Abstract
COVID-19 lockdowns restricted physical activity levels for individuals in many countries. In particular, older adults experienced limited access to their usual activities, including physical exercise programs. How such restrictions and interruptions in physical exercise programs might impact the physical and mental health of older adults has not yet been studied. We sought to analyse changes in the physical and mental health of older adults enrolled in a group-based multicomponent physical exercise (MPE) program that was interrupted due to the COVID-19 pandemic. We followed 17 participants of this program from October 2018 to October 2020, including the interruption of the program during the pandemic. The MPE program included strength, balance, and stretching exercises. We compared anthropometric and cardiovascular parameters, physical fitness, frailty, quality of life, and psychoaffective status of participants before and during the COVID-19 pandemic. Most parameters followed the same pattern, improving after 8 months of the first MPE season (Oct. 2018–Jun. 2019), worsening after 4 months of summer rest, improving from October 2019 to January 2020 in the second MPE season (Oct. 2019–Jan. 2020), and severely worsening after 7 months of program interruption. We show that an MPE program has clear benefits to the physical and psychoaffective health of older adults, and interruption of these programs could adversely impact participants. These results highlight the need to maintain physical exercise programs or facilitate engagement in physical activity and reduce sedentary behaviour in older adults, particularly in situations such as the COVID-19 pandemic.
Abbreviations: BMI, body mass index; IQR, interquartile range; MPE, multicomponent physical exercise; RHR, resting heart rate; RM, repetition maximum; SD, standard deviation; SFT, senior fitness test; SPPB, short physical performance battery
Keywords: Physical activity, Mental health, Physical health, Quality of life, Aging, COVID-19 pandemic
1. Introduction
Physical activity and its structured modality, physical exercise, are important factors in healthy aging (Sasso et al., 2015; Buchner, 2009; Morley et al., 2014; Miljkovic et al., 2015; Concha-Cisternas et al., 2017). Physical activity supports a longer life in good health (May et al., 2015), and physical exercise reduces the effects of aging on functional fitness (Toraman et al., 2004). Among other factors, physical exercise maintains muscle mass levels and cardiovascular health (Concha-Cisternas et al., 2017). As these factors usually limit functional fitness, physical exercise is an optimal and important preventive strategy (Hurst et al., 2019).
A key component of declining health with aging is frailty, a geriatric syndrome characterized by decreasing functional reserves and increasing vulnerability to declining health, leading to dependence on caregivers (Fried et al., 2001). References to frailty usually focus on physical manifestations (Robertson et al., 2013). However, affective psychological aspects, such as anxiety and depression, subjective well-being, and quality of life, are also closely related (Bernal-Lopez et al., 2012; St John et al., 2013; Gale et al., 2014; Mhaolain et al., 2012; Pahor et al., 2014). Importantly, physical exercise improves certain domains of frailty and psychoaffective functions in older adults (Zhang et al., 2020). Thus, physical exercise programs can reverse frailty and improve cognition and emotional and social networking in controlled populations of community-dwelling frail older adults (Tarazona-Santabalbina et al., 2016).
Interventions involving group-based and supervised multicomponent exercise programs (strength, balance, and flexibility) are more effective in tackling frailty in older people than individual programs undertaken at home (Cadore et al., 2013; Kyrdalen et al., 2014). Hence, supervised physical exercise appears to be the best method to increase walking speed, balance, and strength and reduce the risk of falls in frail older adults (Lacroix et al., 2016). In addition, poorer adherence to exercise has been observed in home-based programs than in group programs (Simek et al., 2012).
COVID-19 is a severe acute respiratory syndrome caused by the SARS-CoV-2 virus that emerged in late 2019. Individual countries faced challenges with how to handle the crisis. In March 2020, to stop the spread of SARS-CoV-2, the Government of Spain declared a national state of alarm, establishing a mandatory home “lockdown” from 14 March to 26 April (Alonso-Martínez et al., 2021). During and after this period, many older adults faced limitations in the activities that they could perform because they were considered the group most vulnerable to developing severe COVID-19. As a preventive measure, many community organizations were closed, and visits with family members were limited for older individuals (Sepúlveda-Loyola et al., 2020).
Recent reviews reported that the pandemic caused a radical change in the lifestyles of older people, reducing their levels of physical activity and social interaction (Lippi et al., 2020; Roschel et al., 2020). Such changes have potential to produce negative effects on physical and mental health among older people, especially in those with chronic diseases, disabilities, and geriatric syndromes (Lippi et al., 2020). Restrictions in social interactions and fear of the pandemic could cause higher levels of anxiety and depression and a sense of loneliness (Hwang et al., 2020; Sepúlveda-Loyola et al., 2020). Additionally, limiting physical activity accelerates physical deterioration and may be associated with the development of comorbidities (Roschel et al., 2020). Of note, lower muscle function is a strong, independent risk factor for all-cause mortality in older people (Kirwan et al., 2020).
Effects of the home lockdown on physical and mental health in older adults have been mainly assessed using online questionnaires or making forecasts (Hwang et al., 2020; Kirwan et al., 2020; Lippi et al., 2020; Roschel et al., 2020; Sepúlveda-Loyola et al., 2020). However, to our knowledge, effects of interrupted physical exercise programs during the COVID-19 pandemic on physical and mental health in older adults have not yet been studied through objective physical tests or face-to-face assessments of mental health. The aim of this study was to analyse changes in the physical and mental health of older adults enrolled in a group-based multicomponent physical exercise (MPE) program following its interruption due to the COVID-19 pandemic.
2. Methods
2.1. Study design and participants
We conducted a quasi-experimental trial on a physical exercise program that started in October 2018 and was interrupted by the COVID-19 pandemic in March 2020. Participants included community-dwelling individuals ≥55 years old who were enrolled in MPE sessions offered by Fundación Siel Bleu (https://sielbleu.es) in the Retirement Home of Beasain (Gipuzkoa, Spain) and who were capable of standing up and walking independently for at least ten meters. Participants were not eligible for the study if they had a diagnosis of dementia or any other condition such that participation would not be in their best interests. In addition, participants who did not sign the informed consent were excluded. The trial was approved by the Committee on Ethics in Research at the University of the Basque Country, UPV/EHU (Humans Committee Code M10/2017/189). Because of the COVID-19 pandemic and lockdown, the MPE program was suspended, and a relevant modification of the project was approved by the Committee on Ethics in Research (M10/2017/189MR1) to evaluate the participants 7 months after the program was interrupted. Written informed consent was provided by each participant.
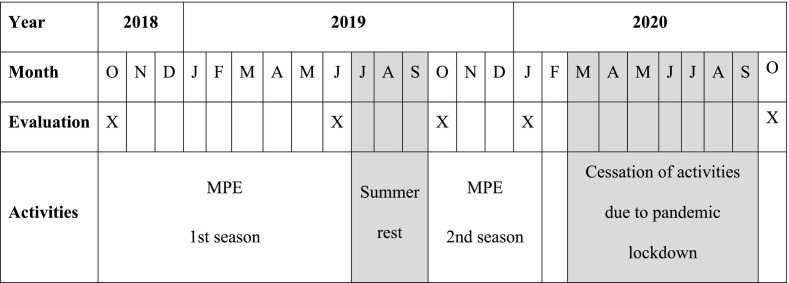
The program consisted of a 1-h supervised group session twice a week, separated by at least 2 days. In the first season, the program occurred without interruption for 8 months (between October 2018 and June 2019). However, in the second season, the program started in October, was interrupted in March due to the COVID-19 pandemic, and did not restart (Table 1 ).
