Abstract
Background
Blastomycosis has been reported from countries in Africa and the Middle East, but a decades-long debate has persisted regarding whether this is the same disease known in North America and caused by Blastomyces dermatitidis and Blastomyces gilchristii.
Methods
We reviewed published cases of human and veterinary blastomycosis from Africa and the Middle East. We abstracted epidemiological and clinical features of cases, including sites of disease, diagnosis, management, outcomes, and, where available, genetic and antigenic typing of case isolates. In addition, we sequenced nucleic acids from 9 clinical isolates from Africa deposited in global collections as B. dermatitidis; for 5, we sequenced the internal transcribed spacer regions, and for the other 4 we sequenced the whole genomes.
Results
We identified 172 unique human patients with blastomycosis, including 159 patients from 25 African countries and 12 patients from 5 Middle Eastern countries, and also identified 7 reports of veterinary blastomycosis. In humans, cutaneous disease predominated (n = 100/137, 73%), followed by pulmonary (n = 73/129, 57%) and osteoarticular involvement (n = 61/128, 48%). Unusual direct microscopy/histopathological presentations included short hyphal fragments in tissues (n = 23/129, 18%). There were 34 genotyped case isolates that comprised 4 species: Blastomyces percursus (n = 22, 65%), from 8 countries throughout all regions; Blastomyces emzantsi (n = 9, 26%), from South Africa; B. dermatitidis (n = 1, 3%), from the Democratic Republic of Congo; and B. gilchristii (n = 2, 6%), from South Africa and Zimbabwe.
Conclusions
Blastomycosis occurs throughout Africa and the Middle East and is caused predominantly by B. percursus and, at least in South Africa, B. emzantsi, resulting in distinct clinical and pathological patterns of disease.
Keywords: Endemic mycosis, dimorphic fungi, emerging infections, neglected tropical disease, blastomyces
Blastomycosis occurs throughout Africa and Middle East and is clinically and microbiologically distinct from the disease in North America. Cutaneous and osteoarticular involvement are common, and causative pathogens are Blastomyces percursus, Blastomyces emzantsi, and, rarely, Blastomyces dermatitidis and Blastomyces gilchristii.
Blastomycosis is a serious fungal disease of humans and other mammals caused by several thermally dimorphic Blastomyces species. Human disease was first reported by Gilchrist in 1894 [1] and B. dermatitidis was described as the etiology soon thereafter [1–3]. The geographic range of blastomycosis was at first thought to be limited to North America (NA), with most cases reported from the US states and Canadian provinces neighboring the Great Lakes and Ohio and Mississippi Rivers [4]. However, sporadic cases have also been reported throughout Africa, stretching from Tunisia to the Republic of South Africa (RSA) [5], as well as from the Middle East [6] and India [7]. Differences between the clinical disease and among fungal isolates implicated in blastomycosis in Africa and/or the Middle East (A/ME) and NA have been noted, leading to decades-long debate about whether the diseases are caused by the same or different pathogens [8].
Recent advances in the methods of genetic analyses of fungi have enabled the reanalysis of recent and archived fungal isolates, resulting in the description of several new species of Blastomyces [9–14]. These include the cryptic species Blastomyces gilchristii (distinguishable from B. dermatitidis only by genetic analyses) [9, 15]; the morphologically distinct taxa, Blastomyces helicus (formerly Emmonsia helica [10, 12, 13], Blastomyces percursus [11, 12, 14], and Blastomyces emzantsi [14]; Blastomyces parvus (formerly Emmonsia parva), associated with soil and the lungs of rodents; and Blastomyces silverae [12], not currently implicated in disease.
Here, we review the literature and global fungal collections for reported cases of human and animal blastomycosis originating from A/ME. We also sequence additional available historical isolates and interpret these findings in light of newly gained insights into the diversity of pathogenic Blastomyces species.
METHODS
We reviewed the literature for human and veterinary cases of blastomycosis diagnosed or putatively acquired in A/ME. We searched for articles and conference abstracts in MEDLINE, EMBASE, Ovid Global Health, Biosis Citation Index, CAB Abstracts and Global Health, Web of Science Core Collection, Google Scholar, and Google using the terms “blastomyc*” or “gilchrist* disease” and current and former names for individual countries in A/ME from 1894 to 2019, inclusive. Epidemiological data for reported cases were reviewed for eligibility: we included cases of blastomycosis if we could confidently surmise that infections were acquired in A/ME, and excluded cases with known travel to NA regions endemic for B. dermatitidis. Details of the clinical manifestations, diagnostic features, management, and outcomes were abstracted. Duplicate cases were excluded. We reviewed descriptions and photographs of histopathological and mycological features of pathogens and characterized Blastomyces isolates using antigen typing and/or nucleic sequence analyses.
In addition, we reevaluated 9 archived clinical isolates from A/ME deposited as B. dermatitidis at the Belgian Coordinated Collection of Microorganisms (IHEM), Institut Pasteur (IP), and American Type Culture Collection (ATCC). Using methods described previously [11], we sequenced the internal transcribed spacer (ITS) region of ribosomal RNA and performed phylogenetic analysis for the following isolates: IP 1898.89 from Tunisia, IP 973.68 from Morocco, ATCC 56214 from Mozambique, and ATCC 56215 and ATCC 56216 from the RSA. For another isolate, ATCC 56220 from Angola, ITS and D1/D2 regions of rRNA were published online [16]. We performed whole-genome sequencing (WGS) for 4 isolates: IHEM 26957 from Morocco, IHEM 26955 from the Democratic Republic of Congo (DRC), IHEM 26951 from Uganda, and IHEM 26956 from the RSA [14]. These strains were cultured on diluted Sabouraud agar at 37°C for 2 weeks. Genomic DNA was extracted using the Genomic-Tip 20/G kit (Qiagen) following manufacturer instructions for yeasts. The concentration and integrity of DNA were assessed with the 4200 TapeStation System (Agilent). Library preparation and sequencing were performed by GATC Biotech AG (Konstanz, Germany). Sequences were compared to those of type or authentic isolates of B. dermatitidis and B. gilchristii, and of recently described Blastomyces species deposited in GenBank [9–12, 14].
We performed a phylogenomic analysis of 28 Blastomyces isolates, including 22 isolates from A/ME (from human cases of blastomycosis) and 6 from NA (4 from human cases of blastomycosis; 1 environmental isolate of B. dermatitidis; and 1 veterinary isolate of B. parvus). Reads were aligned to the B. percursus assembly strain BP222 (GenBank accession GCA_003206225.1_ASM320622v1) [11] using Burrows Wheeler Alignment (BWA-MEM) version 0·7·12 [17]. Variants were then identified using Genome Analysis Toolkit (GATK) version 3·7.9 [18] using the haploid mode. Sites were filtered with GATK Variant Filtration tool using “QD < 2.0 || FS > 60.0 || MQ < 40.0.” Genotypes were filtered if the minimum genotype quality was <50, the percent alternate allele was <0.8, or the depth was <10. For the phylogenetic analysis, a total of 505 069 sites with an unambiguous single nucleotide polymorphism in ≥1 isolate and with ambiguity in a maximum of 10% of isolates were concatenated; insertions or deletions at these sites were treated as ambiguous to maintain the alignment. Maximum likelihood phylogenies were constructed using RAxML version 8.2.4 [19] using the GTRCAT nucleotide substitution model and a bootstrap analysis based on 1000 replicates. Raw sequence data for this project has been deposited in the Sequence Read Archive (SRA) under Bioproject PRJNA603110.
