Abstract
Background
The efficacy of voluntary male medical circumcision (VMMC) for human immunodeficiency virus (HIV) prevention in men was demonstrated in 3 randomized trials. This led to the adoption of VMMC as an integral component of the United States President’s Emergency Plan for AIDS Relief (PEPFAR) combination HIV prevention program in sub-Saharan Africa. However, evidence on the individual-level effectiveness of VMMC programs in real-world, programmatic settings is limited.
Methods
A cohort of initially uncircumcised, non-Muslim, HIV-uninfected men in the Rakai Community Cohort Study in Uganda was followed between 2009 and 2016 during VMMC scale-up. Self-reported VMMC status was collected and HIV tests performed at surveys conducted every 18 months. Multivariable Poisson regression was used to estimate the incidence rate ratio (IRR) of HIV acquisition in newly circumcised vs uncircumcised men.
Results
A total of 3916 non-Muslim men were followed for 17 088 person-years (PY). There were 1338 newly reported VMMCs (9.8/100 PY). Over the study period, the median age of men adopting VMMC declined from 28 years (interquartile range [IQR], 21–35 years) to 22 years (IQR, 18–29 years) (P for trend < .001). HIV incidence was 0.40/100 PY (20/4992.8 PY) among newly circumcised men and 0.98/100 PY (118/12 095.1 PY) among uncircumcised men with an adjusted IRR of 0.47 (95% confidence interval, .28–.78). The effectiveness of VMMC was sustained with increasing time from surgery and was similar across age groups and calendar time.
Conclusions
VMMC programs are highly effective in preventing HIV acquisition in men. The observed effectiveness is consistent with efficacy in clinical trials and supports current recommendations that VMMC is a key component of programs to reduce HIV incidence.
Keywords: circumcision, VMMC, combination HIV prevention, Africa, PEPFAR
In a prospective cohort of uncircumcised men in Uganda, voluntary medical male circumcision through a United States President’s Emergency Plan for AIDS Relief–supported program resulted in a 53% reduction in human immunodeficiency virus risk that was sustained through 5 years of follow-up.
Randomized clinical trials in sub-Saharan Africa demonstrated that voluntary medical male circumcision (VMMC) reduces male human immunodeficiency virus (HIV) incidence by 50%–60% [1–3]. Based on these trials, the Joint United Nations Programme on HIV/AIDS (UNAIDS) and the World Health Organization (WHO) in 2007 recommended inclusion of VMMC in combination HIV prevention programs for countries with high prevalence of HIV and low prevalence of circumcision [4]. With support from the United States President’s Emergency Plan for AIDS Relief (PEPFAR), VMMC programs have been implemented in 15 priority sub-Saharan African countries [5]. As of 2018, >22 million VMMCs have been performed [6]. With this scale-up of VMMC, coupled with broad roll-out of antiretroviral therapy (ART), there have been substantial declines in HIV incidence continent-wide [7].
However, few population-based studies have assessed the individual-level effectiveness of VMMC for HIV prevention. Prior studies conducted in Uganda [8, 9], South Africa [10–12], and Kenya [13] reported significantly lower HIV incidence among circumcised compared with uncircumcised men. However, these studies generally did not exclude men who were circumcised for religious or cultural reasons and did not assess whether the protective effects of VMMC performed through programs diminished with increasing time from surgery. Understanding the longitudinal impact of VMMC programs is vital for developing evidence-based policies for reducing HIV incidence.
Our primary objective was to assess the impact of a PEPFAR-supported VMMC program on the risk of male HIV acquisition over time. Our study included initially HIV-negative, uncircumcised, non-Muslim men participating in the Rakai Community Cohort Study (RCCS), a longitudinal population-based study of HIV incidence in south-central Uganda, which has the highest HIV prevalence nationally [5]. Muslim men in the region, who comprise approximately 15% of the male population, are traditionally circumcised in infancy. Circumcision among non-Muslim men was historically rare but has become increasingly widespread following the implementation of free VMMC programs in 2008 [8]. As of 2017, approximately 60% of male RCCS participants were circumcised [14]. With scale-up of VMMC and ART in Uganda, HIV incidence among men in the RCCS has declined by 54% [8].
Here, we characterized trends in VMMC uptake in the RCCS and the risk of male HIV acquisition among newly circumcised non-Muslim men compared with uncircumcised non-Muslim men. We further assessed whether risk of HIV acquisition changed with estimated time from surgery. We hypothesized that VMMC through PEPFAR-supported programs would provide long-term protective effects against male HIV acquisition.
