in Medically Inoperable Stage I Non-small Cell Lung Cancer. Chest. 2003;124(5):1946-55. [DOI] [PubMed] [Google Scholar]
- 7.Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone D, Fowler J, Gore E, Choy H. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA - J Am Med Assoc. 2010March17;303(11):1070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timmerman RD, Hu C, Michalski J, Straube W, Galvin J, Johnstone D, Bradley J, Barriger R, Bezjak A, Videtic GM, Nedzi L, Werner-Wasik M, Chen Y, Komaki RU, Choy H. Long-term Results of RTOG 0236: A Phase II Trial of Stereotactic Body Radiation Therapy (SBRT) in the Treatment of Patients with Medically Inoperable Stage I Non-Small Cell Lung Cancer. Int J Radiat Oncol. 2014September;90(1):S30. [Google Scholar]
- 9.Bezjak A, Paulus R, Gaspar LE, Timmerman RD, Straube WL, Ryan WF, Garces YI, Pu AT, Singh AK, Videtic GM, McGarry RC, Iyengar P, Pantarotto JR, Urbanic JJ, Sun AY, Daly ME, Grills IS, Sperduto P, Normolle DP, Bradley JD, Choy H. Safety and Efficacy of a Five-Fraction Stereotactic Body Radiotherapy Schedule for Centrally Located Non-Small-Cell Lung Cancer: NRG Oncology/RTOG 0813 Trial.. J Clin Oncol Off J Am Soc Clin Oncol. 2019May;37(15):1316-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritz P, Kraus HJ, Blaschke T, Mühlnickel W, Strauch K, Engel-Riedel W, Chemaissani A, Stoelben E. Stereotactic, high single-dose irradiation of stage I non-small cell lung cancer (NSCLC) using four-dimensional CT scans for treatment planning. Lung Cancer. 2008May;60(2):193-9. [DOI] [PubMed] [Google Scholar]
- 11.Hobbs CJ, Ko SJ, Paryani NN, Accurso JM, Olivier KR, Garces YI, Park SS, Hallemeier CL, Schild SE, Vora SA, Ashman JB, Rule WG, Bowers JR, Heckman MG, Diehl NN, Miller RC. Stereotactic Body Radiotherapy for Medically Inoperable Stage I-II Non-Small Cell Lung Cancer: The Mayo Clinic Experience. Mayo Clin Proc Innov Qual Outcomes. 2018March;2(1):40-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eberhardt WEE, Mitchell A, Crowley J, Kondo H, Kim YT, Turrisi A, Goldstraw P, Rami-Porta R. The IASLC lung cancer staging project: Proposals for the revision of the M descriptors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol. 2015;10(11):1515-22. [DOI] [PubMed] [Google Scholar]
- 13.Immerman SC, Vanecko RM, Fry WA, Head LR, Shields TW. Site of recurrence in patients with stages I and II carcinoma of the lung resected for cure. Ann Thorac Surg. 1981July;32(1):23-7. [DOI] [PubMed] [Google Scholar]
- 14.Doddoli C, D’Journo B, Le Pimpec-Barthes F, Dujon A, Foucault C, Thomas P, Riquet M. Lung cancer invading the chest wall: a plea for en-bloc resection but the need for new treatment strategies. Ann Thorac Surg. 2005December;80(6):2032-40. [DOI] [PubMed] [Google Scholar]
- 15.Wisnivesky JP, Henschke C, McGinn T, Iannuzzi MC. Prognosis of Stage II non-small cell lung cancer according to tumor and nodal status at diagnosis. Lung Cancer. 2005August;49(2):181-6. [DOI] [PubMed] [Google Scholar]
- 16.Gould PM, Bonner JA, Sawyer TE, Deschamps C, Lange CM, Li H. Patterns of failure and overall survival in patients with completely resected T3 N0 M0 non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 1999August;45(1):91-5. [DOI] [PubMed] [Google Scholar]
- 17.Woody NM, Stephans KL, Marwaha G, Djemil T, Videtic GMM. Stereotactic body radiation therapy for non-small cell lung cancer tumors greater than 5 cm: Safety and efficacy. Int J Radiat Oncol Biol Phys. 2015June1;92(2):325-31. [DOI] [PubMed] [Google Scholar]
- 18.Verma V, Shostrom VK, Kumar SS, Zhen W, Hallemeier CL, Braunstein SE, Holland J, Harkenrider MM, S. Iskhanian A, Neboori HJ, Jabbour SK, Attia A, Lee P, Alite F, Walker JM, Stahl JM, Wang K, Bingham BS, Hadzitheodorou C, Decker RH, McGarry RC, Simone CB. Multi-institutional experience of stereotactic body radiotherapy for large (≥5 centimeters) non-small cell lung tumors. Cancer. 2017February15;123(4):688-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson J, Niles C, Patel A, Boujaoude Z, Abouzgheib W, Goldsmith B, Asbell S, Xu Q, Khrizman P, Kubicek GJ. Stereotactic Body Radiotherapy for Large (> 5 cm) Non-Small-Cell Lung Cancer. Clin Lung Cancer. 2017July1;18(4):396-400. [DOI] [PubMed] [Google Scholar]