RESULTS
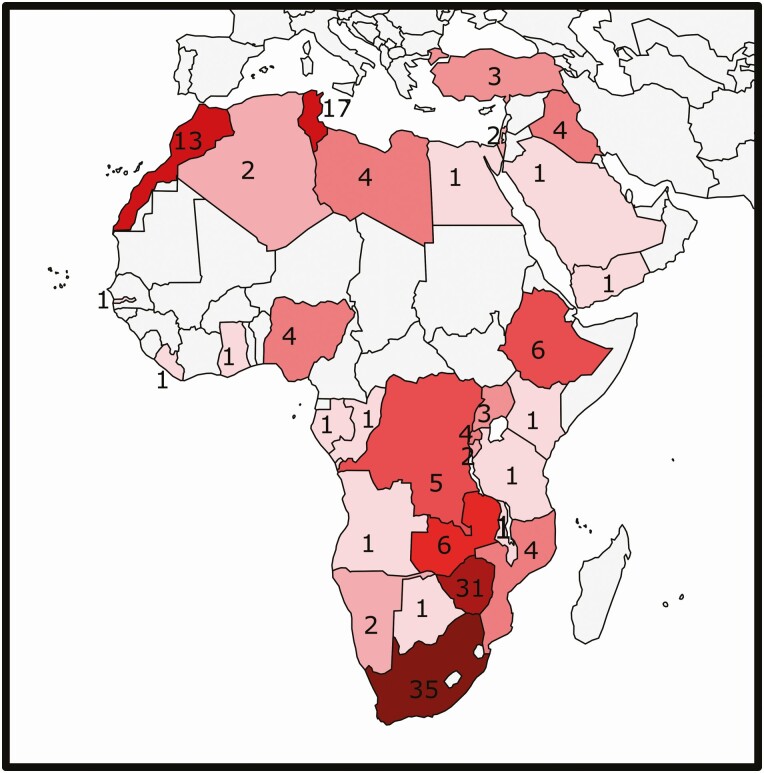
We identified 172 unique human cases of blastomycosis putatively acquired in A/ME. These included 160 patients from 25 African countries, 11 patients from 5 Middle Eastern countries (Figure 1), and 1 patient who had lived in both regions. We excluded 8 additional patients: 5 because of travel to regions of NA considered endemic for B. dermatitidis [20–23] and 3 [6, 24, 25] because the diagnosis of blastomycosis was contested in subsequent publications [26, 27]. Clinical details were available for 143 patients (Supplementary Table 1).
Figure 1.
Distribution of reported cases of human blastomycosis from Africa and the Middle East. Omitted are 3 cases with travel to multiple countries (cases 60, 97, and 121) [28, 29, 30].
There were 21 patients (12.2%) who were diagnosed with blastomycosis outside their country of origin, including 20 in Europe and 1 in the United States (outside of an endemic area). Another 5 were diagnosed in other countries within A/ME. For patients diagnosed abroad, the timing of symptom onset relative to leaving A/ME was noted for 16: symptoms began in Africa for 7 patients, and began up to 29 years (median, 1 year; interquartile range, 1–8 years) after emigrating for 9 others. The median patient age at diagnosis was 38 years (interquartile range, 29–49), ranging from 3 to 75 years. Sex was known for 127 patients; 106 (83.5%) were male. Immunocompromising conditions were reported for 13 patients (Supplementary Table S1).
Pulmonary disease occurred in 73 of 129 patients (56.7%). Radiographic lesions, known for 56 patients, included consolidation (n = 44), reticulonodules (n = 12), other nodules (n = 7), cavitation (n = 10), pleural effusions (n = 4), and/or hilar lymphadenopathy (n = 9). Isolated pulmonary disease occurred in 3 of 136 patients (2.2%).
Cutaneous lesions were present in 100 out of 137 patients (73.0%). Isolated cutaneous disease occurred in 34 of 130 patients (26.2%). Lesions were described for 83 patients (Supplementary Table 2): 57 patients (68.7%) had skin lesions without underlying osteoarticular disease, whereas 37 (44.0%) had skin or subcutaneous lesions contiguous with, and often fistulizing to, bones or joints. Both lesion types were noted in 11 patients. Mucosal lesions were present in 10 patients.
Bones or joints were involved in 61 of 128 patients (47.7%), including 3 with isolated bone disease (Supplementary Table 3). The osteoarticular structures involved included ribs or the sternum (n = 34), vertebrae (n = 28, among whom 11 developed paraplegia), appendicular structures (n = 16), the skull (n = 6), and unknown sites (n = 6). Other involved body sites included the kidneys (n = 11), brain (n = 8), liver (n = 8), spleen (n = 4), prostate (n = 3), adrenals (n = 2), and testes, thyroid, and pancreas (n = 1 each).
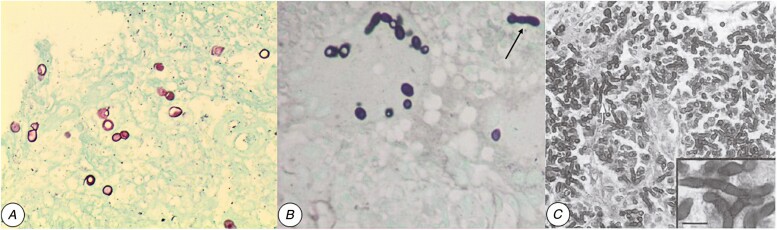
Direct microscopy and/or histopathological examinations of specimens demonstrated fungal elements for 129 patients (Supplementary Table 4). These were usually spherical or ovoid yeast-like cells (~8–12 µm) with thick, refractile walls and single, broad-based buds (Figure 2A). In addition, hyphal fragments (Figure 2B and C) were present in tissues for 23 patients (19.5%). Histopathological examinations (n = 118) usually noted granulomas (n = 59) and/or giant cells (n = 65) containing intracellular fungal cells.
Figure 2.
Histological appearance of yeast-like cells and hyphal fragments (ie, pseudohyphae) in selected cases of blastomycosis acquired in Africa. A, Ovoid yeasts with double-refractile cell walls and broad-based budding seen with GMS staining of endobronchial mass (case 66 from South Africa). The isolate from lung and skin was identified as Blastomyces percursus. B, C, Yeast-like cells and hyphal fragments are often reported in cases of blastomycosis from Africa or the Middle East. B, Bronchial biopsy from a patient with blastomycosis from Tunisia (case 109; GMS x100). Elongated hyphal fragments are highlighted with an arrow. (Modified from Cheikh Rouhou et al [31]). C, Hyphal fragments are seen in the peritoneum of a mouse inoculated with B. percursus (strain IP68.9973/ATCC56214), isolated from a patient with blastomycosis in Mozambique (case 45; GMS, x400). Reproduced with permission from Huerre et al [32], copyright Elsevier Masson SAS. All rights reserved. Abbreviation: GMS, Grocott’s methenamine silver.