METHODS
Study Population
The RCCS is an ongoing open, population-based cohort study of persons aged 15–49 years in Rakai District and surrounding areas of south-central Uganda. The RCCS methodologies have been previously described [15]. In brief, population-based surveys are conducted approximately every 18 months. A household-based census precedes each survey to identify all eligible individuals aged 15–49 years and resident in the community regardless of whether they are present during the census. Participants are assigned unique identifiers to allow follow-up across survey rounds. At each survey, structured questionnaires are administered by same-sex interviewers to ascertain sociodemographic characteristics, health status, sexual risk behaviors, and service utilization, including circumcision. Written informed consent is obtained at enrollment and at each subsequent visit. The RCCS study was reviewed and approved by institutional review boards (IRBs) in Uganda (Research and Ethics Committee of the Uganda Virus Research Institute and the Uganda National Council for Science and Technology) and the Western IRB (Olympia, Washington) in the United States. The study was further reviewed in accordance with the United States Centers for Disease Control and Prevention (CDC) human research protection procedures and was determined to be research. However, CDC investigators did not interact with human subjects or have access to identifiable data or specimens for research purposes.
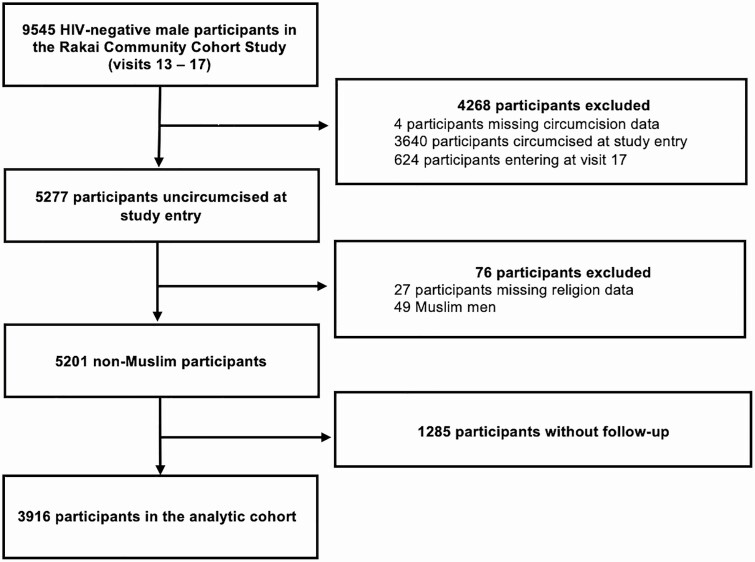
This analysis included 30 agrarian and semiurban trading communities continuously surveyed during 5 survey rounds between 2008 and 2016 (denoted as RCCS study visits 13–17). The analysis was restricted to non-Muslim men who were HIV uninfected and uncircumcised at entry into this study period (Figure 1).
Figure 1.
The analytical cohort.
HIV Testing and Ascertainment of Incident HIV Infection
Free HIV testing and counseling services are offered at each RCCS survey round. Between 2008 and 2011, 2 enzyme immunoassays (EIAs) were used for HIV screening with confirmatory testing by Western blot. Subsequently, a 3-rapid-test algorithm was used for initial screening, with confirmation by 2 EIAs, with Western blot or polymerase chain reaction assays as needed [8]. Our primary study outcome was an incident HIV infection, defined as cases in which a man was determined to be HIV seropositive for the first time after an HIV-seronegative result at the prior visit, allowing for up to 1 missed study visit between consecutive tests.
Assessment of Circumcision Status
VMMC services were initially provided to the participants assigned to the control group of the Rakai male VMMC trial after trial closure [16]. Subsequently, with PEPFAR support, the Rakai Health Sciences Program provided free VMMC services to consenting males aged 13 years and older. VMMC using the dorsal slit procedure is performed under local anesthesia by trained clinicians in mobile camps and at health facilities. VMMC status among male RCCS participants was assessed using self-report. Incident VMMCs were defined as those occurring among men who reported being uncircumcised at the prior study visit.
Information on the exact date of surgery for newly circumcised RCCS participants was unavailable from the RCCS interview. In our primary analysis examining effectiveness of VMMC for HIV prevention, VMMC was assumed to have occurred at the start of the person-interval, t0, preceding the first self-report of VMMC, t1. A participant who first reported being circumcised at t1 was therefore assumed to have been circumcised for the entirety of the person-interval (ie, the time period spanning t0 to t1). We performed 2 sensitivity analyses to assess the impact of this assignment of VMMC timing: (1) We designated the end of the person-period, t1, as the time of VMMC; and (2) we separately analyzed the risk of HIV acquisition during the intervals in which VMMC occurred. In this latter sensitivity analysis, VMMC status was classified as either uncircumcised (uncircumcised at t1), newly circumcised since the last survey (uncircumcised at t0 and circumcised at t1), or previously circumcised (circumcised at t0).
After the first report of VMMC, participants were assumed to be circumcised, even if they subsequently reported being uncircumcised. Sensitivity analyses were performed in which individuals who reported being uncircumcised after a prior report of VMMC were excluded (n = 68 [1.7% of the population of initially uncircumcised non-Muslim men]). Study visits where VMMC status was missing and could not be inferred were excluded from the analysis (n = 2 person-visits [0.02% of total]).
