Abstract
Background
Recurrent Clostridioides difficile infections (rCDI) are a global public health threat. To reduce rCDI, microbiota-restoring therapies are needed, particularly standardized, easy-to-administer formulations.
Methods
This phase I open-label trial assessed the safety, efficacy in preventing rCDI recurrence, and intestinal microbiome effects of RBX7455, a room temperature-stable, orally administered investigational live biotherapeutic. Adult participants with 1 or more prior episodes of rCDI received: 4 RBX7455 capsules twice daily for 4 days (group 1); 4 RBX7455 capsules twice daily for 2 days (group 2); or 2 RBX7455 capsules twice daily for 2 days (group 3). For all groups, the first dose was administered in clinic, with remaining doses self-administered at home. Adverse events were monitored during and for 6 months after treatment. Treatment success was defined as rCDI prevention through 8 weeks after treatment. Participants’ microbiome composition was assessed prior to and for 6 months after treatment.
Results
Nine of 10 group 1 patients (90%), 8 of 10 group 2 patients (80%), and 10 of 10 group 3 patients (100%) were recurrence-free at the 8-week endpoint with durability to 6 months. Seventy-five treatment-emergent adverse events were observed in 27 participants with no serious investigational product-related events. Prior to treatment, participants’ microbiomes were dissimilar from the RBX7455 composition with decreased Bacteroidia- and Clostridia-class bacteria, whereas after treatment, responders’ microbiomes showed increased Bacteroidia and Clostridia.
Conclusions
Three dosing regimens of RBX7455 were safe and effective at preventing rCDI. Responders’ microbiomes converged toward the composition of RBX7455. These results support its continued clinical evaluation.
Clinical Trials Registration
Keywords: Clostridioides difficile infection, recurrence, microbiota-based therapeutic, oral administration, clinical trial
In this open-label phase 1 study, 3 RBX7455 oral dosing regimens were safe and demonstrated an average of 90% efficacy at preventing recurrent Clostridioides difficile infections (rCDI). The intestinal microbiome of treatment responders was altered after treatment to resemble RBX7455 and known healthy populations.
Clostridioides difficile infection (CDI) is the leading hospital-acquired infection in the United States, impacting 500 000 people annually [1] and accounting for 15% of all diagnosed infections [2]. In a recent meta-analysis, the incidence of CDI in the United States was calculated to be 8.3 cases per 10 000 patient days [3]. As such, CDI is associated with significant patient morbidity and mortality as well as a large financial burden, with annual attributable costs of 6.3 billion dollars in the United States [4].
A major risk factor for CDI is systemic antibiotic use, which disrupts normal gut flora, lowering resistance to C. difficile colonization [5]. The standard guideline-based treatment for CDI is antibiotic therapy with either vancomycin or fidaxomicin [6]. However, recurrent CDI (rCDI), defined as a new episode of CDI occurring within 8 weeks of successful treatment, is common [7] and occurs in approximately 15–30% of primary CDI episodes [8, 9] and 45–65% of recurrent CDI episodes [10, 11]. Indeed, given the historic nature of many of the studies, these figures likely underestimate this risk of recurrence, because the incidence of rCDI is on the increase, particularly for multiply recurrent episodes [12].
The recommended treatment of an initial episode of rCDI is antibiotic therapy. However, with further episodes, antibiotic treatment followed by microbiota-based therapies, most commonly fecal microbiota transplantation (FMT), to restore the intestinal microbiome is warranted [6]. A recent systematic review of randomized controlled trials identified 8 heterogeneously designed studies that included FMT, with an aggregate cure rate of 81% and recurrence rates varying from 0 to 56.3% [13]. However, to date there are no standardized Food and Drug Administration (FDA)-approved microbiota treatments for rCDI, and studies of FMT have widely varied in stool quantity, preparation, storage, and administration procedures [14]. To address this, several standardized investigational therapeutics are in formal clinical development [15, 16]. In one example, a randomized, double-blinded, placebo-controlled phase 2B trial indicated that trial participants with rCDI who received at least 1 dose of RBX2660, a liquid suspension investigational microbiota-based therapeutic administered by enema, had a reduced recurrence rate compared to participants who received only placebo [15].
FMT or investigational microbiota-based treatments for rCDI are commonly delivered via colonoscopy or enema by a clinician within a clinical environment, but there are also accumulating data to suggest the effectiveness of oral administration, including frozen [17–19] and lyophilized [20, 21] FMT preparations. Although these trials reported high efficacy, subjects were required to take large numbers of capsules (average 27–40) in 1 “dose,” which may be difficult to adhere for a substantial portion of the eligible rCDI population. Moreover, most oral administrations reported to date have required freezer storage, which necessitates administration in a healthcare setting.
