Abstract
Background
Antibodies to programmed cell death 1 (PD-1) and cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) may perturb human immunodeficiency virus (HIV) persistence during antiretroviral therapy (ART) by reversing HIV latency and/or boosting HIV-specific immunity, leading to clearance of infected cells. We tested this hypothesis in a clinical trial of anti–PD-1 alone or in combination with anti–CTLA-4 in people living with HIV (PLWH) and cancer.
Methods
This was a substudy of the AIDS Malignancy Consortium 095 Study. ART-suppressed PLWH with advanced malignancies were assigned to nivolumab (anti–PD-1) with or without ipilimumab (anti–CTLA-4). In samples obtained preinfusion and 1 and 7 days after the first and fourth doses of immune checkpoint blockade (ICB), we quantified cell-associated unspliced (CA-US) HIV RNA and HIV DNA. Plasma HIV RNA was quantified during the first treatment cycle. Quantitative viral outgrowth assay (QVOA) to estimate the frequency of replication-competent HIV was performed before and after ICB for participants with samples available.
Results
Of 40 participants, 33 received nivolumab and 7 nivolumab plus ipilimumab. Whereas CA-US HIV RNA did not change with nivolumab monotherapy, we detected a median 1.44-fold increase (interquartile range, 1.16–1.89) after the first dose of nivolumab and ipilimumab combination therapy (P = .031). There was no decrease in the frequency of cells containing replication-competent HIV, but in the 2 individuals on combination ICB for whom we had longitudinal QVOA, we detected decreases of 97% and 64% compared to baseline.
Conclusions
Anti–PD-1 alone showed no effect on HIV latency or the latent HIV reservoir, but the combination of anti–PD-1 and anti–CTL-4 induced a modest increase in CA-US HIV RNA and may potentially eliminate cells containing replication-competent HIV.
Clinical Trials Registration
Keywords: HIV, HIV latency, anti–PD-1, anti–CTLA-4
In people living with human immunodeficiency virus (HIV) on antiretroviral therapy receiving immune checkpoint blockade for cancer, anti–PD-1 alone had no effect on the latent HIV reservoir, but the combination of anti–PD-1 and anti–CTLA-4 modestly reversed HIV latency and may potentially eliminate cells containing replication-competent HIV.
Despite the great success of antiretroviral therapy (ART) in reducing human immunodeficiency virus (HIV)–related morbidity and mortality, lifelong treatment is required. HIV persists in long-lived CD4+ T cells by establishing a latent infection that evades immune recognition but can reemerge and lead to viral rebound if ART is discontinued. There is therefore interest in interventions capable of exposing latently infected cells to immune recognition and/or augmenting the immune response against HIV to facilitate elimination of these cells.
The inhibitory receptors programmed cell death 1 (PD-1) and cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) maintain a balance between T-cell activation and autoimmunity, but negative signaling through these molecules can also lead to a state of T-cell exhaustion [1]. Antibodies that block PD-1 and CTLA-4 have demonstrated efficacy against advanced malignancies by enhancing tumor-directed T-cell responses, giving rise to a new paradigm of cancer immunotherapy [2].
During chronic HIV infection, there is increased expression of PD-1 and CTLA-4 on CD4+ and CD8+ T cells, which is reduced but not normalized by ART [3], and this is a key driver of HIV-associated T-cell exhaustion [4–6]. Multiple studies have also shown that HIV is enriched in CD4+ T cells that express inhibitory receptors, including PD-1 and CTLA-4 [7–9], potentially because inhibitory signaling during T-cell infection limits T-cell activation, thereby favoring transition to a latent infection [10, 11]. It has therefore been speculated that blocking PD-1 and/or CTLA-4 may perturb HIV persistence during ART by reversing HIV latency [12] and/or boosting HIV-specific immunity [6, 13, 14], leading to clearance of infected cells.
Due to the frequent exclusion of people living with HIV (PLWH) in oncology trials of anti–PD-1 and anti–CTLA-4 therapies, there is limited knowledge of their role in targeting HIV persistence. Case reports of PLWH on ART receiving anti–PD-1 or anti–CTLA-4 for cancer described a transient increase in either cell-associated or plasma HIV RNA with or without a decrease in the frequency of latently infected CD4+ T cells [11, 15–17], whereas others have not seen this effect [18, 19]. PD-1 and CTLA-4 signaling attenuate T-cell activity through separate pathways [20], which may explain why combined blockade of both PD-1 and CTLA-4 showed superior therapeutic efficacy for metastatic melanoma [21, 22]. We previously showed in vitro that the combination of both anti–PD-1 and anti–CTLA-4 increased the efficiency of latency reversal compared to either antibody alone [23], and similar findings were recently demonstrated in ART-treated rhesus macaques infected with simian immunodeficiency virus [24]. Collectively, these observations suggest that combined blockade of PD-1 and CTLA-4 may have an enhanced effect on reversing HIV latency and targeting HIV persistence on ART.