Statistical Analysis
Baseline sociodemographic characteristics and sexual behaviors were compared between men who adopted VMMC during follow-up and those who did not. Baseline differences were assessed with χ 2 tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. Temporal trends in the incidence of VMMC were evaluated with Poisson regression models. The unit of analysis was person-years (PY) of follow-up. For this analysis only, VMMC was assumed to have occurred at the midpoint of the person-interval during which it was first reported. To assess trends among those adopting VMMC, we examined the sociodemographic characteristics and sexual behaviors of newly circumcised men at each study visit compared to the rest of the male study population. Binomial 95% confidence intervals (CIs) for the prevalence of each characteristic were calculated using the Clopper-Pearson method [17]. Age-adjusted differences over time and between groups defined by VMMC status were assessed with logistic regression.
To evaluate the impact of VMMC status on HIV incidence, we employed adjusted and unadjusted Poisson regression models with generalized estimating equations under an exchangeable correlation structure with robust standard errors. The unit of analysis was PY of follow-up. The timing of incident HIV infection was defined as the midpoint between the last HIV negative test and the first positive result. Person-intervals spanning >2 missed visits were censored from the analysis due to imprecision in measurement of time-varying covariates and the timing of HIV acquisition. Multivariable Poisson regression models were adjusted for study visit, age, marital status, and educational attainment. We further adjusted for high-risk sexual behaviors in the prior year in a separate model as these behaviors may be either mediators or confounders. In these models, we adjusted for self-reported sexual relationships with partners residing in and outside of the community of residence, multiple sex partners, genital ulcer disease, and consistent condom use with casual partners.
To assess the longitudinal risk of HIV acquisition with increasing time from the VMMC surgery, study intervals were classified by the time from the estimated date of VMMC. Time from surgery was defined as the midpoint of each person-interval minus the estimated date of surgery. Analyses were performed using Stata version 14 software (StataCorp, College Station, Texas).
RESULTS
Study Population
The analytical cohort consisted of 3916 men who were followed for a total of 17 088 PY (12 095.1 uncircumcised PY, 4992.8 circumcised PY) over 9469 person-intervals (Figure 1). Of these participants, 34% (n = 1338) were circumcised during follow-up, with an overall incidence of VMMC of 9.80/100 PY (95% CI, 9.26–10.37). The incidence rate of VMMC increased monotonically over the study period from 7.0/100 PY to 11.6/100 PY by 2016 (Supplementary Table 1). The sociodemographic characteristics and sexual behaviors of the population at study entry are presented in Table 1. Men adopting VMMC during follow-up were, at baseline, significantly younger than those who remained uncircumcised throughout follow-up, more likely to be unmarried, not to have initiated sex, to use condoms with casual sexual partners, and be free of genital ulcer disease.
Table 1.
Demographic Characteristics and Sexual Behaviors of the Population at Study Entry
| Characteristic | Uncircumcised Throughout Follow-up (n = 2578) | Circumcised After Study Entry (n = 1338)a | P Value |
|---|---|---|---|
| Age, y | |||
| Median (IQR) | 27 (19–35) | 23 (18–31) | <.001 |
| 15–19 | 676 (26) | 472 (35) | <.001 |
| 20–24 | 433 (17) | 264 (20) | |
| 25–29 | 415 (16) | 225 (17) | |
| 30–34 | 368 (14) | 159 (12) | |
| 35–39 | 294 (11) | 127 (9) | |
| ≥40 | 392 (15) | 91 (7) | |
| Educational attainment | |||
| None | 68 (3) | 23 (2) | .20 |
| Primary | 1653 (64) | 848 (63) | |
| Secondary | 670 (26) | 374 (28) | |
| University or technical | 187 (7) | 93 (7) | |
| Marital status | |||
| Never married | 1117 (43) | 710 (53) | <.001 |
| Currently married | 1267 (49) | 565 (42) | |
| Previously married | 194 (8) | 63 (5) | |
| Religion | |||
| Catholic | 1966 (76) | 1018 (76) | .80 |
| Protestant | 483 (19) | 261 (20) | |
| Saved/born again or Pentecostal | 112 (4) | 52 (4) | |
| None or other | 17 (1) | 7 (1) | |
| Sexual behaviors | |||
| Initiated sex | 2192 (85) | 1074 (80) | <.001 |
| >1 partner in prior year | 767 (30) | 380 (29) | .40 |
| Partners from outside the community in past year | 696 (27) | 333 (25) | .19 |
| Consistent condom useb | 369 (43) | 223 (52) | .002 |
| Genital ulcer disease in past year | 234 (9) | 91 (7) | .014 |
Data are presented as no. (%) unless otherwise indicated.
Abbreviation: IQR, interquartile range.
aIncludes 33 men circumcised during a gap in follow-up.
bAmong those reporting casual sexual partnerships in the past year.