The aim of the present study was to evaluate the safety and efficacy of RBX7455—a standardized, lyophilized, non-frozen, orally administered live biotherapeutic drug candidate—in participants with a history of CDI recurrence. The unique stability of RBX7455 to room temperature storage facilitates the option for patient self-administration in a nonhealthcare setting. In addition, we examined the fecal microbiome profiles of the patients prior to and up to 6 months following therapy with RBX7455.
METHODS
Study Population
Study participants were enrolled between 27 December 2016 and 15 May 2018 at a single tertiary referral center, the Mayo Clinic, Rochester, Minnesota, which served as the regulatory sponsor. The study protocol received Institutional Review Board approval prior to its commencement and was conducted under an FDA Investigational New Drug (IND) application. All patients provided written informed consent.
The study population included patients aged 18 years old or older with documentation of rCDI, defined as at least 1 recurrence after a primary episode, who had completed at least 2 rounds of standard-of-care oral antibiotic therapy. The inclusion of first-recurrent CDI (1 recurrence after a primary episode) differs from recent studies that only included multirecurrent CDI (at least 2 recurrent episodes) [15]. This difference was aimed at assessing the potential for reducing recurrence rates in the first-recurrent CDI population, which has not been as extensively evaluated. For eligibility purposes, patients must have had a positive stool test (either a nucleic acid amplification test or enzyme immunoassay for the C. difficile toxin) for the presence of C. difficile within 30 days prior to enrollment and had to have demonstrated resolution of diarrhea (fewer than 3 watery bowel movements per day) prior to study treatment.
Major exclusion criteria included: continued diarrhea despite antibiotic therapy; requirement of continuous antibiotic therapy for a condition other than CDI; previous fecal transplant; previous treatment with RBX2660; history of inflammatory bowel disease, irritable bowel syndrome, chronic diarrhea, or celiac disease; history of colostomy; evidence of active colitis; intended exposure to antibiotics within 6 months following study enrollment; compromised immune system (white blood cell count < 1000 cells/μL); current or expected use of systemic steroids; and pregnancy.
Study Design and Treatment
This open-label, single arm, dose-ranging study was designed to determine the safety and efficacy of 3 dosing regimens of the investigational RBX7455 drug product. For all participants, antibiotics were discontinued at least 24 and no more than 48 hours prior to the first dose of RBX7455. In group 1, participants received 4 RBX7455 capsules twice daily for 4 days; in group 2, 4 RBX7455 capsules twice daily for 2 days; and in group 3, 2 RBX7455 capsules twice daily for 2 days. Participants were requested but not required to provide stool samples at baseline, defined as after enrollment and prior to assigned treatment, and at day 1, and at weeks 1, 4, 8, 12, and 24 after treatment. These stool samples were provided via a home collection kit for whole stool, shipped overnight under cold conditions to Rebiotix, where upon receipt samples were immediately aliquoted and frozen without stabilizers at −80oC until analysis.
RBX7455 Manufacturing and Administration
RBX7455 was provided for the study by Rebiotix, a Ferring Company (Roseville, MN, USA). RBX7455 is manufactured starting with a microbiota-based suspension (RBX2660) that is prepared from human stool. Donor selection, pathogen screening, and preparation of RBX2660 are previously described [22] and were conducted according to an IND application in compliance with FDA guidelines at the time of manufacture. Multiple aliquots of RBX2660 sourced from a single donor and containing ≥107 live, human-derived bacteria / mL were combined, reduced in volume, and nonviable particulate material was minimized via centrifugation. A proprietary formulation of lyoprotectant and cryoprotectant excipients was added, and the mixture was lyophilized, with the resulting product milled and doubly encapsulated (V-caps® enteric, Capsugel, a Lonza Company, Morristown, NJ, USA) via a proprietary process. These capsules are resistant to opening in gastric pH conditions and release contents in postgastric pH conditions. Finished capsules were packaged into foil bags to limit moisture uptake and were stored at 2 to 8oC at Rebiotix (Roseville, MN, USA) until delivery to the study site pharmacy, where they were stored at 2 to 8oC until dispensation to study participants, at which point it was permitted to store RBX7455 at room temperature. Participants were administered the first assigned dose under supervision in the clinic, with self-administration thereafter permitted at home. Dosing compliance was monitored via study diary and by requesting participants to return empty packaging after dosing was completed.
Using a proprietary assay, the viable bacterial content of RBX7455 (colony forming units per capsule [CFU / capsule]) was measured at the time of manufacture and at specified time points up to 12 months during controlled storage at 2–8oC or 23–27oC. Four different batches of RBX7455 were utilized in the course of this study. All were confirmed to have viable bacterial content that exceeded proprietary minimal specifications at the time of dosing. All doses for each participant were from a single manufacturing batch and the same donor.
