Abstract
Background
Acute vulvovaginal candidiasis (VVC) is common among women, but current azole antifungal treatments are often associated with safety and resistance issues. VT-1161 (oteseconazole) is an oral agent with increased selectivity for fungal CYP51. In this phase 2 clinical study, we evaluated the efficacy and safety of VT-1161 vs fluconazole in participants with moderate to severe acute VVC.
Methods
Participants presenting with an acute episode of VVC (n = 55) were randomized to receive VT-1161 300 mg once daily (q.d.) for 3 days, 600 mg q.d. for 3 days, or 600 mg twice daily (b.i.d.) for 3 days or to receive a single dose of fluconazole 150 mg (FDA-approved dose to treat acute VVC). Participants were followed for 6 months. The primary outcome was the proportion of participants with therapeutic (clinical and mycological) cure at day 28.
Results
A larger proportion of participants in the per-protocol population experienced therapeutic cure in the VT-1161 300 mg q.d. (75.0%), VT-1161 600 mg q.d. (85.7%), and VT-1161 600 mg b.i.d. (78.6%) groups vs the fluconazole group (62.5%); differences were not statistically significant. At 3 and 6 months, no participants in the VT-1161 groups vs 28.5% and 46.1% in the fluconazole group, respectively, had evidence of mycological recurrence. No serious adverse events or treatment-emergent adverse events leading to discontinuation were reported.
Conclusions
The majority of participants across all treatment groups achieved therapeutic cure at day 28. VT-1161 was well tolerated at all dose levels through 6 months of follow-up.
Clinical Trials Registration
Keywords: acute vulvovaginal candidiasis, randomized clinical study, VT-1161
In a phase 2 randomized clinical study, VT-1161, a novel antifungal agent, was shown to be safe and efficacious in women with acute vaginal candidiasis.
Acute vulvovaginal candidiasis (VVC) is typically treated with a single dose or short treatments of either topical or oral azole antifungals, while recurrent VVC requires treatments that can last ≥6 months [1, 2]. Fluconazole remains the current standard of care; however, drug resistance and safety liabilities, including hepatic toxicity, drug–drug interactions (DDIs), pregnancy warnings, and miscarriage concerns, are associated with its use [3–7].
VT-1161 (oteseconazole) was designed to be highly selective for fungal CYP51, thus avoiding off-target toxicities, including pregnancy and miscarriage concerns often associated with the azole drug class [8]. Moreover, VT-1161 has demonstrated greater potency against Candida species, including azole-resistant species, than fluconazole [9]. Our goal in this phase 2a study was to evaluate the safety and efficacy of VT-1161 vs fluconazole in the treatment of patients with moderate to severe acute VVC.
METHODS
Study Design
This was a phase 2, multicenter, randomized, double-blind, active-controlled, parallel-group, dose-ranging trial designed to evaluate the efficacy, safety, and pharmacokinetics (PK) of 3 dose levels of oral VT-1161 compared with a single dose of fluconazole, the current US Food and Drug Administration–approved regimen [10]. The study was conducted at 8 sites in the United States between October 2013 and September 2014.
The available data suggested that VT-1161 plasma exposures of 1–1.5 µg/mL should be clinically effective. This exposure target was therefore chosen to guide the selection of the low-dose arm. Given the safety profile of VT-1161, the mid-dose arm regimen would provide higher exposures (approximately 2-fold greater than the low-dose arm) and would afford the assessment of PK/pharmacodynamic relationships. The study design also afforded the opportunity to escalate to a higher-dose arm.
Participants were randomly assigned to receive 1 of the following dose regimens (Figure 1): VT-1161 300 mg once daily (q.d.) for 3 days (low dose), VT-1161 600 mg q.d. for 3 days (mid dose), or VT-1161 600 mg twice daily (b.i.d.) for 3 days (high dose) or fluconazole 150 mg administered orally in a single dose (administered blinded to match the VT-1161 dose regimens).
