Abstract
Background
Post-treatment Lyme disease symptoms/syndrome (PTLDS) occurs in approximately 10% of patients with Lyme disease following antibiotic treatment. Biomarkers or specific clinical symptoms to identify patients with PTLDS do not currently exist and the PTLDS classification is based on the report of persistent, subjective symptoms for ≥6 months following antibiotic treatment for Lyme disease.
Methods
Untargeted liquid chromatography–mass spectrometry metabolomics was used to determine longitudinal metabolic responses and biosignatures in PTLDS and clinically cured non-PTLDS Lyme patients. Evaluation of biosignatures included (1) defining altered classes of metabolites, (2) elastic net regularization to define metabolites that most strongly defined PTLDS and non-PTLDS patients at different time points, (3) changes in the longitudinal abundance of metabolites, and (4) linear discriminant analysis to evaluate robustness in a second patient cohort.
Results
This study determined that observable metabolic differences exist between PTLDS and non-PTLDS patients at multiple time points. The metabolites with differential abundance included those from glycerophospholipid, bile acid, and acylcarnitine metabolism. Distinct longitudinal patterns of metabolite abundance indicated a greater metabolic variability in PTLDS versus non-PTLDS patients. Small numbers of metabolites (6 to 40) could be used to define PTLDS versus non-PTLDS patients at defined time points, and the findings were validated in a second cohort of PTLDS and non-PTLDS patients.
Conclusions
These data provide evidence that an objective metabolite-based measurement can distinguish patients with PTLDS and help understand the underlying biochemistry of PTLDS.
Keywords: Borrelia burgdorferi, metabolomics, PTLDS, Lyme disease
Objective metabolite-based measurements differ between patients with Lyme disease who develop post-treatment Lyme disease symptoms/syndrome and clinically cured patients. These differences could be useful in classification and understanding of this syndrome.
Lyme disease (LD) is a tick-borne disease that, in the United States, occurs following the bite of an Ixodes scapularis or Ixodes pacificus tick infected with the spirochete Borrelia burgdorferi or Borrelia mayonii [1, 2]. Following treatment with antibiotics, the majority of patients diagnosed with early LD recover without further complications [1]. However, approximately 10% of patients develop post-treatment LD symptoms or syndrome (PTLDS) [3, 4]. These individuals experience persistent, subjective symptoms including fatigue, musculoskeletal pain, and/or cognitive difficulties for 6 or more months following completion of antibiotic treatment. This is classified as a syndrome when persistent symptoms negatively impact life functionality [5, 6].
Specific clinical signs or objective biomarkers to diagnose patients with PTLDS from those with similar subjective symptoms but without LD do not exist. Thus, physicians rely on clinical judgment and the timing of symptoms in the context of previously diagnosed and treated LD, while considering evidence for alternative explanations. Although efforts have been made to standardize the reporting of symptoms [7], a lack of an objective test confounds the ability to diagnose PTLDS and assess its epidemiology or treatment. The limited knowledge regarding PTLDS pathogenesis also complicates clinical care. Several hypotheses exist, including persistence of the pathogen or bacterial products [8, 9], autoimmunity [10], and differential immune/inflammatory responses [11–14]. Studies to develop an objective measure of PTLDS and to elucidate the underlying mechanism(s) of pathogenesis have evaluated specific immune mediators [11, 12, 15] and differential gene expression signatures [16]. However, the variability in type and severity of symptoms complicates the interpretation of results.
Previously, metabolomics studies identified metabolic pathways that are altered during LD and elucidated promising metabolic biomarkers for differentiating LD from other diseases [17–21]. In this study, sera from 2 small cohorts of patients were analyzed by liquid chromatography–mass spectrometry (LC-MS) to identify small-molecule metabolites (SMMs) and metabolic pathways that differ between PTLDS and clinically cured (non-PTLDS) patients with LD at 3 time points (baseline, following treatment completion, and at 1 year post-treatment). This revealed that metabolite classes including glycerophospholipids, bile acids, and acylcarnitines were altered in PTLDS patients as compared with non-PTLDS patients. The metabolic changes were consistent in PTLDS patients regardless of their classification as syndrome or symptoms.
METHODS
Study Participants
Study samples were obtained from 2 separate cohorts. Cohort 1 consisted of retrospective (archived) serum samples from PTLDS (symptoms) and non-PTLDS patients with LD obtained at New York Medical College (Valhalla, NY) from 1991 to 2011 [5]. All patients presented with an erythema migrans skin lesion and were skin culture positive for B. burgdorferi. Cohort 2 consisted of retrospective serum samples from PTLDS (syndrome, individuals with symptoms and impaired life functionality) and non-PTLDS patients with LD obtained at Johns Hopkins University between 2008 and 2012 [11]. All patients presented with an erythema migrans skin lesion. Sample collection, handling, and exclusion for both cohorts are detailed in the Supplementary Material.
