Abstract
Background
Powassan virus (POWV) is a tick-transmitted pathogen that may cause severe encephalitis; experimentally, it can be transmitted within just 15 minutes following a tick bite. The deer tick virus subtype of POWV (DTV) is transmitted by the deer tick and is the likely cause of the increase in the number of POWV cases reported in the United States. However, DTV has only been definitively documented in 6 patients by molecular analysis of the virus.
Methods
Two patients from Connecticut with encephalitis, who had a recent deer tick bite, were evaluated by the relevant serologic tests to determine if they had been infected with POWV. Evaluation also included molecular testing of an adult deer tick that had been removed from one of the patients.
Results
We documented neuroinvasive POWV infection in 2 children from Connecticut. Based on the results of testing the tick removed from case 2, this patient was infected by DTV, representing the 7th reported case and the first documented case of DTV infection in a child. Of note, the duration of the tick bites in both cases was very short.
Conclusions
We provide the first clinical and epidemiologic evidence that POWV/DTV can be rapidly transmitted to a human host, that is, within hours of tick attachment, which is distinctive when compared to other deer tick-transmitted infections such as Lyme disease.
Keywords: Powassan virus, deer tick virus, encephalitis, children, Lyme disease
We report 2 infants with neuroinvasive Powassan virus infection; at least one had been infected with the deer tick virus subtype. We provide epidemiologic evidence that Powassan virus can be transmitted to a human host within hours of tick attachment.
Powassan virus (POWV), a flavivirus in the tick-borne encephalitis (TBE) complex, was first isolated from a fatal case of encephalitis that occurred in 1958 in a 5-year old child from Powassan, Ontario [1]. In the late 1990s a flavivirus, closely related to POWV, was identified in ticks found in sites in New England and the upper Midwestern United States and named “deer tick virus” (DTV) [2, 3]. DTV is genetically distinct from prototypic POWV strains [4], and indeed, subsequent phylogenetic analyses clearly confirmed 2 lineages of POWV [5, 6]. In addition, DTV is ecologically distinct in its maintenance by the deer tick vector of Lyme disease (Ixodes dammini; also, the American clade of Ixodes scapularis) and white-footed mice (Peromyscus leucopus) [2], as opposed to that for prototypic POWV within the tick I. cookei and in woodchucks (or related, narrowly host-specific ticks with sciurid or carnivore mammals).
DTV and POWV are apparently antigenically indistinguishable and thus by taxonomic convention for flaviviruses are considered the same viral species [6]; DTV is considered a POWV subtype, or lineage.
The majority of cases of encephalitis presumably caused by prototype POWV were diagnosed in children (~70%) [7]. Because of the need for genetic data to determine the POWV subtypes, only 6 case reports of POWV infection (at least 5 of whom had encephalitis) have been confirmed as being caused by the DTV subtype, despite the emergence of cases of neuroinvasive POWV infections in Lyme disease endemic areas [5, 8, 9–15]. The 5 proven cases of DTV encephalitis occurred in adult patients. Indeed, recent epidemiologic data from the Centers for Disease Control and Prevention (CDC) document that <12% of the 99 reported cases of POWV virus disease from 2006 through 2016 were in people <18 years old [7, 16]. The explanation for this change in epidemiology remains to be determined.
In this report we describe 2 children with POWV encephalitis with at least 1 caused by DTV. Case 1 was diagnosed in 2016 and is the first case of POWV infection diagnosed in the State of Connecticut; the case history of this patient was previously reported in 2017 in an issue of Morbidity Mortality Weekly Report [17]. The second case was diagnosed in 2019. For this case the infecting tick was analyzed, and DTV was identified in the tick by molecular testing. Animal studies have documented rapid transmission of POWV following a tick bite, as quickly as within 15 minutes [18, 19]. The cases discussed in this report strongly suggest that infected ticks may also rapidly transmit POWV to people.
