Abstract
Background
Sex hormone and preexposure prophylaxis (PrEP) drug interactions among transgender women (TGW), transgender men (TGM), and cisgender men (CGM) are not fully understood.
Methods
TGM and TGW on at least 6 months of stable sex hormone therapy containing testosterone or estradiol (respectively) were enrolled in a 4-week study of directly observed dosing of daily oral coformulated emtricitabine and tenofovir disoproxil fumarate (FTC/TDF). TFV-DP in dried blood spots and sex hormones in serum were measured at weekly intervals. TFV-DP was compared with 2- and 4-week samples from Directly Observed Therapy Dried Blood Spots (DOT-DBS) Study (NCT02022657).
Results
From May 2017 to June 2018, 24 TGM and 24 TGW were enrolled. Testosterone (total and free) and estradiol concentrations were comparable before and after 4 weeks of PrEP use in TGM and TGW, respectively. Historical controls included 17 cisgender women (CGW) and 15 CGM. TFV-DP concentrations at week 4 were comparable between TGW and TGM (mean difference, −6%; 95% confidence interval [CI], −21% to 12%; P = .47), comparable between TGW and CGM (mean difference, −12%; 95% CI, −27% to 7%; P = .21) and were lower among TGM compared with CGW (mean difference, −23%; 95% CI, −36% to −7%; P = .007). All persons in all groups were projected to reach the TFV-DP threshold that has been associated with high protection from human immunodeficiency virus.
Conclusions
CGM, TGM, and TGW had comparable TFV-DP concentrations in dried blood spots after 4 weeks of directly observed daily FTC/TDF PrEP use. Serum hormone concentrations were not affected by FTC/TDF PrEP use.
Clinical Trials Registration
Keywords: HIV, preexposure prophylaxis, transgender, sex hormones, pharmacokinetics
Daily oral tenofovir disoproxil fumarate/emtricitabine yielded comparable drug concentrations in dried blood spots among transgender women using estradiol and transgender men using testosterone compared with men who have sex with men during a 4-week period of directly observed dosing.
Pre-exposure prophylaxis (PrEP) with oral emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) reduces the risk of human immunodeficiency virus (HIV) transmission by 91% in cisgender men who have sex with men when dosed appropriately [reviewed in [1, 2]]. Only 1 randomized trial, the Iniciativa Profilaxis Preexposición (iPrEx) trial (Preexposure prophylaxis initiative), included substantial numbers of transgender women (TGW), and no trial included transgender men (TGM) [3, 4]. The subgroup analysis of iPrEx revealed that TGW were less likely than men who have sex with men to have protective concentrations of PrEP medications in an intention-to-treat analysis, especially if they also reported using feminizing hormone therapy [5]. In a subsequent, more sophisticated transportability analysis, PrEP efficacy among TGW was comparable to efficacy among cisgender men (CGM) who have sex with men when adjusted for the following baseline factors: depression score (using Center for Epidemiologic Studies in Depression Scale [CESD]), number of partners in the previous 3 months, any condomless receptive anal intercourse in the previous 3 months, living situation, any history of transactional sex in the previous 6 months, and any sexually transmitted infection diagnoses in the previous 6 months [6].
Three small pharmacokinetic studies have raised concerns for FTC/TDF interactions with feminizing hormones. These studies suggest that the concurrent use of feminizing hormones may be associated with slightly lower tenofovir concentrations in blood plasma (by approximately 12%) and lower active tenofovir concentrations or tenofovir/natural substrate ratios in rectal cells [7–9]. The number of participants and effect sizes were small in these studies, and PrEP dosing was self-reported in 2 of 3 studies, calling for larger and better controlled pharmacokinetic studies in transgender populations. Studies are also needed that focus on TFV-DP in dried blood spots (DBSs) as this moiety consistently and strongly correlates with TFV exposure, adherence, and PrEP outcomes [4, 10–12]. PrEP research has not been conducted in TGM who use masculinizing hormones.
Tenofovir, emtricitabine, and sex hormones have different metabolic pathways, such that drug–drug interactions are not expected. Tenofovir disoproxil fumarate (TDF) did not affect ethinyl estradiol and 17-deacetyl norgestimate concentrations among 20 women receiving oral contraception over 7 days [13]. TDF-containing PrEP does not affect hormone contraceptive efficacy [14, 15], and progesterone contraception does not affect PrEP efficacy in cisgender women (CGW) [16].
This study was conducted using directly observed dosing of oral FTC/TDF among TGW and TGM not living with HIV on a stable feminizing regimen of estradiol or masculinizing regimen of testosterone to evaluate how such hormone therapy might affect concentrations of TFV-DP in DBSs.