Table 1.
Chronogram with relevant study dates. The year and month of the five performed evaluations are shown as well as the dates when each season of the multicomponent physical exercise (MPE) program occurred. Months without activities due to summer rest or cessation of activities due to the pandemic lockdown are noted in grey.
2.2. Physical exercise program
The MPE program included strength, balance, and stretching exercises. Each session began with 15 min of warm-up by performing range-of-motion exercises. Strength training was individually adapted to each participant and focused on the main muscles of the upper and lower extremities. The Brzycki (Brzycki, 1993) equation was applied to estimate one repetition maximum (1-RM) and adapt adequate load progression of arm-curl exercise for every participant at baseline and every 2 months. The intensity increased 10% every 2 months; therefore, it ranged from 40% to 70% 1-RM across the 8 months of the program. Chair stand, knee flexion, knee extension, hip abduction, and hip adduction exercises were performed without external loads, and the intensity was tailored to the capabilities of each participant by adjusting the number of repetitions and velocity. Balance training consisted of proprioception, agility, and weight-transfer exercises. These exercises were individually adapted and progressed in difficulty, starting with the highest arm support (with two arms at first, then with one hand, and finally no hands if possible), decreasing the base of support, and increasing the complexity of movements to challenge participants' balance as they progressed. In addition, some static exercises were increased in difficulty by sensory reductions (closing eyes). Exercises on stable and unstable surfaces were combined to increase difficulty. A subjective scale (0−10) was used to calculate the intensity and adapt adequate load progression of exercises for every participant at baseline and every 2 months. If participants answered 6 points or fewer, the intensity of the exercises was increased (velocity, number of repetitions, and difficulty of balance exercises); if they answered 7 points or greater, the exercise was not modified because that was considered to be the appropriate intensity at that time. Sessions ended with 5–10 min of stretching exercises. All sessions were conducted by a professional instructor with a degree in physical activity and sports sciences and training in adapted physical activity for older adults. Attendance was determined by participants' presence at physical exercise sessions.
2.3. Physical and psychoaffective evaluation calendar
All enrolled participants were evaluated five times: at the beginning of the first MPE season (October 2018), at the end of the first MPE season (June 2019), after 4 months of summer rest (October 2019), 2 months before the second MPE season was interrupted (January 2020), and 7 months after program interruption because of the COVID-19 pandemic (October 2020). All measurements were collected by the same investigator in the same place where the sessions occurred.
2.4. Anthropometric and cardiovascular parameters
Anthropometric data included body mass index (BMI) and waist–hip ratio. BMI was calculated based on height and mass, and the waist–hip ratio was based on waist and hip circumferences. Body mass was measured with a Beurer (Beurer GmbH, Ulm, Germany) digital scale to the nearest 0.1 kg. Height and waist and hip circumference were measured with non-elastic anthropometric tape to the nearest 0.1 cm. All anthropometric measurements were taken following “The International Society for the Advancement of Kinanthropometry” protocol (Marfell-Jones et al., 2012). Resting heart rate and systolic and diastolic blood pressures were measured with an Omron digital tensiometer.
2.5. Physical fitness
Physical fitness examinations included a senior fitness test (SFT) (Rikli and Jones, 2001), short physical performance battery (SPPB) (Guralnik et al., 2000), handgrip strength test (Jamar dynamometer) (Fess, 1992), and static balance measured with the Berg scale (Berg et al., 1992) (Table 2 ).
Table 2.
Physical fitness tests.
| Test (Reference) |
Functions/parameters | Description |
|---|---|---|
| Senior fitness test (Rikli and Jones, 2001) |
Upper and lower extremity strength and flexibility, static and dynamic balance, and aerobic capacity | Chair stands in 30 s; 2-min step test; arm-curl test (30 s); chair sit-and-reach; back scratch; 8-foot up-and-go test |
| Short physical performance battery (Guralnik et al., 2000) |
Lower extremity function: static balance, gait speed, and getting in and out of a chair | Side-by-side, semi-tandem, and tandem stands (10 s); 4-m walk test at comfortable speed, and 5 quick sit-to-stands from a chair without upper extremity assistance |
| Handgrip strength test (Jamar dynamometer) (Fess, 1992) |
Hand grip strength | Squeeze the dynamometer with maximum isometric effort for about 5 s |
| Berg balance test (Berg et al., 1992) |
Postural stability | Performance of 14 functional tasks |
2.6. Frailty
Frailty was measured with the Tilburg Frailty Indicator (Gobbens et al., 2010), which is divided into two sections: determinants of frailty and components of frailty. Determinants of frailty consist of questions about age, sex, educational level, presence of chronic diseases, and satisfaction with their living conditions, while components of frailty consist of 15 items split into three components: physical (8 items), psychological (4 items), and social (3 items). Total scores range from 0 to 15 points, where an individual is considered frail if they score at least 5 points.
2.7. Quality of life and psychoaffective assessment
The quality of life and psychoaffective assessment examination included the EQ-5D Questionnaire (Herdman et al., 2001), the Goldberg Anxiety and Depression Scale (Goldberg et al., 1988), and the UCLA Loneliness Scale (Velarde-Mayol et al., 2015) (Table 3 ).
Table 3.
Quality of life and psychoaffective assessment tests.
| Test (Reference) |
Functions/parameters | Description |
|---|---|---|
| EQ-5D Questionnaire (Herdman et al., 2001) |
Health-related quality of life | Self-rated quality of life related to health; included dimensions are mobility, self-care, usual activities, pain/discomfort, and anxiety/depression; its total score ranges from 5 to 15, with a higher score corresponding to a lower degree of quality of life |
| Goldberg Anxiety and Depression Scale (Goldberg et al., 1988) |
Affective state | Includes nine depression and nine anxiety items from the past month |
| UCLA Loneliness Scale (Velarde-Mayol et al., 2015) |
Loneliness state | Includes 11 loneliness items |
2.8. Statistical analyses
The IBM SPSS Statistics 21 statistical software package (SPSS, Inc., Chicago, IL) was used to analyse the data. Data normality was evaluated using the Shapiro–Wilk test. Continuous variables are expressed as mean ± standard deviation (SD) when normally distributed and as median with interquartile range (IQR) when not. Intervention-related effects were assessed using either analysis of variance (ANOVA) for repeated measures or a Friedman test according to the type and distribution of the data. Pairwise differences were evaluated by a Bonferroni test or Wilcoxon test. The significance level for all tests was set at P < 0.05.
3. Results
3.1. Study participants
Of the 30 participants who began the study, 17 completed it. Baseline characteristics were similar between participants who completed the study and those who did not, except on the 2-min step test of the SFT (Table 4 ). Average physical exercise session attendance rates were 75.95 ± 11.04% for participants who completed the study and 49.41 ± 22.09% for those who dropped out. Reasons for leaving the study are described in Table 5 . No adverse events associated with the physical exercise program were observed.
Table 4.