CASE 1
A 5-month-old male was hospitalized on 7 November 2016 after a 2-day illness characterized by fever (39.4°C), vomiting, and right sided facial twitching progressing to seizures. Two weeks before the onset of fever, a tick was removed from the infant’s forehead and discarded. Presumably, this was an adult deer tick, because this tick is by far the most common cause of tick bites in humans during the fall in New England [20]. Three hours before the tick’s discovery, the infant’s father had been outside hunting in the Connecticut woods and, while outside, he had brushed multiple ticks off his clothing. The infant’s mother was convinced the tick was attached no more than 3 hours, as her son’s forehead was clear when her husband initially returned from the walk in the woods.
On admission, the infant was afebrile. His seizures were controlled with fosphenytoin and levetiracetam. A complete blood count (12 200 white blood cells [WBCs]/mm3 with 59% neutrophils, 28% lymphocytes, and 12% monocytes, hematocrit = 35%, platelet count 351 000/mm3) and metabolic panel were within normal limits. Cerebrospinal fluid (CSF) examination showed 0 erythrocytes (red blood cells [RBCs])/mm3, 125 WBCs/mm3 with 81% lymphocytes, a protein level of 55 mg/dL, and a glucose level of 57 mg/dL. A brain magnetic resonance imaging (MRI) with contrast revealed edema of the basal ganglia, rostral thalami and left pulvinar, consistent with encephalitis. Viral and bacterial cultures of the CSF were negative. Testing of the CSF by polymerase chain reaction (PCR) for herpes simplex 1 and 2, and enteroviruses was negative. Testing of the blood for antibodies to Epstein-Barr virus, cytomegalovirus (CMV), Bartonella henselae, and Mycoplasma pneumoniae was also negative. Respiratory testing by PCR for respiratory syncytial virus and influenza viruses was negative.
POWV infection was considered because of the recent tick bite. CSF testing was positive for immunoglobulin M (IgM) POWV-specific neutralizing antibody with a titer of 1:32. POWV infection was confirmed by positive plaque reduction neutralization testing (PRNT) performed by the Centers for Disease Control and Prevention. Over the next month, the child’s motor development worsened in that he lost the strength to sit. A second brain MRI with contrast obtained 4 months after the first one revealed gliosis and encephalomalacia of the thalami and basal ganglia bilaterally. At 9 months of age, the child’s motor development began to improve. By age 15 months, his growth and development were normal. At age 3, his motor development was normal; however, there was a speech delay. A brain MRI without contrast done at this time showed no progression of the encephalomalacia.
CASE 2
A 2-month-old male child had fever (38.9° C) and listlessness for 1 day. He then developed left sided focal seizures (rhythmic left arm twitching, facial deviation to the left, and tongue thrusting with lip smacking), and he was hospitalized on 10 November 2019. Thirteen days before the hospitalization, a tick was removed from the infant’s arm and saved. His mother was certain that the tick was not present at the time of his morning bath. The tick was found and removed about 6 hours after the bath. During this 6-hour interval, his father had returned from a long walk with the dog. The family thought it likely that the father or the dog had brought the tick into the house following the walk.
When hospitalized, the infant’s temperature was 39.7° C, and he was having seizures every few hours that lasted 1–2 minutes. Between seizures, he was listless but was able to continue breast-feeding. He was begun on antiseizure medicines (levetiracetam and phenobarbital). A head computed tomography (CT) scan was normal. An electroencephalogram (EEG) showed subclinical seizures. He had a WBC count of 14 800/mm3 with 47% neutrophils, 38% lymphocytes, and 14% monocytes. His hematocrit was 36%, and his platelet count was 399 000/mm3. His CSF contained 127 RBCs/mm3, 215 WBCs/mm3 (10% polys, 52% lymphocytes, 38% monocytes), the protein level was 75 mg/dL, and the glucose level was 56 mg/dL. A brain MRI with contrast showed patchy edema of the thalami, right parietal lobe and right mid brain. Seizures and fever continued for 5 days. Repeat CSF examination done 4 days after admission showed 26 RBCs/mm3, 45 WBCs/mm3, and a protein level of 90 mg/dL. The infant was discharged after an 8-day hospitalization. His seizures had resolved, and he was clinically back to his baseline before he developed encephalitis. At the 2-week post hospitalization follow-up visit, the infant was doing well; an EEG at that time showed no seizure activity. At the 5-month follow-up, the patient was developing normally except there was a paresis of the left upper extremity.