METHODS
Population
TGW and TGM were eligible if they had been on a stable sex hormone therapy regimen that included estradiol or testosterone, respectively, for at least 6 months. Inclusion criteria included age >18 years, willing and able to receive video or in-person directly observed therapy for a 4-week period, no antiretroviral use for the previous 90 days, HIV antibody negative, and willing and able to provide written informed consent. Four weeks accounts for 75% of the accumulation of TFV-DP deemed sufficient to estimate PrEP drug pharmacokinetics. Risk factors for HIV were not required. Enrollment was capped at 24 for both TGW and TGM, sufficient to evaluate drug–drug interactions in each group. The University of California–San Francisco Institutional Review Board approved the protocol. The control populations were from the DOT-DBS Study, which included CGM and CGW receiving directly observed FTC/TDF dosing with specimens collected, processed, and stored as previously reported (NCT02022657) [17, 18].
Intervention
After a screening visit, participants started daily oral FTC/TDF PrEP with a directly observed therapy (DOT) at the enrollment visit. Blood was collected before the dose at enrollment and at weeks 1, 2, 3, and 4. Dosing was directly observed in person or by video on all days, including observation of placing the tablet in the mouth, the swallowing movement, and a check of the mouth for the tablet afterward. On days when a live video connection was not possible, a recorded video could be sent on the same day using a password for encryption that was provided earlier that day.
Measurements
DBSs were prepared, processed, frozen, and shipped to the Colorado Antiviral Pharmacology Laboratory as previously described [17, 19]. Intracellular TFV-DP and emtricitabine-triphosphate (FTC-TP) were quantified with a validated liquid chromatography–tandem mass spectrometry (LC-MS/MS) assay as previously described, with the exception of using a 50:50 methanol:water DBS extraction solution. This 50:50 methanol DBS extraction solution was developed and validated for optimized extraction recovery and improved precision. Because the initial analysis for DOT-DBS samples was done in 2014–2016, this optimized extraction was used to reanalyze DOT-DBS samples at the same time as the current Interactions Between Antiretrovirals And Transgender Hormones (iBrEATHe) samples. The new extraction led to a threshold of 800 fmol/punch for ≥4 doses/week from the DOT-DBS Study (rather than 700 fmol/punch from the previous assay, which is associated with high PrEP efficacy). The serum was frozen and shipped for batch analyses by LC-MS/MS for estradiol, estrone, and testosterone at Brigham Research Assay Core Laboratory at the Brigham and Women’s Hospital (Boston, MA) [20, 21]; these assays have been certified by the Hormone Standardization Program of the Centers for Disease Control and Prevention. Sex hormone binding globulin was measured using an immuno-chemiluminescence assay [20, 21].
Symptoms relating to hormone level fluctuations were measured using the Menopausal Rating Scale (MRS), which has been previously used to describe symptoms associated with perimenopausal hormone level fluctuations and hormone treatment in cisgender women [22, 23]. Adverse events were recorded at every visit.
Statistical Analyses
We compared TFV-DP drug concentrations in trans- vs cisgender individuals after 4 weeks of PrEP use; the dataset of TFV-DP levels in cisgender controls was provided to us from the DOT-DBS Study [17, 18]. While 4 weeks is sufficient to estimate PrEP drug accumulation rates, information emerged after this study was designed that indicates that steady-state concentrations are achieved after 8 weeks. TFV-DP concentrations at steady state (CSS) at 8 weeks were projected using the following formula: CSS = Cday/[1 − exp(−0.04 × day)] where day is the day at which the concentration is obtained. Comparisons of percent differences by groups used regression models of log-transformed TFV-DP controlling for creatinine clearance, weight, age, site of enrollment (San Francisco vs Denver), and study arm (current intervention vs historical control). Comparisons of demographics between groups used the Fisher exact or Kruskal-Wallis test as appropriate. Correlations between hormone and TFV-DP concentrations were based on the Spearman rank correlation. Figures, including box plots, were created and regression models for this analysis were generated using STATA software.