Study Endpoints
Primary Endpoints
Success was defined as the absence of rCDI at 8 weeks following completion of the final treatment. Treatment failure was defined as meeting all of the following criteria: recurrence of diarrhea <8 weeks after completion of RBX7455 therapy, a positive stool test for C. difficile (either a nucleic acid amplification test or enzyme immunoassay for the C. difficile toxin), a need for retreatment for CDI, and no other cause for diarrhea.
Safety was assessed via in-office study visits at 1 and 8 weeks after the start of study treatment, telephone assessments at 2 and 4 days and 3 and 6 months, and a subject diary utilized from enrollment to 7 days after study treatment. The diary solicited for the occurrence of anticipated possible treatment-emergent adverse events (TEAE), including flatulence, abdominal distension or bloating, rectal irritation or pain, chills or severe shivering, abdominal pain or cramping, increased diarrhea, constipation, rectal bleeding, nausea, vomiting, or a fever ≥38.0oC. Assessments beyond 8 weeks included the possibility of CDI recurrence. The in-office and telephone assessments and the study diary were used to assign the frequencies and severity grades of TEAEs in each treatment group from the first day of assigned study treatment through 6 months following the last dose of assigned study treatment. The investigator made a causality assessment for all TEAEs as definitely, probably, possibly, or not related to the investigational product.
Exploratory Endpoints
Comparison of fecal microbial composition and diversity changes was an exploratory endpoint. Participation in the sample collection phase of the trial was optional per consent requirements. To preclude selection bias, all received samples were included in the analysis, with the exception that samples received after treatment failure were not included, because participants received standard-of-care antibiotic and/or FMT at failure determination. Participant fecal samples and representative RBX7455 samples were stored frozen at −80oC with no added stabilizers until extraction and sequencing using a whole genome sequencing method (CoreBiome, Minneapolis, MN, USA). Relative taxonomic abundances and alpha diversity for each sample were determined from operational taxonomic units (OTU) data, and multidimensional scaling analysis (MDS) was used to map all individual samples onto 2-dimensional space with a Bray-Curtis dissimilarity metric with nonparametric scaling [23]. To assess longitudinal changes among patient-matches samples, DMRepeat [24] was used to conduct a repeated measures analysis on the subset of samples from treatment-responsive participants from whom baseline, 1-, 4-, and 8-week samples were received (3, 4, and 6 participants in groups 1, 2, and 3, respectively).
Data Monitoring and Statistical Analysis
Data were monitored by nonstudy personnel for accuracy and completeness using source document verification. Univariate descriptive statistics and frequency distributions were calculated, as appropriate for all variables. Baseline values for demographic, clinical, and outcome variables (primary and secondary) were tabulated for the treatment groups to identify potential confounding variables.
RESULTS
Participants
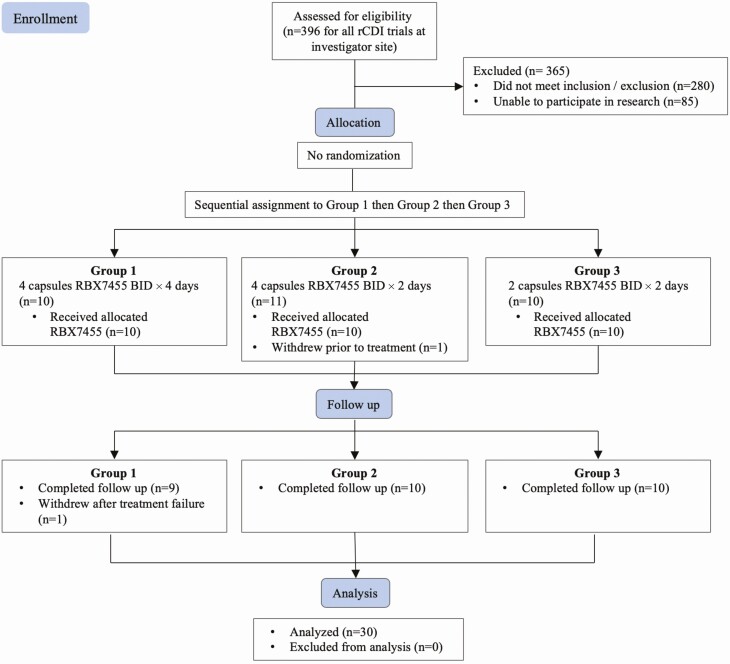
A total of 31 patients were enrolled. One enrollee withdrew prior to treatment, leaving 30 that received RBX7455, with 10 participants assigned to the 3 dosing groups sequentially without randomization (Figure 1). One participant withdrew from follow-up observation after being adjudicated as a treatment failure, and the remaining 29 completed the 6-month follow-up. The demographic and baseline characteristics of the 30 treated participants are summarized in Table 1. There were no statistically significant differences among groups with respect to sex (Fisher exact test), age, baseline antibiotic used, or number or duration of prior CDI episodes (1-way ANOVA).
Figure 1.
