Abstract
Background
In low-resource, malaria-endemic settings, accurate diagnosis of febrile illness in children is challenging. The World Health Organization (WHO) currently recommends laboratory-confirmed diagnosis of malaria prior to starting treatment in stable children. Factors guiding management of children with undifferentiated febrile illness outside of malaria are not well understood.
Methods
This study examined clinical presentation and management of a cohort of febrile Kenyan children at 5 hospital/clinic sites from January 2014 to December 2017. Chi-squared and multivariate regression analyses were used to compare frequencies and correlate demographic, environmental, and clinical factors with patient diagnosis and prescription of antibiotics.
Results
Of 5735 total participants, 68% were prescribed antibiotic treatment (n = 3902), despite only 28% given a diagnosis of bacterial illness (n = 1589). Factors associated with prescription of antibiotic therapy included: negative malaria testing, reporting head, ears, eyes, nose and throat (HEENT) symptoms (ie, cough, runny nose), HEENT findings on exam (ie, nasal discharge, red throat), and having a flush toilet in the home (likely a surrogate for higher socioeconomic status).
Conclusion
In a cohort of acutely ill Kenyan children, prescription of antimalarial therapy and malaria test results were well correlated, whereas antibiotic treatment was prescribed empirically to most of those who tested malaria negative. Clinical management of febrile children in these settings is difficult, given the lack of diagnostic testing. Providers may benefit from improved clinical education and implementation of enhanced guidelines in this era of malaria testing, as their management strategies must rely primarily on critical thinking and decision-making skills.
Keywords: malaria, fever, children, medical decision-making, low resource
Providers in Kenya showed good adherence to testing and treating for malaria in febrile children, but tend to use antibiotics empirically in those who test malaria negative.
Understanding how clinical decisions are made is complicated, especially in resource-poor settings where medical records and tracking of patient outcomes are limited. As of 2010, the World Health Organization (WHO) recommends confirmatory testing to establish a diagnosis of malaria in febrile children prior to prescribing antimalarial therapy [1]. As fever due to malaria continues to decrease in sub-Saharan Africa [2, 3], providers must evaluate large numbers of patients who may not always fit the algorithms they were trained to use [4], creating even more diagnostic uncertainty [5–7]. As concern for antibiotic resistance increases, understanding management of febrile illness, including factors influencing antibiotic prescription in these environments is essential to promoting antibiotic stewardship worldwide [8].
Most government-funded clinics in Kenya do not have consistent access to diagnostic testing beyond point-of-care tests for malaria; thus, clinicians must rely on history and physical exam [9–11] and the use of Integrated Management of Childhood Illness (IMCI)-based clinical guidelines [12–14]. In prior studies, adherence to clinical guidelines in the management of malaria and other pediatric febrile illnesses has been poor [5, 15–18]. Studies demonstrate overuse of antibiotic treatment in some cases, and under-use in others, indicating that there are insufficient resources to accurately diagnose febrile illness outside of malaria and significant risk of antibiotic overuse [15, 19–21].
Goals of this study were to characterize which variables are associated with higher odds of prescription of antibiotic therapy in both malaria-negative and malaria-positive participants and evaluate concordance of diagnosis of bacterial illness with prescription of antibiotic treatment [21]. Rather than focusing on adherence to IMCI guidelines, our study population allowed us to evaluate the management of children with undifferentiated febrile illness using detailed clinical data in a resource-limited setting.
Our study focused on 5 sites in malaria-endemic areas of Kenya: 2 sites in Kwale County, Kenya (Coastal region) and 3 sites in Kisumu County, Kenya (Nyanza Region). These regions have higher under-5 mortality rates than the Kenyan national rate (52/1000 live births), with the Coastal region at 57/1000 live births and the Nyanza region at 82/1000 live births [11]. In the regions studied, 40% of caregivers of children recently evaluated for fever reported that they had received antibiotic treatment, with increasing frequency of caregivers presenting to a health provider for evaluation of a febrile child in comparison to previous years [11]. Preliminary findings demonstrate children in Kenya are commonly exposed to viral illnesses, such as chikungunya and dengue infections [22–25], however, these are rarely diagnosed [23].
METHODS
Study Sites
This sub-study on provider decision-making is part of a larger arbovirus study (NIH R01AI102918, SSC No. 2611) assessing the burden of chikungunya- and dengue-related disease in children in urban and rural areas of coastal (Ukunda-Diani Beach) and western (Kisumu) Kenya. Child health clinics at study site locations were used for recruitment as previously described [21, 24, 26], with the addition of the Mbaka Oromo Dispensary (a small clinic) in June of 2015 (−0.091 648, 34.767 978), located in Chulaimbo village, about 20 km west of Kisumu, added to increase recruitment due to difficulty from competing studies at Chulaimbo County Hospital. All facilities are government funded, public health facilities with the capability to obtain either laboratory or rapid diagnostic testing (RDT) for malaria, hemoglobin, and HIV. The two larger referral hospitals (Msambweni and Obama Children’s) and the district hospital (Chulaimbo) have capability for inpatient admission and some laboratory testing; however, results would not be available to the clinician at time of enrollment. The other 2 facilities (Mbaka Oromo and Diani Health Centre) must refer pediatric patients for admission. No facility had microbiology testing available, and radiographs were not available except for admitted patients.