RESULTS
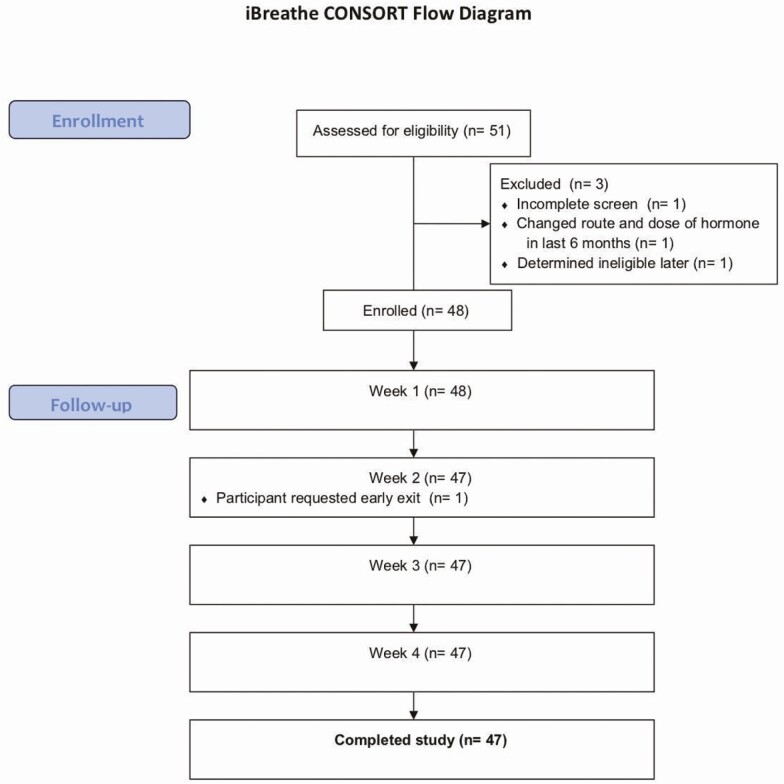
Between June 2017 and June 2018, 51 people were screened and 48 were enrolled (Figure 1). Of these, 1 withdrew consent at week 2 (not due to an adverse event) and 47 completed all study visits. Among the enrolled cohort, 24 were TGM and 24 were TGW, and the median age was 31 years (interquartile range [IQR], 28 to 40; Table 1). Race/ethnicity (nonexclusive) was 27 (57%) white, 10 (21%) black, and 15 (32%) Latinx. In the week before enrollment, 24 (50%) reported using alcohol, 39 (81%) reported using caffeine, 17 (35%) reported using nicotine (including tobacco), and 27 (56%) reported using marijuana. There were no differences in these characteristics between TGM and TGW.
Figure 1.
Interactions Between Antiretrovirals And Transgender Hormones Study (iBrEATHe) consort diagram. There were 24 transgender women and 24 transgender men enrolled in a stratified enrollment design.
Table 1.
Demographic and Selected Characteristics, Overall and by Birth Sex and Gender
| Interactions Between Antiretrovirals And Transgender Hormones (iBrEATHEe) Study | Directly Observed Therapy Dried Blood Spots (DOT-DBS) Study | |||||
|---|---|---|---|---|---|---|
| Overall | Transgender Men | Transgender Women | Cisgender Women | Cisgender Men | ||
| (n = 74) | (n = 23) | (n = 24) | (n = 17) | (n = 15) | ||
| Characteristic | N | N | N | N | N | P Valuea |
| Race/ethnicity | ||||||
| White | 46 (58%) | 11 (48%) | 16 (67%) | 9 (53%) | 10 (67%) | .5115 |
| Black/African American | 15 (19%) | 6 (26%) | 4 (17%) | 4 (24%) | 1 (7%) | .5067 |
| Hispanic/Latino | 23 (29%) | 9 (39%) | 6 (25%) | 4 (24%) | 4 (27%) | .6757 |
| Age (y)b | 30 (27–38) | 34 (29–40) | 29 (26–40) | 30 (27–32) | 28 (26–39) | .1738 |
| Weight (kg)b | 75 (66–94) | 75 (65–85) | 74 (68–100) | 63 (59–94) | 82 (76–102) | .0524 |
| Height (cm)b | 172 (163–177) | 169 (164–172) | 175 (175–181) | 162 (158–164) | 177 (170–183) | <.0001 |
| Body mass index (kg/m2)b | 27 (23–30) | 27 (24–30) | 25 (22–30) | 24 (22–33) | 27 (24–30) | .6314 |
| Serum creatinine (mg/dL)b | 0.8 (0.8–1.0) | 1.0 (0.9–1.0) | 0.8 (0.7–0.9) | 0.8 (0.7–0.8) | 1.0 (0.8–1.0) | <.0001 |
| Creatinine clearance (MDRD)b | 89 (76–110) | 71 (63–79) | 116 (101–142) | 96 (84–102) | 88 (81–108) | <.0001 |
| Creatinine clearance (CG) sex at birthb | 119 (98–165) | 95 (87–110) | 165 (119–197) | 116 (100–140) | 135 (104–152) | <.0001 |
| Creatinine clearance (CG) gender identityb | 121 (103–155) | 112 (103–129) | 141 (101–168) | 116 (100–140) | 135 (104–152) | .3687 |
Abbreviations: CG, Cockcroft-Gault Equation; MDRD, Modified Diet in Renal Disease Study equation.
aFisher exact P values for frequency data; Kruskal-Wallis P values for continuous variables.
bMedian with interquartile range.