Characteristics of Newly Circumcised Men
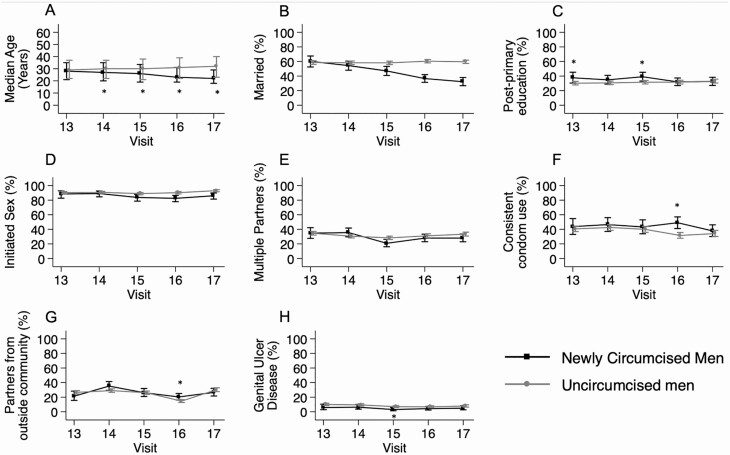
Figure 2 shows longitudinal trends in age, marital status, education level, and sexual behaviors among men first adopting VMMC. The age of men first reporting VMMC decreased consistently over time (P for trend < .001). Newly circumcised men were significantly younger than uncircumcised men at all visits except the first study visit. After age adjustment, the proportion of newly circumcised men in marital relationships declined over time (P for trend < .001; Figure 2B). After age adjustment, there were no significant temporal trends in the prevalence of sexual behaviors or postprimary education among newly circumcised men (Figure 2C–H).
Figure 2.
Demographic characteristics and sexual behaviors of newly circumcised men compared to uncircumcised men. A, Error bars denote interquartile range of participants’ age. B–H, Error bars denote 95% confidence interval for attribute prevalence. *Significant age-adjusted difference in prevalence between groups at the given study visit (P < .05).
Circumcision and HIV Incidence
Over the analysis period, there were 138 incident HIV infections detected. HIV incidence was 0.40/100 PY among circumcised men (20/4992.8 PY), and 0.98/100 PY) among uncircumcised men (118/12 095.1 PY). After adjusting for age, education, and marital status, VMMC was associated with a 51% lower risk of incident HIV infection in men (adjusted incidence rate ratio [IRR], 0.49 [95% CI, .30–.81]). Further adjustment for sexual behaviors did not markedly affect risk estimates (IRR, 0.47 [95% CI, .28–.78]). Despite limited statistical power, we observed similar effectiveness of VMMC in preventing HIV acquisition across age groups and strata of calendar time (Table 2). Sensitivity analyses (1) excluding men who had not initiated sex; (2) excluding those with contradictory self-reported VMMC status (ie, men who reported being circumcised and subsequently reported being uncircumcised); and (3) assuming that VMMC occurred at the end of the person-interval during which it was first reported all had no impact on risk estimates and the protective effect of circumcision (Supplementary Tables 2–4).
Table 2.
Human Immunodeficiency Virus Incidence by Voluntary Medical Male Circumcision Status and Demographic Factors
| Characteristic | Circumcision Status | No. of Incident Infections/PY at Risk | Incidence Rate per 100 PY (95% CI) | Crude IRR (95% CI) | Adjusted IRR: Demographics | Adjusted IRR: Demographics and Sexual Behaviors | |
|---|---|---|---|---|---|---|---|
| Overall | Uncircumcised | 118/12 095.09 | 0.98 (.81–1.17) | Ref | Ref | Ref | |
| Circumcised | 20/4992.78 | 0.40 (.26–.62) | 0.41 (.25–.66) | 0.49 (.30–.81) | 0.47 (.28–.78) | ||
| Age, y | 15–24 | Uncircumcised | 25/3934.02 | 0.64 (.43–.94) | Ref | Ref | Ref |
| Circumcised | 5/1921.50 | 0.26 (.11–.63) | 0.41 (.16–1.07) | 0.54 (.18–1.65) | 0.34 (.09–1.33) | ||
| 25–34 | Uncircumcised | 60/3813.10 | 1.57 (1.22–2.03) | Ref | Ref | Ref | |
| Circumcised | 11/1619.42 | 0.68 (.37–1.22) | 0.43 (.23–.82) | 0.48 (.25–.93) | 0.55 (.29–1.06) | ||
| ≥35 | Uncircumcised | 33/4347.98 | 0.76 (.54–1.07) | Ref | Ref | Ref | |
| Circumcised | 4/1451.85 | 0.28 (.10–.74) | 0.36 (.13–1.03) | 0.49 (.17–1.43) | 0.47 (.16–1.35) | ||
| Study visits | 13–14 (2008–2011) | Uncircumcised | 64/4820.95 | 1.33 (1.04–1.70) | Ref | Ref | Ref |
| Circumcised | 8/935.55 | 0.86 (.43–1.72) | 0.64 (.31–1.35) | 0.68 (.32–1.44) | 0.53 (.23–1.26) | ||
| 15–17 (2011–2016) | Uncircumcised | 54/7274.14 | 0.74 (.57–.97) | Ref | Ref | Ref | |
| Circumcised | 12/4057.22 | 0.30 (.17–.52) | 0.40 (.21–.75) | 0.41 (.22–.76) | 0.44 (.23–.83) |
Demographic adjustment only: adjusted for study visit, age in years, marital status, and educational attainment. Sexual behavior adjustment: additionally adjusted for sex with partners residing outside of the community in the prior year, sex with multiple partners in the past year, history of genital ulcer disease in the past year, and consistent condom use with casual partner.