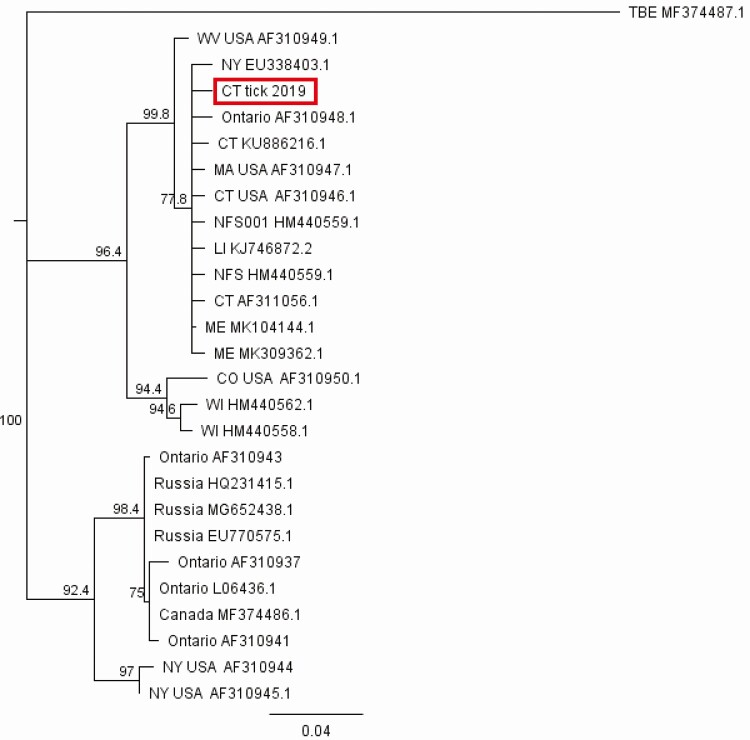
Testing of the CSF for arbovirus antibodies (including the California encephalitis panel, West Nile virus, eastern equine encephalitis virus, western equine encephalitis virus, St. Louis encephalitis virus) was negative. Testing the CSF by the Biofire PCR meningitis/encephalitis panel (which includes the viruses CMV, enteroviruses, herpes simplex 1 and 2, human herpes virus 6, human parechovirus, and varicella-zoster) was negative. Testing of the serum and CSF by the CDC was positive for IgM POWV antibodies. POWV infection was confirmed by a positive PRNT on both serum and CSF. The tick removed from the infant’s arm was a nonengorged adult female deer tick (Figure 1) that had not fed for more than 24 hours. A portion of the tick was analyzed for evidence of infection; it had been desiccated (held at room temperature within a plastic bag) for >2 weeks and hence virus was not recovered by intracerebral inoculation of tick homogenate into suckling mice, or by culture on VeroE6 cells. Reverse transcription polymerase chain reaction (RT-PCR) for POWV from this tick was positive, and sequencing of the 290bp NS5 gene target demonstrated that the infecting tick contained DTV (Figure 2). PCR targeting a 240bp Borrelia spp. flagellin target demonstrated that the tick also contained DNA of Borrelia burgdorferi. However, the child did not develop erythema migrans, and acute phase serologic testing for antibodies to Borrelia burgdorferi was negative.
Figure 1.
Left image, Portion of adult female Ixodes dammini tick received from patient’s family. The posterior third of the alloscutum was recovered and demonstrates a prominent marginal groove (black arrow), which is present on ticks that have not fed or have fed <24 hours. The posterior margin of the scutum (shield) is also seen (red arrow). In addition, defined gut diverticulae (dark branches under the tegument) are present; these diverticulae swell with an increasing amount of blood). Right image, Nonfed adult female I. dammini collected from vegetation, demonstrating marginal groove (black arrow), intact scutum (red arrow), and prominent gut diverticulae. Scale: Arrow is approximately 3 mm in length.
Figure 2.