Among 24 TGW, all used estradiol including oral (20) or injected (4) routes. Oral progesterone was used by 3, oral medroxyprogesterone acetate by 1, and spironolactone (used as an antiandrogen) by 9. Among 24 TGM, testosterone included injected testosterone cypionate (18), injected testosterone enanthate (2), topical testosterone gel (2), and implanted testosterone pellets (1). Finasteride, an antiandrogen, was used by 3.
TGM and TGW had similar weight (median, 74 kg; IQR, 67 to 91; P = .45), body mass index (BMI; median, 26; IQR, 23 to 30; P = .28). Serum creatinine was higher among TGM (median, 1.0 mg/dL vs 0.8 mg/dL; P = .0001), such that the estimated creatinine clearance (eCrCl) was lower among TGM compared with TGW (P < .0001) regardless of the estimating equation (Modification of Diet in Renal Disease vs Cockcroft-Gault) or whether sex at birth or current gender was used.
Among the 47 participants followed through 28 days of dosing, 1309 of 1316 (99.5%) PrEP doses were reported to have been taken. Of these, dosing was directly observed by video for 1271 (96.9%) and in person for 20 (1.5%). Nonobserved doses included 12 (0.9%) by text message, 2 (0.15%) by telephone, and 6 (0.5%) by patient report.
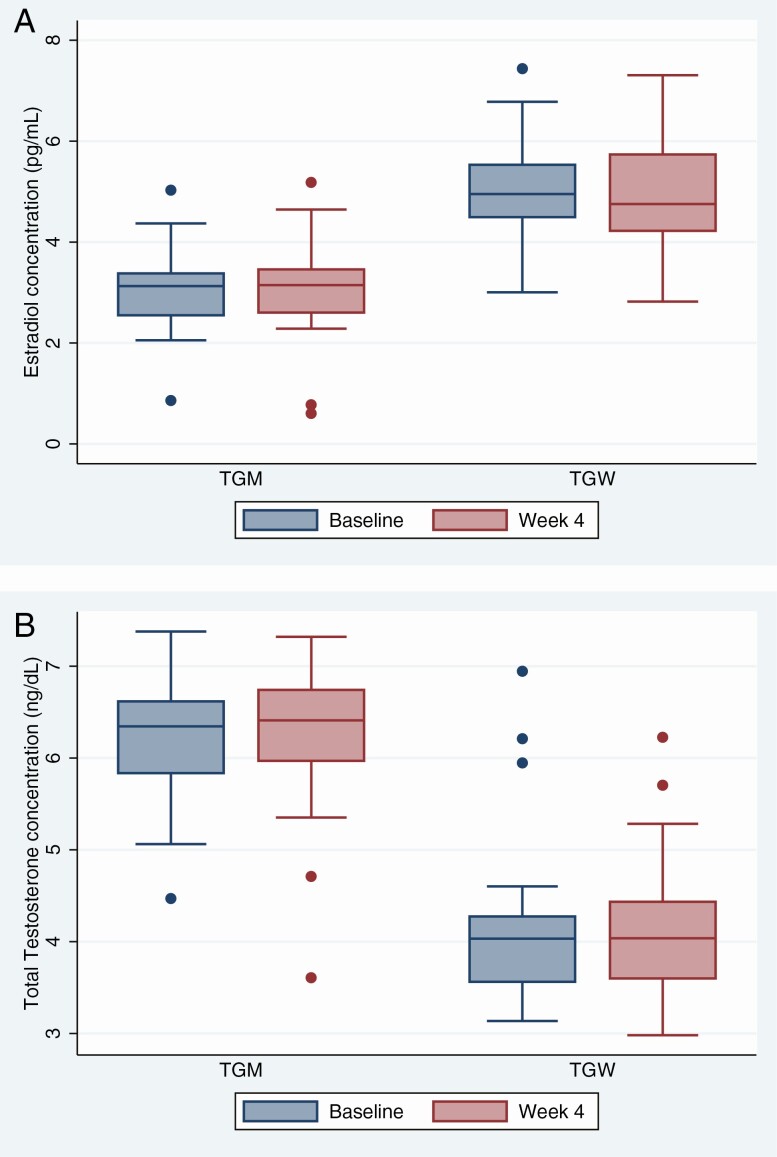
Serum estradiol concentrations were higher among TGW (Figure 2A), and serum testosterone concentrations were higher among TGM (Figure 2B; P < .0001, both comparisons) as expected based on self-reported exogenous use. Comparing visits on the day PrEP was started and on the day of completing 4 weeks of daily oral FTC/TDF, there were no differences in estradiol, estrone, total or free testosterone, dihydrotestosterone, or sex hormone binding globulin.