The AIDS Malignancy Consortium (AMC) 095 study is a phase 1 clinical trial of nivolumab (anti–PD-1) with or without ipilimumab (anti–CTLA-4) in PLWH with cancer on ART. In this prospectively designed substudy, we aimed to determine the effect of anti–PD-1 alone or in combination with anti–CTLA-4 on HIV latency and HIV persistence. We hypothesized that blocking PD-1 with or without CTLA-4 would activate expression of latent HIV and that, combined with its immune-enhancing effects on CD8+ T cells, this would facilitate elimination of latently infected cells, thus reducing the size of the HIV reservoir.
METHODS
Study Design and Participants
We conducted a prospectively designed substudy of the AMC-095 study, a phase 1 clinical trial of ipilimumab and nivolumab in advanced HIV-associated solid tumors and classical Hodgkin lymphoma (cHL). Study participants who contributed samples to this substudy were recruited at sites in the United States between January 2016 and April 2019. The study enrolled adult PLWH on ART with histologically confirmed metastatic or unresectable solid tumor malignancy, including uncontrolled Kaposi sarcoma (KS), into several separate cohorts without randomization. Participants were enrolled in 2 strata based on CD4+ T-cell count ≥200 cells/μL (stratum 1) or 100–200 cells/μL (stratum 2). Additional key inclusion criteria were Eastern Cooperative Oncology Group performance status ≤1, preserved end-organ and bone marrow function, and plasma HIV RNA suppressed below the limit of detection within 4 weeks of enrollment. The study excluded individuals with prior immune checkpoint blockade (ICB), pregnancy, active autoimmune disease, active intercurrent disease, grade 2 or higher diarrhea, opportunistic infection within 3 months of enrollment, or evidence of pancreatitis. Complete inclusion/exclusion criteria are available at ClinicalTrials.gov (NCT02408861). The study was approved by the institutional review boards at all recruiting sites and was conducted in accordance with the principles of the Declaration of Helsinki (1996) and the principles described in the Food and Drug Administration regulations and the Department of Health and Human Services regulations for the protection of human participants. Each participant provided written informed consent.
Procedures
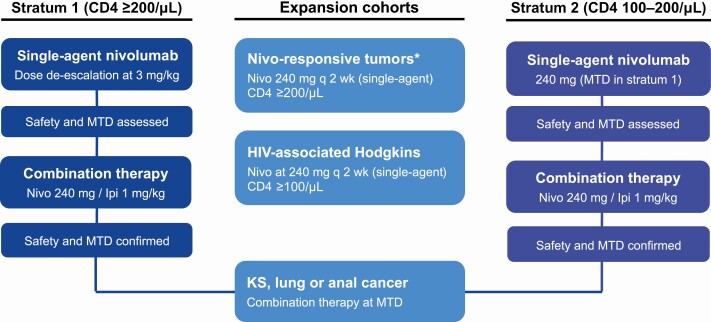
The study initially enrolled participants with CD4+ T-cell counts ≥200 cells/μL (stratum 1) to receive nivolumab monotherapy 3 mg/kg every 2 weeks, corresponding to 1 cycle of study therapy. After confirming safety in this cohort, the study enrolled stratum 1 participants into a combination cohort of nivolumab 240 mg every 2 weeks in combination with ipilimumab 1 mg/kg every 6 weeks. Stratum 2 participants with CD4+ T-cell counts 100–200 cells/μL were subsequently enrolled in a similar approach to nivolumab monotherapy or combination therapy cohorts (Figure 1). Finally, the study also enrolled participants into expansion cohorts of either nivolumab monotherapy (HIV-associated cHL and advanced solid tumors) or combination therapy (KS, lung cancer, and anal cancer). Participants continued study therapy until death, progressive disease, or unacceptable adverse events.
Figure 1.
Study profile. *Excludes pancreas, prostate and microsatellite stable colorectal cancers. Abbreviations: Ipi, ipilimumab; KS, Kaposi sarcoma; MTD, maximal tolerated dose; Nivo, nivolumab.
We collected plasma and peripheral blood mononuclear cells at baseline, within 24 hours, and 7 days after infusion of immune checkpoint antibodies at cycles 1, 4, 7, 10, and so forth until the participant was off study. These timepoints corresponded to cycles that included both nivolumab and ipilimumab for participants on combination therapy. Samples were also obtained at termination of study therapy. For participants who provided an additional consent, a large volume blood draw was done at baseline, cycle 16, and/or at termination of study therapy for a quantitative viral outgrowth assay (QVOA).
Outcomes
Key outcome measures to assess the latency-reversing effect of the antibodies was the level of cell-associated unspliced (CA-US) HIV RNA in peripheral blood CD4+ T cells [25] and plasma HIV RNA as measured by a sensitive assay [26]. To assess effects on the frequency of latently infected cells, we quantified total HIV DNA in peripheral blood CD4+ T cells [27] and replication-competent HIV using QVOA and expressed as infectious units per million (IUPM) [28, 29]. See the Supplementary Data for detailed methods on virological assays.