Participant Recruitment and Enrollment
Participants were recruited continuously from January 6, 2014—December 31, 2017. Consent was provided by the parent or guardian of the child, with assent obtained for children 7 years and older. Inclusion criteria included: children aged 1 year to <18 years presenting for medical evaluation for an acute febrile illness with temperature 38 degrees Celsius or above recorded at triage (temperature could have normalized by time of assessment) and meeting any criteria for a possible arboviral infection (ie, nonspecific fever with or without signs of meningoencephalitis and/or hemorrhagic disease), without a readily diagnosable source of illness after routine clinical evaluation as judged by the clinician. Participants were not required to have any specific arboviral symptoms such as rash, myalgias, arthralgias, or headache. Participants with possible or suspected malarial illness were included, given its variable presentation, uniform availability of malaria testing at each site, and the recommendation by WHO against the diagnosis of malaria based on history or exam alone.
Data Collection
All child participants with fever identified at triage were evaluated by one of 13 study clinical officers who were part of the clinical team evaluating patients during their shift at their respective sites. These clinical officers had varying degrees of pediatric experience, none of whom had pediatric specialty training (two had IMCI and Emergency Triage Assessment and Treatment training). A study clinical officer completed a survey form for each participant using Open Data Tool Kit Survey (Open Data Kit 2.0 Toolkit. 2017. URL https://opendatakit.org/, see Supplementary File 1: Survey Form) and provided a primary diagnosis (malaria, viral, or bacterial) for the participant, and secondary (differential diagnosis) as necessary. All children were tested for malaria by blood smear microscopy, or RDT if microscopy was not available. Post-hoc validation for all RDT results was performed by laboratory study staff using blood smear review.
Data Analysis
Data were stored securely using REDCap electronic data capture tools hosted at the Stanford Center for Clinical Informatics [27]. Variations in frequency among categorical variables were compared by χ 2 analysis. Univariate logistic regression analysis was performed initially, followed by multivariate analysis to predict the main outcome of prescription of antibiotic therapy (variables listed in Table 1). Variables determined to have a priori clinical significance and those determined to have statistical significance (P < .05) in univariate analysis were then used to create a multivariate model. As there was great overlap among reported symptoms on history and clinical exam findings, only exam findings were included in the multivariate model. A P-value of < .05 was considered significant. All analyses were performed using R [28].
Table 1.
Prescription of Antibiotics. Comparison of Characteristics of Participants Prescribed Antibiotics Versus Those Who Were Not Prescribed Antibiotics
| Antibiotic prescribed | ||||
|---|---|---|---|---|
| Yes (row %) | No (row %) | P-valuea | ||
| Hospital site | 1 | 1054 (93) | 84 (7) | <.001b |
| 2 | 241 (60) | 160 (40) | ||
| 3 | 1214 (65) | 658 (35) | ||
| 4 | 1226 (63) | 708 (37) | ||
| 5 | 167 (43) | 223 (57) | ||
| Sex | Female | 1845 (68) | 881 (32) | .6 |
| Male | 2057 (68) | 952 (32) | ||
| Age group (years) | 1 to 4 | 2129 (73) | 789 (27) | <.001b |
| 5 to 9 | 1105 (62) | 663 (38) | ||
| 10 to 14 | 376 (55) | 302 (45) | ||
| 15 or older | 96 (64) | 54 (36) | ||
| Season | Jan-Mar (dry) | 1035 (69) | 470 (31) | <.001b |
| Apr-Jun (rainy) | 1057 (67) | 517 (33) | ||
| Jul-Sept (dry) | 1172 (63) | 690 (37) | ||
| Oct-Dec (less rainy) | 638 (80) | 156 (20) | ||
| Bed net use | Always | 2907 (66) | 1526 (34) | <.001b |
| Sometimes | 810 (79) | 209 (21) | ||
| Never | 181 (66) | 94 (34) | ||
| Roof type | Natural | 1355 (60) | 888 (40) | <.001b |
| Other | 2544 (73) | 941 (27) | ||
| Toilet type | None/bush | 838 (64) | 474 (36) | <.001b |
| Latrine | 2608 (67) | 1294 (33) | ||
| Flush | 453 (88) | 63 (12) | ||