Figure 2.
Box plots of hormone concentrations before and after emtricitabine and tenofovir disoproxil fumarate (FTC/TDF) preexposure prophylaxis dosing. Serum estradiol concentrations (A) and total testosterone concentrations (B) are depicted on a natural logarithmic scale before FTC/TDF dosing and after 4 weeks of directly observed daily dosing among TGM and TGW. The boxes represent median values and the interquartile range, and the whiskers represent the 95% confidence intervals; dots beyond the whiskers are outliers. There are no statistically significant differences. Abbreviations: TGM, transgender men; TGW, transgender women.
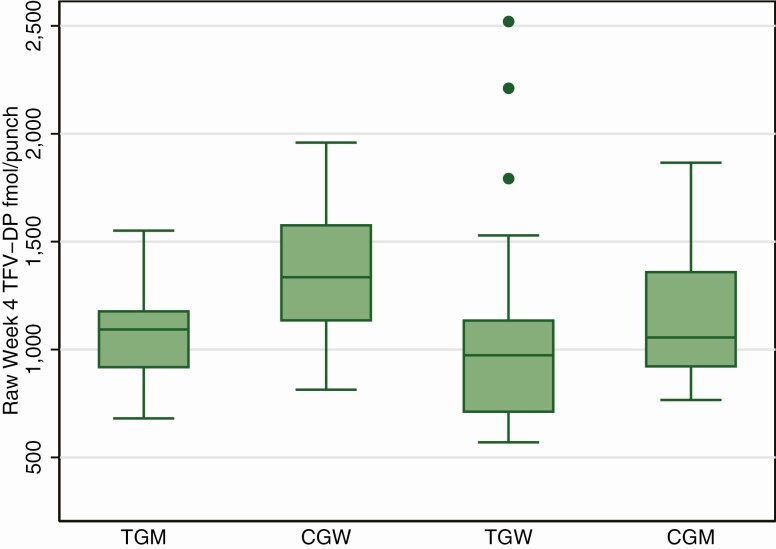
For the analysis of TFV-DP, comparison groups were 17 CGW and 15 CGM who had completed 4 weeks of directly observed daily FTC/TDF PrEP dosing. There were no differences in TFV-DP concentrations during the first 2 weeks of PrEP comparing TGW, TGM, and CGM (P = .87). The median fold increase in TFV-DP from week 2 to week 4 overall was 1.54 (IQR, 1.34–1.74) and was slightly higher among CGM at 1.63 (IQR, 1.55 vs 1.84; P = .05). TFV-DP concentrations at week 4 were comparable between TGW and CGM (Figure 3; mean difference, −12%; 95% CI, −27% to 7%; P = .21) and were lower among TGM compared with CGW (mean difference, −23%; 95% CI, −36% to −7%; P = .007). These comparisons were not substantially altered after adjusting for eCrCl, age, weight, spironolactone use, height, and BMI (comparing TGW with CGM: mean difference, −17%; 95% CI, −36% to 8; P = .17; comparing TGM with CGW: mean difference, −24%; 95% CI, −41% to −2%; P = .04). All persons in all groups were projected to reach steady-state TFV-DP >800 fmol/punch by 8 weeks, comparable to the 700 fmol/punch level using prior extraction methods reported to be highly protective in prior studies [4].
Figure 3.
TFV-DP concentrations in dried blood spots after 4 weeks of directly observed daily dosing. The boxes represent median values and the interquartile range, and the whiskers represent the 95% confidence intervals; dots beyond the whiskers are outliers. Abbreviations: CGM, cisgender men; CGW, cisgender women; TFV-DP, tenofovir diphosphate; TGM, transgender men; TGW, transgender women.
There was no association between serum estradiol concentration and TFV-DP among TGW (P = .35) or TGM (P = .15). Similarly, there was no association between total or free testosterone concentrations and TFV-DP among TGW (P = .60 and P = .08, respectively) and TGM (P = .21 and P = .73, respectively).
There were 23 treatment emergent adverse events reported among 18 participants, including 11 (46%) TGM and 7 (29%) TGW (P = .4). The most common complaints were nausea (10 events), diarrhea or soft stools (5 events), fatigue (4 events), and abdominal pain (3 events). The numbers of adverse events reported decreased over time (12 at week 1, 3 at week 2, 5 at week 3, and 3 at week 4). No one discontinued PrEP due to adverse events. No HIV acquisitions were detected.