Sera (both cohorts) were collected at 3 time points: diagnosis (baseline); at the completion of antibiotic therapy, approximately 2 to 3 weeks following diagnosis (post-treatment); and at 1 year post-treatment (1-year follow-up). A detailed description of each patient population is provided in Table 1. All participating institutions obtained Institutional Review Board approval, and all patients gave informed consent. All methods were carried out in accordance with the guidelines and regulations of human subjects.
Table 1.
Patient Characteristics
| Characteristic | Cohort 1 | Cohort 2 | ||
|---|---|---|---|---|
| Non-PTLDS | PTLDSa | Non-PTLDS | PTLDSb | |
| No. | 10 | 11 | 13 | 13 |
| Mean age ± SD at baseline visit (range), years | 50 ± 11 (26–64) | 48 ± 13 (32–70) | 50 ± 14 (25–73) | 44 ± 16 (20–75) |
| Males, n (%) | 2 (20) | 6 (55) | 8 (62) | 4 (31) |
| Single EM at baseline,c n (%) | 8 (80) | 8 (73) | 8 (62) | 10 (77) |
| Symptomatic at study entry, n (%) | 10 (100) | 11 (100) | 13 (100) | 12 (92) |
| Mean no. of symptoms ± SD at baseline visit (range) | 6.9 ± 1.45 (5–10) | 5.18 ± 1.99 (3–9) | 5.15 ± 2.03 (2–8) | 5.07 ± 3.25 (0–10) |
| Mean no. of symptoms ± SD at 1-year visit (range) | 0.2 ± 0.42 (0–1) | 2.09 ± 1.14 (1–4) | 0.62 ± 1.19 (0–4) | 2.46 ± 2.73 (0–10) |
| Mean treated with doxycycline,d n (%) | 9 (90) | 11 (100) | 13 (100) | 13 (100) |
| Treatment duration ± SD (range),e days | 14.33 ± 2.83 (10–21) | 14.91 ± 3.62 (10–20) | 21 ± 0 (21) | 21 ± 0 (21) |
| Follow treatment, n (%) | 0 (0) | 2 (18) | 1 (8) | 4 (31) |
| Prior Lyme disease,f n (%) | 1 (10) | 1 (9.1) | 0 | 0 |
| Two-tier positive,g n (%) | 7 (70) | 7 (64) | 13 (100) | 7 (54) |
Abbreviations: EIA, enzyme immunoassay; ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobulin G; IgM, immunoglobulin M; PTLDS, post-treatment Lyme disease symptoms/syndrome; EM, erythema migrans; WB, western blot.
aPTLDS (symptoms) patients, classified as such if symptoms developed at or within 6 months following diagnosis and persisted for at least 6 months following the completion of antibiotic therapy [5, 11].
bPTLDS (syndrome) patients, classified as such if they met the PTLDS (symptoms) criteria and reported daily life functional impairment [5, 22].
cAll other patients had multiple EMs.
dIn cohort 1, 3 PTLDS and 1 non-PTLDS patients received an additional 1–4 doses of ceftriaxone; treatment for 1 non-PTLDS patient is unknown.
eTreatment duration for 1 non-PTLDS patient is unknown.
fPrior history of Lyme disease diagnosis and successful treatment not including current episode.
gPositive or equivocal EIA/ELISA and positive IgM and/or IgG WB at baseline and/or post-treatment time point.
Sample Preparation and Evaluation of Small-molecule Metabolites
Serum samples were randomized prior to extraction of SMMs with 75% methanol as previously described [18]. Extracted SMMs were analyzed by LC-MS using reversed-phase chromatography and electrospray ionization operated in the positive-ion mode (Supplementary Material).
Biosignature Selection and Analyses
The LC-MS data were processed with MassHunter Profinder version B.08.00 and Mass Profiler Professional version B.14.9.01 (Agilent Technologies) to identify SMMs that differed between PTLDS and non-PTLDS patients at baseline, post-treatment, and 1-year follow-up in cohort 1 (Supplementary Material). These SMMs comprised time-point–specific biosignatures.
To reduce the number of SMMs that differentiated PTLDS and non-PTLDS patients at each time point, elastic net regularization modeling with the glmnet package version 2.0–16 in R version 3.5 was applied [23]. Elastic net tuning and regularization parameters were chosen by leave-one-out cross-validation implemented in the caret package version 6.0–7.0 [24, 25]. Elastic net regularization is a variable selection technique that works by imposing a penalty on the coefficients of a linear regression model, so that those variables that contribute least to the classifier are removed. The separation of PTLDS and non-PTLDS patients based on time-point–specific biosignature SMMs as well as elastic net–selected SMMs were visualized using partial least-squares discriminate analysis.