| Mother’s education level | Primary school | 2261 (67) | 1137 (33) | <.001b |
| Secondary school | 803 (80) | 206 (20) | ||
| Technical college | 171 (90) | 18 (10) | ||
| Professional degree | 18 (75) | 6 (25) | ||
| Other | 18 (38) | 29 (62) | ||
| Number of days ill | 0–1 day | 239 (53) | 212 (47) | <.001b |
| 2 days | 1660 (70) | 726 (30) | ||
| 3 days | 1415 (66) | 727 (34) | ||
| 4 days | 209 (74) | 72 (26) | ||
| 5+ days | 223 (78) | 65 (22) | ||
| Fever on exam (°C) | None (<38) | 611 (28) | 1551 (72) | <.001b |
| 38–40 | 2996 (68) | 1421 (32) | ||
| >40 | 147 (53) | 130 (47) | ||
| Positive physical exam findings | HEENT exam | 762 (91) | 72 (9) | <.001b |
| Lymph node exam | 547 (90) | 63 (10) | <.001b | |
| Joint exam | 548 (86) | 87 (14) | <.001 | |
| Abdominal exam | 140 (76) | 45 (24) | .03 | |
| Malaria test positive | Yes | 1615 (53) | 1450 (47) | <.001b |
| No | 2245 (87) | 332 (13) | ||
| Primary diagnosis of malaria | Yes | 1753 (55) | 1450 (45) | <.001b |
| No | 2078 (85) | 359 (15) | ||
| Prescribed antimalarials | Yes | 1701 (45) | 1467 (55) | <.001b |
| No | 2201 (85) | 366 (15) | ||
| Primary diagnosis bacterial | 700 (86) | 112 (14) | <.001b | |
| Differential diagnosis bacterial | 515 (67) | 258 (33) | ||
| No diagnosis bacterial | 2515 (66) | 1324 (34) | ||
| Missing diagnosis bacterial | 172 (55) | 139 (45) | ||
| Primary diagnosis viral | Yes | 1281 (84) | 245 (16) | <.001b |
| No | 2607 (62) | 1578 (38) | ||
aχ 2 test.
bsignificant on univariate analysis.
Ethical Considerations
This study was approved by the Stanford University IRB (34649) and the Kenya Medical Research Institute (KEMRI) Scientific and Ethical Review Unit (SCC No. 2611).
RESULTS
Population Characteristics
A total of 5737 children were enrolled. Two participants with temperature values recorded at the time of assessment that were outside of the range of 34–42°C were excluded due to physiologic improbability, leaving a total of 5735 participants for analysis. As all but one participant had outcomes data for antibiotic treatment prescribed, no one was excluded due to missing data. There was a total of 5735 participants included in the analysis. Approximately 50% of participants were male. Most were under the age of 10 years and presented with an illness duration of 2 days (Table 2). Malaria was the most common primary diagnosis (n = 3203, 56%), followed by viral (n = 1526, 27%), and bacterial illness (n = 812, 14%) (Table 2).
Table 2.
Demographic and Clinical Characteristics for All Study Participants, n = 5735
| Variable | n(%) | |
|---|---|---|
| Hospital Site | 1 | 1138 (20) |
| 2 | 401 (7) | |
| 3 | 1872 (33) | |
| 4 | 1934 (34) | |
| 5 | 390 (7) | |
| Sex | Male | 3009 (52) |
| Age Group (years) | 1 to 4 | 2918 (53) |
| 5 to 9 | 1768 (32) | |
| 10 to 14 | 678 (12) | |
| 15 plus | 150 (3) | |
| Season | Jan-Mar (dry) | 1505 (26) |
| Apr-Jun (rainy) | 1574 (27) | |
| Jul-Sept (dry) | 1471 (26) | |
| Oct-Dec (less rainy) | 1185 (21) | |
| Number of days ill | 0–1 | 451 (8) |
| 2 | 2386 (43) | |
| 3 | 2142 (38) | |
| 4 | 281 (5) | |
| 5 or more | 298 (5) | |
| Fever on exam (°Celsius) | <38 | 862 (16) |
| 38–40 | 4417 (80) | |
| Over 40 | 277 (5) | |
| Malaria test positive | Yes | 3065 (53) |
| No | 2577 (45) | |
| Other/Missing | 93 (2) | |
| Primary diagnosis | Malaria | 3203 (56) |
| Viral | 1526 (27) | |
| Bacterial | 812 (14) | |
| Other/Missinga | 194 (3) | |
| Differential diagnosis | Viral | 1213 (22) |
| Bacterial | 777 (14) | |
| Malaria | 153 (3) | |
| Other | 30 (0) | |
| None/Missing | 3562 (62) | |
| Prescribed antibiotics | Yes | 3902 (68) |
| Prescribed antimalarials | Yes | 3168 (55) |
| Hospitalized | Yes | 162 (7) |
aOther diagnoses included: fungal infection, acute upper respiratory infection, gastroenteritis (2nd diagnosis), dysentery (2nd diagnosis), furunculosis, amebiasis, HIV/oral thrush, musculoskeletal injury, sickle cell disease, parasitic infection, otitis media, pharyngitis, tinea capitis, enteric fever, chicken pox, asthma, soft tissue infection (2nd diagnosis), scabies, congestive heart failure, measles, mumps, dental problem, tonsillitis, sepsis (many also listed as “None”).