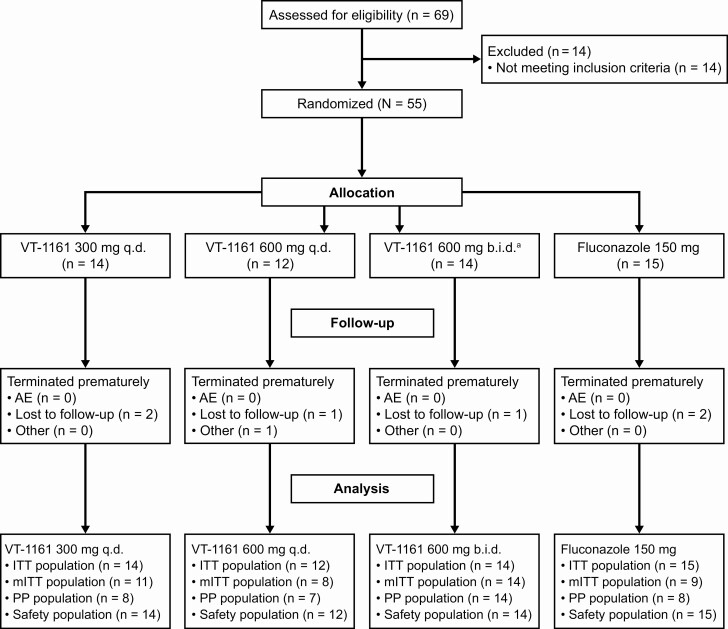
Figure 1.
Flow diagram of participant disposition. aAfter all participants in the 2 lower VT-1161 dose regimens completed their test-of-cure visit (day 28), an interim analysis was conducted to determine randomization into the VT-1161 600 mg b.i.d. group. Abbreviations: AE, adverse event; b.i.d., twice daily; ITT, intent-to-treat; mITT, modified intent-to-treat; PP, per-protocol; q.d., once daily; VT-1161, oteseconazole.
An interactive web response system was used to initially randomize participants in a 3:3:2 ratio to 300 mg q.d. or 600 mg q.d. VT-1161 or fluconazole. Participants were stratified by composite vulvovaginal signs and symptoms severity score (6–8 and ≥9) at screening and monitored to ensure that ≥50% of the participants had a VVC severity score of ≥9.
An interim safety analysis was performed after all participants randomized to receive VT-1161 300 mg q.d. and 600 mg q.d. (first randomization) had completed their test-of-cure (TOC) visit on day 28. Demographics and baseline characteristics, adverse events (AEs), laboratory assessments, electrocardiograms (ECGs), and efficacy. PK data were summarized and reviewed by the independent medical monitor. Based on this analysis, the VT-1161 600 mg b.i.d. group was added. After the interim analysis, participants were randomized in a 3:1 ratio to 600 mg b.i.d. VT-1161 or fluconazole. A second interim analysis was performed to review safety, efficacy, and PK when the last participant of the VT-1161 600 mg b.i.d. group completed their TOC visit. The duration of individual participant participation was approximately 6 months.
Participants returned to the clinic on days 7, 14, 28, 84 (3 months), and 168 (6 months) for efficacy, safety, laboratory, and PK assessments. TOC was assessed on day 28.
The trial was conducted in accordance with the Declaration of Helsinki and the Guidelines for Good Clinical Practice. The study protocol was reviewed and approved by the appropriate institutional review board. All participants gave written informed consent before participating in the study.
Study Population
Healthy, nonpregnant female participants aged ≥18 years and <65 years with a clinical diagnosis of symptomatic acute VVC were enrolled in the study. Participants had a positive baseline potassium hydroxide (KOH) wet mount from a vaginal smear revealing filamentous hyphae/pseudohyphae or budding yeast cells, presence of ≥1 vulvovaginal sign, presence of ≥1 vulvovaginal symptom, and a composite severity score of ≥6. Vaginal swabs were obtained at the screening visit and at subsequent study visits. Candida species were characterized and stored frozen for susceptibility testing at study completion. Inclusion criteria required participants to present with a clinical diagnosis of acute VVC and did not differentiate between acute and recurrent VVC participants.
Participants did not have any clinically significant major organ system disease or infection (with the exception of VVC) and were not taking concomitant medications that would have interfered with study assessments.
Participants completed a review of pertinent medical history, vital signs, and body measurements; a symptom/sign evaluation; a physical examination including speculum examination of the vagina; and a KOH wet mount test from a vaginal smear.
Outcomes
Efficacy measurements included collection of clinical signs (erythema, edema, excoriation) and symptoms (itching, burning, irritation) of vulvovaginitis using a scoring scale of 0–3 (0 = none, 1 = mild, 2 = moderate, 3 = severe) for each sign or symptom to give a total severity score of 0–18 [11]. In addition, a KOH wet mount test and vaginal fungal culture were performed at each visit.
The primary efficacy outcome was the proportion of participants with therapeutic cure, defined as both clinical and mycological cure, at the TOC day 28 visit. Mycological cure was defined as a negative fungal culture for Candida species. Clinical cure was defined as follows: complete resolution of signs and symptoms pertaining to VVC and any new sign or symptom observed at the TOC visit determined by the investigator not to be related to VVC.