Statistical Analyses
Based on our prior work, we assumed a mean and standard deviation of the within-person change in CA-US HIV RNA of 21.5 and 32.8 copies per 106 CD4+ T cells, respectively [27]. Using these estimates, 40 participants yielded 80% power to detect a change in CA-US HIV RNA of 15 copies or more (corresponding to >70% change) at a 2-sided .05 significance level. We used logarithmic transformation to achieve normal distribution whenever possible. We analyzed changes from baseline in virological measures using paired t test or Wilcoxon matched-pairs signed rank test, depending on data distribution. To compare changes from baseline across treatment groups, we performed unpaired t test or Mann-Whitney rank-sum test, depending on data distribution. We also applied a generalized negative binomial regression model where all replicate data were employed in the analysis as recently described [30]. In this model, the number of measured HIV copies (CA-US HIV RNA or HIV DNA) was included as the outcome variable and the input quantity for the polymerase chain reaction analysis (total RNA or total DNA) as an exposure variable. This model adjusted for variation in the amount of input RNA, DNA, or plasma volume such that specimens with higher input quantity provided more weight than specimens with lower input quantity.
RESULTS
Study Participants and Study Design
We included samples from 40 study participants (36 males and 4 females). Of those, 33 received nivolumab alone and 7 received nivolumab plus ipilimumab. Baseline characteristics are provided in Table 1. Cancer diagnoses constituted a heterogenous group of tumors including 15 with KS, 4 with anal cancer, and 3 with HIV-associated cHL (Table 2). As an increasing number of study participants withdrew from the study over time due to death, confirmed disease progression, or toxicity, samples were primarily available for timepoints in cycle 1 (n = 38) and cycle 4 (n = 29), but we also analyzed effects in multiple samples collected at later timepoints in participants who remained on study for additional cycles. Ten participants provided consent to large volume blood draw for QVOA at baseline and during follow-up.
Table 1.
Baseline Characteristics of Study Participants
| Variable | Total | Nivolumab | Nivolumab + Ipilimumab |
|---|---|---|---|
| Study drugs and dosage | Nivolumab at 3 mg/kg or 240 mg | Nivolumab at 240 mg plus ipilimumab at 1 mg/kg | |
| No. of participants included | 40 | 33 | 7 |
| Median (IQR) age, y | 53.0 (47.0–58.5) | 52.0 (47.0–57.0) | 56.4 (51.0–61.0) |
| Median (IQR) CD4+ T-cell count, cells/μL | 315 (227–465) | 315.0 (225.0–434.0) | 496.0 (231.0–600.0) |
| Sex, No. (%) | |||
| Male | 36 (90) | 31 (93.9) | 5 (71.4) |
| Female | 4 (10) | 2 (6.1) | 2 (28.6) |
| Race, No. (%) | |||
| White | 25 (62.5) | 21 (63.6) | 4 (57.1) |
| African American | 11 (27.5) | 10 (30.3) | 1 (14.3) |
| Other/unknown | 4 (10.0) | 2 (6.1) | 2 (28.6) |
| Enrolled through stratum 1 (CD4 ≥200 cells/μL), No. (%) | 33 (82.5) | 26 (78.8) | 7 (100.0) |
| Enrolled through stratum 2 (CD4 100–200 cells/μL), No. (%) | 7 (17.5) | 7 (21.2) | 0 (0.0) |
Abbreviation: IQR, interquartile range.
Table 2.
Malignancies of Enrolled Participants
| Malignancy | No. |
|---|---|
| Kaposi sarcoma | 15 |
| Anal cancer | 4 |
| Hodgkin lymphoma | 3 |
| Colon cancer | 3 |
| Head and neck squamous cell carcinoma | 2 |
| Non-small-cell lung cancer | 1 |
| Follicular dendritic cell sarcoma to lung bone | 1 |