Diagnosis and Prescription of Antibiotics
Of all participants enrolled in the study, 68% (n = 3902) were prescribed antibiotic therapy (Table 2). A primary diagnosis of bacterial illness was given to 812 (14%) participants, and of these, 86% were prescribed antibiotic therapy (n = 700) (Table 1). For 777 (14%) participants, bacterial illness was reported as the differential diagnosis. Most of these participants were also prescribed antibiotic therapy (n = 515, 67%). Combined, all participants in whom bacterial illness was at least considered accounted for only a third of all participants prescribed antibiotic treatment (1215 of 3902, 31%). The majority given antibiotic treatment had neither a primary nor a differential diagnosis of bacterial illness (n = 2515, 64%) (Table 1).
Participants given a primary diagnosis of viral disease were frequently prescribed antibiotic treatment (n = 1281, 84%) (Table 1), with only 6% (n = 83) given a concomitant differential diagnosis of bacterial illness. In contrast, antibiotics were prescribed less frequently in participants with a primary diagnosis of malaria (55%, 1753 of 3203, Table 1). Conversely, antibiotic therapy was prescribed to 87% (n = 2245) of those who were malaria test negative (Table 1). Both antimalarial and antibiotic treatments were prescribed to 30% of all participants (n = 1701) (Table 1).
Demographic and Environmental Factors Associated With Prescription of Antibiotic Therapy
On multivariate analysis, factors significantly associated with lower odds of prescription of antibiotic therapy included: older children (age 5–14 years), owning a latrine, high fever (>40°C) at time of exam, and abnormal joint (including warmth, redness, swelling, and tenderness) or abdominal physical exam findings (including tenderness to palpation, splenomegaly, and hepatomegaly).
Higher odds of prescription of antibiotic therapy were significantly associated with negative malaria testing, which increased the odds of antibiotic prescription by 7-fold (OR 7.1, 95% CI 5.6, 9.1). Other factors associated with increased odds of antibiotic therapy included: younger age (ages 1–4 years), evaluation during the months of October through December, owning a flush toilet, presentation earlier in the course of illness (day 2 of illness), positive head, ears, eyes, nose, and throat (HEENT) exam findings (including nasal discharge, red throat, or cervical lymphadenopathy), primary diagnosis of bacterial illness, and Hospital 1 (Table 3). There was concern that both “natural roof” and “latrine type” were correlated, thus, a sensitivity analysis was conducted to determine if excluding them would have a significant effect on the model, which it did not.
Table 3.
Multivariate Analysis of Factors Associated With the Prescription of Antibiotics
| Characteristic | OR (CI) | P-value | |
|---|---|---|---|
| Hospital Site | 1 | referent | n/a |
| 2 | 0.1 (.1, .2) | <.001 | |
| 3 | 0 (0, .1) | <.001 | |
| 4 | 0.1 (0, .1) | <.001 | |
| 5 | 0 (0, .1) | <.001 | |
| Age Group | 1 to 4 years | referent | n/a |
| 5 to 9 years | 0.8 (.6, .9) | .01 | |
| 10 to 14 years | 0.5 (.4, .6) | <.001 | |
| 15+ years | 0.8 (.5, 1.4) | .45 | |
| Season | Jan-Mar (dry) | referent | n/a |
| Apr-Jun (rainy) | 0.7 (.6, .9) | .01 | |
| Jul-Sept (dry) | 0.9 (.7, 1.1) | .16 | |
| Oct-Dec (less rainy) | 1.6 (1.3, 2.0) | <.001 | |
| Sleep with a bed net | Always | referent | n/a |
| Sometimes | 1.2 (.9, 1.5) | .27 | |
| Never | 1.1 (.7, 1.6) | .67 | |
| Natural roof | No | referent | n/a |
| Yes | 0.8 (.7, 1.0) | .09 | |
| Latrine Type | None/bush | referent | n/a |