Radar plots were developed using the ggplot2 package in R [26]. Simple longitudinal patterns (12 patterns) were identified for each patient by evaluating the elastic net SMMs at baseline, post-treatment, and 1-year follow-up and indicating whether an SMM was high, low, or missing (ie, patient sample missing for that time point). Rényi indices, which calculate diversity for 11 scales ranging from 0 to ∞ for each SMM, were determined using the vegan package version 2.5.6 [27]. An SMM was considered more diverse in 1 group if the Rényi indices were higher across all scales in that group [28].
To evaluate the robustness of the time-point–specific biosignature SMMs defined using cohort 1 were targeted in the LC-MS data files of patient samples from cohort 2 using MassHunter Profinder (Supplementary Material). Linear discriminant analyses (LDA) were performed using the MASS package in R [29].
Metabolite Identification and Pathway Analyses
The experimental accurate mass values of each SMM were interrogated against the Human Metabolome Database (HMDB) using a 30-ppm mass tolerance and exclusion of nonendogenous metabolites to obtain putative metabolite identifications [30]. The chemical structures of selected metabolites were confirmed by LC–tandem MS (LC-MS/MS) using previously published parameters [19]. The level of structural identification followed refined Metabolomics Standards Initiative guidelines proposed by Schymanski et al [31]. Chemical standards used are listed in Supplementary Material. Metabolic pathway analyses were performed via Mummichog version 2 under python program version 2.7.14 [32]. Default parameters were used, except the mass error was 20 ppm, the P-value cutoff was .05, and the analytical mode was set for positive ionization.
RESULTS
Small-molecule Metabolite Abundances Differ Between PTLDS and Non-PTLDS Patients at Multiple Time Points
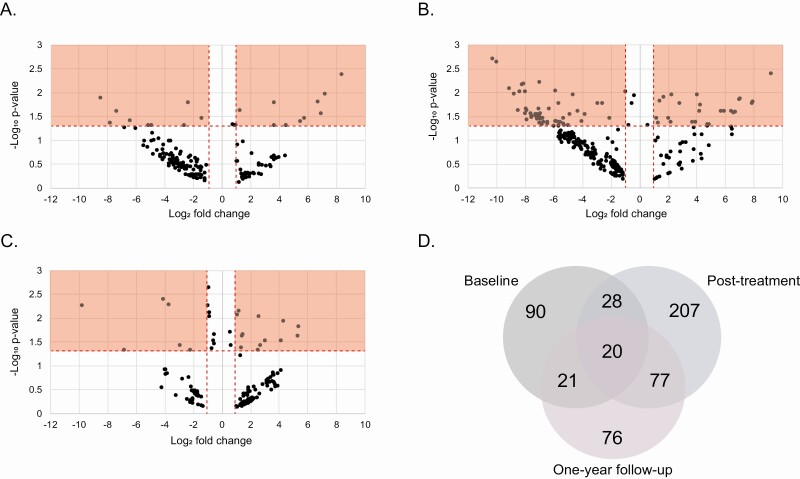
The evaluation of baseline, post-treatment, and 1-year follow-up sera by LC-MS detected 940 SMMs, 1086 SMMs, and 1073 SMMs, respectively, that were present in at least 60% of either PTLDS or non-PTLDS patients of cohort 1. Pairwise comparisons between PTLDS and non-PTLDS patients generated metabolic biosignatures composed of 160, 335, and 198 SMMs for baseline, post-treatment, and 1-year follow-up time points, respectively (Supplementary Table 3). All biosignature SMMs differed significantly (P < .05, Welch’s 2-tailed t test or Mann-Whitney U 2-tailed test) and/or produced a 2-fold abundance change. At baseline and post-treatment, 91% and 94% SMMs, respectively, that differed significantly between PTLDS and non-PTLDS also had at least a 2-fold abundance difference (Supplementary Table 3, Figure 1A and 1B). This consistency between significance and fold change dropped to 65.5% of the SMMs for the 1-year follow-up biosignature (Figure 1C). A total of 20 SMMs were present in the biosignatures at all 3 time points (Figure 1D).
Figure 1.
Metabolic differences between PTLDS and non-PTLDS. Volcano plots for PTLDS vs non-PTLDS at baseline (A), post-treatment (B), and 1-year follow-up (C). D, Venn diagram using the biosignature SMMs from all 3 time points. Abbreviations: PTLDS, post-treatment Lyme disease symptoms/syndrome; SMM, small-molecule metabolite.