The secondary efficacy outcome measures were the proportion of participants with the following: clinical cure at day 28; mycological cure at days 7, 14, and 28; clinical improvement (every individual VVC signs and symptoms score that was 0, 1, or 2 at baseline was a score of 0 at day 28, and scores of 3 at baseline were scores of 0 or 1 at day 28); clinical relapse (clinical cure at day 28 and signs and symptoms total severity score >0 at day 84 or day 168); mycological reinfection or recurrence (negative fungal culture at day 28 and positive fungal culture for Candida species at day 84 or day 168); modified clinical cure (total VVC severity score of ≤1 at day 28); modified therapeutic cure (modified clinical cure and mycological cure at day 28); and therapeutic improvement (clinical improvement and mycological cure at day 28). Additional evaluations included the following: number of participants with a change in clinical signs and symptoms from baseline to day 28; number of participants with positive culture and KOH test at days 28, 84, and 168; and PK analyses.
PK samples were obtained from all participants prior to dosing of the study drug on day 1 and at each subsequent visit. Serial sampling was obtained from consenting participants at participating sites on day 1 (pre-dose and 1, 2, 3, 4, 5, 6, and 8 hours after dosing).
AEs were collected throughout the study. Physical examination findings, vital signs, ECGs, and safety laboratory tests (hematology, chemistry, and urinalysis) were recorded at baseline and throughout the study.
Determination of Minimum Inhibitory Concentration
The minimum inhibitory concentration (MIC) of VT-1161 and fluconazole against each clinical isolate was determined according to Clinical and Laboratory Standards Institute M27-A3 [12]. Briefly, 1640 Roswell Park Memorial Institute medium was inoculated and incubated at 35ºC for 24 hours. The MIC end points were recorded at 50% inhibition as compared to the growth control.
Statistical Analyses
A sample size of 12 participants per treatment group was based on clinical judgment; no formal power calculations were performed. Treatment differences were compared using the Cochran–Mantel–Haenszel test. All tests were 2-sided, and inferential analyses used an alpha level of 0.05. All statistical analyses were conducted using SAS version 9.2.
AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA), version 16.0. The number and percentage of participants who had treatment-emergent AEs (TEAEs) were tabulated by system organ class and MedDRA preferred term with a breakdown by treatment group. Mean changes from pretreatment in vital signs, ECGs, and clinical laboratory variables were summarized by treatment group.
The following PK parameters were derived from the plasma concentration vs time data from participants who underwent serial PK sampling after dosing on day 1: maximum measured plasma concentration (Cmax), area under the plasma concentration vs time curve (AUC), and time to Cmax (tmax).
Three analysis populations were defined as follows: the per-protocol (PP) population included all randomized participants who met all inclusion/exclusion criteria, had a positive baseline vaginal fungal culture Candida species, received all doses of study drug, were compliant with the assigned study treatment, completed the TOC visit within an acceptable time window, had no major protocol violations, and had not received another systemic antifungal drug that has documented activity against the causative organism while on study before the TOC assessment, unless the participant had a therapeutic response of failure. The intent-to-treat (ITT)/safety population included all randomized participants who received ≥1 dose of study drug. The modified ITT (mITT) population included all randomized participants who met the inclusion/exclusion criteria, had a positive baseline vaginal fungal culture for Candida species, received ≥1 dose of study drug, and returned for ≥1 postbaseline visit.
RESULTS
Participant Demographics and Disposition
Between October 2013 and September 2014, 55 female participants were randomized into the study (Figure 1). Participants were followed for approximately 6 months including up to 3 days for screening, 3 days for study drug administration, and a final follow-up visit 6 months after starting treatment.
The study had a high rate of completion across all groups: VT-1161 300 mg q.d., 12/14, 85.7%; VT-1161 600 mg q.d., 10/12, 83.3%; VT-1161 600 mg b.i.d., 13/14, 92.9%; and fluconazole 150 mg single dose, 13/15, 86.7%. No participant withdrew from the study due to an AE. All protocol deviations were considered to be minor and did not affect study outcomes.
Baseline participant demographics are summarized in Table 1. Overall, the average age of participants was 32.7 years. Baseline characteristics were similar across the groups.
Table 1.