Fungal cultures were positive from 80 patients; 78 isolates were identified phenotypically as B. dermatitidis by methods then available. In 1 case, experts disagreed on whether the organism was B. dermatitidis or Emmonsia crescens [33]; that isolate was since designated as the type strain of B. percursus [11]. In another case, the fungus was identified phenotypically as a Chytridiales [28], but was later reclassified as B. dermatitidis based on an examination of histopathology [4].
Information was available about treatment and outcomes for 99 and 105 patients, respectively (Supplementary Table S1). Of those with treatment data, 79 patients received antifungals and 20 who did not. Of those with outcome data, 31 patients (29.8%) died; 74 patients reportedly survived, including 5 not treated with antifungals. However, follow-up evaluations after therapy discontinuation were reported for just 20 patients (3 experienced relapses). Despite initial improvement, 6 patients had disease progression on antifungal therapy: 5 received triazoles, among whom 4 responded favorably by changing to amphotericin B (n = 1) or an alternative triazole (n = 3). Despite receiving no or negligible antifungal therapy, 3 patients with isolated cutaneous disease recovered: 1 each was treated with cryotherapy [34], local excision [35], and traditional medicine [36]. Follow-up data were reported for only the first, who had no recurrence after a year.
We identified 7 reports describing veterinary blastomycosis from A/ME [37–43] (Supplementary Table 5), and 3 reported instances of B. dermatitidis detection from the environment [44–47]. Helal et al [44] reported B. dermatitidis isolation from Egyptian wastewater. Additionally, Lahmiti et al [45] and Rais et al [46] reported from Morocco on a case of blastomycosis in a used clothing trader; Blastomyces spores were reportedly identified on his wares, but further details were absent. Abubakari [47] reported isolating B. dermatitidis from peanut paste acquired from a market in Ghana. No isolates or voucher material from veterinary cases or environmental detection were available for verification.
Genetic analyses have been published for 25 human clinical isolates from A/ME, and were obtained for 9 additional human clinical isolates described here. Using multilocus sequence typing (MLST), Brown et al [9] analyzed 78 human clinical and environmental Blastomyces isolates; 3 were from Africa, including 1 each from the DRC, Zimbabwe, and the RSA. Dukik et al [11] used MLST for 2 isolates from the RSA (1 was also examined with WGS) and 1 isolate from Israel, for which an ITS-partial large subunit (LSU) sequence had been obtained [48]. Maphanga et al [14] performed MLST on 18 isolates and WGS analyses for 20 RSA isolates from cases of blastomycosis (which included the 2 RSA strains also analyzed by Dukik et al [11]). In addition to these, sequences for ITS and D1/D2 were published online for a strain from Angola [16]. We newly sequenced ITS for 5 isolates (1 each from Tunisia, Morocco, and Mozambique and 2 from the RSA) and whole genomes for 4 others (1 each from Morocco, Uganda, the DRC, and the RSA). Species identification was therefore determined for 34 clinical isolates from A/ME (Supplementary Table 6). We identified 22 isolates (65%) as B. percursus: 13 from the RSA and 1 each from Angola, the DRC, Israel, Morocco, Mozambique, Tunisia, and Uganda. There were 9 RSA isolates (26%) identified as B. emzantsi, a recently described species [14]. A single isolate (3%) from the DRC was identified as B. dermatitidis, and 2 isolates (6%) from the RSA and Zimbabwe were B. gilchristii [9]. Epidemiological and clinical information was available to complement sequence-based identification of isolates for 16 patients (Table 1).
Table 1.
Demographic and Clinical Details
| Species | Country | Case ID, Author, year (case no.)a | Year, depositor (where known), Collection or isolate number (NCBI Accession) | Patient age, sex, comorbidities | Sites affected | Description of fungi in tissue | Treatment, Outcome |
|---|---|---|---|---|---|---|---|
| Blastomyces percursus | Angola | 3, Bleeker and Haanen, 1979 [49] | 1974, ATCC 56220,b CBS 514.74,c CDC B3464d | 36, M, Lymphoma (in remission) | Skin, lung | Double-contoured yeast cells, single broad-based buds | Miconazole (3 g daily for 14 weeks), Survived |
| Democratic Republic of Congo (then Zaire) | 7, Bregant et al, 1973 (case 2) [50]; Huerre et al, 2002 (case 3) [32] | 1972, Vandepitte Raymond Vanbreuseghem collection (RV) 28217, IHEM 26955, ATCC 48089 (SAMN13949613; AF071949) | 33, M, None | Rib, skin | Spherical or oval yeasts (9–12 µm), with single buds at broad bases | Amphotericin B (2 g), Survived | |
| Israel | 25, Kemna et al, 1994 [33]; Dukik et al, 2017 [11]; Jiang et al, 2018 [12] | 1993, Polachek UAMH 7425, Centraalbureau voor Schimmelcultures (CBS) 139878, UAMH 7426 (NR 153647; KY195964; KY195971; KY195949; AF038323; AF038324) | 53, M, None | Oral mucosa, palate, skin | Spherical cells, 8–10 µm budding at broad-bases | Fluconazole 400 mg daily for 6 months, Survived | |
| Morocco | 34, Sekkat et al, 1981 [51]; Huerre et al, 2002 (African case 5) [32] | 1978, Collection de L’Institut Pasteur (CIP) 1898.89 (MK521438) | 30, M, None | Skin | Spores resembling B. dermatitidis, measuring 10 µm on average with broad-based budding. 2.5% of fungal cells resembled hyphae | Ketoconazole for 82 days, Survived | |
| Mozambique | 45, Campos Magalhaes et al, 1968 [52]; Huerre et al, 2002 (African case 2) [32] | CDC B838, ATCC 56214 (MK521440) | 18, M None | Bone, skin, soft tissue (foot), lung, prostate | Round yeasts, 8–10 µm, with thick walls and broad-based budding. No hyphal forms | Amphotericin B (2.79 g), prednisone, Survived | |
| South Africa | 65, Heys et al, 2014 (case 1) [53]; Schwartz et al, 2015 (case 34) [54]; Dukik et al, 2017 [10]; Maphanga et al, 2020 (case 2) [14] | 2008, Govender BP222 (LGTZ00000000) | 58, M, None (HIV−) | Brain, lungs | Broad-based budding yeasts with double contoured refractile walls, some forming pseudohyphae | Amphotericin B for 14 days, followed by itraconazole, Survived | |