| Inguinal squamous cell carcinoma | 1 |
| Metastatic lung adenocarcinoma | 1 |
| Metastatic cholangiocarcinoma | 1 |
| Rectosigmoid colon | 1 |
| Small cell lung cancer | 1 |
| Squamous cell carcinoma | 1 |
| Squamous cell carcinoma of unknown origin | 1 |
| Adenocarcinoma of gall bladder | 1 |
| Adenocarcinoma of the lung | 1 |
| Liposarcoma | 1 |
| Ovarian cancer | 1 |
Combined Blockade of PD-1 and CTLA-4 Modestly Reversed HIV Latency
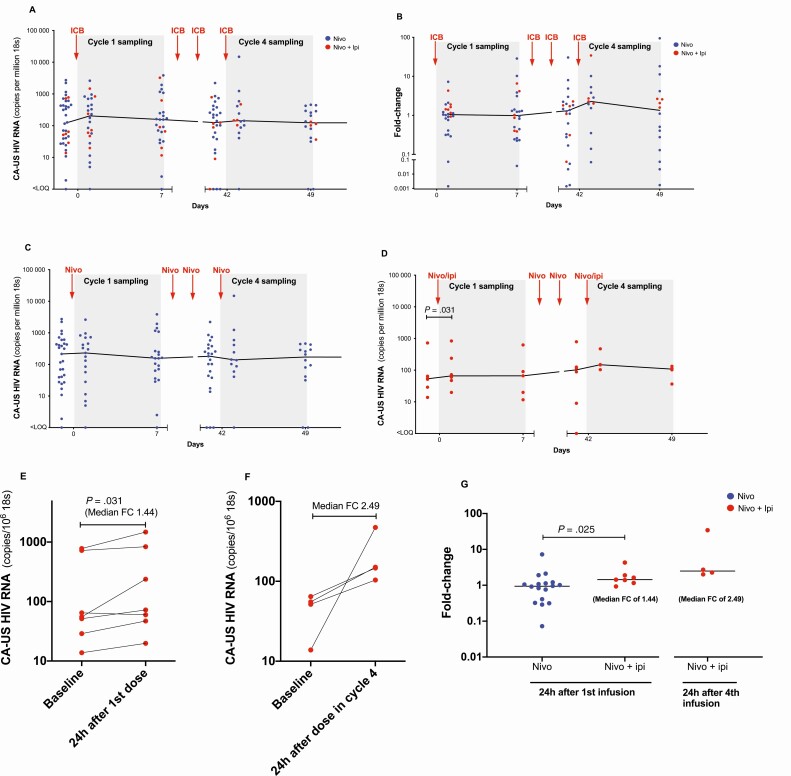
We analyzed HIV latency reversal by quantifying levels of CA-US HIV RNA at baseline and sampling timepoints in cycles 1 and 4. There was no change from baseline to any timepoint in cycle 1 or 4 when we analyzed the entire cohort (Figure 2A and 2B). We furthermore found that CA-US HIV RNA did not change from baseline in participants receiving nivolumab alone (Figure 2C), but there was a median 1.44 fold-increase (interquartile range [IQR], 1.16–1.89) within 24 hours of the first dose in participants on ipilimumab plus nivolumab (P = .031) (Figure 2D). This increase was detected in 6 of 7 participants at this time point (Figure 2E). CA-US HIV RNA appeared to increase even further at the timepoint 24 hours after infusion of ipilimumab plus nivolumab in cycle 4, but with samples from only 4 participants at this timepoint, a formal statistical analysis was not possible (Figure 2F). The increase in CA-US HIV RNA in cycle 1 was also significantly higher compared to the corresponding change from baseline in participants receiving nivolumab alone (P = .025; Figure 2G). Generalized negative binomial regression confirmed the finding of a statistically significant increase with ipilimumab plus nivolumab at day 1 in the first cycle (P = .045) and also at day 7 in cycle 1 (P = .023). We did not detect any increase in CA-US HIV RNA with nivolumab alone; in fact, the negative binomial regression model suggested a marginal decrease over time for the first 4 cycles of nivolumab alone but with a very low effect size (P = .037).
Figure 2.
Cell-associated unspliced human immunodeficiency virus (HIV) RNA during immune checkpoint blockade (ICB). The level of cell-associated unspliced HIV RNA during ICB shown in absolute numbers and as fold-changes from baseline for the entire cohort (A and B) and for groups receiving nivolumab alone (C) or nivolumab plus ipilimumab (D). Data points are connected for the same participant receiving nivolumab plus ipilimumab in cycle 1 (E) and cycle 4 (F). G, Fold-changes from baseline are displayed and compared across groups receiving nivolumab or nivolumab plus ipilimumab. Abbreviations: CA-US, cell-associated unspliced; FC, fold-change; HIV, human immunodeficiency virus; ICB, immune checkpoint blockade; Ipi, ipilimumab; Nivo, nivolumab.
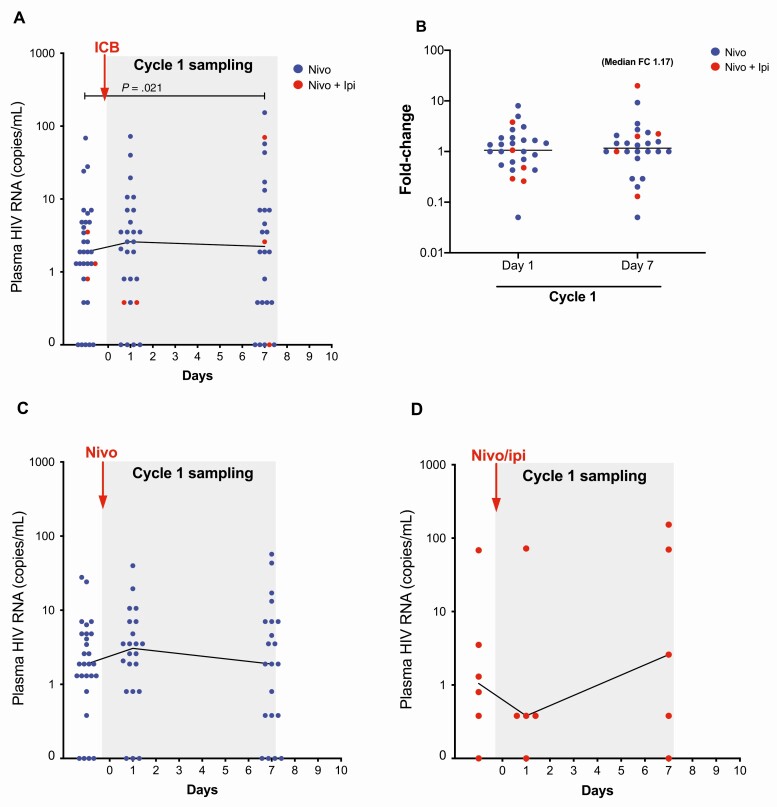
We then investigated whether ICB increased levels of plasma HIV RNA. This was analyzed in samples from cycle 1 in participants with samples at baseline and at least 1 follow-up timepoint. We were able to quantify changes in plasma HIV RNA for 33 of the 40 participants and found that ICB led to a modest but significant increase in plasma HIV RNA at day 7 after ICB but not at the 24-hour timepoint (P = .021; Figure 3A). The median fold-increase from baseline at day 7 was 1.17 (IQR, 0.93–2.12) (Figure 3B). We analyzed effects in participants receiving either nivolumab alone or ipilimumab plus nivolumab, but did not detect any significant changes from baseline (Figure 3C and 3D).