POW NS5 gene sequence recovered from the infecting tick sample demonstrating identity with deer tick virus sequences. A 290bp portion of the NS5 gene, amplified with primers POW1/POW2 [2], was sequenced and aligned with related DTV and POW sequences downloaded from GenBank (accession numbers on tree). A neighbor-joining consensus tree was constructed using Tamura-Nei algorithm for genetic distances with 500-bootstrap replicates using Geneious v10 (Biomatters). Central European encephalitis virus was used as the outgroup. Abbreviations: DTV, deer tick virus; POW, Powassan.
DISCUSSION
In Connecticut, deer ticks can serve as vector for at least 5 pathogens, most frequently: Borrelia burgdorferi, Babesia microti, and Anaplasma phagocytophilum [21]. In animal studies, deer ticks typically need >36 hours of the tick feeding to transmit B. burgdorferi or B. microti, because these pathogens undergo a period of reactivation during the up to 5–7 day tick feeding process [19, 22]. POWV infected ticks, like those containing the closely related tick-borne encephalitis virus (TBEV), however, may infect mice by feeding for as little time as 15 minutes [18, 23]. POWV and the TBEV invade tick salivary glands very rapidly after infection and persist there in unfed ticks for at least 120 days [24], consistent with the observations in animal studies of rapid transmission during the first hours of tick salivation during feeding. Upon attachment to a host, a tick will immediately start to salivate into the bite site to introduce antihemostatic, anti-inflammatory, and immunosuppressive agents to promote blood feeding [25]; virus would be present within the first boluses of salivary secretion. Both of our reported cases appear to have been rapidly infected by the bite of infected deer ticks, although we cannot exclude another tick bite that had gone unnoticed.
POWV infection of humans has been notable for the severity of both the acute disease and the long-term sequelae. The case-fatality rate is at least 10%, and long-term sequelae are common (~50% of survivors) that may include hemiplegia, wasting, personality changes, and headaches [14, 26]. One of our 2 cases had residual paresis of the left upper extremity.
Within the last decade severe neurologic disease attributed to POWV infection has been increasingly reported from Lyme disease endemic areas of New England, New York, and the upper Midwest [14, 27, 28]. DTV has been definitively incriminated as the agent in only a few of these cases, but it is very likely that virtually all of the reported POWV cases within the last decade were caused by DTV infection given the geographic areas in which cases are now occurring [14]. This increasing incidence may be explained, in part, by intensive surveillance for encephalitis following the 1999 introduction of the West Nile virus into North America [29], as well as by increased accessibility of relevant testing methods. It is also likely that there has been cumulative slow amplification of DTV in local enzootic cycles. In Connecticut, from 2016 to 25 November 2019, 8 POWV cases were identified [30] (our case 1 occurred in 2016 and is the first case to have been diagnosed in this state).
TBEV infections, as well as the related West Nile virus, have large asymptomatic to symptomatic ratios of infected cases, and it is possible that POWV/DTV infection is similar. The asymptomatic to symptomatic ratio for POWV, however, remains to be formally determined. The incubation period for those who have developed clinical neurologic disease is 7–28 days [15].
The circumstances under which the 2 children reported here acquired infection require some comments that are pertinent to prevention of future cases. Infants typically would not be exposed to tick bites, but in both of our cases, parents presumably brought ticks into their homes after outdoor activities. Because of searching for an optimal skin site, ticks will not immediately attach to a person. Outdoor clothing may prevent access to skin, but the ticks may remain undetected and will crawl off the person when body heat is reduced, such as when a coat is removed. Parents should be educated about the need to treat outdoor clothing with permethrin, an effective mode of preventing tick bites. Permethrin binds tightly to fabric, lasting for at least a dozen wash cycles [31]. Although there is some repellent action from permethrin, contact with treated fabric will kill all ticks within 2 hours and thus reduce the risk that ticks brought into the home will seek hosts there. Parents should also be educated about the possibility that dogs could bring ticks into homes, and that these animals should be inspected after every outdoor exposure. Most anti-tick preventives only work after a tick has attached to a dog (the tick needs to ingest the chemical), although there are collars that are impregnated with permethrin or similar products that might repel or kill ticks.