Baseline descriptive characteristics of participants who completed the study and participants who dropped outa.
| Completed the study |
P | ||
|---|---|---|---|
| Yes (n = 17) |
No (n = 13) |
||
| Age (y) | 80.48 ± 4.64 | 80.6 ± 9.40 | 0.963 |
| Sex, n (%) | |||
| Female | 15 (88.2) | 11 (84.6) | 0.782 |
| Male | 2 (11.8) | 2 (15.4) | |
| Anthropometry | |||
| BMI (kg/m2) | 27.67 ± 3.23 | 27.28 ± 4.33 | 0.780 |
| WHR | 0.91 ± 0.05 | 0.90 ± 0.05 | 0.518 |
| Cardiovascular parameters | |||
| RHR | 70.18 ± 9.17 | 67.62 ± 10.43 | 0.481 |
| SBP | 149.59 ± 23.18 | 136.38 ± 20.79 | 0.118 |
| DBP | 71.94 ± 9.49 | 67.77 ± 10.89 | 0.272 |
| Physical fitness | |||
| SFT | |||
| Chair stands (reps. in 30 s) | 12.53 ± 2.45 | 12.31 ± 2.59 | 0.813 |
| Arm-curl test (reps. in 30 s) | 12.94 ± 3.45 | 13.85 ± 2.38 | 0.426 |
| 2-Min step test (reps. in 2 min) | 76.35 ± 16.65 | 64.15 ± 11.58 | 0.032 |
| CSR (cm) | −15.06 ± 6.30 | −15.77 ± 6.64 | 0.767 |
| Back scratch test (cm) | −15.18 ± 9.25 | −14.23 ± 12.04 | 0.809 |
| FUG (s) | 8.67 ± 0.46 | 8.90 ± 2.32 | 0.766 |
| SPPB | |||
| Total (0–12 points) | 9.71 ± 1.65 | 8.85 ± 2.58 | 0.275 |
| Static balance (0–4 points) | 2.00 (2.00) | 2.00 (2.00) | 0.440 |
| Gait speed (s) | 4.59 ± 0.67 | 4.77 ± 0.93 | 0.557 |
| CST (s) | 11.60 ± 1.96 | 12.50 ± 2.80 | 0.310 |
| Hand grip (kg) | 20.53 ± 4.30 | 23.54 ± 7.88 | 0.191 |
| Berg balance test (0–56 points) | 44.29 ± 6.47 | 44.38 ± 8.69 | 0.974 |
| Frailty, quality of life, and psychoaffective parameters | |||
| TFI (0–15 points) | 7.65 ± 2.03 | 6.77 ± 2.59 | 0.306 |
| EQ-5D-3L (5–15 points) | 8.29 ± 1.49 | 8.31 ± 1.75 | 0.982 |
| AGS (0–9 points) | 2.94 ± 1.85 | 1.69 ± 1.25 | 0.046 |
| DGS (0–9 points) | 2.00 (4.00) | 0.00 (2.50) | 0.178 |
| UCLA (0–11 points) | 2.82 ± 2.30 | 2.92 ± 2.69 | 0.914 |
Abbreviations: BMI = body mass index; WHR = waist–hip ratio; RHR = resting heart rate; SBP = systolic blood pressure; DBP = diastolic blood pressure; SFT = senior fitness test; CSR = chair sit and reach test; FUG = foot up-and-go test; SPPB = short physical performance battery; CST = chair-stand test; TFI = Tilburg Frailty Indicator; EQ-5D-3L = EQ-5D-3L Questionnaire; AGS = Goldberg Anxiety Scale; DGS = Goldberg Depression Scale; UCLA = UCLA Loneliness Scale; reps, repetitions.
Mean ± standard deviation values are presented for parametric data, while median (interquartile range) values are presented for non-parametric data.
Table 5.
Reasons for leaving the study.
| n (%) | |
|---|---|
| Death | 3 (23.08) |
| Evolution of chronic disease | 7 (53.85) |
| Admission to residence or day centre | 2 (15.38) |
| Other reasons | 1 (7.69) |
3.2. Anthropometry and cardiovascular parameters
During the study, all anthropometry and cardiovascular parameters except waist–hip ratio followed similar patterns (Table 6 ). BMI decreased significantly (P < 0.05 to P < 0.001) after the first MPE season, increased significantly (P < 0.05 to P < 0.001) after 4 months of summer rest, and did not change significantly after the second MPE season or after 7 months of program interruption.
Table 6.
Anthropometry and cardiovascular parameters of participants who completed the study (n = 17)a.
| 1st MPE season |
2nd MPE season |
COVID-19 |
ANOVA P value |
|||
|---|---|---|---|---|---|---|
| Beginning (October 2018) |
End (June 2019) |
Beginning (October 2019) |
Pre-interruption (January 2020) | Follow-up (October 2020) |
||
| BMI (kg/m2) | 27.67 ± 3.23## | 27.05 ± 3.31$$$ | 27.67 ± 3.47 | 27.67 ± 3.43 | 27.81 ± 3.87 | <0.001 |
| WHR | 0.91 ± 0.05 | 0.91 ± 0.06 | 0.91 ± 0.06 | 0.90 ± 0.05 | 0.90 ± 0.05 | 0.336 |
| RHR | 70.18 ± 9.17 | 68.06 ± 6.48$ | 71.82 ± 5.33&&& | 64.35 ± 3.22^^^ | 79.12 ± 8.16€€ | <0.001 |
| SBP | 149.59 ± 23.18 | 144.94 ± 16.43$$ | 151.12 ± 13.59&&& | 142.82 ± 8.73^ | 154.88 ± 16.66 | <0.001 |
| DBP | 71.94 ± 9.49 | 70.00 ± 6.96 | 72.29 ± 6.28&& | 67.88 ± 3.76^ | 76.00 ± 8.14€ | <0.001 |
Abbreviations: MPE = multicomponent physical exercise; BMI = body mass index; WHR = waist–hip ratio; RHR = resting heart rate; SBP = systolic blood pressure; DBP = diastolic blood pressure.
October 2018 vs June 2019: #P < 0.05, ##P < 0.01, ###P < 0.001.
June 2019 vs October 2019: $P < 0.05, $$P < 0.01, $$$P < 0.001.
October 2019 vs January 2020: &P < 0.05, &&P < 0.01, &&&P < 0.001.
January 2020 vs October 2020: ^P < 0.05, ^^P < 0.01 ^^^P < 0.001.
October 2018 vs October 2020: €P < 0.05, €€P < 0.01 €€€P < 0.001.
Data are represented as mean ± standard deviation.
Both resting heart rate (RHR) and systolic blood pressure demonstrated nonsignificant reductions during the first MPE season and increased significantly (P < 0.05 to P < 0.001) during the summer rest. RHR, systolic blood pressure, and diastolic blood pressure decreased significantly (P < 0.05 to P < 0.001) after the second MPE season. By contrast, these parameters significantly increased (P < 0.05 to P < 0.001) during program interruption caused by the COVID-19 pandemic. Resting heart rate and diastolic blood pressure also increased significantly between the beginning of the study and the two-year follow up (P < 0.05 to P < 0.001).
3.3. Physical fitness
All physical fitness parameters except gait speed of SPPB followed the same pattern (Table 7 ), improving after the first MPE season, worsening after 4 months of summer rest, improving after the second MPE season, and severely worsening after 7 months of program interruption by the COVID-19 pandemic.
Table 7.