Participant Demographics and Baseline Characteristics
| Parameter | VT-1161 300 mg q.d. (n = 14) | VT-1161 600 mg q.d. (n = 12) | VT-1161 600 mg b.i.d. (n = 14) | Fluconazole 150 mg (n = 15) | Overall (N = 55) |
|---|---|---|---|---|---|
| Age (y) | 34.4 ± 12.01 | 38.2 ± 11.77 | 27.6 ± 7.37 | 31.3 ± 8.22 | 32.7 ± 10.40 |
| Race | |||||
| White | 7 (50.0) | 9 (75.0) | 4 (28.6) | 9 (60.0) | 29 (52.7) |
| Black | 7 (50.0) | 3 (25.0) | 9 (64.3) | 6 (40.0) | 25 (45.5) |
| Asian | 0 | 0 | 1 (7.1) | 0 | 1 (1.8) |
| Ethnicity | |||||
| Hispanic or Latino | 1 (7.1) | 4 (33.3) | 1 (7.1) | 3 (20.0) | 9 (16.4) |
| Not Hispanic or Latino | 13 (92.9) | 8 (66.7) | 13 (92.9) | 12 (80.0) | 46 (83.6) |
| Height (cm) | 162.4 ± 4.46 | 159.3 ± 5.89 | 162.9 ± 4.42 | 160.5 ± 5.80 | 161.3 ± 5.23 |
| Weight (kg) | 72.2 ± 16.55 | 72.6 ± 17.35 | 71.4 ± 20.70 | 73.9 ± 14.49 | 72.6 ± 16.90 |
| Body mass index (kg/m2) | 27.3 ± 5.52 | 28.7 ± 7.06 | 26.9 ± 7.63 | 28.7 ± 5.22 | 27.9 ± 6.27 |
Data are mean ± standard deviation or n (%).
Abbreviation: b.i.d., twice daily; q.d., once daily; VT-1161, oteseconazole.
Efficacy
The primary efficacy outcome was the proportion of participants with therapeutic cure at the day 28 TOC visit. These data are summarized for all populations (PP, mITT, and ITT) in Table 2. A larger proportion of participants in the PP population experienced therapeutic cure in the VT-1161 300 mg q.d. (6/8, 75.0%), VT-1161 600 mg q.d. (6/7, 85.7%), and VT-1161 600 mg b.i.d. (11/14, 78.6%) treatment groups vs the fluconazole 150 mg group (5/8, 62.5%). No statistically significant difference was observed for any of the VT-1161 treatment groups vs the fluconazole group. The secondary efficacy outcome measures included the proportion of participants with clinical cure, mycological cure, clinical improvement, clinical relapse, and mycological relapse. These data are summarized for the PP population in Table 3.
Table 2.
Percentage of Participants With Therapeutic Cure at Test-of-Cure Visit Day 28
| Population | VT-1161 300 mg q.d., n (%) | VT-1161 600 mg q.d., n (%) | VT-1161 600 mg b.i.d., n (%) | Fluconazole 150 mg, n (%) |
|---|---|---|---|---|
| Per-protocol | n = 8 | n = 7 | n = 14 | n = 8 |
| Yes | 6 (75.0) | 6 (85.7) | 11 (78.6) | 5 (62.5) |
| No | 2 (25.0) | 1 (14.3) | 3 (21.4) | 3 (37.5) |
| 95% CI | 34.9–96.8 | 42.1–99.6 | 49.2–95.3 | 24.5–91.5 |
| P valuea | .956 | .371 | .363 | |
| Modified intent-to-treat | n = 11 | n = 8 | n = 14 | n = 9 |
| Yes | 7 (63.6) | 6 (75.0) | 11 (78.6) | 6 (66.7) |
| No | 4 (36.4) | 2 (25.0) | 3 (21.4) | 3 (33.3) |
| 95% CI | 30.8–89.1 | 34.9–96.8 | 49.2–95.3 | 29.9–92.5 |
| P valuea | .691 | .657 | .506 | |
| Intent-to-treat | n = 14 | n = 12 | n = 14 | n = 15 |
| Yes | 9 (64.3) | 9 (75.0) | 11 (78.6) | 10 (66.7) |
| No | 5 (35.7) | 3 (25.0) | 3 (21.4) | 5 (33.3) |
| 95% CI | 35.1–87.2 | 42.8–94.5 | 49.2–95.3 | 38.4–88.2 |
| P valuea | .521 | .496 | .296 |
Abbreviation: b.i.d., twice daily; CI, confidence interval; q.d., once daily; VT-1161, oteseconazole.
aP values are from Cochran–Mantel–Haenszel tests comparing each VT-1161 treatment group to fluconazole, stratified by baseline composite vulvovaginal signs and symptoms severity score (6–8 and ≥9).