| 66, Schwartz et al, 2015 (case 45) [54]; Maphanga et al, 2020 (case 3) [14] | 2014, SA-NICD-06 (QGQT00000000) | 52, M, None | Lung, endobronchial mass, skin | Broad-based budding yeast with double refractile walls suggestive of B. dermatitidis | Amphotericin B, followed by itraconazole, Survived | ||
| 67, Maphanga et al, 2020 (case 4) [14] | 2014, SA-NICD-08 (QGQR00000000) | 34, M, None (HIV−) | Skin | Large intracellular fungal cells with thick walls resembling B. dermatitidis | Itraconazole, Survived | ||
| 62, Simon et al, 1977 [55]; Maphanga et al, 2020 (case 5) [14] | 1975, SA-NICD-05 CDC. B3013 (QGQS00000000) | 63, M, None | Tongue, lung | Broad-based budding yeasts resembling B. dermatitidis | None, Died | ||
| 64, Frean et al, 1989 [56]; Carman et al, 1989 [5]; Maphanga et al, 2020 (case 6) [14] | 1983, SA-NICD-04 (QGQQ00000000) | 65, M, None | Skin, lung, kidney, liver, spleen, thyroid | Yeast-like cells resembling B. dermatitidis | Unknown, Died | ||
| 61, Martin and Berson, 1973 (case 2) [57]; Maphanga et al, 2020 (case 7) [14] | 1967, SA-NICD-18 (MH571861, MH644816) | 27, M | Bone (skull, iliac, clavicle, vertebrae), skin | Hour-glass shaped yeasts resembling B. dermatitidis | Amphotericin B, griseofulvin, Died | ||
| 75, Bayles, 1975 [58]; Frean et al, 1989 [56]; Carman et al, 1989 [5] | 1975, Bayles RV 33150, IHEM 26956 (SAMN13949615) | 17, F | Lungs, skin, bone (nonvertebral) | Not done | Unknown, Survived | ||
| Uganda | 118, Jelliffe et al, 1964 [59]; Emmons et al, 1964 (case 1) [60] | 1964, Murray RV 15455, IHEM 26951, CDC B832, ATCC 56213 (SAMN13949612) | 12, M, None | Joints, skin, bone, lungs, liver, lymph nodes, kidney, spleen, thyroid. | Spherical or ovoid fungi (9–12µm) with thick-walls and single buds at broad bases. | None, Died | |
| Blastomyces emzantsi | South Africa | 69, Frean, 1993 [61]; Maphanga et al, 2020 (case 8) [14] | 1989, SA-NICD-13 (QGQJ00000000) | 40, M, None (HIV−) | Lung, vertebrae, and paravertebral abscess fistulising to skin | First interpreted as chromoblastomycosis, but on reexamination, broad-based budding yeasts resembling B. dermatitidis were seen | Amphotericin B, Died |
| 70, Frean et al, 1993 [61]; Maphanga et al, 2020 (case 9) [14] | 1993, SA-NICD-15 (QGQI00000000) | 31, M, None (HIV−) | Lung, ribs, thoracic vertebrae, subcutaneous abscesses | Yeasts resembling B. dermatitidis | Amphotericin B, Survived | ||
| 68, Frean et al, 1989 [56]; Maphanga et al, 2020 (case 10) [14] | 1975, SA-NICD-14 (QGQK00000000) | Adult, M, Severe combined immune deficiency | Skin, subcutaneous abscesses | Unknown | Unknown, Died |
Data are from patients with blastomycosis acquired in Africa or the Middle East for whom the infecting species identity is determined. For 3 additional cases of infection with B. percursus, the skin was involved but no other details are available. These include 1 case from Morocco (RV 52435/ IHEM 26957 [SAMN13949614], deposited by Rollier in 1983) and 2 from South Africa (CBS 142605/ NCPF 4091 [QGQM00000000] deposited by K. Stead in 1986; and NICD-9 [QGQF00000000] from 1978). Limited information is known for 2 cases of infection with B. gilchristii (UAMH Centre for Global Microfungal Diversity [UAMH] 10245 [EF592163] from South Africa, isolated from lung biopsy; and UAMH 10251 [EF592160] from Zimbabwe [then Rhodesia], isolated from sputum); and for 1 case of B. dermatitidis (UAMH 10246/CDC B3003 [EF592159], obtained by Vandepitte from a patient in the Democratic Republic of Congo [then Belgian Congo]).
Abbreviations: ATCC, American Type Culture Collection; CDC, Centers for Disease Control and Prevention; F, female; HIV−, documented seronegative for human immunodeficiency virus; IHEM, Belgian Coordinated Collection of Microorganisms; M, male; NCBI, National Center for Biotechnology Information.
aEach row represents 1 case, although multiple publications discussing the case or the isolate may be referenced.
bSequences are not published to NCBI, but have been published at the following website: https://www.atcc.org/products/all/56220.aspx#specifications.
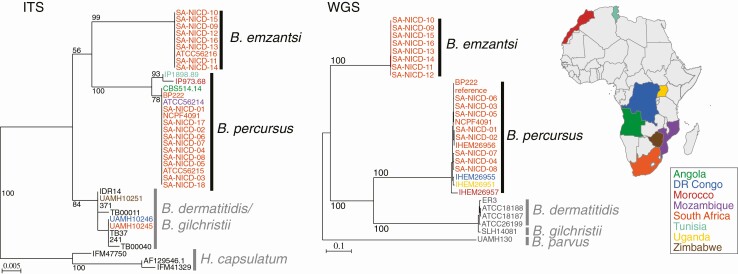
A whole-genome phylogenetic analysis using 505 069 variants sites placed Blastomyces isolates from A/ME into 2 highly divergent groups, B. emzantsi and B. percursus (Figure 3). Blastomyces percursus isolates appeared more closely related to B. dermatitidis and B. gilchristii isolates from North America, and B. emzantsi appeared more distantly related as a sister clade of this group of species. Isolates of B. percursus from the RSA clustered together, while those from Uganda (IHEM 26951) and the DRC (IHEM 26955) were more similar to each other; an isolate from Morocco (IHEM26957) was an outgroup. A phylogenetic analysis using ITS sequences clustered IP 1898.89 (Tunisia), IP 973.68 (Morocco), ATCC 56214 (Mozambique), ATCC 56215 (RSA), and ATCC 56220 (Angola) with B. percursus and ATCC 56216 (RSA) within B. emzantsi. Blastomyces dermatitidis and B. gilchristii could not be distinguished by ITS sequencing.
Figure 3.
Phylogenetic relationships of African isolates of Blastomyces spp. (Left) Maximum likelihood phylogenetic tree inferred from ITS sequences of 32 isolates of Blastomyces spp. and 3 isolates of Histoplasma capsulatum (outgroup). (Right) Maximum likelihood phylogenetic tree inferred from whole-genome SNPs of 28 isolates of Blastomyces spp. mapped to the genome of B. percursus BP222. African isolate labels are color-coded by country and represented in the map. Additional Blastomyces isolates from outside Africa or the Middle East are included in the trees for context (ITS: IDR14, 371, TB00011, 241, TB37, TB00040; and WGS: ER3, ATCC18188, ATCC18187, ATCC26199, SLH14081, UAMH130). All nodes were supported by 100% of bootstrap replicates. UAMH 10246 was determined as B. dermatitidis, and UAMH10245 and UAMH10251 were determined as B. gilchristii by Brown et al [9,14]. Abbreviations: DR Congo, Democratic Republic of Congo; ITS, internal transcribed spacer; SNP, single nucleotide polymorphism; WGS, whole-genome sequences.