In conclusion, we report 2 of the first cases of neuroinvasive POWV infection in the state of Connecticut. We report the first child in the United States who was presumed to have DTV infection based upon the presence of this virus subtype in a tick removed from the child. We also provide the first clinical and epidemiologic evidence that POWV can be rapidly transmitted to a human host, that is, within hours of tick attachment.
Notes
Acknowledgments. The authors thank Kristy L. Burkhalter of Centers for Disease Control and Prevention (CDC) Ft. Collins for laboratory information and for sharing a portion of the tick recovered from case 2. The authors also thank Dr Nickolas Bennett for his assistance.
Financial support. S. T. and H. K. G. are supported by National Institutes of Health (NIH) R01 AI 137424 and by NIH R01 AI 130105.
Potential conflicts of interest. G. P. W. reports receiving research grants from NIH/Immunetics, Inc., Institute for Systems Biology, Rarecyte, Inc., NIH/Tufts, CSU/NIH, and Quidel Corporation. He owns equity in Abbott/AbbVie; has been an expert witness in malpractice cases involving Lyme disease; and is an unpaid board member of the American Lyme Disease Foundation. He claims US Patent no. 10,669,567 B2. He reports employment with New York Medical College and unpaid lecturing for various medical centers and professional organizations. S. T. owns equity in Abbott; he is a consultant for Meridian Biosciences and Fuller Laboratories, and was a consultant last year to Takeda. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.McLean DM, Donohue WL. Powassan virus: isolation of virus from a fatal case of encephalitis. Can Med Assoc J 1959; 80:708–11. [PMC free article] [PubMed] [Google Scholar]
- 2.Telford SR III, Armstrong PM, Katavolos P, et al. A new tick-borne encephalitis-like virus infecting New England deer ticks, Ixodes dammini. Emerg Infect Dis 1997; 3:165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebel GD, Foppa I, Spielman A, Telford SR 2nd. A focus of deer tick virus transmission in the north central United States. Emerg Infect Dis 1999; 5:570–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebel GD, Spielman A, Telford SR. Phylogeny of North American Powassan virus. J Gen Virol 2001; 82:1657–65. [DOI] [PubMed] [Google Scholar]
- 5.Kuno G, Artsob H, Karabatsos N, Tsuchiya KR, Chang GJ. Genomic sequencing of deer tick virus and phylogeny of Powassan-related viruses of North America. Am J Trop Med Hyg 2001; 65:671–6. [DOI] [PubMed] [Google Scholar]
- 6.Beasley DW, Suderman MT, Holbrook MR, Barrett AD. Nucleotide sequencing and serological evidence that the recently recognized deer tick virus is a genotype of Powassan virus. Virus Res 2001; 79:81–9. [DOI] [PubMed] [Google Scholar]
- 7.Kemenesi G, Banyai K. Tick-borne flaviruses, with a focus on Powassan virus. Clin Microbiol Rev 2019; 32:e00106-17. 10.1128/CMR.00106-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gholam BI, Puksa S, Provias JP. Powassan encephalitis: a case report with neuropathology and literature review. CMAJ 1999; 161:1419–22. [PMC free article] [PubMed] [Google Scholar]
- 9.Tavakoli NP, Wang H, Dupuis M, et al. Fatal case of deer tick virus encephalitis. N Engl J Med 2009; 360:2099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Khoury MY, Hull RC, Bryant PW, et al. Diagnosis of acute deer tick virus encephalitis. Clin Infect Dis 2013; 56:e40–7. [DOI] [PubMed] [Google Scholar]
- 11.Neitzel DF, Lynfield R, Smith K. Powassan virus encephalitis, Minnesota, USA. Emerg Infect Dis 2013; 19:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavanaugh CE, Muscat PL, Telford SR 3rd, et al. Fatal deer tick virus infection in Maine. Clin Infect Dis 2017; 65:1043–6. [DOI] [PubMed] [Google Scholar]