Consort diagram showing participant enrollment, allocation, follow-up, and analysis. Abbreviation: rCDI, recurrent Clostridioides difficile infection.
Table 1.
Demographic Characteristics of Participants Receiving RBX7455
| Group | |||
|---|---|---|---|
| 1 | 2 | 3 | |
| Median age (range) years | 67.5 (27–83) | 55 (22–81) | 63.2 (22–85) |
| Female sex (n) | 9 | 5 | 8 |
| Ethnicity, White (%) | 100 | 100 | 100 |
| Antibiotic administered at screening | |||
| Vancomycin | 9 | 9 | 9 |
| Fidaxomicin | 0 | 1 | 1 |
| Metronidazole | 1 | 0 | 0 |
| Mean number of CDI episodes (range) | 2.3 (2–3) | 2.8 (2–4) | 2.5 (2–4) |
| 3 or more CDI episodes (n) | 3 | 6 | 4 |
| Median duration of qualifying CDI episodes (range) | 15.5 (10–26) | 18 (12–29) | 15 (10–29) |
| With history of CDI hospitalization (n) | 4 | 1 | 1 |
Abbreviation: CDI, Clostridioides difficile infection.
Clinical Outcomes
All assigned doses were recorded in subject diaries as successfully taken as prescribed. Nine of 10 participants in group 1, 8 of 10 in group 2, and 10 of 10 group 3 were recurrence-free at the 8-week endpoint, with an overall success rate of 90% (Table 2). There was not a significant difference in clinical effectiveness among the 3 dose levels (P > .05; 1-way ANOVA) or between first recurrent (17 of 17 responded) and multirecurrent participants (10 of 13 responded; P > .05, Fisher exact test). Among the 3 nonresponders (1 in group 1 and 2 in group 2), the time between completing treatment and recurrence was 6, 2, and 44 days. Among 27 treatment responders, there were no additional rCDI episodes between 8 weeks and 6 months.
Table 2.
Efficacy and Safety Following Administration of RBX7455
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| Success at 8 weeks, n (%) | 9 (90) | 8 (80) | 10 (100) |
| Recurrences at 8 weeks, n (%) | 1 (10) | 2 (20) | 0 (0) |
| Recurrence-free at 6 months, n (%) | 9 (90) | 8 (80) | 10 (100) |
| Safety events, n/participants (%) | |||
| Total TEAEs | 34/9 (90) | 15/10 (100) | 26/8 (80) |
| Possibly/probably related to treatmenta | 7/5 (50) | 5/5 (50) | 14/6 (60) |
| Related to CDI | 5/5 (50) | 5/5 (50) | 12/5 (50) |
| Related to preexisting condition | 4/4 (40) | 5/4 (40) | 7/3 (30) |
| Serious | 5/1 (10)b | 1/1 (10)c | 0/0 (0) |
| Most prevalent organ classes | |||
| Gastrointestinal disorders | 13/7 (70) | 7/7 (70) | 13/6 (60) |
| Infections | 4/2 (20) | 2/2 (20) | 7/3 (30) |
Abbreviations: CDI, Clostridioides difficile infection; TEAE, treatment-emergent adverse event.
aPossibly or probably related to treatment. None reported as definitely related to RBX7455 treatment.
bNot related to treatment. Participant hospitalized 96 days after treatment for conditions unrelated to treatment.
cNot related to treatment. Participant hospitalized 111 days after treatment for condition unrelated to treatment.
A total of 75 TEAEs were observed in 27 participants (Table 2). The most common TEAEs were gastrointestinal including transient diarrhea (n = 10), abdominal discomfort (n = 3), and constipation (n = 5). There was no significant difference in TEAE frequency among treatment groups (χ 2 test). Serious TEAE were reported for 2 participants. One was hospitalized 96 days after treatment for chest and abdominal pain and shortness of breath, possibly related to prior diagnosis of urinary tract infection (UTI) and not related to RBX7455 treatment. A second was hospitalized 111 days after treatment for a diverticulitis-associated fistula not related to RBX7455, which resolved without sequelae. No deaths occurred in the duration of the study.