Table 3.
Secondary Outcome Results for Per-Protocol Population
| Secondary Outcome | VT-1161 300 mg q.d., n (%) | VT-1161 600 mg q.d., n (%) | VT-1161 600 mg b.i.d., n (%) | Fluconazole 150 mg, n (%) |
|---|---|---|---|---|
| Clinical cure (day 28) | n = 8 | n = 7 | n = 14 | n = 8 |
| Yes | 6 (75.0) | 7 (100.0) | 12 (85.7) | 5 (62.5) |
| No | 2 (25.0) | 0 | 2 (14.3) | 3 (37.5) |
| 95% CI | 34.9–96.8 | 59.0–100.0 | 57.2–98.2 | 24.5–91.5 |
| P valuea | .956 | .109 | .182 | |
| Mycological cure (day 7) | n = 8 | n = 7 | n = 14 | n = 8 |
| Yes | 8 (100.0) | 6 (85.7) | 14 (100.0) | 8 (100.0) |
| No | 0 | 1 (14.3) | 0 | 0 |
| 95% CI | 63.1–100.0 | 42.1–99.6 | 76.8–100.0 | 63.1–100.0 |
| P valuea | – | .414 | – | |
| Mycological cure (day 14) | n = 8 | n = 7 | n = 14 | n = 8 |
| Yes | 8 (100.0) | 6 (85.7) | 14 (100.0) | 5 (62.5) |
| No | 0 | 1 (14.3) | 0 | 3 (37.5) |
| 95% CI | 63.1–100.0 | 42.1–99.6 | 76.8–100.0 | 24.5–91.5 |
| P valuea | .073 | .260 | .021 | |
| Mycological cure (day 28) | n = 8 | n = 7 | n = 14 | n = 8 |
| Yes | 8 (100.0) | 6 (85.7) | 13 (92.9) | 6 (75.0) |
| No | 0 | 1 (14.3) | 1 (7.1) | 2 (25.0) |
| 95% CI | 63.1–100.0 | 42.1–99.6 | 66.1–99.8 | 34.9–96.8 |
| P valuea | .285 | .624 | .224 | |
| Clinical improvement (day 28) | n = 8 | n = 7 | n = 14 | n = 8 |
| Yes | 7 (87.5) | 7 (100.0) | 12 (85.7) | 6 (75.0) |
| No | 1 (12.5) | 0 | 2 (14.3) | 2 (25.0) |
| 95% CI | 47.3–99.7 | 59.0–100.0 | 57.2–98.2 | 34.9–96.8 |
| P valuea | .621 | .221 | .495 | |
| Clinical relapseb | n = 6 | n = 7 | n = 12 | n = 5 |
| Yes | 1 (16.7) | 0 | 1 (8.3) | 1 (20.0) |
| No | 5 (83.3) | 7 (100) | 11 (91.7) | 4 (80.0) |
| Mycological relapsec | n = 8 | n = 6 | n = 13 | n = 6 |
| Yes | 0 | 2 (33.3) | 0 | 4 (66.7) |
| No | 8 (100.0) | 4 (66.7) | 13 (100.0) | 2 (33.3) |
Abbreviation: b.i.d., twice daily; CI, confidence interval; q.d., once daily; VT-1161, oteseconazole.
aP values are from Cochran–Mantel–Haenszel tests comparing each VT-1161 treatment group to fluconazole.
bn is the number of participants who experienced clinical cure at day 28.
cn is the number of participants who experienced mycological cure at day 28.
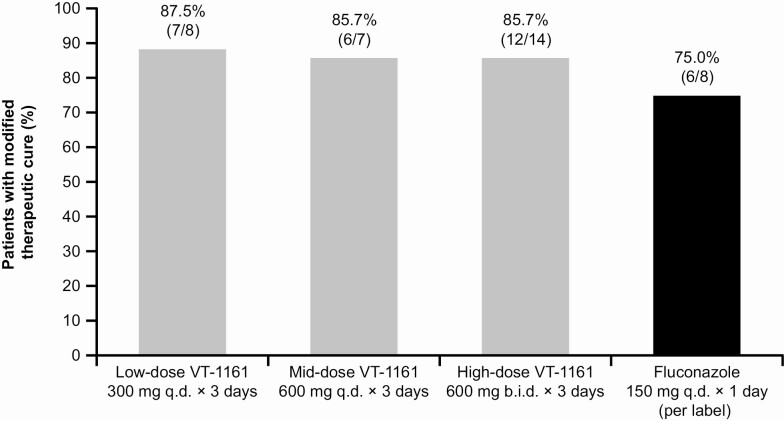
At day 28, a larger proportion of participants in the PP population experienced clinical cure, mycological cure, clinical improvement, and no clinical or mycological relapse in the 3 VT-1161 treatment groups vs the fluconazole group. At day 28, a larger proportion of participants in the PP population experienced modified therapeutic cure in the 3 VT-1161 treatment groups vs the fluconazole group (Figure 2).