Abbreviations: CI, confidence interval; IRR, incidence rate ratio; PY, person-years; Ref, reference group.
Of note, HIV incidence declined over the study period both in circumcised men (0.86 infections/100 PY for study visits 13–14 vs 0.30 infections/100 PY for study visits 15–17; a 65% decline), and in men who remained uncircumcised (1.33 infections/100 PY for study visits 13–14 vs 0.74 infections/100 PY for study visits 15–17; a 44% decline).
Risk of HIV Acquisition With Estimated Time From Surgery
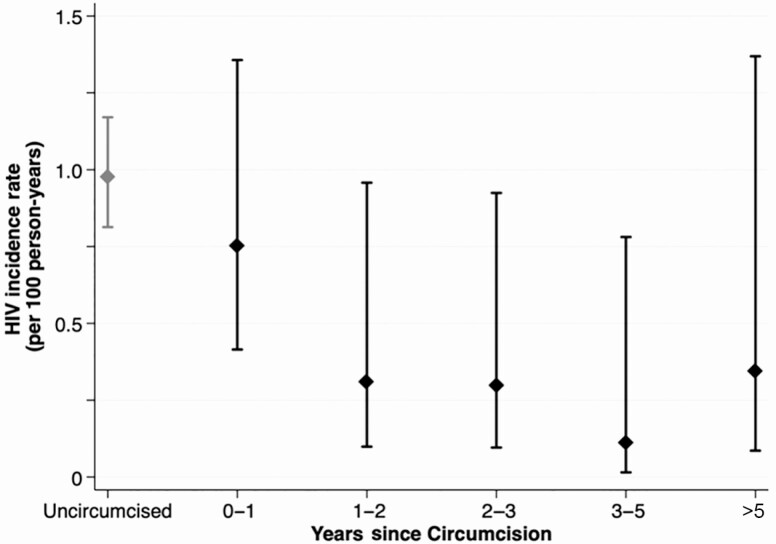
Among circumcised men, 60% of HIV infections (12/20) were detected at the same visit at which VMMC was first reported. Figure 3 presents the effectiveness of VMMC for HIV prevention by the estimated time since surgery. HIV incidence was 0.75/100 PY (95% CI, .41–1.36) during the year immediately following VMMC. This was nonsignificantly lower than the HIV incidence among uncircumcised men (adjusted IRR, 0.69 [95% CI, .35–1.39]). HIV incidence declined substantially to 0.31/100 PY (95% CI, .09–.95) 1–2 years following VMMC and remained low with increasing years from surgery.
Figure 3.
Longitudinal effectiveness of voluntary male medical circumcision for human immunodeficiency virus (HIV) prevention. HIV incidence per 100 person-years among non-Muslim uncircumcised and circumcised men by estimated time since surgery in years. Time since surgery was defined as the interval between the start of the person-period in which circumcision was reported to the midpoint of a given person-period.
DISCUSSION
In this population-based study in Uganda, we found widespread adoption of VMMC with 34% of eligible non-Muslim men newly circumcised between 2008 and 2016. We estimate the effectiveness of VMMC for prevention of HIV acquisition among newly circumcised men to be 53%, similar both to the efficacy observed in clinical trials of VMMC [1–3] and the effectiveness reported in observational studies of prevalent VMMC in sub-Saharan Africa [9, 11, 13]. We also found that men adopting VMMC were younger and more likely to be unmarried, as observed in other PEPFAR-supported VMMC programs in sub-Saharan Africa, and that these differences have become more accentuated over time [10, 11, 13, 18, 19]. Notably, we found that the protective effects of VMMC appear to be sustained for >5 years postsurgery. We found no significant differences in the protective effects of VMMC over calendar time, with a trend toward increased effectiveness in more recent years. These findings strongly support the continued inclusion of VMMC in combination HIV prevention strategies.
With the leadership and support of national ministries of health and PEPFAR, VMMC programs have been implemented and expanded throughout sub-Saharan Africa [6]. These efforts have provided lasting partial protection against HIV to millions of circumcised men, and likely have contributed to declines in HIV incidence among both men and women [20]. Emerging data from Uganda, Kenya, and South Africa show sharply declining HIV incidence with scale-up of VMMC, with the lowest overall HIV incidence among circumcised men [8–10, 13]. In Zimbabwe, VMMC programs are estimated to have averted up to 12 000 infections as of 2016 with future projections of >100 000 infections averted by 2030 [21]. It is likely that expanded VMMC programs will result in similar numbers of averted infections across sub-Saharan Africa, making it among the most effective HIV prevention tools for control of generalized heterosexual epidemics. In addition to HIV prevention, VMMC programs provide a secondary benefit of decreasing the risk of other sexually transmitted infections among both circumcised men and their female partners [22–25].