The A exoantigen was present on just 5 of 22 A/ME isolates [33, 62–65]. There were 11 strains for which species identity and exoantigen were characterized: the A exoantigen was present for 3 A/ME strains confirmed as B. dermatitidis or B. gilchristii, and absent for 7 strains of B. percursus and 1 strain of B. emzantsi (Supplementary Table 6). Macroscopic and microscopic characteristics of Blastomyces species from A/ME are depicted in Figure 3. Because B. dermatitidis and B. gilchristii are morphologically indistinguishable, only the latter is shown, in comparison to B. percursus and B. emzantsi, morphologically distinct species that have been isolated only from A/ME.
DISCUSSION
Until the middle of the 20th century, blastomycosis was thought to occur only in NA. The disease was widely referred to as North American blastomycosis, primarily for distinction from South American blastomycosis, a term then used for paracoccidioidomycosis. However, cases have been reported sporadically from 25 countries from Africa, 5 countries from the Middle East, from India [7], and occasionally elsewhere. Based on differences in the morphology, serology, and clinical features of infection with Blastomyces from A/ME compared to NA, mycologists debated whether these cases were caused by the same taxa [8, 32, 50, 60, 66–71]. Based on our review of 172 human cases and 7 veterinary reports of blastomycosis from A/ME, and on genetic analyses of 34 historic case isolates, we conclude that blastomycosis in A/ME is caused by at least 4 pathogens: B. percursus, with the currently known geographic range from Israel to the RSA; B. emzantsi, to date known only from the RSA; and, rarely, B. dermatitidis and B. gilchristii.
In light of this new understanding of the genetic diversity among isolates from A/ME, it is worth revisiting the observed differences between NA and A/ME blastomycosis and causative fungi. Clinical differences have been noted between cases of blastomycosis occurring on different continents. Vandepitte and Gatti [70] reviewed 17 cases of blastomycosis diagnosed in Africa up to 1972, and found that osteoarticular and cutaneous cases predominated, occurring in 14 (82%) and 12 patients (71%), respectively, whereas pulmonary disease affected just 9 patients (53%). Later, Carman et al [5] reviewed 59 cases of African blastomycosis reported until 1987, and similarly found that bone disease predominated (64%), followed closely by pulmonary (59%) and cutaneous involvement (54%). In our review of 143 patients reported to 2019, disease most frequently involved the skin (73.0%), followed by the lungs (56.7%), and then bones/joints (46.9%). In contrast, among large blastomycosis case series from NA, pulmonary disease occurred in 82–93% of patients 72–74], with cutaneous and osteoarticular involvement in 18–21% and 4–15% of patients, respectively [73, 75]. It is possible that some cases of pulmonary blastomycosis may be under- or misdiagnosed in A/ME because of reduced accessibility to invasive lung sampling (eg, via bronchoscopy) and higher prevalences of tuberculosis, which it can resemble clinically. Among A/ME cases, isolated cutaneous blastomycosis occurred in approximately a quarter of all cases, a proportion far higher than observed in NA. For example, isolated cutaneous disease was noted in just 4% of patients in a large series from Wisconsin [76]. Veterinary blastomycosis has been reported much less frequently from A/ME than from NA, where canine cases are estimated to outnumber those in humans by 10-fold [77].
The observation of hyphal fragments or forms (short segments of varying width) in tissue associated with cases of blastomycosis from A/ME is notable. Hyphal forms were present in the histopathology of 23 cases from A/ME (19.5%), and occurred in pulmonary as well as extrapulmonary tissue (Figure 2B). By comparison, hyphal forms are uncommonly observed in patients with blastomycosis from NA [78]. Huerre et al [32] observed hyphal forms in tissues of 12 of 14 African patients with blastomycosis (comprising up to 42% of fungal elements seen) and 5 of 10 NA patients (but never comprising >3% of fungal elements). These hyphal forms were reproducible in experimental murine blastomycosis with 5/5 African strains (Figure 2C), versus just 1/5 NA strains [32]. Similar hyphal forms were observed in culture for B. percursus by Dukik et al [11] and by us here (Figure 4F). Although Kaufman et al [62] reported that yeast-like cells of African strains were smaller (8–12 µm vs 9–18 µm for NA strains), our review suggests the size of yeast-like cells in A/ME cases are variable (in vivo and in vitro), limiting the value of this characteristic for distinguishing groups. However, if yeast-like cells are accompanied by hyphal fragments, B. percursus should be considered.
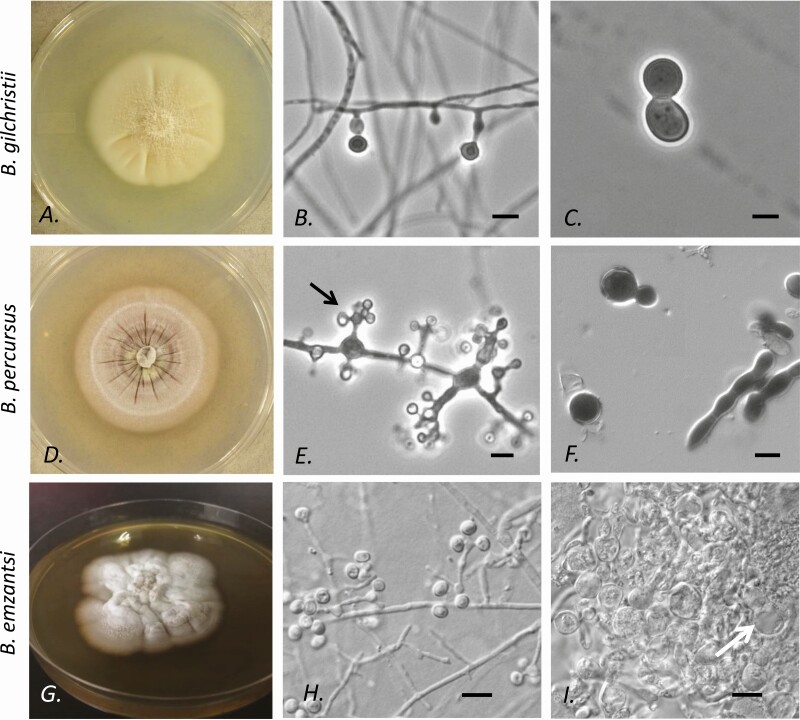
Figure 4.
Colonial and microscopic morphologies of Blastomyces gilchristii, Blastomyces percursus, and Blastomyces emzantsi isolates originating from patients in Africa or the Middle East. A–C, Blastomyces gilchristii (UAMH 10245 from South Africa [https://www.uamh.ca] used with permission); the appearance of Blastomyces dermatitidis is indistinguishable. A, Colony grown at 30℃. B, Conidia produced singly on slightly swollen stalks at 30℃. C, Yeast cell with single broad-based bud at 35℃. D–F, Blastomyces percursus (UAMH 7425 from Israel). D, Colony grown at 30℃. E, Conidia borne in clusters (florets; arrow) from slightly swollen cells or singly on slender stalks. F, Hyphal fragments and a single budding yeast in culture at 35℃. G–I, Blastomyces emzantsi isolate (South Africa National Institute for Communicable Diseases [SA-NICD-15] from South Africa). G, Colony grown at 25℃. H, Conidia borne singly on slender stalks or in florets from slightly swollen cells. I, Swollen hyphal filaments and single budding yeast (arrow) at 35℃. All scale bars = 5 µm. Cultures were killed prior to photography (UAMH 10245 and UAMH 7425 by exposure to formalin vapor in a desiccator jar; SA-NICD-15 with 2.5% glutaraldehyde).