Microbiome Outcomes
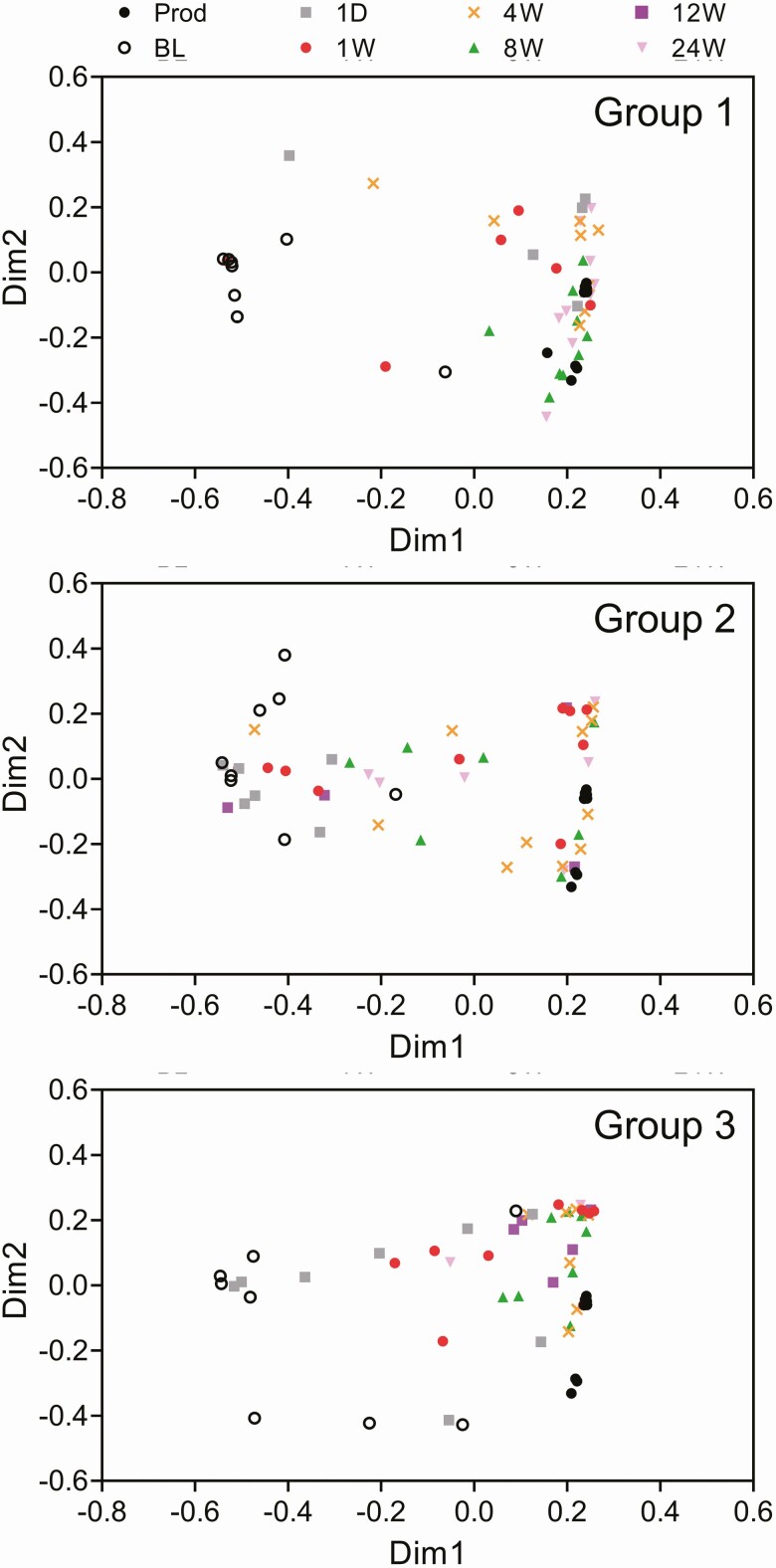
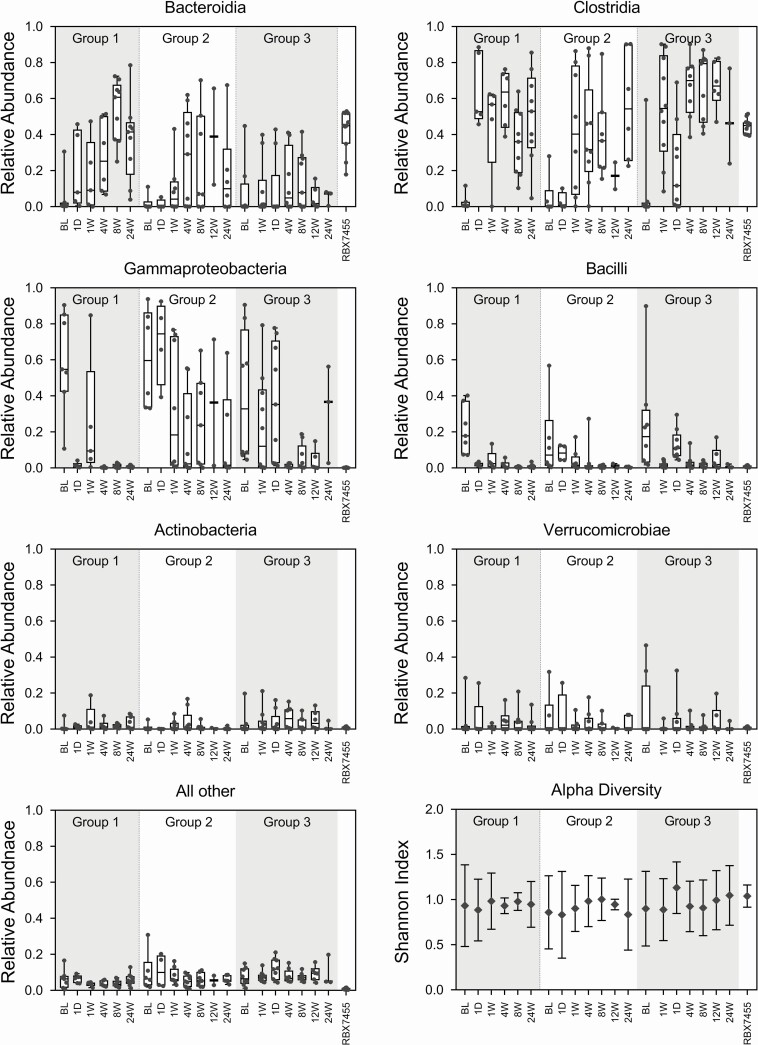
All 30 treated participants provided fecal samples that were included in this analysis (Table S1), for a total of 148 samples including 11 RBX7455 product samples representing all 4 batches dosed. Nonparametric multidimensional scaling analysis and relative abundance analyses indicated that treatment responsive participants’ bacterial microbiome compositions were divergent from the RBX7455 composition prior to treatment, with Gammaproteobacteria- and Bacilli-class bacteria predominating (Figures 2 and 3). Posttreatment microbiomes converged toward the RBX7455 composition after treatment within days and remained so through the 24-week period, with Bacteroidia and Clostridia predominating and a decrease in Gammaproteobacteria, Bacilli, and Verrucomicrobiae. At the genus level, restoration of Bacteroidia appeared to be driven by Bacteroides and Alistipes genera; restoration of Clostridia included multiple genera (Figure S1). Decreases in Bacilli were predominantly Lactobacillus and decreases in Gammaproteobacteria were exclusively genera in the Enterobacteriaceae family. There was not a clear trend of alpha diversity changes from before to after treatment (Figure 3), possibly due to small sample numbers. In the 3 nonresponders, baseline compositions were similar to those of responders, and although compositional changes were observed after treatment, little Bacteroidia restoration was observed (Figure S2).
Figure 2.
Nonparametric multidimensional similarity analysis for microbiome compositions of RBX7455 (Prod) and participant microbiome compositions before treatment (BL) and 1 day (1D) or 1, 4, 8, 12, and 24 weeks (1W, 4W, 8W, 12W, and 24W) after treatment.
Figure 3.
Taxonomic compositions at the class level and alpha diversity of responder microbiomes and RBX7455. Relative abundance data are shown in box-and-whiskers format, and alpha diversity is shown as a mean with standard deviations. Classes with <5% total relative abundances are groups together as “Other”.