There were no changes before and after PrEP use in hormone-related symptoms on the MRS. Question 10, which was related to vaginal dryness, was excluded from the analysis for TGW. The mean MRS score among 24 TGW was 5.5 (standard deviation [SD], 5.4) before PrEP and 5.9 (SD, 5.3) after PrEP (P = .5); among 22 TGM, the mean MRS score was 4.9 (SD, 3.4) before PrEP and 4.9 (SD, 4.1) after PrEP (P = .7). Vaginal dryness did not change before and after PrEP among TGM (score 0.3 before PrEP, 0.2 after PrEP; P = .7).
DISCUSSION
This drug–drug interaction study demonstrated that 4 weeks of directly observed daily oral use of FTC/TDF does not affect concentrations of estradiol or estrone in TGW or concentrations of free or total testosterone in TGM. No changes in hormone dosing were reported during the study. There were no clinical or self-reported signs of hormone withdrawal during the first 4 weeks of PrEP use.
TGW, TGM, and CGM had similar TFV-DP concentrations in DBSs at 2 and 4 weeks. As reported previously in the DOT-DBS Study, CGW had higher TFV-DP concentrations compared with CGM. This difference has not been explained and could reflect differences in drug transport and metabolism in red blood cells or differences in blood cell clearance. A limitation of this analysis was that the DOT-DBS samples were collected and stored 3–4 years before reanalysis concurrent with iBrEATHe analysis. However, the laboratory has demonstrated stability of TFV-DP measurement in samples stored at −80ºC for >5 years (1835 days), so TFV-DP degradation is not expected in this 3- to 4-year time frame. Initially, the iBrEATHe samples were analyzed with the original extraction procedure in an attempt to match the initial analysis of DOT-DBS conducted 3–4 years prior. This approach could be biased by assay drift. To control for assay drift, we contemporaneously reanalyzed both the DOT-DBS and the iBrEATHe specimens with the new extraction procedure to allow the most rigorous comparison with minimal confounding.
These results support current recommendations for PrEP dosing using regimens that contain TDF [24]. Although men who have sex with men and TGW are different in multiple important respects, dosing recommendations are similar based on findings that trends in differences in efficacy in the intention-to-treat subgroup analyses did not reach statistical significance (P = .09), that the relationships between blood concentrations and protective benefits are similar, and that baseline characteristics explain trends in observed efficacy [5, 6]. These findings of comparable accumulation kinetics in these 2 groups support application of similar dosing recommendations across these groups. Our findings also suggest that previously observed lower concentrations of TFV-DP in DBSs among TGW in the iPrEx trial were due to less use of the medication, rather than differences in metabolism [4].
All participants in all populations were projected to achieve concentrations of >800 fmol/punch by week 8 (analogous to >700 fmol/punch for the original extraction procedure); this concentration was associated with high levels of protection from HIV acquisition [4]. Our findings comparing TFV-DP between TGW and CGM was −11% with a 95% CI of −27% to 10% (P = .27), indicating similar pharmacokinetics in TGW. A strength of our study was the use of DOT to control for bias in self-reported adherence and evaluation of TFV-DP in DBSs, a parameter that has been robustly associated with adherence and clinical outcomes. The results confirm that the effects of hormones are small and not expected to erode clinical protection from PrEP. A prior study could be confounded by biased reports of adherence [8]. Another study used DOT but did not adjust plasma drug concentrations for substantial differences in body weight (mean weight was 98 kg for TGW and 83 kg for CGM) [9]. The observed plasma concentrations in both of these studies among TGW were 2200–2500 hr × ng/mL (area under the curve), which is well within plasma tenofovir concentrations observed in regulatory pharmacokinetic studies (approximately 2000–3000 hr × ng/mL), further supporting the conclusion that pharmacokinetics are similar in TGW [13].
Messaging about PrEP efficacy has used correct and compelling qualitative terms, emphasizing that protection can be expected if PrEP is used as directed, and this message applies to all populations studied so far, including transgender people [25]. There are remarkably few cases of HIV infection that have occurred when PrEP is used as directed, and all have occurred among CGM who have sex with men [26–28]. Large cohorts of TGM and TGW taking PrEP have been and are being studied; HIV infections in these cohorts would be expected if PrEP were not highly effective [5, 29].