In culture, Vermeil et al [68] found that African B. dermatitidis strains had more complex conidial arrangements, comprising clusters of solitary conidia. These complex “florets” are noted with B. percursus (Figure 4E) and B. emzantsi (Figure 4H), but not in B. dermatitidis or B. gilchristii (Figure 4B) [11, 12].
Antigenic differences exist for Blastomyces strains from different continents [62, 79]. The A exoantigen is present on B. dermatitidis and B. gilchristii—strains that predominate in NA, but which together comprised just 2 of the 34 speciated A/ME isolates (9%)—but is absent on B. percursus and B. emzantsi. The A exoantigen is related to the virulence factor Blastomyces adhesin-1 (BAD-1) [65], which is present on some African Blastomyces strains (since confirmed as B. dermatitidis and B. gilchristii [9]) but lacking on others (including some reclassified as B. percursus) [65]. Indeed, Maphanga et al [14] found that both B. percursus and B. emzantsi isolates lacked the full orthologue of the BAD-1 gene. The absence of BAD-1 among species that predominate in A/ME may contribute to the observed clinical differences in NA and A/ME cases of blastomycosis.
Our study has limitations. Because not all cases were reported, incidence rates cannot be inferred. The ability to determine species-level identification by DNA sequencing for only 34 case isolates limited our comparisons of infections caused by different Blastomyces species. In addition, some cases may have been misclassified as being from A/ME because of incompletely reported travel histories. We cannot exclude the possibility that some cases reported as blastomycosis were African histoplasmosis caused by Histoplasma capsulatum var. duboisii, and vice versa. This disease is also characterized by the predominant involvement of skin and sometimes bone [80], and in tissue the yeast-like cells can be confused for Blastomyces because of similarities in the size and presence of thick refractile walls. However, whereas Blastomyces yeasts are multinucleate and bud at broad bases, H. capsulatum var. duboisii yeasts are uninucleate and bud at narrow bases [80]. Moreover, H. capsulatum var. duboisii is found primarily in Western and Central Africa [81], whereas Blastomyces species appear to span the continent. Finally, heterogeneity in the availability of antifungals and the scarcity of follow-up data limit analyses of outcomes, and comparisons with data from other settings.
In conclusion, blastomycosis has been reported throughout A/ME, although the disease likely remains underdiagnosed and underappreciated. Clinical and histopathological features may differ from the disease in North America, although overlap does exist. There are mycological differences; through modern molecular tools and genetic analyses, we have confirmed the predictions of some prescient mycologists that the pathogens that cause blastomycosis in A/ME are largely distinct from those causing the disease in NA.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Linda Slater and library staff at the University of Alberta John W. Scott Library for help with the search strategy and retrieval of articles; Dr Erin Amerson, Dr Kim Forrestel, and Dr Ernest Sung for sharing unpublished details of cases; and Dr Natalia Stavila for assistance with translation of Russian reports.
Financial support. This work was supported by Fonds voor Wetenschappelijk Onderzoek–Vlaanderen, which funded whole-genome sequencing of 3 isolates from the Belgian Coordinated Collection of MicroOrganisms, and Public Health Ontario, which funded internal transcribed spacer sequencing of 5 isolates.
Potential conflicts of interest. N. P. G. reports grants from the National Institutes of Health, US Centers for Disease Control and Prevention, and the Bill and Melinda Gates Foundation, and nonfinancial support from Gilead. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gilchrist TC.Protozoan dermatitis. J Cutan Gen Dis 1894; 12:496–9. [Google Scholar]
- 2.Gilchrist TC, Stokes WR. A case of pseudo-lupus vulgaris caused by a blastomyces. J Exp Med 1898; 3:53–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Hoog GS, Redhead SA, Feng P, Jiang Y, Dukik K, Sigler L. (2465–2466) Proposals to conserve Blastomyces Gilchrist & W.R. Stokes against Blastomyces Constantin & Rolland and Ajellomycetaceae against Paracoccidioidaceae (Ascomycota: Onygenales). Taxon 2016; 65:1167–9. [Google Scholar]
- 4.DiSalvo AF. The ecology of Blastomyces dermatitidis. In: Al-Doory Y, DiSalvo AF, eds. Blastomycosis. Boston, Massachusetts: Springer US, 1992:43–73. [Google Scholar]
- 5.Carman WF, Frean JA, Crewe-Brown HH, Culligan GA, Young CN. Blastomycosis in Africa. A review of known cases diagnosed between 1951 and 1987. Mycopathologia 1989; 107:25–32. [DOI] [PubMed] [Google Scholar]
- 6.Malak JA, Farah FS. Blastomycosis in the Middle East. Report of a suspected case of North American blastomycosis. Br J Dermatol 1971; 84:161–6. [DOI] [PubMed] [Google Scholar]
- 7.Randhawa HS, Chowdhary A, Kathuria S, et al. . Blastomycosis in India: report of an imported case and current status. Med Mycol 2013; 51:185–92. [DOI] [PubMed] [Google Scholar]
- 8.Blastomycosis —one disease or two? Lancet 1989; 333:25–6. [PubMed] [Google Scholar]
- 9.Brown EM, McTaggart LR, Zhang SX, Low DE, Stevens DA, Richardson SE. Phylogenetic analysis reveals a cryptic species Blastomyces gilchristii, sp. nov. within the human pathogenic fungus Blastomyces dermatitidis. PLoS One 2013; 8:e59237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sigler L, et al. Emmonsia helica Sigler sp. nov. Index Fungorum 2015:237. Available at: http://www.indexfungorum.org/Publications/Index%20Fungorum%20no.237.pdf. [Google Scholar]
- 11.Dukik K, Muñoz JF, Jiang Y, et al. . Novel taxa of thermally dimorphic systemic pathogens in the Ajellomycetaceae (Onygenales). Mycoses 2017; 60:296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang YP, Dukik K, Munoz JF, et al. . Phylogeny, ecology and taxonomy of systemic pathogens and their relatives in Ajellomycetaceae (Onygenales): Blastomyces, Emergomyces, Emmonsia, Emmonsiellopsis. Fungal Divers 2018; 90:245–91. [Google Scholar]
- 13.Schwartz IS, Wiederhold NP, Hanson KE, Patterson TF, Sigler L. Blastomyces helicus, a new dimorphic fungus causing fatal pulmonary and systemic disease in humans and animals in Western Canada and the United States. Clin Infect Dis 2019; 68:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maphanga TG, Birkhead M, Munoz JF, et al. . Human blastomycosis in South Africa caused by Blastomyces percursus and Blastomyces emzantsi sp. nov., 1967– 2014. J Clin Microbiol 2020; 58: e01661–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown EM, McTaggart LR, Zhang SX, Low DE, Stevens DA, Richardson SE. Correction: phylogenetic analysis reveals a cryptic species Blastomyces gilchristii, sp. nov. within the human pathogenic fungus Blastomyces dermatitidis. PLoS One 2016; 11:e0168018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Type Culture Collection. Emmonsia parva (Emmons et Ashburn) Ciferri et Montemartini, teleomorph (ATCC® 56220™). Available at: https://www.atcc.org/products/all/56220.aspx#specifications. Accessed 27 November 2018.