Figure 3.
Plasma human immunodeficiency virus (HIV) RNA during immune checkpoint blockade (ICB). Plasma HIV RNA prior to and following the ICB in cycle 1 is shown in absolute numbers and as fold-change from baseline for the entire cohort (A and B) and for groups receiving nivolumab monotherapy (C) or combination therapy with nivolumab plus ipilimumab (D). Abbreviations: FC, fold-change; HIV, human immunodeficiency virus; ICB, immune checkpoint blockade; Ipi, ipilimumab; Nivo, nivolumab.
Effects of Immune Checkpoint Blockade on the Latent HIV Reservoir
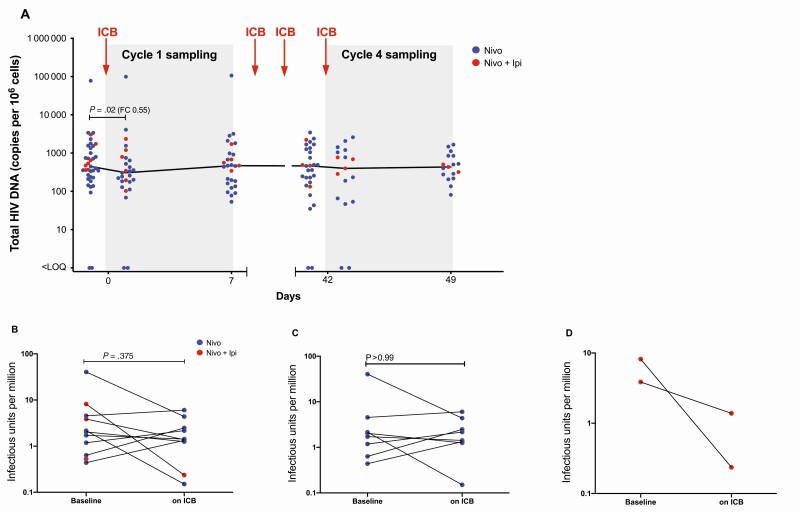
We quantified total HIV DNA in CD4+ T cells and performed QVOA to determine the frequency of replication-competent HIV. Although we detected a minor decrease in HIV DNA of around 45% within 24 hours of the first dose of ICB (P = .02) using a paired t test, this decrease was not sustained (Figure 4A) beyond this time point. Using generalized negative binomial regression, we found that both nivolumab alone (P = .005) and nivolumab plus ipilimumab (P = .001) resulted in a statistically significant decrease in HIV DNA over the duration of the first 4 cycles of ICB, but the effect size of this decrease was very small.
Figure 4.
Effects on the frequency of human immunodeficiency virus (HIV)–infected cells. The level of cell-associated total HIV DNA during immune checkpoint blockade in cycles 1 and 4 is shown in absolute numbers for the entire cohort (A). The frequency of cells containing replication-competent HIV measured by a quantitative viral outgrowth assay and expressed as infectious units per million shown for the entire cohort (B) and for groups receiving nivolumab alone (C) or nivolumab plus ipilimumab (D). Abbreviations: FC, fold-change; HIV, human immunodeficiency virus; ICB, immune checkpoint blockade; Ipi, ipilimumab; LOQ, limit of quantification; Nivo, nivolumab.
Given that total HIV DNA includes defective virus which is not replication competent [31], we also assessed QVOA in a subset of 10 of the 40 participants on blood collected at baseline and at cycle 16 and/or at termination of study therapy. The time on ICB therapy when these samples were collected ranged from 2 to 35 weeks (average, 20.6 weeks). Overall, we did not detect a significant decrease in IUPM (Figure 4B), nor was there any change in participants on nivolumab monotherapy (Figure 4C). Of note, in 2 individuals who received nivolumab only, QVOA was performed using total CD4+ T cells at baseline (due to a limited number of cells available) but in resting CD4+ T cells at the end of treatment. IUPM increased slightly in both participants but excluding these participants from the analysis did not alter the overall conclusion of no change with nivolumab in IUPM following nivolumab.
In contrast, in 2 individuals who received ipilimumab and nivolumab and who had longitudinal samples available for QVOA, replication-competent HIV decreased considerably during study therapy (Figure 4D). One of these individuals received nivolumab plus ipilimumab for 17 cycles and had an almost 2 log10 decrease in IUPM over this period. We explored characteristics that might distinguish participants who had a decrease (n = 4) compared to no decrease (n = 6) in IUPM following ICB. We found no differences in baseline levels or change from baseline in CA-US HIV RNA or plasma HIV RNA, and also detected no significant correlations between HIV DNA or IUPM and CA-US HIV RNA or plasma HIV RNA.