While VMMC uptake was high in this population, the overall prevalence of VMMC still fell short of the international targets of 80% population-level coverage [26]. We found that younger men were increasingly more likely to adopt VMMC over time. Findings from modeling studies suggest that increasing VMMC coverage among young men will result in significant reductions in HIV incidence over the long term. However, the short-term impact may be less because male HIV incidence is concentrated in older men [27]. Future strategies to increase VMMC uptake should focus on all age groups with consideration for both short- and long-term reductions in HIV incidence [28, 29].
Similar to the findings from extended follow-up of participants from VMMC trials [19, 30], we found no diminution in the protective effects of VMMC with increasing time from surgery, This suggests that the benefits of VMMC for HIV prevention are likely lifelong. We further found that HIV incidence was higher in the period immediately following VMMC relative to later time points, though lower than among uncircumcised men. This may be due to misclassification of timing of VMMC relative to HIV acquisition because the precise dates of surgery and HIV acquisition were unknown and both were estimated based on a priori assumptions. However, prior studies have found increased HIV risk among men who resume sexual activity prior to wound healing, which may also partly explain these findings [31, 32].
The largest reductions in HIV incidence due to VMMC have been observed in high HIV incidence communities [33, 34]. However, in our study population, we found that VMMC remained protective despite declining HIV incidence. This suggests that VMMC should remain a central component of HIV prevention in settings with low HIV incidence and receding epidemics. Results from large randomized trials of universal ART for HIV prevention show limitations to achieving reductions in incidence through the lowering of community viral load alone [35–37]. Thus, interventions that afford direct protective benefits, including VMMC and oral preexposure prophylaxis (PrEP), continue to be essential for epidemic control. In contrast to PrEP, where roll-out has proved challenging in some settings due to poor uptake and adherence [38, 39], VMMC is a 1-time intervention with lasting benefit.
This observational study has limitations. Circumcision status and sexual behaviors were ascertained via self-report and may be subject to social desirability and recall biases. Circumcision status was ascertained at intervals of approximately 18 months between study visits, and the exact date of VMMC was unknown. This limited our ability to detect short-term changes in HIV risk immediately following VMMC. For participants who first report VMMC at the same interval in which HIV was detected, it was not possible to establish the relative timing of these 2 events (ie, did VMMC precede or succeed HIV acquisition). However, the results of sensitivity analyses suggest little impact of assumptions regarding the relative timing on the estimated effectiveness of VMMC for HIV prevention, and HIV incidence was stable and consistently low during subsequent follow-up intervals in which surgery clearly preceded incident infections. We could not determine exposure to HIV-infected female partners, and men who accepted VMMC were younger, more likely to be unmarried, less likely to have initiated sex, and more likely to use condoms after sexual debut, all of which are characteristics associated with lower risk of heterosexual HIV acquisition. While we adjusted for demographic characteristics and sexual risk behaviors, we cannot exclude the possibility of residual confounding. Finally, data on Oral PrEP use among study participants were not collected. However, PrEP availability was scarce in the region during the study period, and we believe any resulting bias to be minimal.
In conclusion, we find that men circumcised through PEPFAR-supported VMMC programs are at significantly lower risk of HIV acquisition than uncircumcised men, consistent with findings from randomized trials and observational studies of prevalent VMMC. These findings strongly support the promotion of VMMC in combination HIV prevention programs in order to achieve the goal of epidemic control by 2030 [40].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
Financial support. This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID); the NIAID (grant numbers R01AI128779, R01AI110324, U01AI100031, U01AI075115, and K01AI125086-01); the National Institute of Mental Health (grant number R01MH107275); the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant numbers RO1HD070769 and R01HD050180); the Johns Hopkins University Center for AIDS Research (grant number P30AI094189); and the President’s Emergency Plan for AIDS Relief through the Centers for Disease Control and Prevention under the terms of NU2GGH000817.
Potential conflicts of interest. M. J. W. and R. G. report consulting fees as part of the Rakai Health Sciences Program. Contributions to this publication/presentation from M. J. W. and R. G. were as members of the Board of Directors of the Rakai Health Sciences Program. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed. Rakai Health Sciences Program Study Team Contributors: Dorean Nabukalu, Anthony Ndyanabo, Joseph Ssekasanvu, Hadijja Nakawooya, Jessica Nakukumba, Grace N. Kigozi, Betty S. Nantume, Nampijja Resty, Jedidah Kambasu, Margaret Nalugemwa, Regina Nakabuye, Lawrence Ssebanobe, Justine Nankinga, Adrian Kayiira, Gorreth Nanfuka, Ruth Ahimbisibwe, Stephen Tomusange, Ronald M. Galiwango, Sarah Kalibbali, Margaret Nakalanzi, Joseph Ouma Otobi, Denis Ankunda, Joseph Lister Ssembatya, John Baptist Ssemanda, Robert Kairania, Emmanuel Kato, Alice Kisakye, James Batte, James Ludigo, Abisagi Nampijja, Steven Watya, Kighoma Nehemia, Sr. Margaret Anyokot, Joshua Mwinike, George Kibumba, Paschal Ssebowa, George Mondo, Francis Wasswa, Agnes Nantongo, Rebecca Kakembo, Josephine Galiwango, Geoffrey Ssemango, Andrew D. Redd, John Santelli, Caitlin E. Kennedy, Jennifer Wagman,Tom Lutalo, Fred Makumbi Nelson K. Sewankambo, Oliver Laeyendecker.