To assess whether longitudinal microbiome changes were different among treatment groups, a repeated measures analysis was conducted with patient-matched samples from baseline, 1, 4, and 8 weeks after treatment, including 3, 4, and 6 participants from groups 1, 2, and 3, respectively, using a DMRepeat algorithm designed for Dirichlet multinomial relative abundance data [25]. Repeated measures examine an aggregate time course of posttreatment microbiome changes within individual patients and, as such, may be considered a pharmacodynamic measure of RBX7455 treatment. In this analysis, there was a significant difference between groups 2 and 3 at 4 and 8 weeks (P < .05), with more Clostridia restoration and Gammaproteobacteria decrease in group 3, but the difference was not significant at 1 week after treatment (Figure S3). Likewise, there was no significant difference at any time point between groups 1 and 2 or groups 1 and 3. Thus, there was no consistent trend among dosing groups that would confirm a dose-responsiveness with respect to microbiome changes, which is accordant with clinical outcomes.
DISCUSSION
The primary finding in this study was that 3 different dosing regimens of RBX7455 were effective at preventing rCDI, with success rates at 8 weeks of between 80% and 100% and no dose dependence. The efficacy demonstrated with RBX7455 is highly consistent with published studies of FMT reported by Madoff et al. at 81% [13], within which the 2 RCTs that examined oral preparations reported a mean efficacy of 87% [17–21]. Moreover, there were no additional recurrences between the 8-week and 6-month assessment points, indicating durable efficacy of RBX7455, which has not been examined in controlled studies of oral FMT. Finally, the safety profile of RBX7455 for rCDI was acceptable in this study, comparable to other reports for FMT, and possibly advantageous compared to administration by colonoscopy, for which there are known procedure-related risks [25].