- 17.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009; 25:1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKenna A, Hanna M, Banks E, et al. . The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 2010; 20:1297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014; 30:1312–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarez GG, Burns BF, Desjardins M, Salahudeen SR, AlRashidi F, Cameron DW. Blastomycosis in a young African man presenting with a pleural effusion. Can Respir J 2006; 13:441–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al Jahdali H, Memish Z, Bannatyne RM, Bamefleh H. First case of combined blastomycosis and tuberculosis in a patient from Saudi Arabia. J Chemother 2001; 13(Suppl 1):65–8. [DOI] [PubMed] [Google Scholar]
- 22.Assaly RA, Hammersley JR, Olson DE, et al. . Disseminated blastomycosis. J Am Acad Dermatol 2003; 48:123–7. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein S, Brunner HI, Summerbell R, Allen U, Babyn P, Richardson SE. Blastomycosis acquired by three children in Toronto. Can J Infect Dis 2002; 13:259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conor A, Marchetti C. Un nouveau cas de blastomycose observe en Tunisie. Bul Soc Path Exot 1913; 6:557–9. [Google Scholar]
- 25.Conor A, Bruch A. Un cas de blastomycose humaine observe en Tunisie. Bul Soc Path Exot 1911; iv:366–8. [Google Scholar]
- 26.Kuttin ES, Beemer AM, Levij J, Ajello L, Kaplan W. Occurrence of Blastomyces dermatitidis in Israel. First autochthonous Middle Eastern case. Am J Trop Med Hyg 1978; 27:1203–5. [DOI] [PubMed] [Google Scholar]
- 27.Chadli A, Juminer B, Najah S. Deuxième cas tunisien de blastomycose a Blastomyces dermatitidis Gilchrist & Stokes, 1898. Arch Inst Pasteur, Tunis 1969; 46:1–16. [Google Scholar]
- 28.Bianchi L, Della Torre B, Martinazzi M. Fatal pancreatic necrosis in human phycomycosis. Pathol Microbiol 1967; 30:15–26. [DOI] [PubMed] [Google Scholar]
- 29.Nelson MR, Barton SE, Hawkins DA, Gazzard BG. Blastomycosis in an HIV antibody positive male in the UK. Int J STD AIDS 1993; 4:176–7. [DOI] [PubMed] [Google Scholar]
- 30.Emerson PA, Higgins E, Branfoot A. North American blastomycosis in Africans. Br J Dis Chest 1984; 78:286–91. [PubMed] [Google Scholar]
- 31.Cheikh Rouhou S, Racil H, Ismail O, et al. . Pulmonary blastomycosis: a case from Africa. Sci World J 2008; 8:1098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huerre M, Carloz E, Boubaker MS, et al. . Blastomycosis: the morphology of Blastomyces dermatitidis in tissue sections from African and American cases suggests that different varieties of the fungus prevail in the old and new worlds. J Mycol Med 2002; 12:5–11. [Google Scholar]
- 33.Kemna ME, Weinberger M, Sigler L, Zeltser R, Polachek I, Salkin IF. A primary oral blastomycosis-like infection in Israel. In: Program and abstracts of the American Society of Microbiology 93rd General Meeting (Las Vegas, NV). Washington, D.C: ASM, 1994:601.
- 34.El Euch D, Cherif F, Aoun K, et al. . Cutaneous blastomycosis: description of two cases in Tunisia. Med Trop 2004; 64:183–6. [PubMed] [Google Scholar]
- 35.Fragoyannis S, van Wyk G, de Beer M. North American blastomycosis in South Africa: a case report. S Afr Med J 1977; 51:169–71. [PubMed] [Google Scholar]
- 36.Rollier R, Berrada M. A case of African blastomycosis. Bull Soc Fr Dermatol Syphiligr 1969; 76:194–5. [PubMed] [Google Scholar]
- 37.Itodo AE, Ezeh AO. Blastomyces dermatitidis from lesions associated with Dermatophilus congolensis in cattle: case report. Bull Anim Health Prod Afr 1983; 31:191–2. [Google Scholar]
- 38.Alaka OO, Jarikre TA, Ogunro BN, et al. . A case of pulmonary blastomycosis in a common eland (Taurotragus oryx). Bulg J Vet Med 2017; 22:114–21. [Google Scholar]
- 39.Kuria JN, Gathogo SM. Concomitant fungal and Mycobacterium bovis infections in beef cattle in Kenya. Onderstepoort J Vet Res 2013; 80:585. [DOI] [PubMed] [Google Scholar]
- 40.Berry HH.Behavioural and eco-physiological studies on blue wildebeest (Connochaetes taurinus) at the Etosha National Park. Cape Town, South Africa: University of Cape Town, 1980. [Google Scholar]
- 41.Tavakoli A, Kazemi Mehrjerdi H. Pulmonary bulla in a dog secondary to blastomycosis. Iran J Vet Surg 2010; 5:109–14. [Google Scholar]
- 42.Dalis JS, Kazeem H. Concurrent infections of a goat with Dermatophilus congolensis and Blastomyces dermatitides. J Anim Vet Adv 2007; 6:773–5. [Google Scholar]
- 43.Adeyefa CAO. Survey of zoophilic dermatophytes from symptomatic and asymptomatic horses in Nigeria. Bull Anim Health Prod Afr 1992; 40:219–23. [Google Scholar]
- 44.Helal GA, Mostafa MH, El-Said MA. Fungi in the sewage-treatment Zeinein plant, Cairo, Egypt. J Basic Appl Mycol 2011; 2:69–82. [Google Scholar]
- 45.Lahmiti S, Baki S. Thoracic blastomycosis from Morocco. Sci World J 2009; 9:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rais H, Jghaimi F, Baalal H, et al. . Blastomycosis in Morocco: imported mycosis. Rev Pneumol Clin 2012; 68:45–9. [DOI] [PubMed] [Google Scholar]
- 47.Abubakari Y.Evaluation of peanut paste in selected markets in northern Ghana. Kumasi, Ghana: Kwame Nkrumah University of Science and Technology, 2016. [Google Scholar]
- 48.Peterson SW, Sigler L. Molecular genetic variation in Emmonsia crescens and Emmonsia parva, etiologic agents of adiaspiromycosis, and their phylogenetic relationship to Blastomyces dermatitidis (Ajellomyces dermatitidis) and other systemic fungal pathogens. J Clin Microbiol 1998; 36:2918–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bleeker PA, Haanen C. Blastomycosis treated successfully with miconazole. Trop Geogr Med 1979; 31:305–8. [PubMed] [Google Scholar]
- 50.Bregant S, Gigase P, Bastin JP, Vandepitte J. North American blastomycosis in the Republic of Zaire (apropos of 2 further cases). Bull Soc Pathol Exot Filiales 1973; 66:77–92. [PubMed] [Google Scholar]