Contributor Information
Rakai Health Sciences Program:
Dorean Nabukalu, Anthony Ndyanabo, Joseph Ssekasanvu, Hadijja Nakawooya, Jessica Nakukumba, Grace N Kigozi, Betty S Nantume, Nampijja Resty, Jedidah Kambasu, Margaret Nalugemwa, Regina Nakabuye, Lawrence Ssebanobe, Justine Nankinga, Adrian Kayiira, Gorreth Nanfuka, Ruth Ahimbisibwe, Stephen Tomusange, Ronald M Galiwango, Sarah Kalibbali, Margaret Nakalanzi, Joseph Ouma Otobi, Denis Ankunda, Joseph Lister Ssembatya, John Baptist Ssemanda, Robert Kairania, Emmanuel Kato, Alice Kisakye, James Batte, James Ludigo, Abisagi Nampijja, Steven Watya, Kighoma Nehemia, Sr Margaret Anyokot, Joshua Mwinike, George Kibumba, Paschal Ssebowa, George Mondo, Francis Wasswa, Agnes Nantongo, Rebecca Kakembo, Josephine Galiwango, Geoffrey Ssemango, Andrew D Redd, John Santelli, Caitlin E Kennedy, Jennifer Wagman, Tom Lutalo, Fred Makumbi, Nelson K Sewankambo, and Oliver Laeyendecker
References
- 1.Gray RH, Kigozi G, Serwadda D, et al. . Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet 2007; 369:657–66. [DOI] [PubMed] [Google Scholar]
- 2.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 trial. PLoS Med 2005; 2:e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey RC, Moses S, Parker CB, et al. . Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet 2007; 369:643–56. [DOI] [PubMed] [Google Scholar]
- 4.Male circumcision for HIV prevention: research implications for policy and programming. WHO/UNAIDS technical consultation, 6–8 March 2007. Conclusions and recommendations (excerpts). Reprod Health Matters 2007; 15:11–4. [DOI] [PubMed] [Google Scholar]
- 5.Uganda Ministry of Health. Uganda population-based HIV impact assessment (UPHIA) 2016–2017: final report. Kampala, Uganda: Ministry of Health, Uganda, 2019. [Google Scholar]
- 6.Joint United Nations Programme on HIV/AIDS/World Health Organization. Voluntary medical male circumcision: remarkable progress in the scale up of VMMC as an HIV prevention intervention in 15 ESA countries. Geneva, Switzerland: UNAIDS/WHO, 2019. [Google Scholar]
- 7.Joint United Nations Programme on HIV/AIDS. Global HIV statistics. Geneva, Switzerland: UNAIDS, 2019. [Google Scholar]
- 8.Grabowski MK, Serwadda DM, Gray RH, et al. . HIV prevention efforts and incidence of HIV in Uganda. N Engl J Med 2017; 377:2154–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kagaayi J, Chang LW, Ssempijja V, et al. . Impact of combination HIV interventions on HIV incidence in hyperendemic fishing communities in Uganda: a prospective cohort study. Lancet HIV 2019; 6:e680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auvert B, Taljaard D, Rech D, et al. . Association of the ANRS-12126 male circumcision project with HIV levels among men in a South African township: evaluation of effectiveness using cross-sectional surveys. PLoS Med 2013; 10:e1001509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lissouba P, Taljaard D, Rech D, et al. . Adult male circumcision as an intervention against HIV: an operational study of uptake in a South African community (ANRS 12126). BMC Infect Dis 2011; 11:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandormael A, Akullian A, Siedner M, de Oliveira T, Bärnighausen T, Tanser F. Declines in HIV incidence among men and women in a South African population–based cohort. Nat Commun 2019; 10:5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borgdorff MW, Kwaro D, Obor D, et al. . HIV incidence in western Kenya during scale-up of antiretroviral therapy and voluntary medical male circumcision: a population-based cohort analysis. Lancet HIV 2018; 5:e241–9. [DOI] [PubMed] [Google Scholar]
- 14.Kong X, Kigozi G, Ssekasanvu J, et al. . Medical male circumcision coverage in Rakai, Uganda. AIDS 2017; 31:735–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang LW, Grabowski MK, Ssekubugu R, et al. . Heterogeneity of the HIV epidemic in agrarian, trading, and fishing communities in Rakai, Uganda: an observational epidemiological study. Lancet HIV 2016; 3:e388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kong X, Kigozi G, Nalugoda F, et al. . Assessment of changes in risk behaviors during 3 years of posttrial follow-up of male circumcision trial participants uncircumcised at trial closure in Rakai, Uganda. Am J Epidemiol 2012; 176:875–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934; 26:404–13. [Google Scholar]