| Latrine | 0.6 (.4, .8) | <.001 | |
| Flush Toilet | 1.8 (1.2, 2.8) | <.001 | |
| Mother’s education level | Primary school | referent | n/a |
| Secondary school | 0.9 (.8, 1.2) | .54 | |
| Technical college | 0.8 (.4, 1.5) | .46 | |
| Professional degree | 0.5 (.1, 1.7) | .24 | |
| Other | 0.3 (.2, .7) | <.001 | |
| Number of days ill | 0–1 days | referent | n/a |
| 2 days | 1.7 (1.3, 2.3) | <.001 | |
| 3 days | 1.3 (1.0, 1.7) | .09 | |
| 4 days | 1.2 (.7, 1.8) | .54 | |
| 5+ days | 1.2 (.8, 2.0) | .38 | |
| Fever on exam | None (<38 deg) | referent | n/a |
| 38–40 deg | 0.9 (.8, 1.2) | .56 | |
| >40 deg | 0.7 (.4, 1.0) | .05 | |
| Physical exam findings | Negative exam | referent | n/a |
| HEENT exam | 3.0 (2.0, 4.7) | <.001 | |
| Lymph node exam | 0.4 (.3, .8) | <.001 | |
| Joint exam | 0.4 (.2, .7) | <.001 | |
| Abdominal exam | 0.2 (.1, .5) | <.001 | |
| Malaria test result | Positive | referent | n/a |
| Negative | 7.1 (5.3, 9.1) | <.001 | |
| Primary diagnosis bacterial | No | referent | n/a |
| Yes | 3.1 (2.2, 4.3) | <.001 | |
| Primary diagnosis viral | No | referent | n/a |
| Yes | 0.5 (.4, .7) | <.001 |
Specific Antibiotic Therapies and Antibiotic Use Over Time
Amoxicillin, was the most frequently prescribed antibiotic treatment, given to 40% of all participants, and 59% (n = 2302/3902) of participants who were prescribed antibiotic therapy. Amoxicillin-clavulanate was the second most prescribed antibiotic treatment (13% of all participants, 764/3902 or 20% of those prescribed antibiotic therapy). Several other antibiotic medications were prescribed at lower frequencies including, but not limited to: trimethoprim-sulfamethoxazole, penicillin, ciprofloxacin, and erythromycin. Ceftriaxone was the most frequent parenteral antibiotic agent used (n = 88, 2% of all participants, 2% of those prescribed antibiotic therapy).
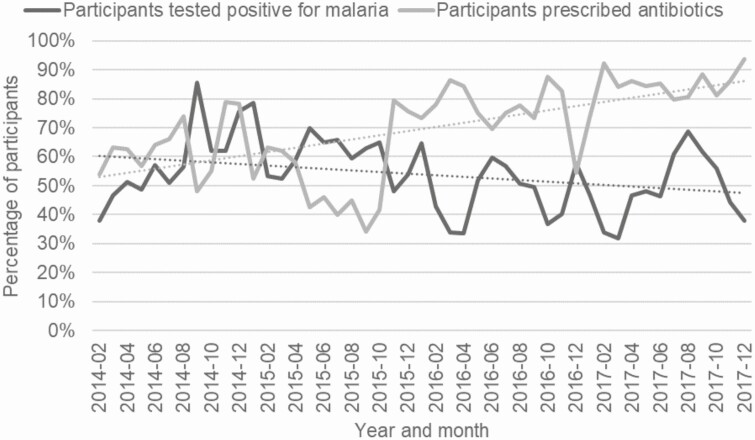
Overall, frequency of antibiotic therapy use increased over time (Figure 1) with this trend most prominent in Hospitals 3 and 5 (Supplementary File 2).
Figure 1.
Percentage of participants prescribed antibiotics and percentage of participants tested positive for malaria by blood smear or RDT over timeµ. µData prior to 02–2014 omitted because tablets were not used for first month of study and malaria test results were not fully documented.
Malaria Testing
Most participants who tested positive for malaria were treated with antimalarials (n = 2940, 95%), and diagnosed with a primary diagnosis of malaria (n = 2714, 87%) (Supplementary File 3). In contrast to prescription of antibiotic therapy, diagnosis of malaria during the study period showed a slight decline overall among all sites combined, though this trend varied among individual sites (Figure 1 and Supplementary File 4). Validation of RDT results using post hoc blood smears as a gold standard was performed, and there were no false positive or false negative RDTs.
DISCUSSION
This study is one of the few to examine provider behaviors when evaluating children with undifferentiated fever in a resource-limited, malaria-endemic setting. Despite a minority of participants with a diagnosis of bacterial illness (14% with a primary diagnosis; 28% with either primary or differential diagnosis), a high percentage of all participants (68%) were prescribed antibiotic therapy. A negative malaria test result had the most dramatic effect on antibiotic prescription, increasing odds by 7-fold. Based on these results and given the degree of diagnostic uncertainty, providers seem highly reluctant to discharge febrile children without empiric antibiotic treatment.