Figure 2.
Modified therapeutic cure of VT-1161 dose regimens vs fluconazole for acute vulvovaginal candidiasis (day 28) in the per-protocol population. Note: Modified therapeutic cure = total VVC signs and symptoms severity score of ≤1 and mycological cure at day 28. Abbreviations: b.i.d., twice daily; q.d., once daily; VT-1161, oteseconazole.
All participants treated with VT-1161 demonstrated mycological cure at both the day 84 (3-month) and day 168 (6-month) follow-up visits, while almost half of the participants treated with fluconazole showed a mycological recurrence at the 6-month visit. The percentage of participants in the fluconazole-treated group who experienced mycological recurrence increased with time since treatment.
Additional efficacy analyses included the number of participants with a positive culture for Candida species and KOH test evaluated at days 28, 84, and 168 (Table 4). All participants treated with VT-1161 demonstrated mycological cure at both the day 84 (3-month) and day 168 (6-month) follow-up visits, while almost half of the participants treated with fluconazole showed a mycological recurrence at the 6-month visit.
Table 4.
Number of Participants With Positive Culture and Potassium Hydroxide Test During Follow-up for Intent-to-Treat Population (Mycological Recurrence)
| Visit | VT-1161 300 mg q.d., n/N (%) | VT-1161 600 mg q.d., n/N (%) | VT-1161 600 mg b.i.d., n/N (%) | Fluconazole 150 mg, n/N (%) |
|---|---|---|---|---|
| Day 28 | 0/14 (0) | 1/12 (8.3) | 0/14 (0) | 2/14 (14.3) |
| Day 84 | 0/14 (0) | 0/11 (0) | 0/14 (0) | 4/14 (28.6) |
| Day 168 | 0/12 (0) | 0/11 (0) | 0/13 (0) | 6/13 (46.1) |
Abbreviation: b.i.d., twice daily; CI, confidence interval; q.d., once daily; VT-1161, oteseconazole.
At screening, 41 isolated cultures (89%) were Candida albicans, 9 isolated cultures (7%) were Candida parapsilosis, 1 culture (2%) was Candida tropicalis, and 1 culture (2%) was Candida lusitanea. Candida glabrata was isolated from a single participant while on-study. Based on the MIC susceptibility results, VT-1161 was shown to have potent activity against the Candida strains obtained in the study. When all 60 Candida spp. isolates collected throughout the study were considered, the MIC ranges for VT-1161 and fluconazole were <0.001–1.0 and <0.125–64 µg/mL, respectively. The MIC50 (defined as the minimum concentration to inhibit 50% of the strains tested) was 0.002 µg/mL for VT-1161 and 0.25 µg/mL for fluconazole, whereas the MIC90 (defined as the minimum concentration to inhibit 90% of the strains tested) was 0.004 µg/mL for VT-1161 and 1.0 µg/mL for fluconazole. Overall, the VT-1161 MIC values were approximately 100-fold lower than those of fluconazole. Importantly, the MIC of VT-1161 against the fluconazole-resistant C. glabrata was 64-fold lower than the fluconazole MIC.
Pharmacokinetics
The median tmax of VT-1161 was 4.0 hours (range, 3.1–5.0) and 5.5 hours (range, 4.0–8.0) for the 300- and 600-mg doses, respectively. On day 1, Cmax was 407 ng/mL for the 300-mg dose and 968 ng/mL for the 600-mg dose. The AUC(0–8) values on day 1 averaged 1870 and 4230 hours × ng/mL for the 300- and 600-mg doses, respectively.
VT-1161 concentrations on day 168 remained measurable (127, 409, and 1300 ng/mL for the 300 mg q.d., 600 mg q.d., and 600 mg b.i.d. treatment groups, respectively). Median half-lives for the current study were calculated as 76 days for the low dose to 160 days for the high dose cohort.