DISCUSSION
This is the first study to investigate effects of combined anti–PD-1 and anti–CTLA-4 on HIV persistence in PLWH on ART. Contrary to other reports, we found no latency reversing effect of nivolumab alone but there was a modest yet significant increase in HIV transcription during combined blockade of PD-1 and CTLA-4. Overall, we detected no change in the frequency of cells containing replication-competent HIV, but in the 2 individuals on combination ICB with samples available to perform QVOA, the frequency of replication-competent HIV decreased dramatically. These data indicate a latency-reversing effect of nivolumab and ipilimumab but no effect with nivolumab monotherapy.
The key strength of this study lies in its prospective comparison of single and combination blockade of PD-1 and CTLA-4 in PLWH on ART and the number of participants assessed. Targeting HIV persistence through ICB has been investigated in multiple nonhuman primate studies [13, 14, 24, 32, 33], but outside case reports this is the first prospective clinical trial to quantify these effects following anti-PD-1 and anti-CTLA-4.
Anti–PD-1 in the presence of a submaximal T-cell stimulus was recently shown to augment latency reversal ex vivo [12], which contrasts with our findings with anti–PD-1 monotherapy. We believe this could be explained by differences in T-cell co-stimulation, given that participants in this study were given no additional stimulus beyond ICB. We also note that a single low-dose anti–PD-L1 in ART-treated PLWH without cancer did not induce increases in HIV expression with a limited number of study participants [34].
The enhanced effect of combination ICB in our study is consistent with the improved therapeutic efficacy of combined blockade of PD-1 and CTLA-4 in metastatic melanoma [21, 22], but the reasons behind this are not clear. Immunological and genetic profiling revealed that combined blockade of PD-1 and CTLA-4 led to a distinct genomic and functional signature compared to either therapy alone, suggesting a possible synergistic effect [35]. In an in vitro model, we recently demonstrated differential expression of PD-1 and CTLA-4 on resting and proliferating CD4+ T cells, and that anti–PD1 reversed latency in nonproliferating T-cells and anti–CTLA-4 in proliferating T-cells [23]. Also, during melanoma treatment, anti–CTLA-4 specifically induced a subset of inducible T-cell co-stimulator+ Th1-like CD4+ effector T-cells [36, 37]. Collectively, these data suggest that engagement of distinct T-cell populations by anti–CTLA-4 and anti–PD-1 may play an important role in the enhanced effect seen with combined blockade of PD-1 and CTLA-4.
Several limitations of our study require attention. First, assignment to single or combination ICB was based on sequential enrollment and not prospective randomization. Second, it is possible that cancer-associated T-cell exhaustion is not comparable to that in PLWH on ART without cancer. This difference may affect cellular control of HIV latency and/or the capacity of cytotoxic T cells to eliminate virus-expressing cells. This is a key consideration with regard to extrapolating our findings to PLWH on ART without cancer. Third, due to limitations in cell numbers and the timing of performing reservoir analyses, we were unable to quantify intact proviral HIV DNA, which was subsequently described and is now possible [31]. Finally, as participants frequently declined to contribute large volume blood samples, QVOA analyses were limited to 10 participants with longitudinal data including only 2 receiving both anti–PD-1 and anti–CTLA-4. In these 2 individuals we observed substantial decreases in IUPM of 97% and 64%, respectively, but this must be interpreted in the context of the considerable variation of the QVOA, where 95% confidence intervals for individual IUPM estimates are around ±0.7 log IUPM or 5-fold [29].
Although the current risks associated with ICB, particularly when used in combination, will limit their use in PLWH without cancer as a strategy for cure, ongoing efforts to find safer ways to interrupt these pathways are in progress. Lower ICB doses given for brief periods of time might be better tolerated and potentially suitable for clinical trials for HIV or other chronic viral infections, as recently demonstrated for chronic hepatitis B infection [38].
In conclusion, we found no evidence for a latency-reversing effect or an impact on the latent HIV reservoir in individuals receiving anti–PD-1 alone; however, we demonstrated reversal of latency following administration of anti–PD-1 and anti–CTLA-4 in combination. We found a small but statistically significant decrease in HIV DNA across the whole cohort and a decline in the frequency of cells containing replication-competent HIV in individuals receiving anti–PD-1 and anti–CTLA-4. The impact of combination ICB on HIV persistence warrants further investigation.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. S. R. L., S. G. D., M. H. E., and C. D. conceived of and designed the study. L. R., M. H. E., E. C., and D. P. D. oversaw all aspects of the clinical trial including study protocol, ethics submission, and management of study participants. T. A. R., S. R. L., and C. D. coordinated all data generation and performed data analysis and interpretation. T. A. R., C. D., S. R. L., D. R., and R. R. coordinated sample collection and planned sample analyses. C. D. and D. R. oversaw quantitative viral outgrowth assay (QVOA) analyses. A. R., A. D., S. T., and S. C. performed polymerase chain reaction analyses of cell-associated unspliced HIV RNA and HIV DNA. S. B. and M. B. oversaw and coordinated analyses of plasma HIV RNA. T. S., S. L., and T. A. R. performed statistical analyses. The manuscript was drafted by T. A. R. and S. R. L. All authors reviewed and provided input to the manuscript and approved the final version.