Antibiotic therapy was prescribed to a substantial number of participants with a diagnosis of viral illness and was more likely to be prescribed to children with positive HEENT exam findings (ie, nasal discharge), suggesting that upper respiratory symptoms with fever are inappropriately treated as bacterial illness, as previously reported [29]. High fever or abnormal joint exam were associated with lower odds of antibiotic prescription, suggesting that these findings are less likely to be considered signs of bacterial illness or a more severe disease process. These behaviors directly correspond with results of our qualitative study in which clinicians reported hesitancy discharging patients with a presumed viral infection without antibiotic therapy due to fear that these patients may go on to worsen clinically, supporting a strong belief that early empiric antibiotic treatment can and should be used in order to prevent progression to severe disease [21].
Antibiotics were also prescribed more frequently at Hospital 1 (which is a more central, urban hospital) and to families with ownership of a flush toilet (which may be a measure of higher socioeconomic status). These findings may indicate that antibiotic use is correlated with increased availability and reliance upon laboratory testing, increased fear of repercussion by wealthier families should their child’s health worsen, higher perceived likelihood that these families can afford treatment, or variation in practice patterns at the regional or provider level, as supported by the associated qualitative study [21]. Hospital 1 is a referral center, so it is possible that patients are more acutely ill on presentation, however, patients at this facility did not have higher rates of hospitalization. In contrast, Hospital 2 demonstrated a decreased frequency of antibiotic prescription over time. This hospital is in a more rural area and this effect may be attributable to the increased percentage of malaria-positive tests during the course of the study, or it may be due to other factors not explained by this study.
Our results cannot be directly compared to prior studies of IMCI adherence or provider behaviors when evaluating acutely ill children, as these outcomes were not examined directly. However, cases with overlap in symptoms (ie, both malarial and respiratory symptoms), those who tested negative for malaria, or those who presented with concurrent illness deviating from IMCI pathways were included, all of which have been previously shown to be challenging diagnostically for providers [15, 20, 30–33]. Given that most malaria-endemic countries are highly limited in available diagnostics to aid in clinical decision-making, adding specific RDTs for diseases other than malaria (either viral or bacterial) is an attractive concept. The ideal laboratory test(s) for predicting bacterial diagnosis is an ongoing debate, though there may be more utility for RDTs in areas where certain diseases are common or peak during seasonal periods (ie, influenza season) [34, 35]. Increased availability of broad scope viral testing and other less specific laboratory tests such as inflammatory markers (eg, C-reactive protein, procalcitonin) have shown some benefit when used in conjunction with refined clinical algorithms in risk stratification and determining whether antibiotics should be prescribed [36–40].
Further studies are needed to directly observe provider behaviors and track test results and management decisions in the evaluation of children with febrile illness, as bacterial testing becomes more commonplace.
Limitations
This study was dependent on accurate reporting by providers. Outside of malaria testing, there was no laboratory validation of diagnosis. Though this would not be considered an all-cause fever study given the requirement for the possibility of arboviral disease, with the high degree of overlap between arboviral disease presentation and that of other infections, the possibility of co-infection, and the lack of available confirmatory testing, in practice, children were almost never excluded for an obvious, nonarboviral source of fever. Providers used a survey tool and had the option of choosing a primary and secondary (differential) diagnosis, and given the study included different providers with varying degrees of training and experience, this may have introduced inter-operator variability. Though this study was based in 5 government-funded clinical centers, participants were evaluated by a limited group of providers and their practice patterns may not be generalizable to the practices of all other providers at health centers elsewhere in the country. Providers may have prescribed medications that were in stock and readily available, which may have skewed the proportion of specific antibiotics prescribed. However, the focus of this study was not on the specific antibiotic treatment used, but overall frequency of antibiotic therapy prescribed. To maintain anonymity, we did not compare or control for practice patterns of individual providers; however, we used multivariate analysis to control for facility-specific tendencies. Finally, the providers in this study were aware of the larger umbrella study on arboviral disease prevalence and presentation, which may have influenced their management. This should have, if anything, caused an increased awareness and frequency of diagnosis of viral illness.