Physical fitness of the participants who completed the study (n = 17)a.
| 1st MPE season |
2nd MPE season |
COVID-19 |
ANOVA P value |
|||
|---|---|---|---|---|---|---|
| Beginning (October 2018) |
End (June 2019) |
Beginning (October 2019) |
Pre-interruption (January 2020) |
Follow-up (October 2020) |
||
| SFT | ||||||
| Chair stands (reps. in 30s) | 12.53 ± 2.45### | 15.06 ± 2.08$$ | 12.76 ± 2.46 | 13.71 ± 2.31^^^ | 10.06 ± 2.19€€ | <0.001 |
| Arm-curl test (reps. in 30 s) | 12.94 ± 3.45### | 17.76 ± 2.19$$ | 15.82 ± 2.72&& | 18.18 ± 2.30^^^ | 12.35 ± 3.48 | <0.001 |
| 2-Min step test (reps. in 2 min) | 76.35 ± 16.65 | 81.76 ± 13.58$$$ | 71.06 ± 10.56 | 74.35 ± 12.77^^^ | 57.65 ± 11.94€€€ | <0.001 |
| CSR (cm) | −15.06 ± 6.30## | −12.06 ± 4.74 | −14.94 ± 5.95 | −12.94 ± 6.12^^^ | −23.00 ± 6.93€€€ | <0.001 |
| Back scratch test (cm) | −15.18 ± 9.25 | −11.41 ± 7.78 | −14.18 ± 9.81 | −13.59 ± 9.47^^^ | −23.76 ± 11.31€€€ | <0.001 |
| FUG (s) | 8.67 ± 0.46## | 8.14 ± 0.44$$ | 10.16 ± 0.66 | 9.44 ± 0.49^^ | 13.16 ± 0.90€€€ | <0.001 |
| SPPB | ||||||
| Total (0–12 points) | 9.71 ± 1.65## | 11.41 ± 0.80$$$ | 9.24 ± 1.95 | 9.94 ± 1.52^^^ | 5.76 ± 1.99€€€ | <0.001 |
| Static balance (0–4 points) | 2.00 (2.00) ## | 4.00 (1.00) $$$ | 2.00 (2.00) & | 3.00 (0.00) ^^^ | 1.00 (1.00)€€€ | <0.001 |
| Gait speed (s) | 4.59 ± 0.67### | 4.01 ± 0.55 | 4.40 ± 0.73 | 4.41 ± 0.60^^^ | 6.16 ± 1.00€€€ | <0.001 |
| CST (s) | 11.60 ± 1.96 | 10.18 ± 1.44$ | 11.68 ± 1.96 | 11.44 ± 1.80^^^ | 14.93 ± 3.00€€€ | <0.001 |
| Hand grip (kg) | 20.53 ± 4.30# | 21.82 ± 4.32$ | 19.18 ± 4.85 | 20.06 ± 3.44^^^ | 16.00 ± 2.83€€€ | <0.001 |
| Berg balance test (0–56 points) | 44.29 ± 6.47### | 50.12 ± 3.79$$$ | 43.76 ± 4.89 | 44.94 ± 4.42^^^ | 33.47 ± 5.30€€€ | <0.001 |
Abbreviations: MPE = multicomponent physical exercise; SFT = senior fitness test; CSR = chair sit-and-reach test; FUG = foot up-and-go test; SPPB = short physical performance battery; CST = chair-stand test; reps, repetitions.
October 2018 vs June 2019: #P < 0.05, ##P < 0.01, ###P < 0.001.
June 2019 vs October 2019: $P < 0.05, $$P < 0.01, $$$P < 0.001.
October 2019 vs January 2020: &P < 0.05, &&P < 0.01, &&&P < 0.001.
January 2020 vs October 2020: ^P < 0.05, ^^P < 0.01 ^^^P < 0.001.
October 2018 vs October 2020: €P < 0.05, €€P < 0.01 €€€P < 0.001.
Mean ± standard deviation values are presented for parametric data, while median (interquartile range) values are presented for non-parametric data.
In the arm-curl test of the SFT and in the balance test of the SPPB, all changes were statistically significant (P < 0.05 to P < 0.001). Changes in chair stands of the SFT, total score in the SPPB, and in handgrip and Berg balance tests were significant (P < 0.05 to P < 0.001) after the first MPE season, after 4 months of summer rest, and after 7 months of program interruption. Changes in the chair-stand test of the SPPB were significant (P < 0.05 to P < 0.001) after 4 months of summer rest and after 7 months of program interruption.
Gait speed of the SPPB also improved after the first MPE season and severely worsened after 7 months of program interruption (P < 0.05 to P < 0.001). Although gait speed demonstrated similar changes to the other physical fitness parameters after 4 months of summer rest, these changes were not significant. Gait speed remained stable after the second MPE season.
All physical parameters except the arm-curl test of the SFT worsened significantly (P < 0.05 to P < 0.001) from the beginning of the study to the end of the follow-up.
3.4. Frailty, quality of life, and psychoaffectivity
All parameters of frailty, quality of life, and psychoaffective status except the Goldberg Depression Scale demonstrated similar statistically significant changes (P < 0.05 to P < 0.001) (Table 8 ), improving after the first MPE season, worsening after 4 months of summer rest, improving after the second MPE season, and severely worsening after 7 months of program interruption.
Table 8.
Frailty, quality of life, and psychoaffective parameters of participants who completed the study (n = 17)a.
| 1st MPE season |
2nd MPE season |
COVID-19 |
ANOVA P value |
|||
|---|---|---|---|---|---|---|
| Beginning (October 2018) |
End (June 2019) |
Beginning (October 2019) |
End (January 2020) |
Follow-up (October 2020) |
||
| TFI (0–15 points) | 7.65 ± 2.03### | 2.06 ± 1.03$$$ | 4.41 ± 2.15&&& | 1.06 ± 0.90^^^ | 9.29 ± 1.96€€ | <0.0001 |
| EQ-5D-3L (5–15 points) | 8.29 ± 1.49### | 5.12 ± 0.33$ | 5.94 ± 0.90&& | 5.06 ± 0.24^^^ | 9.47 ± 1.46 | <0.0001 |
| AGS (0–9 points) | 2.94 ± 1.85### | 0.35 ± 0.86 $ | 1.24 ± 1.64& | 0.06 ± 0.24^^^ | 3.41 ± 2.21 | <0.0001 |
| DGS (0–9 points) | 2.00 (4.00) ## | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) ^^ | 4.00 (5.00) | <0.0001 |
| UCLA (0–11 points) | 2.82 ± 2.30## | 0.94 ± 1.09$ | 2.00 ± 2.03& | 0.47 ± 0.62^^^ | 5.82 ± 2.19€€€ | <0.0001 |
Abbreviations: MPE = multicomponent physical exercise; TFI = Tilburg Frailty Indicator; EQ-5D-3L = EQ-5D-3L Questionnaire; AGS = Goldberg Anxiety Scale; DGS = Goldberg Depression Scale; UCLA = UCLA Loneliness Scale.
October 2018 vs June 2019: #P < 0.05, ##P < 0.01, ###P < 0.001.
June 2019 vs October 2019: $P < 0.05, $$P < 0.01, $$$P < 0.001.
October 2019 vs January 2020: &P < 0.05, &&P < 0.01, &&&P < 0.001.
January 2020 vs October 2020: ^P < 0.05, ^^P < 0.01 ^^^P < 0.001.
October 2018 vs October 2020: €P < 0.05, €€P < 0.01 €€€P < 0.001.
Mean ± standard deviation values are presented for parametric data, while median (interquartile range) values are presented for non-parametric data.
However, the Goldberg Depression Scale demonstrated statistically significant improvements (P < 0.05 to P < 0.001) after the first MPE season, when all participants obtained zero points. This assessment remained stable until after the second MPE season; however, after 7 months of program interruption, the Goldberg Depression Scale worsened significantly (P < 0.05 to P < 0.001). The Tilburg frailty indicator and UCLA loneliness scale worsened significantly from the beginning of the study to the end of the follow-up (P < 0.05 to P < 0.001).