- 51.Sekkat A, Benhayoune S, Benomar S, Derdabi D, Drouhet E, Heid E. La blastomycose cutanee et son traitement. A propos d’un nouveau cas marocain. Ann Dermatol Vener 1981; 108:877–82. [PubMed] [Google Scholar]
- 52.Campos Magalhaes M, Drouhet E, Destombes P. Premier cas de blastomycose à Blastomyces dermatitidis observe au Mozambique. Guérison par l’amphotéricine B. Bull Soc Pathol Exot 1968; 61:210–8. [PubMed] [Google Scholar]
- 53.Heys I, Taljaard J, Orth H. An emmonsia species causing disseminated infection in South Africa. N Engl J Med 2014; 370:283–4. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz IS, Govender NP, Corcoran C, et al. . Clinical characteristics, diagnosis, management and outcomes of disseminated emmonsiosis: a retrospective case series. Clin Infect Dis 2015; 61:1004–12. [DOI] [PubMed] [Google Scholar]
- 55.Simon GB, Berson SD, Young CN. Blastomycosis of the tongue: a case report. S Afr Med J 1977; 52:82–3. [PubMed] [Google Scholar]
- 56.Frean JA, Carman WF, Crewe-Brown HH, Culligan GA, Young CN. Blastomyces dermatitidis infections in the RSA. S Afr Med J 1989; 76:13–6. [PubMed] [Google Scholar]
- 57.Martin PM, Berson SD. Fungus diseases in Southern Africa. Mycopathol Mycol Appl 1973; 50:1–84. [DOI] [PubMed] [Google Scholar]
- 58.Bayles MAH. North American blastomycosis—atypical clinical presentation in a young African female. S Afr Med J 1975; 49: 2168. [Google Scholar]
- 59.Jelliffe DB, Hutt MSR, Connor DH, King MH, Lunn HF. Report of a clinico-pathological conference from Mulago. July 30, 1963. E Afr Med J 1964; 41:79–87. [PubMed] [Google Scholar]
- 60.Emmons CW, Murray IG, Lurie HI, King MH, Tulloch JA, Connor DH. North American blastomycosis: two autochthonous cases from Africa. Sabouraudia 1964; 3:306–11. [DOI] [PubMed] [Google Scholar]
- 61.Frean J, Blumberg L, Woolf M. Disseminated blastomycosis masquerading as tuberculosis. J Infect 1993; 26:203–6. [DOI] [PubMed] [Google Scholar]
- 62.Kaufman L, Standard PG, Weeks RJ, Padhye AA. Detection of two Blastomyces dermatitidis serotypes by exoantigen analysis. J Clin Microbiol 1983; 18:110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blume U, de Almeida H, Seibold M, Gollnick H, Orfanos CE. Mucocutaneous blastomycosis with epididymitis and orchitis blastomycetica: successful long-term treatment with ketoconazole. Eur J Dermatol 1992; 2:31–4. [Google Scholar]
- 64.Taillan B, Ferrari E, Cosnefroy JY, et al. . Favourable outcome of blastomycosis of the brain stem with fluconazole and flucytosine treatment. Ann Med 1992; 24:71–2. [DOI] [PubMed] [Google Scholar]
- 65.Klein BS, Aizenstein BD, Hogan LH. African strains of Blastomyces dermatitidis that do not express surface adhesin WI-1. Infect Immun 1997; 65:1505–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Summerbell RC, Kane J, Pincus DH. Enzymatic activity profiling as a potential biotyping method for Ajellomyces dermatitidis. J Clin Microbiol 1990; 28:1054–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vermeil C, Gordeeff A, Haddad N. Sur un cas tunisien de mycose generalisee mortelle. Ann Inst Pasteur 1954; 86:636–46. [PubMed] [Google Scholar]
- 68.Vermeil C, Morin O, Miegeville M, Marjolet M, Gordeeff A. Gilchrist’s disease and fungal taxonomy: a thorny problem. Bull Soc Pathol Exot Filiales 1981; 74:37–45. [PubMed] [Google Scholar]
- 69.Symmers WSC. Aspects of contributions of histopathology to the study of deep-seated fungal infections. In: Wolstenholme GEW and Ruth P, eds. Systemic Mycoses–a Ciba Foundation Symposium in commemoration of William Balfour Baikie. London, UK: J. & A. Churchill, 1968:37–45. [Google Scholar]
- 70.Vandepitte J, Gatti F. A case of North American blastomycosis in Africa. Its existence in Republic of Zaire. Ann Soc Belg Med Trop 1972; 52:467–79. [PubMed] [Google Scholar]
- 71.Guého E, Leclerc MC, de Hoog GS, Dupont B. Molecular taxonomy and epidemiology of Blastomyces and Histoplasma species. Mycoses 1997; 40:69–81. [DOI] [PubMed] [Google Scholar]
- 72.Chapman SW, Lin AC, Hendricks KA, et al. . Endemic blastomycosis in Mississippi: epidemiological and clinical studies. Semin Respir Infect 1997; 12:219–28. [PubMed] [Google Scholar]
- 73.Crampton TL, Light RB, Berg GM, et al. . Epidemiology and clinical spectrum of blastomycosis diagnosed at Manitoba hospitals. Clin Infect Dis 2002; 34:1310–6. [DOI] [PubMed] [Google Scholar]
- 74.Proctor ME, Davis JP. Blastomycosis--Wisconsin, 1986–1995. MMWR Morb Mortal Wkly Rep 1996; 45:601–3. [PubMed] [Google Scholar]
- 75.Lemos LB, Baliga M, Guo M. Blastomycosis: the great pretender can also be an opportunist. Initial clinical diagnosis and underlying diseases in 123 patients. Ann Diagn Pathol 2002; 6:194–203. [DOI] [PubMed] [Google Scholar]
- 76.Baumgardner DJ, Buggy BP, Mattson BJ, Burdick JS, Ludwig D. Epidemiology of blastomycosis in a region of high endemicity in north central Wisconsin. Clin Infect Dis 1992; 15:629–35. [DOI] [PubMed] [Google Scholar]
- 77.Baumgardner DJ, Paretsky DP, Yopp AC. The epidemiology of blastomycosis in dogs: north central Wisconsin, USA. J Med Vet Mycol 1995; 33:171–6. [DOI] [PubMed] [Google Scholar]
- 78.Hardin HF, Scott DI. Blastomycosis. Occurence of filamentous forms in vivo. Am J Clin Pathol 1974; 62:104–6. [DOI] [PubMed] [Google Scholar]
- 79.Sudman MS, Kaplan W. Antigenic relationship between American and African isolates of Blastomyces dermatitidis as determined by immunofluorescence. Appl Microbiol 1974; 27:496–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gugnani HC, Muotoe-Okafor F. African histoplasmosis: a review. Rev Iberoam Micol 1997; 14:155–9. [PubMed] [Google Scholar]
- 81.Oladele RO, Ayanlowo OO, Richardson MD, Denning DW. Histoplasmosis in Africa: an emerging or a neglected disease? PLoS Negl Trop Dis 2018; 12:e0006046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.