CONCLUSION
In the era of test and treat for malaria [1], a subset of Kenyan providers commonly uses antibiotic treatment as empiric therapy in febrile children, especially in those who are malaria negative and/or diagnosed with a viral illness. As in other malaria-endemic areas, providers in this study face a high degree of diagnostic uncertainty evaluating children in the absence of additional laboratory or point-of-care testing beyond malaria. As providers are now able to rule-in or rule-out malaria with more certainty, the various factors that influence the choice of whether to prescribe antibiotic treatment in both malaria-positive and malaria-negative patients need to be evaluated. Further studies are necessary to characterize the epidemiology of febrile illness and to determine the true risk of serious bacterial illness, the rationale for provision of antibiotic therapy, and the disconnect that exists between diagnosis and treatment. This information can then be used to inform medical education curricula and improve guidelines for clinical decision-making in low resource settings.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors would like to acknowledge and remember the late Dr John Vulule who was an important partner in this study and will be missed greatly. We would also like to acknowledge the laboratory technicians and clinical and field staff working at our sites in Kenya who worked tirelessly collecting data and treating patients. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Financial support. This work was supported by National Institutes of Health [grant number R01-AI102918 to ADL], a travel grant from UCSF to AMH, and National Institutes of Health/National Center for Research Resources funding to Stanford University [grant UL1 TR001085].
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.World Health Organization. Guidelines for the treatment of malaria. 2nd edn. Geneva: World Health Organization, 2010. Available at: http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf. Accessed 5 May 2020. [Google Scholar]
- 2.Johansson EW, Gething PW, Hildenwall H, et al. Diagnostic testing of pediatric fevers: meta-analysis of 13 national surveys assessing influences of malaria endemicity and source of care on test uptake for febrile children under five years. PLoS One 2014; 9:e95483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. World Malaria Report 2015. WHO Press, Geneva: World Health Organization, 2015. Available at: https://www.who.int/malaria/publications/world-malaria-report-2015/report/en/. Accessed 5 May 2020. [Google Scholar]
- 4.World Health Organization. Updated guideline: paediatric emergency triage, assessment and treatment. Geneva: World Health Organization, 2016. Available at: https://www.who.int/maternal_child_adolescent/documents/paediatric-emergency-triage-update/en/. Accessed 5 May 2020. [PubMed] [Google Scholar]
- 5.Lange S, Mwisongo A, Mæstad O. Why don’t clinicians adhere more consistently to guidelines for the integrated management of childhood illness (IMCI)? Soc Sci Med 2014; 104:56–63. [DOI] [PubMed] [Google Scholar]
- 6.Senn N, Rarau P, Salib M, et al. Use of antibiotics within the IMCI guidelines in outpatient settings in Papua New Guinean children: an observational and effectiveness study. PLoS One 2014; 9:e90990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansson EW, Gething PW, Hildenwall H, et al. Effect of diagnostic testing on medicines used by febrile children less than five years in 12 malaria-endemic African countries: a mixed-methods study. Malar J 2015; 14:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Global action plan on antimicrobial resistance. Geneva: World Health Organization, 2015. Available at: https://www.who.int/antimicrobial-resistance/global-action-plan/en/. Accessed 5 May 2020. [DOI] [PubMed] [Google Scholar]
- 9.Crump JA, Morrissey AB, Nicholson WL, et al. Etiology of severe non-malaria febrile illness in Northern Tanzania: a prospective cohort study. PLoS Negl Trop Dis 2013; 7:e2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petti CA, Polage CR, Quinn TC, Ronald AR, Sande MA. Laboratory medicine in Africa: a barrier to effective health care. Clin Infect Dis 2006; 42:377–82. [DOI] [PubMed] [Google Scholar]
- 11.Kenya National Bureau of Statistics (KNBS). Kenya Demographic and Health Survey, 2014. Nairobi: KNBS, 2015. Available at: https://dhsprogram.com/pubs/pdf/fr308/fr308.pdf. Accessed 5 May 2020. [Google Scholar]
- 12.Kenyan Ministry of Medical Services and Ministry of Public Health and Sanitation. Clinical management and referral guidelines, volume III: clinical guidelines for management and referral of common conditions at levels 2–3: primary care. Nairobi, Republic of Kenya.2009.
- 13.Kenyan Ministry of Medical Services and Ministry of Public Health and Sanitation. Clinical management and referral guidelines, volume III: clinical guidelines for management and referral of common conditions at levels 4–6: hospitals. Nairobi, Republic of Kenya, 2009.
- 14.World Health Organization. Pocket book of hospital care for children: second edition.2017. Available at: https://www.who.int/maternal_child_adolescent/documents/child_hospital_care/en/. Accessed 20 July 2020.