4. Discussion
Here, we report anthropometric and cardiovascular parameters, physical fitness, frailty, quality of life, and psychoaffective status of older adults who participated in an MPE program before and during the COVID-19 pandemic. During the study, most parameters followed the same pattern and improved after 8 months of the first MPE season (October 2018–June 2019), worsened after 4 months of summer rest (June 2019–October 2019), improved after 4 months of the second MPE season (October 2019–January 2020), and severely worsened after 7 months of interruption caused by the COVID-19 pandemic (January 2020–October 2020).
Our results agree with other studies that demonstrated that MPE programs are effective in reducing BMI (Concha-Cisternas et al., 2017) and blood pressure (Buchner, 2009), improving physical fitness (Henwood and Taaffe, 2008; Toraman et al., 2004; Hurst et al., 2019), reducing frailty (Bernal-Lopez et al., 2012; Gale et al., 2014; Mhaolain et al., 2012; Tarazona-Santabalbina et al., 2016), improving quality of life (Pahor et al., 2014; Rizzoli et al., 2013), and reducing anxiety (Bernal-Lopez et al., 2012), depression (St John et al., 2013), and loneliness in older adults (Tarazona-Santabalbina et al., 2016). We also observed differences between the effects of the first and second seasons. Upon first season completion, we found significant improvements in almost all parameters analysed. However, in the second season, which was interrupted by the COVID-19 pandemic, fewer parameters changed significantly. This could be due to the shorter time between the beginning and the assessment in this season. In this regard, a study by Theou et al. (2011) reported that MPE programs of long duration (≥5 months) generally have superior outcomes to shorter ones.
We also observed significant worsening of the analysed parameters after the end of the first season of the program due to the summer rest, and all parameters of physical fitness analysed were reversed during this period, returning to values similar to the beginning of the season. Other studies reported maintenance or slight decreases in muscular strength following 12–14 weeks of detraining in older adults (Padilha et al., 2015; Yasuda et al., 2015; Nascimineto et al., 2014; Correa et al., 2013). One possible explanation for this discrepancy might be that the individuals who participated in our program were on average more than 10 years older than the individuals who participated in the prior studies. In support of this, recent studies proposed that older individuals might require a higher minimum stimulus to maintain physical performance (Spiering et al., 2021).
Fewer studies have examined the effects of detraining on the other parameters herein analysed. Nascimineto et al. (2014) reported that reductions in blood pressure after participating in a physical exercise program were maintained 12 weeks after program completion. By contrast, other studies showed that reductions in blood pressure caused by physical exercise were reversed after program cessation (Moker et al., 2014). This discrepancy may be explained by interindividual variability in the effects of training and detraining on blood pressure.
Bocalini et al. (2010) reported that improvement in quality of life after participating in a physical exercise program was reversed after a period of 4–6 weeks of detraining. In addition, Esain et al. (2017) reported that after a 3-month period of detraining, quality of life decreased significantly, especially in women. Romero-Zurita et al. (2012) reported that after a 3-month period of detraining, levels of anxiety worsened significantly. The results of these studies were in agreement with ours, as our participants also demonstrated significant declines in quality of life, worsened levels of anxiety, and maintained levels of depression after 4 months of summer rest. By contrast, Ansai and Rebelatto (2015) reported no changes in depressive symptoms after 6 weeks of detraining. However, in this program, the authors also did not observe improvements during training, something that, according to the authors, was perhaps due to low program adherence.
The second season of the MPE program in our study was interrupted by the lockdown caused by the spread of SARS-CoV-2. For 7 weeks, individuals were mandated to remain at home. Some studies reported effects of the home lockdown on physical activity and psychoaffective status in older adults. Overall, these studies found that physical activity was reduced (Wilke et al., 2021), and psychoaffective status worsened (Sepúlveda-Loyola et al., 2020; Hwang et al., 2020). Among the few reports on physical fitness in older adults during the COVID-19 pandemic, one was based on studies showing the effects of exercise cessation in other circumstances (Kirwan et al., 2020). However, Makizako et al. (2021) showed that 43% of older adults under study perceived declining physical fitness during the COVID-19 pandemic; this study used a wide sample of participants of similar ages to the participants in our study.
Nevertheless, little is known about the effects of physical exercise program interruption because of the COVID-19 pandemic on the health of older adults using both questionnaires and objective measures. Our results demonstrated that during program interruption, participant physical and psychoaffective status severely worsened and frailty increased. These findings are congruent with those of Makizako et al. (2021), where a higher perception of declining physical and cognitive fitness during the state of emergency was observed in older adults who had participated in an exercise class before the COVID-19 pandemic. These changes could be caused by legal restrictions, fear of going out related to the COVID-19 pandemic, and/or program interruption. However, taking into account the declines in physical and psychoaffective status observed during the summer rest after the first season, a relevant role of program interruption on physical and mental health in older individuals is highly probable.
Increases in blood pressure observed during the COVID-19 pandemic may reflect increased cardiovascular risk of the participants (Lippi et al., 2020). Similarly, decreases in physical fitness and increases in frailty may augment the risk of developing chronic diseases and suffering adverse events (Roschel et al., 2020; Kirwan et al., 2020). Furthermore, these alterations may increase the risk of complications from COVID-19. There are studies that associate high hypertension (Clark et al., 2021), frailty (Hewitt et al., 2020), and low physical fitness with a worse prognosis for COVID-19 (Ekiz et al., 2020) and concluded that active people have a lower risk for severe COVID-19 (Salgado-Aranda et al., 2021; Wang et al., 2020). Therefore, physical activity and exercise should be strongly recommended for older adults in the context of the pandemic not only to reduce the risk of developing chronic diseases, but also to reduce hospitalizations and deaths due to COVID-19.
Being physically active in these kinds of situations also could be beneficial from a psychoaffective point of view. Physical exercise is effective in improving quality of life (Rizzoli et al., 2013) and in reducing anxiety, depression, and loneliness in older people (Tarazona-Santabalbina et al., 2016; Arrieta et al., 2019). A positive relationship exists between physical activity and psychoaffective state in older people during the COVID-19 pandemic (Carriedo et al., 2020). In particular, there is a link between physical activity, loneliness, anxiety, and depression (Giuntella et al., 2021; Creese et al., 2020). Therefore, exercise program interruption likely affects psychoaffective status.
Notably, almost all parameters worsened during the two-year follow-up. In particular, physical tests, with the exception of the arm-curl test of the SFT, had a significant 20–40% decrease at the end of the follow-up (7 months after interruption of the program) from baseline values. This is much more than the expected age-related reduction in two years. Normative SPPB values in the Spanish population are reduced around 17% per decade after age 80 (Río et al., 2021). However, in our sample, the SPPB score was reduced by 40% in only two years. These data suggest that the COVID-19 pandemic had a high impact on physical deterioration of the older population.
Frailty and loneliness also increased significantly during the analysed period. This finding is especially worrying because there is a bidirectional relationship between these factors: loneliness increases the risk of developing frailty (Yamada et al., 2021) and the risk of death of frail individuals (Hoogendijk et al., 2020) and greater frailty increases the likelihood of high levels of loneliness in the future (Gale et al., 2018). This interaction could cause a vicious circle with putative bad consequences.