This study has also importantly demonstrated that the microbiome compositions of RBX7455 treatment responders shifted after receiving therapy. Participants’ microbiomes normalized from Gammaproteobacteria and Bacilli predominance (before treatment) to Bacteroidia and Clostridia predominance (after treatment), demonstrating profiles similar to the RBX7455 composition and to recognized healthy compositions [26–28]. This is consistent with microbiome shifts measured after treatment with RBX2660, an enema-administered investigational live biotherapeutic [29]. The observed changes after RBX7455 may underpin its mechanism for reducing rCDI, because Bacteroidia and commensal Clostridia are associated with increased resistance to C. difficile colonization [30, 31], and because Gammaproteobacteria, which are reduced after treatment, are associated with increased CDI susceptibility and gastrointestinal inflammation [32, 33].
A key potential benefit of RBX7455 over existing FMT formulations is that it is produced using standardized good manufacturing practices (GMP) processes under strict quality-controlled conditions. This provides greater batch-to-batch consistency and ensures a documented, high quantity of viable bacteria per dose. Furthermore, the process for RBX7455 yields a formulation in which bacteria remain viable even during storage at room temperature throughout dosing. This feature allowed for participants in this trial to self-administer RBX7455 outside of a healthcare setting and still achieve a positive clinical outcome. This patient feasibility improvement has the potential to broaden the population that could benefit from RBX7455.
The main limitations of the study are the size of the population under investigation, lack of a control arm, and the possibility that the encouraging results may be related to a type-2 error. However, given the fact that many previous studies have reported comparable results for microbiome restorative therapies, this is unlikely.
CONCLUSIONS
In this study, 3 dosing regimens of RBX7455 were shown to be safe and effective at preventing rCDI for 8 weeks after treatment, with no additional recurrences for up to 6 months posttreatment. In addition, treatment with RBX7455 appeared to modify the microbiome in patients responding to therapy such that it converged toward the composition of RBX7455. Although this study is small, these results are encouraging and support the continued clinical evaluation of RBX7455 in randomized controlled trials.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The Data Safety Monitoring Board (DSMB) for this study comprised: Teena Chopra, MD, MPH (Chairperson, Wayne State University), Monika Fischer, MD, MSCR (Indiana University), and Adam Hamm, PhD (Chiltern International Limited). This manuscript was written with the assistance of Amy Moore, an independent medical writer funded by Rebiotix.
Financial support. The work was supported by Rebiotix Inc, a Ferring company, Roseville, MN, USA.
Potential conflicts of interest. S. K. has received research support from Rebiotix. K. B. and C. J. are employees of Rebiotix. K. B. has patents WO2019213595 and WO2019075426A1 (licensed to Rebiotix, A Ferring Company) pending. C. J. has the following patents issued: 9675648, 9433651, 10603341, 9642880, 10610547, 10624932, 10471107, 10493111, 2014275025, 2017201330, 2018220114, 3003330, 242934, 6330032, 6363259, KR102093537, KR102099503, 9511099, 10434124, 9511100, 10434125, 10434126, 9782445, 10688137, 10226431, 10391064, 20180243349, JP6616431, KR102066242, RU2714841C1, 9694039, 10383901, and 20180289750; and the following patents issued: CN111247254A, 2952500, 2019-0365831, 3455373, 109415760, 3022847, 2017/0327862, 2020037573, 3307289, 107921072, 2986915, 20180078586, 2928652, 3375447, 105473152, and 2914636. S. K. reports stock options from Jetson; and fees paid to Mayo Clinic from Vedanta, outside the submitted work. D. P. reports consulting fees from Seres Therapeutics, Assembly Biosciences, and Vedanta, outside the submitted work. All other authors have no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Guh AY, Mu Y, Winston LG, et al. ; Emerging Infections Program Clostridioides difficile Infection Working Group . Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med 2020; 382:1320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magill SS, O’Leary E, Janelle SJ, et al. ; Emerging Infections Program Hospital Prevalence Survey Team . Changes in prevalence of health care-associated infections in U.S. Hospitals. N Engl J Med 2018; 379:1732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marra AR, Perencevich EN, Nelson RE, et al. . Incidence and outcomes associated with Clostridium difficile infections: a systematic review and meta-analysis. JAMA Netw Open 2020; 3:e1917597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S, Palazuelos-Munoz S, Balsells EM, Nair H, Chit A, Kyaw MH. Cost of hospital management of Clostridium difficile infection in United States: a meta-analysis and modelling study. BMC Infect Dis 2016; 16:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eze P, Balsells E, Kyaw MH, Nair H. Risk factors for Clostridium difficile infections—an overview of the evidence base and challenges in data synthesis. J Glob Health 2017; 7:010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald LC, Gerding DN, Johnson S, et al. . Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:987–94. [DOI] [PubMed] [Google Scholar]