Safety
A total of 19 TEAEs were reported in the participant group that received 300 mg q.d. VT-1161 (n = 14); 17 TEAEs were reported in the participant group that received 600 mg q.d. VT-1161 (n = 12); 4 TEAEs were reported in the participant group that received 600 mg b.i.d. VT-1161 (n = 14), and 22 TEAEs were reported in the participant group that received fluconazole.
The most common TEAEs were in the infections and infestations system organ class (16 participants, 29.1%), including the preferred terms nasopharyngitis, urinary tract infection, and vaginitis bacterial, which were reported across all treatment groups. Most TEAEs were mild or moderate in intensity and resolved during the study. A total of 7 participants (12.7%) reported 15 treatment-related TEAEs: 3 participants each in the 300 mg q.d. and 600 mg q.d. VT-1161 treatment groups and 1 participant in the fluconazole treatment group. Three participants reported treatment-related events of nausea: 1 participant in the VT-1161 300 mg q.d. group and 2 participants in the VT-1161 600 mg q.d. group. All other TEAEs were reported for only 1 participant each. No serious AEs, deaths, or TEAEs leading to study discontinuation were reported. Two pregnancies were reported during the study for participants in the fluconazole treatment group, which resulted in a full-term infant and a miscarriage. There were no clinically significant treatment-emergent changes in vital signs, physical findings, ECGs, or laboratory parameters.
Discussion
The primary efficacy outcome, therapeutic cure at day 28, was observed in the majority of participants across all treatment groups. Secondary outcome analyses showed similar results as the primary efficacy outcome. Generally, there was no statistical difference between VT-1161 and fluconazole treatment groups; however, the study was not prospectively powered for the primary end point, and sample sizes were small. While the current preliminary study did not differentiate between acute or recurrent VVC participants, requiring only an acute episode at presentation, analyses at the 3-month and 6-month follow-up visits found no indication of mycological recurrence in the VT-1161 groups, while 29% and 46% of participants in the fluconazole group had mycological recurrence at 3 and 6 months, respectively. During the 6-month trial, a higher percentage of fluconazole-treated participants reported TEAEs than VT-1161–treated participants.
Currently, women with uncomplicated acute VVC are treated with single-dose or short-course treatments; those with complicated, resistant, or recurring VVC (up to 20% of women) require therapy for ≥5–7 days and possibly maintenance therapy [13]. Topical agents are limited by local side effects, especially burning, and are messy to apply; therefore, most women prefer oral regimens. Systemically administered available azoles, such as fluconazole, can exhibit toxicity and DDI issues [3, 4] and come with reproductive warnings [14]. Furthermore, resistance surveillance studies have found that current azole therapies can have poor activity against C. glabrata [15, 16], and cross-resistance among current azole antifungal agents has been observed [17].
In a previous study that compared a 6-month weekly maintenance fluconazole regimen to placebo in patients with recurrent VVC, the percentage of fluconazole-treated patients who had a recurrence (defined as a clinical severity score of ≥3 and vaginal culture positive for yeast) at the 6-month end of maintenance phase visit was 9%. At the 9-month and 12-month observation phase visits, this percentage increased to 27% and 57%, respectively [2]. This trend of increasing recurrence rates in the 6 months following fluconazole treatment is similar to the mycological recurrence rates observed in fluconazole-treated participants in this trial and highlights the unmet medical need for these patients. We have previously reported the potential clinical utility of VT-1161 in women with recurrent VVC [18]. Overall, recurrence rates of culture-proven VVC were only 4% in the 169 VT-1161–treated participants at 9 months after ending the study drug compared with 52% in the 46 women who received placebo. Our current study shows that VT-1161 may also be an effective treatment for acute VVC.
During this trial, VT-1161 was safe and well tolerated at all dose levels through 6 months of follow-up. As an initial phase 2 study with a small number of participants, its main limitation was that it was not powered to permit meaningful comparisons between different treatment groups. Nevertheless, our findings suggest that VT-1161 is worthy of further investigation for both acute and recurrent VVC.
Notes
Acknowledgments. The authors acknowledge the following principal investigators and study sites for their participation in this study: Melvin Seid, MD, Lyndhurst Clinical Research, Winston-Salem, NC; Robert Littleton, MD, Lyndhurst Clinical Research, Raleigh, NC; Jeff Livingston, MD, Brownstone Clinical Trials, LLC, Irving, TX; Samuel Lederman, MD, Altus Research, Lake Worth, FL; Steve Chavoustie, MD, Healthcare Clinical Data, Inc, North Miami, FL; Jeanne Marrazzo, MD, Harborview ID Research Clinic, Seattle, WA; Michael Augenbraun, MD, SUNY Downstate Medical Center, Brooklyn, NY; and Paul Nyirjesy, MD, Drexel University College of Medicine, Philadelphia, PA. Editorial assistance was provided by Cello Health and was funded by Mycovia, Inc.