Taking into account all these data, there is an urgent need to promote and implement physical activity proposals adapted to older people when face-to-face activities are prohibited or limited. Social media and new technologies have been especially successful in promoting physical activity in the younger population (Rodríguez-Larrad et al., 2021). In addition, there are many online physical activity support systems aiming to encourage adults to perform physical activity at home (Sport santé chez soi - silver. Fédération Française d'Education Physiqye et de Gymnastique Volontaire, 2020; Ministère Français des Sports, 2020; National Health Service, 2020). However, a recent study suggested that older adults are reluctant to use these online tools (Goethals et al., 2020). Synchronous physical exercise sessions, which allow real time visual and auditory contact between the participants and the professional by video conference, could be a useful alternative for the older population (Jennings et al., 2020) because they could facilitate social interaction and correct execution of the exercises. To our knowledge, there is very scarce literature demonstrating the putative benefits of using synchronous online physical exercise to improve physical and mental health (Jennings et al., 2020). However, in a recent work performed in patients with spinal cord injury, workload, adherence and exercise recording were better with synchronous compared to asynchronous tele-exercises (Costa et al., 2021). Further studies are needed to ascertain the best methodology to help older adults integrate simple, safe and effective ways to stay physically active at home.
A major strength of the present study is that it includes an objective and face-to-face multidimensional analysis before and during the COVID-19 pandemic, including physical performance, anthropometry, frailty, psychoaffective status, and quality of life. To our knowledge, this is the first study with these characteristics. Moreover, a 2-year follow-up of the participants was performed, including the 7 months of program interruption. Potential limitations of the present study include that it lacks a control group, the inclusion of which would have allowed comparative data from a group of non-exercising older individuals. Specifically, it is not known whether the worsening of participants after 7 months of program interruption is due to cessation of the MPE intervention or is a consequence of the COVID-19 pandemic. Although the sample size was not large enough to reach clear conclusions, the drastic changes related to the program and its interruption may counterbalance the small sample size. Information on comorbidities and medication was also not available. Therefore, the changes in blood pressure and resting heart rate observed during the study should be interpreted with caution. Finally, more accurate data on body composition and physical activity of daily life would allow us to interpret some of the results more accurately.
5. Conclusions
Our study demonstrated that an MPE program had clear benefits for the physical and psychoaffective health of older adults. Interruption of these programs due to the COVID-19 pandemic could have significant impacts on participant physical and mental health. These results highlight the need to maintain physical exercise programs or facilitate engagement in physical activity and reduce sedentary behaviour in older adults, especially in situations such as the COVID-19 pandemic.
Funding
This work was supported by Siel Bleu and Basque Government (SAN 20/12).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
We thank the study participants for their willingness to participate. We also thank the staff of Retirement Home and Council of Beasain, and Fundación Siel Bleu involved in our study for their support and cooperation. Open Access funding provided by University of Basque Country.
Section Editor: Li-Ning Peng
References
- Alonso-Martínez A.M., Ramírez-Vélez R., García-Alonso Y., Izquierdo M., García-Hermoso A. Physical, sedentary behaviour, sleep and self-regulation in Spanish preschoolers during the COVID-19 lockdown. Int. J. Environ. Res. Public Health. 2021;18:693. doi: 10.3390/ijerph18020693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansai J.H., Rebelatto J.R. Effect of two physical exercise protocols on cognition and depressive symptoms in oldest-old people: a randomized controlled trial. Geriatr Gerontol Int. 2015;15(9):1127–1134. doi: 10.1111/ggi.12411. [DOI] [PubMed] [Google Scholar]
- Arrieta H., Rezola-Pardo C., Gil S.M., Virgala J., Iturburu M., Antón I., Rodriguez-Larrad A.… Effects of multicomponent exercise on frailty in long-term nursing homes: a randomized controlled trial. J.Am.Geriatr. Soc. 2019;67(6):1145–1151. doi: 10.1111/jgs.15824. [DOI] [PubMed] [Google Scholar]
- Berg K.O., Wood-Dauphinee S.L., Williams J.I., Maki B. Measuring balance in the elderly: validation of an instrument. Can. J. Public Health Rev. Can. Sante Publique. 1992;83:S7–S11. [PubMed] [Google Scholar]
- Bernal-Lopez C., Potvin O., Avila-Funes J.A. Frailty is associated with anxiety in community-dwelling elderly adults. J.Am.Geriatr. Soc. 2012;60(12):2373–2374. doi: 10.1111/jgs.12014. [DOI] [PubMed] [Google Scholar]
- Bocalini D.S., Serra A.J., Rica R.L., Dos Santos L. Repercussions of training and detraining by water-based exercise on functional fitness and quality of life: a short-term follow-up in healthy older women. Clinics. 2010;65(12):1305–1309. doi: 10.1590/S1807-59322010001200013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzycki M. Strength testing—predicting a one-rep max from reps-to-fatigue. J. Phys. Educ. Recreat. Dance. 1993;64(1):88–89. doi: 10.1080/07303084.1993.10606684. [DOI] [Google Scholar]
- Buchner D.M. Physical activity and prevention of cardiovascular disease in older adults. Clin.Geriatr. Med. 2009;25:661–675. doi: 10.1016/j.cger.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Cadore E.L., Rodríguez-Mañas L., Sinclair A., Izquierdo M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: a systematic review. Rejuvenation Res. 2013;16(2):105–114. doi: 10.1089/rej.2012.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriedo A., Cecchini J.A., Fernandez-Rio J., Méndez-Giménez A. COVID-19, psychological well-being and physical activity levels in older adults during the nationwide lockdown in Spain. Am.J.Geriatr.Psychiatry. 2020;28(11):1146–1155. doi: 10.1016/j.jagp.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C.E., McDonagh S.T.J., McManus R.J., Martin U. COVID-19 and hypertension: risks and management. A scientific statement on behalf of the British and Irishhypertensionsociety. J.Hum. Hypertens. 2021;35:304–307. doi: 10.1038/s41371-020-00451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha-Cisternas Y., Valdés-Badilla P., Guzmán-Muñoz E., Ramírez-Campillo R. Comparación de marcadores antropométricos de salud entre mujeres de 60–75 años físicamente activas e inactivas. Rev. Esp. Nutr. Hum. Diet. 2017;21(3) doi: 10.14306/renhyd.21.3.367. [DOI] [Google Scholar]