- 18.Davis SM, Hines JZ, Habel M, et al. . Progress in voluntary medical male circumcision for HIV prevention supported by the US President’s Emergency Plan for AIDS Relief through 2017: longitudinal and recent cross-sectional programme data. BMJ Open 2018; 8:e021835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray R, Kigozi G, Kong X, et al. . The effectiveness of male circumcision for HIV prevention and effects on risk behaviors in a posttrial follow-up study. AIDS 2012; 26:609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GBD 2017 HIV Collaborators. Global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2017, and forecasts to 2030, for 195 countries and territories: a systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV 2019; 6:e831–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGillen JB, Stover J, Klein DJ, et al. . The emerging health impact of voluntary medical male circumcision in Zimbabwe: an evaluation using three epidemiological models. PLoS One 2018; 13:e0199453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray RH, Serwadda D, Tobian AA, et al. . Effects of genital ulcer disease and herpes simplex virus type 2 on the efficacy of male circumcision for HIV prevention: analyses from the Rakai trials. PLoS Med 2009; 6:e1000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tobian AA, Serwadda D, Quinn TC, et al. . Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med 2009; 360:1298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wawer MJ, Tobian AA, Kigozi G, et al. . Effect of circumcision of HIV-negative men on transmission of human papillomavirus to HIV-negative women: a randomised trial in Rakai, Uganda. Lancet 2011; 377:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sobngwi-Tambekou J, Taljaard D, Lissouba P, et al. . Effect of HSV-2 serostatus on acquisition of HIV by young men: results of a longitudinal study in Orange Farm, South Africa. J Infect Dis 2009; 199:958–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joint United Nations Programme on HIV/AIDS/World Health Organization. Joint strategic action framework to accelerate the scale-up of voluntary medical male circumcision for HIV prevention in eastern and southern Africa: 2012–2016. Geneva, Switzerland: UNAIDS/WHO, 2011. [Google Scholar]
- 27.Kripke K, Opuni M, Schnure M, et al. . Age targeting of voluntary medical male circumcision programs using the decision makers’ program planning toolkit (DMPPT) 2.0. PLoS One 2016; 11:e0156909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaufman MR, Smelyanskaya M, Van Lith LM, et al. . Adolescent sexual and reproductive health services and implications for the provision of voluntary medical male circumcision: results of a systematic literature review. PLoS One 2016; 11:e0149892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel EU, Kaufman MR, Dam KH, et al. . Age differences in perceptions of and motivations for voluntary medical male circumcision among adolescents in South Africa, Tanzania, and Zimbabwe. Clin Infect Dis 2018; 66:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta SD, Moses S, Agot K, et al. . The long-term efficacy of medical male circumcision against HIV acquisition. AIDS 2013; 27:2899–907. [DOI] [PubMed] [Google Scholar]
- 31.Njeuhmeli E, Hatzold K, Gold E, et al. . Lessons learned from scale-up of voluntary medical male circumcision focusing on adolescents: benefits, challenges, and potential opportunities for linkages with adolescent HIV, sexual, and reproductive health services. J Acquir Immune Defic Syndr 2014; 66:S193–9. [DOI] [PubMed] [Google Scholar]
- 32.Ediau M, Matovu JK, Byaruhanga R, Tumwesigye NM, Wanyenze RK. Risk factors for HIV infection among circumcised men in Uganda: a case-control study. J Int AIDS Soc 2015; 18:19312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavreys L, Rakwar JP, Thompson ML, et al. . Effect of circumcision on incidence of human immunodeficiency virus type 1 and other sexually transmitted diseases: a prospective cohort study of trucking company employees in Kenya. J Infect Dis 1999; 180:330–6. [DOI] [PubMed] [Google Scholar]
- 34.Gray RH, Kiwanuka N, Quinn TC, et al. . Male circumcision and HIV acquisition and transmission: cohort studies in Rakai, Uganda. Rakai Project Team. AIDS 2000; 14:2371–81. [DOI] [PubMed] [Google Scholar]
- 35.Hayes RJ, Donnell D, Floyd S, et al. . Effect of universal testing and treatment on HIV incidence—HPTN 071 (PopART). N Engl J Med 2019; 381:207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Havlir DV, Balzer LB, Charlebois ED, et al. . HIV testing and treatment with the use of a community health approach in rural Africa. N Engl J Med 2019; 381:219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makhema J, Wirth KE, Pretorius Holme M, et al. . Universal testing, expanded treatment, and incidence of HIV infection in Botswana. N Engl J Med 2019; 381:230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marrazzo JM, Ramjee G, Richardson BA, et al. . Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015; 372:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koss CA, Ayieko J, Mwangwa F, et al. . Early adopters of human immunodeficiency virus preexposure prophylaxis in a population-based combination prevention study in rural Kenya and Uganda. Clin Infect Dis 2018; 67:1853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization. HIV/AIDS: framework for action in the WHO African region, 2016–2020. Geneva, Switzerland: WHO, 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.