There are multiple limitations of this study. As described above, there was a difference of several years in the enrollment of historical control groups comprising cisgender men and women to compare with TGM and TGW. Additionally, cisgender participants were recruited from both Denver and San Francisco, while transgender participants were studied only in San Francisco. The control study had broader visit windows, although the actual date of the visit was used in the analysis. The 17-day half-life minimizes the effect of small differences in collection days. Recruitment patterns would necessarily differ between these populations. Specimen-handling protocols were identical, although different study sites and staff were used. Importantly, a DBS is not a target tissue for HIV replication or transmission. A DBS is a convenient specimen for monitoring long-term exposure and adherence, and this study confirms similar relationships between FTC/TDF PrEP use and DBS drug concentrations over the first 4 weeks among TGW, TGM, and CGM. However, the relationships between TFV-DP concentrations in DBSs and HIV acquisition risk are most confidently known for CGM. The tissues that are most relevant for HIV transmission are CD4-bearing cells in the rectal and genital mucosa and lymphatics, and concentrations of FTC-TP and TFV-DP in these tissues were not studied [30]. Two small studies found no statistical difference in rectal tissue TFV-DP among TGW vs CGM (P = .53), although 1 study reported a lower TFV-DP:dATP (natural substrate) in TGW [7, 9]. Finally, drug accumulation did not reach steady state in this 4-week study, so steady-state projections were estimated. The MRS has not been validated for use among transgender populations. The results of this study may not apply to tenofovir alafenamide, a different formulation of tenofovir that has different pharmacokinetics in tissues [31].
FTC/TDF PrEP was demonstrated to be safe and effective in an ambitious and diverse phase 3 program [1, 2]. The trials involved nearly 20 000 men and women in Asia, Africa, South America, Europe, and the United States. Subgroup analysis and meta-analysis found that the only significant mitigator of efficacy was adherence; age, sex at birth, current gender, hormone use, condom use, concomitant sexually transmitted infections, and other factors did not significantly interact with PrEP efficacy once adherence was controlled [1, 5]. Pharmacokinetic studies should use directly observed therapy given that self-reported adherence is highly affected by social desirability bias, and such biases differ by setting and population. Given that HIV acquisition during PrEP use is rare, assurance of nearly complete clinical protection among transgender populations calls for active surveillance in large samples.
Notes
Acknowledgments. The Interactions Between Antiretrovirals And Transgender Hormones (iBrEATHe) Study Team thanks all of the participants and community who made this study possible, as well as the leadership and staff of the San Francisco AIDS Foundation where the study was implemented. They also thank Kathryn Jee for specimen handling and shipping. The study drug was donated by Gilead Sciences.
Financial support. This work was supported by a University of California, California HIV/AIDS Research Program grant to M. D., R. M. G, J. S., M. Y., M. P., and D. V. G and by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant R01AI118575) to R. M. G. and P. D.
Potential conflicts of interest. P. L. A. has received grants and consulting fees from Gilead Sciences. R. M. G. and P. L. A. have led studies in which Gilead Sciences donated the study drug. D. V. G. has received consulting fees from Gilead Sciences and Merck and Company. S. B. reports grants from AbbVie, FPT LCC, Transition Therapeutics, Alivegen, and MIB LLC; travel expenses and speaker fees from AbbVie; consulting fees from OPKO; has equity interest in FTP LLC; and is co-holder of a patent on calculating free testosterone outside the submitted work. R. M. G. reports grants to the institution from Gilead Sciences outside the submitted work. P. L. A. reports grants and personal fees from Gilead Sciences outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fonner VA, Dalglish SL, Kennedy CE, et al. . Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS 2016; 30:1973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owens DK, Davidson KW, Krist AH, et al. ; US Preventive Services Task Force . Preexposure prophylaxis for the prevention of HIV infection: US Preventive Services Task Force recommendation statement. JAMA 2019; 321:2203–13. [DOI] [PubMed] [Google Scholar]
- 3.Grant RM, Lama JR, Anderson PL, et al. ; iPrEx Study Team . Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant RM, Anderson PL, McMahan V, et al. ; iPrEx Study Team . Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14:820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deutsch MB, Glidden DV, Sevelius J, et al. ; iPrEx Investigators . HIV pre-exposure prophylaxis in transgender women: a subgroup analysis of the iPrEx trial. Lancet HIV 2015; 2:e512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehrotra ML, Westreich D, McMahan VM, et al. . Baseline characteristics explain differences in effectiveness of randomization to daily oral TDF/FTC PrEP between transgender women and cisgender men who have sex with men in the iPrEx trial. J Acquir Immune Defic Syndr 2019; 81:e94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cottrell ML, Prince HMA, Schauer AP, et al. . Decreased tenofovir diphosphate concentrations in a transgender female cohort: implications for human immunodeficiency virus preexposure prophylaxis. Clin Infect Dis 2019; 69:2201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiransuthikul A, Janamnuaysook R, Himmad K, et al. ; iFACT Study Team . Drug-drug interactions between feminizing hormone therapy and pre-exposure prophylaxis among transgender women: the iFACT Study. J Int AIDS Soc 2019; 22:e25338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shieh E, Marzinke MA, Fuchs EJ, et al. . Transgender women on oral HIV pre-exposure prophylaxis have significantly lower tenofovir and emtricitabine concentrations when also taking oestrogen when compared to cisgender men. J Int AIDS Soc 2019; 22:e25405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosek SG, Landovitz RJ, Kapogiannis B, et al. . Safety and feasibility of antiretroviral preexposure prophylaxis for adolescent men who have sex with men aged 15 to 17 years in the United States. JAMA Pediatr 2017; 171:1063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu AY, Cohen SE, Vittinghoff E, et al. . Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med 2015; 176:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer KH, Molina J-M, Thompson MA, et al. . Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet: 2020; 396:239–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Package insert: viread (tenofovir disoproxil fumarate) tablets. Foster City, CA: Gilead Sciences, 2004. [Google Scholar]
- 14.Mugo NR, Hong T, Celum C, et al. ; Partners PrEP Study Team . Pregnancy incidence and outcomes among women receiving preexposure prophylaxis for HIV prevention: a randomized clinical trial. JAMA 2014; 312:362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murnane PM, Heffron R, Ronald A, et al. ; Partners PrEP Study Team . Pre-exposure prophylaxis for HIV-1 prevention does not diminish the pregnancy prevention effectiveness of hormonal contraception. AIDS 2014; 28:1825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heffron R, Mugo N, Were E, et al. ; Partners PrEP Study Team . Preexposure prophylaxis is efficacious for HIV-1 prevention among women using depot medroxyprogesterone acetate for contraception. AIDS 2014; 28:2771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson PL, Liu AY, Castillo-Mancilla JR, et al. . Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother 2018; 62. doi: 10.1128/AAC.01710-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson PL, Liu AY, Castillo-Mancilla J, et al. . TFV-DP in dried blood spots (DBS) following directly observed therapy: DOT-DBS Study. Intern Med 2015; 1:11. [Google Scholar]
- 19.Zheng JH, Rower C, McAllister K, et al. . Application of an intracellular assay for determination of tenofovir-diphosphate and emtricitabine-triphosphate from erythrocytes using dried blood spots. J Pharm Biomed Anal 2016; 122:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jasuja GK, Travison TG, Davda M, et al. . Age trends in estradiol and estrone levels measured using liquid chromatography tandem mass spectrometry in community-dwelling men of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci 2013; 68:733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhasin S, Pencina M, Jasuja GK, et al. . Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab 2011; 96:2430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider HP, Heinemann LA, Rosemeier HP, Potthoff P, Behre HM. The Menopause Rating Scale (MRS): reliability of scores of menopausal complaints. Climacteric 2000; 3:59–64. [DOI] [PubMed] [Google Scholar]
- 23.Dinger J, Zimmermann T, Heinemann LA, Stoehr D. Quality of life and hormone use: new validation results of MRS scale. Health Qual Life Outcomes 2006; 4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. WHO implementation tool for pre-exposure prophylaxis (PrEP) of HIV infection: module 1: clinical.2017. Available at: https://apps.who.int/iris/bitstream/handle/10665/255889/WHO-HIV-2017.17-eng.pdf. Accessed 1 June 2020.
- 25.Grant RM, Koester KA. What people want from sex and preexposure prophylaxis. Curr Opin HIV AIDS 2016; 11:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knox DC, Anderson PL, Harrigan PR, Tan DH. Multidrug-resistant HIV-1 infection despite preexposure prophylaxis. N Engl J Med 2017; 376:501–2. [DOI] [PubMed] [Google Scholar]
- 27.Cohen SE, Sachdev D, Lee SA, et al. . Acquisition of tenofovir-susceptible, emtricitabine-resistant HIV despite high adherence to daily pre-exposure prophylaxis: a case report. Lancet HIV 2018. doi: 10.1016/S2352-3018(18)30288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markowitz M, Grossman H, Anderson PL, et al. . Newly acquired infection with multidrug-resistant HIV-1 in a patient adherent to preexposure prophylaxis. J Acquir Immune Defic Syndr 2017; 76:e104–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grinsztejn B, Hoagland B, Moreira RI, et al. ; PrEP Brasil Study Team . Retention, engagement, and adherence to pre-exposure prophylaxis for men who have sex with men and transgender women in PrEP Brasil: 48 week results of a demonstration study. Lancet HIV 2018; 5:e136–45. [DOI] [PubMed] [Google Scholar]
- 30.Ronen K, Sharma A, Overbaugh J. HIV transmission biology: translation for HIV prevention. AIDS 2015; 29:2219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cottrell ML, Garrett KL, Prince HMA, et al. . Single-dose pharmacokinetics of tenofovir alafenamide and its active metabolite in the mucosal tissues. J Antimicrob Chemother 2017; 72:1731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]