- Correa C.S., Baroni B.M., Radaelli R., Lanferdini F.J., Dos Santos-Cunha G., Reischak-Oliveira A., Silveira-Pinto R.… Effects of strength training and detraining on knee extensor strength, muscle volume and muscle quality in elderly women. Age. 2013;35:1899–1904. doi: 10.1007/s11357-012-9478-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R.R.G., Dorneles J.R., Veloso J.H., Gonçalves C.W., Neto F.R. Synchronous and asynchronous tele-exercise during the coronavirus disease 2019 pandemic: comparisons of implementation and training load in individuals with spinal cord injury. J. Telemed. Telecare. 2021 doi: 10.1177/1357633X20982732. 2021 Jan 18:1357633X20982732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese B., Khan Z., Henley W., O’Dwyer S., Corbett A., Vasconcelos Da Silva M., Ballard C. Loneliness, physical activity, and mental health during COVID-19: A longitudinal analysis of depression and anxiety in adults over the age of 50 between 2015 and 2020. Int. Psychogeriatr. 2020 doi: 10.1017/S1041610220004135. Published online December 17, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekiz T., Kara M., Özçakar L. Measuring grip strength in COVID-19: a simple way to predict overall frailty/impairment. Heart Lung. 2020;49(6):853–854. doi: 10.1016/j.hrtlng.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esain I., Rodriguez-Larrad A., Bidaurrazaga-Letona I., Gil S.M. Health-related quality of life, handgrip strength and falls during detraining in elderly habitual exercisers. Health Qual. Life Outcomes. 2017;15:226. doi: 10.1186/s12955-017-0800-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fess E. In: Clinical Assessment Recommendations. Grip Strength. 2nd ed. Casanova J.S., editor. American Society of Hand Therapists; Chicago: 1992. pp. 41–45. [Google Scholar]
- Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J., McBurnie M.A.… Frailty in older adults: evidence for a phenotype. J. Gerontol. Ser. A. 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- Gale C., Cooper C., Deary I., Aihie-Sayer A. Psychological well-being and incident frailty in men and women: theenglishlongitudinalstudy of ageing. Psychol.Med. 2014;44(4):697–706. doi: 10.1017/S0033291713001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale C., Westbury L., Cooper C. Social isolation and loneliness as risk factors for the progression of frailty: the englishlongitudinalstudy of ageing. Age Ageing. 2018;47(3):392–397. doi: 10.1093/ageing/afx188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuntella O., Hyde K., Saccardo S., Sadoff S. Lifestyle and mental health disruptions during COVID-19. Proc.Natl.Acad.Sci. 2021;118(9) doi: 10.1073/pnas.2016632118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbens R.J., Van Assen M.A., Luijkx K.G., Wijnen-Sponselee M.T., Schols J.M. The Tilburg frailtyindicator: psychometric properties. J.Am.Med.Dir. Assoc. 2010;11:344–355. doi: 10.1016/j.jamda.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Goethals L., Barth N., Guyot J., Hupin D., Celarier T., Bongue B. Impact of home quarantine on physical activity among older adults living at home during the COVID-19 pandemic: qualitative interview study. JMIR Aging. 2020;3(1) doi: 10.2196/19007. Published 2020 May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D., Bridges K., Duncan-Jones P., Grayson D. Detecting anxiety and depression in general medical settings. Br.Med. J. 1988;297(6653):897–899. doi: 10.1136/bmj.297.6653.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik J.M., Ferrucci L., Pieper C.F., Leveille S.G., Markides K.S., Ostir G.V., Wallace R.B.… Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J. Gerontol. Ser. A. 2000;55(4):M221–M231. doi: 10.1093/gerona/55.4.M221. [DOI] [PubMed] [Google Scholar]
- Henwood T.R., Taaffe D.N. Detraining and retraining in older adults following long-term muscle power or muscle strength specific training. J. Gerontol. Med. Sci. 2008;65(7):751–758. doi: 10.1093/gerona/63.7.751. [DOI] [PubMed] [Google Scholar]
- Herdman M., Badia X., Berra S. El EuroQol-5D: una alternativa sencilla Para la medición de la calidad de Vida relacionada con la salud en atención primaria. Aten. Primaria. 2001;28(6):425–429. doi: 10.1016/S0212-6567(01)70406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt J., Carter B., Vilches-Moraga A., Quinn T.J., Braude P., Verduri A., Pearce L., McCarthy K.… The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, european, observational cohort study. Lancet Public Health. 2020;5(8):e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendijk E.O., Smit A.P., Van Dam C., Schuster N.A., De Breij S., Holwerda T.J., Andrew M.K.… Frailty combined with loneliness or socialisolation:anelevatedrisk for mortality in laterlife. J.Am.Geriatr.Soc. 2020;68:2587–2593. doi: 10.1111/jgs.16716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst C., Weston K.L., McLaren S.J., Weston M. The effects of same-session combined exercise training on cardiorespiratory and functional fitness in older adults: a systematic review and meta-analysis. Aging Clin.Exp. Res. 2019;31(12):1701–1717. doi: 10.1007/s40520-019-01124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang T., Rabheru K., Peisah C., Reichman W., Ikeda M. Loneliness and social isolation during the COVID-19 pandemic. Int.Psychogeriatr. 2020;32(10):1217–1220. doi: 10.1017/S1041610220000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings S.C., Manning K.M., Bettger J.P., Hall K.M., Pearson M., Mateas C., Morey M.C. Rapid transition to telehealth group exercise and functional assessments in response to COVID-19. Gerontol. Geriatr. Med. 2020;6 doi: 10.1177/2333721420980313. 2020 Dec 14. 2333721420980313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan R., McCullough D., Butler T., Perez de Heredia F., Davies I.G., Stewart C. Sarcopenia during COVID-19 lockdown restrictions: long-term health effects of short-term muscle loss. GeroScience. 2020;42:1547–1578. doi: 10.1007/s11357-020-00272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrdalen I.L., Moen K., Røysland A.S., Helbostad J.L. The Otago exercise program performed as group training versus home training in fall-prone older people: a randomized controlled trial. Physiother.Res.Int. 2014;19(2):108–116. doi: 10.1002/pri.1571. [DOI] [PubMed] [Google Scholar]
- Lacroix A., Kressig R.W., Muehlbauer T., Gschwind Y.J., Pfenninger B., Bruegger O., Granacher U. Effects of a supervised versus an unsupervised combined balance and strength training program on balance and muscle power in healthy older adults: a randomized controlled trial. Gerontology. 2016;62(3):275–288. doi: 10.1159/000442087. [DOI] [PubMed] [Google Scholar]
- Lippi G., Henry B.M., Sanchis-Gomar F. Physical inactivity and cardiovascular disease at the time of coronavirus disease 2019 (COVID-19) Eur.J.Prev.Cardiol. 2020;27(9):906–908. doi: 10.1177/2047487320916823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makizako H., Nakai Y., Shiratsuchi D., Akanuma T., Yokoyama K., Matsuzaki-Kihara Y., Yoshida H. Perceived declining physical and cognitive fitness during the COVID-19 state of emergency among community-dwelling japanese old-old adults. GeriatrGerontolInt. 2021;21:364–369. doi: 10.1111/ggi.14140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfell-Jones M.J., Stewart A.D., De Ridder J.H. International Society for the Advancement of Kinanthropometry; Wellington, New Zealand: 2012. International Standards for Anthropometric Assessment. [Google Scholar]
- May A.M., Struij E.A., Frasen H.P., Onland-Moret N.C., De Wit G.A., Boer J.M.A., Beulens J.W.… The impact of a healthy lifestyle on disability-adjusted life years: a prospective cohort study. BMC Med. 2015;13:39. doi: 10.1186/s12916-015-0287-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhaolain A.M., Gallagher D., Crosby L., Ryan D., Lacey L., Coen R.F., Lawlor B.… Frailty and quality of life for people with Alzheimer’s dementia and mild cognitive impairment. Am.J. Alzheimers Dis. Other Dement. 2012;27(1):48–54. doi: 10.1177/1533317511435661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miljkovic N., Lim J.Y., Miljkovic I., Frontera W.R. Aging of skeletal muscle fibers. Ann.Rehabil. Med. 2015;39(2):155–162. doi: 10.5535/arm.2015.39.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministère Français des Sports Bouger Chez Vous. https://bougezchezvous.fr/