Acknowledgments. The authors thank all the study participants for donating their time and samples to contribute to the study. The authors also give huge thanks to Danielle Rigau, William Ford, Adam Capoferri, and Sarah Jabour, who all contributed to performing QVOA analyses and managing sample inventory and logistics.
Disclaimer. As the funding source for the clinical trial, the National Cancer Institute (NCI) was involved in clinical trial design. The funding sources did otherwise not have any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. The study was funded by the American Foundation for AIDS Research (amfAR; grant number 109226-58-RGRL to S. R. L.); the Australian National Health and Medical Research Council (NHMRC; grant number GNT1149990 to S. R. L.); the Australian Centre for HIV and Hepatitis Research (ACH2; Rasmussen 2019 to T. A. R.); and the National Institutes of Health funded Delaney AIDS Research Enterprise to find a cure (UM1AI126611) to S. R. L. The clinical trial was supported by a grant from the NCI to the AIDS Malignancy Consortium (grant number UM1 CA121947). D. P. D. was supported by public health service grants CA239583 and DE018304. C.D. was supported by the National Cancer Institute (grant number 5K23CA177321-05).
Potential conflicts of interest. S. R. L. has received funding from the NHMRC, the National Institutes of Health (NIH), and amfAR, during the conduct of the study, and grants from Gilead Sciences, Merck, ViiV, Wellcome Trust, ACH2, Melbourne HIV Cure Consortium, Department of Health and Human Services, Medical Research Future Fund, and Leidos, outside the submitted work. T. A. R. reports salary support from NHMRC (fellowship GNT1135851), current and paid to their institution, during the conduct of the study. T. A. R. has also received funding from the Danish Research Council, Region Midt Denmark, the Australian Centre for HIV and Hepatitis Research, the Melbourne HIV Cure Consortium, and Gilead, outside the submitted work; T. A. R. also reports personal fees from Gilead, outside the submitted work. D. P. D. was funded by NCI (grant number RO1-CA228172). S. G. D. receives grant support from Gilead, Merck, and ViiV; is a member of the scientific advisory boards for BryoLogyx and Enochian Biosciences; and has consulted for AbbVie, Biotron, and Eli Lilly. S. B. and M. C. report that Hologic, Inc, provided a Panther instrument and Aptima VL reagents through a grant to V. R. I. that included support for the replicate Aptima testing performed for this study. T. S. reports personal fees from Biogen for advisory board/scientific leadership group service, outside the submitted work. M. H. E. reports consulting with remuneration to his institution from Photocure, Papivax, Cynvec, PDS, Becton Dickinson, Merck, Hologix, Pfizer, Inovio, AstraZeneca, Advaxis, and Johnson & Johnson, outside the submitted work; M. H. E. also reports advising or participating in educational speaking activities, but does not receive an honorarium from any companies listed above. In specific cases, his employers have received payment for his time spent for these activities from Merck, Hologic, Papivax, Cynvec, and Altum Pharma. If travel is required for meetings with industry, the company pays for his travel expenses. Rutgers has received grant funding for research-related costs of clinical trials for which M. E. has been the overall or local principal investigator within the past 12 months from Roche, Johnson & Johnson, Pfizer, AstraZeneca, Advaxis, and Inovio. D. P. D. and S. L. report grants from NIH, during the conduct of the study. E. C. reports grants from NIH, outside the submitted work. C. D. reports grants from GlaxoSmithKline and AbbVie and has served on a grant review committee for Gilead Sciences, outside the submitted work. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol 2019; 37:457–95. [DOI] [PubMed] [Google Scholar]
- 2.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018; 359:1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cockerham LR, Siliciano JD, Sinclair E, et al. . CD4+ and CD8+ T cell activation are associated with HIV DNA in resting CD4+ T cells. PLoS One 2014; 9:e110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macatangay BJC, Gandhi RT, Brad Jones R, et al. . PD-1HI T cells are associated with lower HIV-specific immune responses despite long-term antiretroviral therapy. AIDS 2019; 34:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trautmann L, Janbazian L, Chomont N, et al. . Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med 2006; 12:1198–202. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann DE, Kavanagh DG, Pereyra F, et al. . Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol 2007; 8:1246–54. [DOI] [PubMed] [Google Scholar]
- 7.Fromentin R, Bakeman W, Lawani MB, et al. . CD4+ T cells expressing PD-1, TIGIT and LAG-3 contribute to HIV persistence during ART. PLoS Pathog 2016; 12:e1005761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banga R, Procopio FA, Noto A, et al. . PD-1 and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat Med 2016; 22:754–61. [DOI] [PubMed] [Google Scholar]