- 13.Solomon IH, Spera KM, Ryan SL, et al. Fatal Powassan encephalitis (deer tick virus, lineage II) in a patient with fever and orchitis receiving rituximab. JAMA Neurol 2018; 75:746–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Khoury MY, Camargo JF, White JL, et al. Potential role of deer tick virus in Powassan encephalitis cases in Lyme disease-endemic areas of New York, USA. Emerg Infect Dis 2013; 19:1926–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ei Khoury MY, Camargo JF, Wormser GP. Changing epidemiology of Powassan encephalitis in North America suggests the emergence of the deer tick virus subtype. Expert Rev Anti Infect Ther 2013; 11:983–5. [DOI] [PubMed] [Google Scholar]
- 16.Krow-Lucal ER, Lindsey NP, Fischer M, Hills SL. Powassan virus disease in the United States, 2006–2016. Vector Borne Zoonotic Dis 2018; 18:286–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tutolo JW, Staples E, Sosa L, Bennett N. Powassan virus disease in an infant – Connecticut, 2016. MMWR Morbid Mortal Wkly Rep 2017; 66:408–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ebel GD, Kramer LD. Short report: duration of tick attachment required for transmission of Powassan virus by deer ticks. Am J Trop Med Hyg 2004; 71:268–71. [PubMed] [Google Scholar]
- 19.Eisen L. Pathogen transmission in relation to duration of attachment by Ixodes scapularis ticks. Ticks Tick Borne Dis 2018; 9:535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rand PW, Lacombe EH, Dearborn R, et al. Passive surveillance in Maine, an area emergent for tick-borne diseases. J Med Entomol 2007; 44:1118–29. [DOI] [PubMed] [Google Scholar]
- 21.Wormser GP, McKenna D, Scavarda C, et al. Co-infections in persons with early Lyme disease, New York, USA. Emerg Infect Dis 2019; 25:748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katavolos P, Armstrong PM, Dawson JE, Telford SRIII. Duration of tick attachment required for transmission of granulocytic ehrlichiosis. J Infect Dis 1998; 177:1422–5. [DOI] [PubMed] [Google Scholar]
- 23.Alekseev AN, Burenkova LA, Vasilieva IS, Dubinina HV, Chunikhin SP. Preliminary studies on virus and spirochete accumulation in the cement plug of ixodid ticks. Exp Appl Acarol 1996; 20:713–23. [DOI] [PubMed] [Google Scholar]
- 24.Slovák M, Kazimírová M, Siebenstichová M, et al. Survival dynamics of tick-borne encephalitis virus in Ixodes ricinus ticks. Ticks Tick Borne Dis 2014; 4:962–9. doi: 10.1016/j.ttbdis.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro JM, Makoul GT, Levine J, Robinson DR, Spielman A. Antihemostatic, antiinflammatory, and immunosuppressive properties of the saliva of a tick, Ixodes dammini. J Exp Med 1985; 161:332–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fatmi SS, Zehra R, Carpenter DO. Powassan virus: a new reemerging tick-borne disease. Front Public Health 2017; 5:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piantadosi A, Rubin DB, McQuillen DP, et al. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis 2016; 62:707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Courtney T, Sears S, Woytowicz J, et al. Outbreak of Powassan encephalitis: Maine and Vermont, 1999–2001. MMWR Morb Mortal Wkly Rep 2001; 50:761–4. [PubMed] [Google Scholar]
- 29.Hinten S, Beckett GA, Gensheimer KF, et al. Increased recognition of Powassan encephalitis in the United States, 1999–2005. Vector-Borne Zoonotic Dis 2008; 8:733–40. [DOI] [PubMed] [Google Scholar]
- 30.Mullins J, Esponda-Morrison B. Powassan virus: Connecticut, 2016–2019. Connecticut Epidemiologist 2019; 39:16. [Google Scholar]
- 31.Connally NP, Rose DA, Breuner NE, et al. Impact of wearing and washing/drying of permethrin-treated clothing on their contact irritancy and toxicity for nymphal Ixodes scapularis (Acari: Ixodidae) ticks. J Med Entomol 2019; 56:199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]