- Moker E.A., Bateman L.A., Kraus W.E., Pescatello L.S. The relationship between the blood pressure responses to exercise following training and detraining periods. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0105755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley J.E., Von H.S., Anker S.D., Vellas B. From sarcopenia to frailty: a road less traveled. J.Cachexia. Sarcopenia Muscle. 2014;5(1):5–8. doi: 10.1007/s13539-014-0132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimineto D.C., Tibana R.A., Benik F.M., Fontana K.E., Ribeiro-Neto F., Santos-De Santana F., Prestes J.… Sustained effect of resistance training on blood pressure and hand grip strength following a detraining period in elderly hypertensive women: a pilot study. Clin.Interv. Aging. 2014;20:219–225. doi: 10.2147/CIA.S56058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHS: National Health Service Physical activity guidelines for older adults. 2020. https://www.nhs.uk/live-well/exercise/physical-activity-guidelines-older-adults/
- Padilha C.S., Ribeiro A.S., Fleck S.J., Nascimento M.A., Pina F.L.C., Miyuki-Okino A., Cyrino E.S.… Effect of resistance training with different frequencies and detraining on muscular strength and oxidative stress biomarkers in older women. Age. 2015;37:104. doi: 10.1007/s11357-015-9841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahor M., Guralnik J.M., Ambrosius W.T., Blair S., Bonds D.E., Church T.S., Williamson J.D.… Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikli R.E., Jones C.J. 2001. Senior Fitness Test. Human Kinetics, Champaign (IL) (ISBN 0-7360-3356-4) [Google Scholar]
- Río X., Guerra-Balic M., González-Pérez A., Larrinaga-Undabarrena A., Coca A. Valores de referencia del SPPB en personas mayores de 60 años en el País Vasco [Reference values for SPPB in people over 60 years of age in the Basque Country] Aten. Primaria. 2021;53(8) doi: 10.1016/j.aprim.2021.102075. 2021 May 15. Spanish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli R., Reginster J.Y., Arnal J.F., Bautmans I., Beaudart C., Bischoff-Ferrari H., Bruyère O.… Quality of life in sarcopenia and frailty. Calcif. Tissue Int. 2013;93(2):101–120. doi: 10.1007/s00223-013-9758-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D.A., Savva G.M., Kenny R.A. Frailty and cognitive impairment–a review of the evidence and causal mechanisms. Ageing Res. Rev. 2013;12(4):840–851. doi: 10.1016/j.arr.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Larrad A., Mañas A., Labayen I., González-Gross M., Espin A., Aznar S., Irazusta J. Impact of COVID-19 confinement on physical activity and sedentary behaviour in spanish university students: role of gender. Int. J. Environ. Res. Public Health. 2021;18(2):369. doi: 10.3390/ijerph18020369. 2021 Jan 18:1357633X20982732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Zurita A., Carbonell-Baeza A., Aparicio V.A., Ruiz J.R., Tercedor P., Delgado-Fernández M. Effectiveness of a tai-chi training and detraining on functional capacity, symptomatology and psychological outcomes in women with fibromyalgia. Evid. Based Complement.Alternat. Med. 2012;2012 doi: 10.1155/2012/614196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roschel H., Artioli G.G., Gualano B. Risk of increased physical inactivity during COVID-19 outbreak in older people: a call for actions. J.Am.Geriatr.Soc. 2020;68(6):1126–1128. doi: 10.1111/jgs.16550. [DOI] [PubMed] [Google Scholar]
- Salgado-Aranda R., Pérez-Castellano N., Núñez-Gil I., Orozco A.J., Torres-Esquivel N., Flores-Soler J., Pérez-Villacastín J. Influence of baseline physical activity as a modifying factor on COVID-19 mortality: a single-center, retrospective study. Infect. Dis. Ther. 2021 doi: 10.1007/s40121-021-00418-6. Published online March 14, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasso J.P., Eves N.D., Christensen J.F., Koelwyn G.J., Scott J., Jones L.W. A framework for prescription in exercise-oncology research. J.Cachexia. Sarcopenia Muscle. 2015;6(2):115–124. doi: 10.1002/jcsm.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepúlveda-Loyola W., Rodríguez-Sánchez I., Pérez-Rodríguez P., Ganz F., Torralba R., Oliveira D.V., Rodríguez-Mañas L. Impact of social isolation due to COVID-19 on health in older people: mental and physical effects and recommendations. J.Nutr. Health Aging. 2020;24(9):938–947. doi: 10.1007/s12603-020-1469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simek E.M., McPhate L., Haines T.P. Adherence to and efficacy of home exercise programs to prevent falls: a systematic review and meta-analysis of the impact of exercise program characteristics. Prev.Med. 2012;55(4):262–275. doi: 10.1016/j.ypmed.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Spiering B.A., Mujika I., Sharp M.A., Foulis S.A. Maintaining physical performance: the minimal dose of exercise needed to preserve endurance and strength over time. J. Strength Cond. Res. 2021;35:1449–1458. doi: 10.1519/JSC.0000000000003964. [DOI] [PubMed] [Google Scholar]
- Sport santé chez soi - silver. Fédération Française d'Education Physiqye et de Gymnastique Volontaire SportSanté.fr. 2020. https://www.sport-sante.fr/fr/adherents/seances-sport-sante-chez-soi/actus/22831-silver.html
- St John P.D., Tyas S.L., Montgomery P.R. Depressive symptoms and frailty. Int J Geriatr Psychiatry. 2013;28(6):607–614. doi: 10.1002/gps.3866. [DOI] [PubMed] [Google Scholar]
- Tarazona-Santabalbina F.J., Gómez-Cabrera M.C., Pérez-Ros P., Martínez-Arnau F.M., Cabo H., Tsaparas, Viña J. A multicomponent exercise intervention that reverses frailty and improves cognition, emotion, and social networking in the community-dwelling frail elderly: a randomized clinical trial. J. Am. Med. Dir. Assoc. 2016;17(5):426–433. doi: 10.1016/j.jamda.2016.01.019. [DOI] [PubMed] [Google Scholar]
- Theou O., Stathokostas L., Roland K.P., Jakobi J.M., Patterson C., Vandervoort A.A., Jones G.R. The effectiveness of exercise interventions for the management of frailty: a systematic review. J. Aging Res. 2011;2011 doi: 10.4061/2011/569194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toraman N.F., Eman A., Agyar E. Effects of multi-component training on functional fitness in older adults. J. Aging Phys. Act. 2004;26:448–454. doi: 10.1123/japa.12.4.538. [DOI] [PubMed] [Google Scholar]
- Velarde-Mayol C., Fragua Gil S., García de Cecilia J.M. Validación de la escala de soledad de UCLA y perfil social en la población anciana que vive sola. SEMERGENMed. Fam. 2015;42(3):177–183. doi: 10.1016/j.semerg.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Yamada M., Kimura Y., Ishiyama D., Otobe Y., Suzuki M., Koyama S., Arai H.… The influence of the COVID-19 pandemic on physicalactivity and newincidence of frailty among initiallynon-frailolderadults in Japan: afollow-uponlinesurvey. J.Nutr. Health Aging. 2021;25:751–756. doi: 10.1007/s12603-021-1634-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T., Fukumura K., Iida H., Nakajima T. Effects of detraining after blood flow-restricted low-load elastic band training on muscle size and arterial stiffness in older women. Springerplus. 2015;4:348. doi: 10.1186/s40064-015-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Baker J.S., Quan W., Shen S., Fekete G., Gu Y. A preventive role of exercise across the coronavirus 2 (SARS-CoV-2) pandemic. Front.Physiol. 2020;11:1139. doi: 10.3389/fphys.2020.572718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke J., Mohr L., Tenforde A.S., Edouard P., Fossati C., González-Gross M., Hollander K.… A pandemic within the pandemic? Physical activity levels substantially decreased in countries affected by COVID-19. Int.J.Environ.Res. Public Health. 2021;18(5):2235. doi: 10.3390/ijerph18052235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Tan S.S., Franse C.B., Bilajac L., Alhambra-Borrás T., Garcés-Ferrer J., Raat H.… Longitudinal association between physical activity and frailty among community-dwelling older adults. J.Am.Geriatr. Soc. 2020;68(7):1484–1493. doi: 10.1111/jgs.16391. [DOI] [PMC free article] [PubMed] [Google Scholar]