Financial support. This work was supported by Viamet Pharmaceuticals, Inc.
Potential conflicts of interest. S. R. B., T. P. D., and R. J. S. were employees of Viamet Pharmaceuticals at the time of the study. S. R. B and T. P. D. are employees at Mycovia Pharmaceuticals, Inc. J. D. S. is a consultant for Mycovia Pharmaceuticals, Inc and has received research funding from Cidara Therapeutics, Symbiomix Therapeutics, NovaDigm, Scynexis, and Perrigo. P. N. has received research funds from Hologic Inc, Curatek Pharmaceuticals, Viamet Pharmaceuticals, Mycovia Pharmaceuticals, and Scynexis, Inc and has served as a consultant for Mycovia Pharmaceuticals, Viamet Pharmaceuticals, Lupin Pharmaceuticals, Hologic, Inc, Scynexis, Inc, Daré Bioscience, Inc, and Becton, Dickinson. M. A. G. received funding from Cidara, Scynexis, Mycovia, and Amplyx. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Sobel JD. Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol 2016; 214:15–21. [DOI] [PubMed] [Google Scholar]
- 2.Sobel JD, Wiesenfeld HC, Martens M, et al. . Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med 2004; 351:876–83. [DOI] [PubMed] [Google Scholar]
- 3.Bates DW, Yu DT. Clinical impact of drug-drug interactions with systemic azole antifungals. Drugs Today (Barc) 2003; 39:801–13. [DOI] [PubMed] [Google Scholar]
- 4.Lewis RE. Current concepts in antifungal pharmacology. Mayo Clin Proc 2011; 86:805–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pappas PG, Kauffman CA, Andes DR, et al. . Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62:e1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchaim D, Lemanek L, Bheemreddy S, Kaye KS, Sobel JD. Fluconazole-resistant Candida albicans vulvovaginitis. Obstet Gynecol 2012; 120:1407–14. [DOI] [PubMed] [Google Scholar]
- 7.Mølgaard-Nielsen D, Svanström H, Melbye M, Hviid A, Pasternak B. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA 2016; 315:58–67. [DOI] [PubMed] [Google Scholar]
- 8.Hoekstra WJ, Garvey EP, Moore WR, Rafferty SW, Yates CM, Schotzinger RJ. Design and optimization of highly-selective fungal CYP51 inhibitors. Bioorg Med Chem Lett 2014; 24:3455–8. [DOI] [PubMed] [Google Scholar]
- 9.Warrilow AG, Hull CM, Parker JE, et al. . The clinical candidate VT-1161 is a highly potent inhibitor of Candida albicans CYP51 but fails to bind the human enzyme. Antimicrob Agents Chemother 2014; 58:7121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diflucan (Fluconazole Tablets). Package insert. Pfizer; 2011. [Google Scholar]
- 11.Sobel JD, Kapernick PS, Zervos M, et al. . Treatment of complicated Candida vaginitis: comparison of single and sequential doses of fluconazole. Am J Obstet Gynecol 2001; 185:363–9. [DOI] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. 3rd ed. Wayne, PA: Clinical and Laboratory Standards Institute, 2008. [Google Scholar]
- 13.Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. Recommendations Rep 2015; 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 14.Zarn JA, Brüschweiler BJ, Schlatter JR. Azole fungicides affect mammalian steroidogenesis by inhibiting sterol 14 alpha-demethylase and aromatase. Environ Health Perspect 2003; 111:255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 2007; 20:133–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaller MA, Messer SA, Hollis RJ, et al. . Variation in susceptibility of bloodstream isolates of Candida glabrata to fluconazole according to patient age and geographic location in the United States in 2001 to 2007. J Clin Microbiol 2009; 47:3185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kontoyiannis DP, Lewis RE. Antifungal drug resistance of pathogenic fungi. Lancet 2002; 359:1135–44. [DOI] [PubMed] [Google Scholar]
- 18.Brand SR, Degenhardt TP, Person K, et al. . A phase 2, randomized, double-blind, placebo-controlled, dose-ranging study to evaluate the efficacy and safety of orally administered VT-1161 in the treatment of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol 2018; 218:624.e1–e9. [DOI] [PubMed] [Google Scholar]