- 9.McGary CS, Deleage C, Harper J, et al. . CTLA-4(+)PD-1(-) memory CD4(+) T cells critically contribute to viral persistence in antiretroviral therapy-suppressed, SIV-infected rhesus macaques. Immunity 2017; 47:776–88.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wykes MN, Lewin SR. Immune checkpoint blockade in infectious diseases. Nat Rev Immunol 2018; 18:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans VA, van der Sluis RM, Solomon A, et al. . Programmed cell death-1 contributes to the establishment and maintenance of HIV-1 latency. AIDS 2018; 32:1491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fromentin R, DaFonseca S, Costiniuk CT, et al. . PD-1 blockade potentiates HIV latency reversal ex vivo in CD4(+) T cells from ART-suppressed individuals. Nat Commun 2019; 10:814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velu V, Titanji K, Zhu B, et al. . Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 2009; 458:206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mylvaganam GH, Chea LS, Tharp GK, et al. . Combination anti-PD-1 and antiretroviral therapy provides therapeutic benefit against SIV. JCI Insight 2018; 3:e122940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guihot A, Marcelin AG, Massiani MA, et al. . Drastic decrease of the HIV reservoir in a patient treated with nivolumab for lung cancer. Ann Oncol 2018; 29:517–8. [DOI] [PubMed] [Google Scholar]
- 16.Uldrick TS, Fling S, Adams SV, et al. . Pembrolizumab induces HIV latency reversal in HIV+ individuals on ART with cancer. In: Conference on Retroviruses and Opportunistic Infections, Seattle, WA, 2019. [Google Scholar]
- 17.Wightman F, Solomon A, Kumar SS, et al. . Effect of ipilimumab on the HIV reservoir in an HIV-infected individual with metastatic melanoma. AIDS 2015; 29:504–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scully EP, Rutishauser RL, Simoneau CR, et al. . Inconsistent HIV reservoir dynamics and immune responses following anti-PD-1 therapy in cancer patients with HIV infection. Ann Oncol 2018; 29:2141–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bui JK, Cyktor JC, Fyne E, Campellone S, Mason SW, Mellors JW. Blockade of the PD-1 axis alone is not sufficient to activate HIV-1 virion production from CD4+ T cells of individuals on suppressive ART. PLoS One 2019; 14:e0211112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 2018; 8:1069–86. [DOI] [PubMed] [Google Scholar]
- 21.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. . Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019; 381:1535–46. [DOI] [PubMed] [Google Scholar]
- 22.Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373:1270–1. [DOI] [PubMed] [Google Scholar]
- 23.Van der Sluis RM, Kumar NA, Pascoe RD, et al. . Combination immune checkpoint blockade to reverse HIV latency. J Immunol 2020; 204:1242–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harper J, Gordon S, Chan CN, et al. . CTLA-4 and PD-1 dual blockade induces SIV reactivation without control of rebound after antiretroviral therapy interruption. Nat Med 2020; 26:519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elliott JH, Wightman F, Solomon A, et al. . Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog 2014; 10:e1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakkour S, Deng X, Bacchetti P, et al. . Replicate aptima assay for quantifying residual plasma viremia in individuals on antiretroviral therapy. J Clin Microbiol 2020; 58:e01400–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasmussen TA, McMahon JH, Chang JJ, et al. . The effect of antiretroviral intensification with dolutegravir on residual virus replication in HIV-infected individuals: a randomised, placebo-controlled, double-blind trial. Lancet HIV 2018; 5:e221–30. [DOI] [PubMed] [Google Scholar]
- 28.Siliciano JD, Siliciano RF. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol Biol 2005; 304:3–15. [DOI] [PubMed] [Google Scholar]
- 29.Laird GM, Eisele EE, Rabi SA, et al. . Rapid quantification of the latent reservoir for HIV-1 using a viral outgrowth assay. PLoS Pathog 2013; 9:e1003398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elliott JH, McMahon JH, Chang CC, et al. . Short-term administration of disulfiram for reversal of latent HIV infection: a phase 2 dose-escalation study. Lancet HIV 2015; 2:e520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruner KM, Wang Z, Simonetti FR, et al. . A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 2019; 566:120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hryniewicz A, Boasso A, Edghill-Smith Y, et al. . CTLA-4 blockade decreases TGF-beta, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood 2006; 108:3834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dyavar Shetty R, Velu V, Titanji K, et al. . PD-1 blockade during chronic SIV infection reduces hyperimmune activation and microbial translocation in rhesus macaques. J Clin Invest 2012; 122:1712–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gay CL, Bosch RJ, Ritz J, et al. ; AIDS Clinical Trials 5326 Study Team . Clinical trial of the anti-PD-L1 antibody BMS-936559 in HIV-1 infected participants on suppressive antiretroviral therapy. J Infect Dis 2017; 215:1725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das R, Verma R, Sznol M, et al. . Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol 2015; 194:950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei SC, Levine JH, Cogdill AP, et al. . Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell 2017; 170: 1120–33.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei SC, Anang NAS, Sharma R, et al. . Combination anti-CTLA-4 plus anti-PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc Natl Acad Sci U S A 2019; 116:22699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gane E, Verdon DJ, Brooks AE, et al. . Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: a pilot study. J Hepatol 2019; 71:900–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.