- 20.Tekatli H, van ’t Hof S, Nossent EJ, Dahele M, Verbakel WFAR, Slotman BJ, Senan S. Use of Stereotactic Ablative Radiotherapy (SABR) in Non-Small Cell Lung Cancer Measuring More Than 5 cm.. J Thorac Oncol. 2017June1;12(6):974-82. [DOI] [PubMed] [Google Scholar]
- 21.Jumeau R, Bahig H, Filion É, Campeau M-P, Lambert L, Roberge D, Gorgos A-B, Vu T. Assessing the Need for Adjuvant Chemotherapy After Stereotactic Body Radiation Therapy in Early-stage Non-small Cell Lung Carcinoma. Cureus. 2016November;8(11):e901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senthi S, Lagerwaard FJ, Haasbeek CJA, Slotman BJ, Senan S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: A retrospective analysis. Lancet Oncol. 2012August;13(8):802-9. [DOI] [PubMed] [Google Scholar]
- 23.Allibhai Z, Taremi M, Bezjak A, Brade A, Hope AJ, Sun A, Cho BCJ. The impact of tumor size on outcomes after stereotactic body radiation therapy for medically inoperable early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013December1;87(5):1064-70. [DOI] [PubMed] [Google Scholar]
- 24.Parker SM, Siochi RA, Wen S, Mattes MD. Impact of Tumor Size on Local Control and Pneumonitis After Stereotactic Body Radiation Therapy for Lung Tumors. Pract Radiat Oncol. 2019January1;9(1):e90-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onimaru R, Onishi H, Shibata T, Hiraoka M, Ishikura S, Karasawa K, Matsuo Y, Kokubo M, Shioyama Y, Matsushita H, Ito Y, Shirato H. Phase I study of stereotactic body radiation therapy for peripheral T2N0M0 non-small cell lung cancer (JCOG0702): Results for the group with PTV≥100cc. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2017February;122(2):281-5. [DOI] [PubMed] [Google Scholar]
- 26.Miyakawa A, Shibamoto Y, Baba F, Manabe Y, Murai T, Sugie C, Yanagi T, Takaoka T. Stereotactic body radiotherapy for stage I non-small-cell lung cancer using higher doses for larger tumors: Results of the second study. Radiat Oncol. 2017September11;12(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitsuyoshi T, Matsuo Y, Shintani T, Iizuka Y, Ueki N, Nakamura M, Mizowaki T. Pilot Study of the Safety and Efficacy of Dose Escalation in Stereotactic Body Radiotherapy for Peripheral Lung Tumors. Clin Lung Cancer. 2018May1;19(3):e287-96. [DOI] [PubMed] [Google Scholar]
- 28.McGarry RC, Papiez L, Williams M, Whitford T, Timmerman RD. Stereotactic body radiation therapy of early-stage non-small-cell lung carcinoma: Phase I study. Int J Radiat Oncol Biol Phys. 2005November15;63(4):1010-5. [DOI] [PubMed] [Google Scholar]
- 29.Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, Ewing M, Abdulrahman R, DesRosiers C, Williams M, Fletcher J. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006October20;24(30):4833-9. [DOI] [PubMed] [Google Scholar]
- 30.Fakiris AJ, McGarry RC, Yiannoutsos CT, Papiez L, Williams M, Henderson MA, Timmerman R. Stereotactic Body Radiation Therapy for Early-Stage Non-Small-Cell Lung Carcinoma: Four-Year Results of a Prospective Phase II Study. Int J Radiat Oncol Biol Phys. 2009November1;75(3):677-82. [DOI] [PubMed] [Google Scholar]
- 31.Ernani V, Appiah AK, Marr A, Zhang C, Zhen W, Smith LM, Ganti AK. Adjuvant Systemic Therapy in Patients With Early-Stage NSCLC Treated With Stereotactic Body Radiation Therapy. J Thorac Oncol. 2019March1;14(3):475-81. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Guo W, Lu Y, Zou B. Dose-individualized stereotactic body radiotherapy for T1-3N0 non-small cell lung cancer: Long-term results and efficacy of adjuvant chemotherapy. Radiother Oncol. 2008September;88(3):351-8. [DOI] [PubMed] [Google Scholar]
- 33.Strauss GM, Herndon JE, Maddaus MA, Johnstone DW, Johnson EA, Harpole DH, Gillenwater HH, Watson DM, Sugarbaker DJ, Schilsky RL, Vokes EE, Green MR. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the cancer and leukemia group B, radiation therapy oncology group, and North Central cancer treatment group study groups. J Clin Oncol. 2008November1;26(31):5043-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, De Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, De Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017November16;377(20):1919-29. [DOI] [PubMed] [Google Scholar]