- 15.Baltzell K, Elfving K, Shakely D, et al. Febrile illness management in children under five years of age: a qualitative pilot study on primary health care workers’ practices in Zanzibar. Malar J 2013; 12:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baiden F, Owusu-Agyei S, Bawah J, et al. An evaluation of the clinical assessments of under-five febrile children presenting to primary health facilities in rural Ghana. PLoS One 2011; 6:e28944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acácio S, Verani JR, Lanaspa M, et al. Under treatment of pneumonia among children under 5 years of age in a malaria-endemic area: population-based surveillance study conducted in Manhica district, rural, Mozambique. Int J Infect Dis 2015; 36:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krüger C, Heinzel-Gutenbrunner M, Ali M. Adherence to the integrated management of childhood illness guidelines in Namibia, Kenya, Tanzania and Uganda: evidence from the national service provision assessment surveys. BMC Health Serv Res 2017; 17:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goverment of Kenya 2014. Kenya Service Availability and Readiness Assessment Mapping (SARAM). Nairobi, Republic of Kenya. 2014. Available at: http://apps.who.int/healthinfo/systems/datacatalog/index.php/catalog/42/download/145. Accessed 5 May 2020.
- 20.Lunze K, Biemba G, Lawrence JJ, et al. Clinical management of children with fever: a cross-sectional study of quality of care in rural Zambia. Bull World Health Organ 2017; 95:333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hooft AM, Ripp K, Ndenga B, et al. Principles, practices and knowledge of clinicians when assessing febrile children: a qualitative study in Kenya. Malar J 2017; 16:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vu DM, Banda T, Teng CY, et al. Dengue and West Nile Virus transmission in children and adults in Coastal Kenya. Am J Trop Med Hyg 2017; 96:141–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vu DM, Mutai N, Heath CJ, et al. Unrecognized dengue virus infections in children, Western Kenya, 2014–2015. Emerg Infect Dis 2017; 23:1915–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waggoner J, Brichard J, Mutuku F, et al. Malaria and chikungunya detected using molecular diagnostics among febrile Kenyan children. Open Forum Infect Dis 2017; 4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutherland LJ, Cash AA, Huang YJ, et al. Serologic evidence of arboviral infections among humans in Kenya. Am J Trop Med Hyg 2011; 85:158–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah MM, Krystosik AR, Ndenga BA, et al. Malaria smear positivity among Kenyan children peaks at intermediate temperatures as predicted by ecological models. Parasit Vectors 2019; 12:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R Core Team. R: a language and environment; statistical computing.2017. Available at: https://www.r-project.org/. Accessed 20 July 2020.
- 29.Risk R, Naismith H, Burnett A, Moore SE, Cham M, Unger S. Rational prescribing in paediatrics in a resource-limited setting. Arch Dis Child 2013; 98:503–9. [DOI] [PubMed] [Google Scholar]
- 30.Walter ND, Lyimo T, Skarbinski J, et al. Why first-level health workers fail to follow guidelines for managing severe disease in children in the Coast Region, the United Republic of Tanzania. Bull World Health Organ 2009; 87:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrer BE, Webster J, Bruce J, et al. Integrated community case management and community-based health planning and services: a cross sectional study on the effectiveness of the national implementation for the treatment of malaria, diarrhoea and pneumonia. Malar J 2016; 15:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nichols C, Cruz Espinoza LM, von Kalckreuth V, et al. bloodstream infections and frequency of pretreatment associated with age and hospitalization status in sub-Saharan Africa. Clin Infect Dis 2015; 61 Suppl 4:S372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johansson EW, Selling KE, Nsona H, et al. Integrated paediatric fever management and antibiotic over-treatment in Malawi health facilities: Data mining a national facility census. Malar J 2016; 15:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manyando C, Njunju EM, Chileshe J, Siziya S, Shiff C. Rapid diagnostic tests for malaria and health workers’ adherence to test results at health facilities in Zambia. Malar J 2014; 13:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van den Bruel A, Thompson MJ, Haj-Hassan T, et al. Diagnostic value of laboratory tests in identifying serious infections in febrile children: systematic review. BMJ 2011; 342:d3082. [DOI] [PubMed] [Google Scholar]
- 36.Phommasone K, Althaus T, Souvanthong P, et al. Accuracy of commercially available c-reactive protein rapid tests in the context of undifferentiated fevers in rural Laos. BMC Infect Dis 2015; 16:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lubell Y, Althaus T, Blacksell SD, et al. Modelling the impact and cost-effectiveness of biomarker tests as compared with pathogen-specific diagnostics in the management of undifferentiated fever in remote tropical settings. PLoS One 2016; 11:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lubell Y, Blacksell SD, Dunachie S, et al. Performance of C-reactive protein and procalcitonin to distinguish viral from bacterial and malarial causes of fever in Southeast Asia. BMC Infect Dis 2015; 15:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keitel K, Kagoro F, Samaka J, et al. A novel electronic algorithm using host biomarker point-of-care tests for the management of febrile illnesses in Tanzanian children (e-POCT): a randomized, controlled non-inferiority trial. PLoS Med 2017; 14:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Acremont V, Kilowoko M, Kyungu E, et al. Beyond malaria–causes of fever in outpatient Tanzanian children. N Engl J Med 2014; 370:809–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.