- 7.Surawicz CM, Brandt LJ, Binion DG, et al. . Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013; 108:478–98. [DOI] [PubMed] [Google Scholar]
- 8.Doh YS, Kim YS, Jung HJ, et al. . Long-term clinical outcome of Clostridium difficile infection in hospitalized patients: a single center study. Intest Res 2014; 12:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McFarland LV, Surawicz CM, Rubin M, Fekety R, Elmer GW, Greenberg RN. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol 1999; 20:43–50. [DOI] [PubMed] [Google Scholar]
- 10.Barbut F, Richard A, Hamadi K, Chomette V, Burghoffer B, Petit JC. Epidemiology of recurrences or reinfections of Clostridium difficile-associated diarrhea. J Clin Microbiol 2000; 38:2386–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect 2012; 18:21–7. [DOI] [PubMed] [Google Scholar]
- 12.Ma GK, Brensinger CM, Wu Q, Lewis JD. Increasing incidence of multiply recurrent Clostridium difficile infection in the United States: a cohort study. Ann Intern Med 2017; 167:152–8. [DOI] [PubMed] [Google Scholar]
- 13.Madoff SE, Urquiaga M, Alonso CD, Kelly CP. Prevention of recurrent Clostridioides difficile infection: a systematic review of randomized controlled trials. Anaerobe 2020; 61:102098. [DOI] [PubMed] [Google Scholar]
- 14.Bafeta A, Yavchitz A, Riveros C, Batista R, Ravaud P. Methods and reporting studies assessing fecal microbiota transplantation: a systematic review. Ann Intern Med 2017; 167:34–9. [DOI] [PubMed] [Google Scholar]
- 15.Dubberke ER, Lee CH, Orenstein R, Khanna S, Hecht G, Gerding DN. Results from a randomized, placebo-controlled clinical trial of a RBX2660—A microbiota-based drug for the prevention of recurrent Clostridium difficile infection. Clin Infect Dis 2018; 67:1198–204. [DOI] [PubMed] [Google Scholar]
- 16.McGovern BH, Ford CB, Henn MR, et al. . SER-109, an investigational microbiome drug to reduce recurrence after Clostridioides difficile infection: lessons learned from a phase 2 trial. Clin Infect Dis 2020; 7:ofaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirsch BE, Saraiya N, Poeth K, Schwartz RM, Epstein ME, Honig G. Effectiveness of fecal-derived microbiota transfer using orally administered capsules for recurrent Clostridium difficile infection. BMC Infect Dis 2015; 15:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kao D, Roach B, Silva M, et al. . Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial. JAMA 2017; 318:1985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 2014; 312:1772–8. [DOI] [PubMed] [Google Scholar]
- 20.Jiang ZD, Jenq RR, Ajami NJ, et al. . Safety and preliminary efficacy of orally administered lyophilized fecal microbiota product compared with frozen product given by enema for recurrent Clostridium difficile infection: a randomized clinical trial. PLoS One 2018; 13:e0205064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staley C, Hamilton MJ, Vaughn BP, et al. . Successful resolution of recurrent Clostridium difficile infection using freeze-dried, encapsulated fecal microbiota: pragmatic cohort study. Am J Gastroenterol 2017; 112:940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orenstein R, Dubberke E, Hardi R, et al. ; PUNCH CD Investigators . Safety and durability of RBX2660 (microbiota suspension) for recurrent Clostridium difficile infection: results of the PUNCH CD Study. Clin Infect Dis 2016; 62:596–602. [DOI] [PubMed] [Google Scholar]
- 23.La Rosa PS, Brooks JP, Deych E, et al. . Hypothesis testing and power calculations for taxonomic-based human microbiome data. PLoS One 2012; 7:e52078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shannon WD. Repeated measures method for microbial count data. (BioRankings Technical Report 3). Available at: https://biorankings.com/technical-series. Accessed 1 October 2020
- 25.Baxter M, Colville A. Adverse events in faecal microbiota transplant: a review of the literature. J Hosp Infect 2016; 92:117–27. [DOI] [PubMed] [Google Scholar]
- 26.The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012; 486:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med 2016; 8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin J, Li R, Raes J, et al. ; MetaHIT Consortium . A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010; 464:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blount KF, Shannon WD, Deych E, Jones C. Restoration of bacterial microbiome composition and diversity among treatment responders in a phase 2 trial of RBX2660: an investigational microbiome restoration therapeutic. Open Forum Infect Dis 2019; 6:ofz095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khanna S, Montassier E, Schmidt B, et al. . Gut microbiome predictors of treatment response and recurrence in primary Clostridium difficile infection. Aliment Pharmacol Ther 2016; 44:715–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schubert AM, Sinani H, Schloss PD. Antibiotic-induced alterations of the murine gut microbiota and subsequent effects on colonization resistance against Clostridium difficile. mBio 2015; 6:e00974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol 2013; 13:790–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garrett WS, Gallini CA, Yatsunenko T, et al. . Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe 2010; 8:292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.