Small-molecule Metabolite Alterations Are Associated With Classes of Metabolites
HMDB was used to obtain putative metabolite identification. Approximately half of the SMMs in each time-point biosignature (56% baseline, 44% post-treatment, and 51% 1-year follow-up) were putatively identified as endogenous metabolites (Supplementary Table 2). MS/MS fragmentation of the putative metabolites provided full structural validation for 24 of the metabolites based on a retention time and MS/MS spectrum match to a commercial standard (level 1 identification). Twenty metabolites were validated based on a match of experimental MS/MS data to MS/MS spectra in the NIST (National Institute of Standards and Technology) database or generation of diagnostic fragment ions (level 2 identification). The validated metabolites (level 1 and 2 identification) belonged to the following classes of compounds: bile acids, acylcarnitines, glycerophospholipids, dipeptides, fatty acids, and dioic acids (Supplementary Table 1). More comprehensive metabolic pathway analyses via Mummichog were unsuccessful due to the small number of SMMs that differentiated PTLDS and non-PTLDS patients [32].
Small-molecule Metabolite Profiles Discriminate Between PTLDS and Non-PTLDS
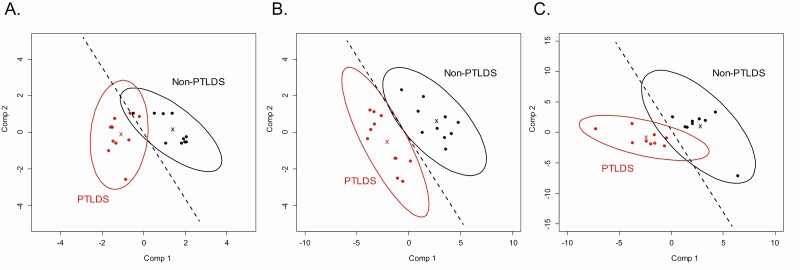
Supervised separation of PTLDS and non-PTLDS patients based on biosignature SMMs was observed at each time point (Supplementary Figure 2A–C). A more prominent separation was observed at baseline and 1-year follow-up as compared with post-treatment. Elastic net regularization was utilized to select those SMMs that most strongly contributed to separation of the PTLDS and non-PTLDS patients at each time point. This resulted in 6, 28, and 40 SMMs for baseline, post-treatment, and 1-year follow-up, respectively (Supplementary Table 1). The elastic net models classified PTLDS and non-PTLDS patients with accuracies of .90 (95% confidence interval [CI], .7–.99), 1.0 (95% CI, .84–1), and 1.0 (95% CI, .81–1) at baseline, post-treatment, and 1-year follow-up, respectively (Figure 2A–C).
Figure 2.
Discrimination of PTLDS and non-PTLDS using SMMs down-selected via elastic net regularization. Partial least-squares discriminant analysis plots of PTLDS (red) and non-PTLDS (black) patients at baseline using the 6 SMMs selected at baseline (A), the 28 SMMs selected post-treatment (B), and the 40 SMMs selected at 1-year follow-up (C). Ellipses represent the 95% confidence interval for each group. Dashed lines are drawn to aid in visualization of group separation. Abbreviations: PTLDS, post-treatment Lyme disease symptoms/syndrome; SMM, small-molecule metabolite; Comp, component.
The SMMs selected by elastic net for each time point were combined. Of these 72 SMMs, 26 had validated identities belonging to bile acid, amino acid, fatty acid, sphingolipid, microbiota, retinol, acylcarnitine, and glycerophospholipid metabolite classes (Supplementary Table 1). The majority (93%) of the SMMs with confirmed identities met the 2-fold abundance change criterion; a smaller percentage (70%) met the significance criterion (Supplementary Tables 1 and 3).
Behavior of Small-molecule Metabolites Differs in PTLDS and Non-PTLDS Over Time
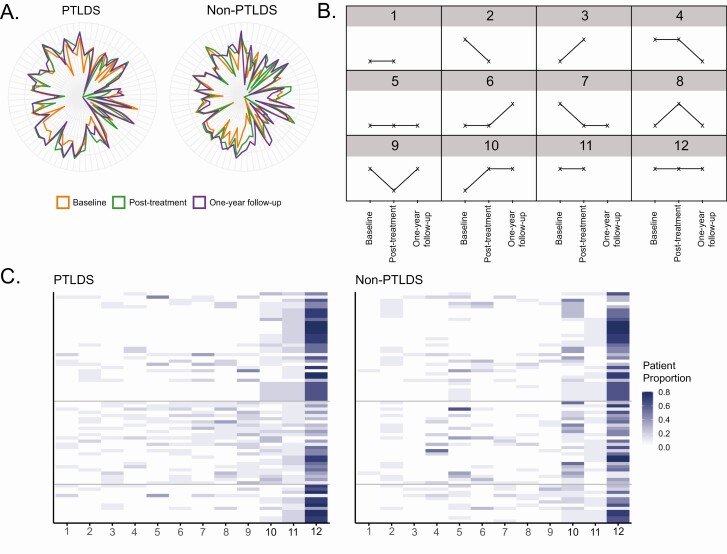
An alternative means of evaluating metabolic differences was to visualize the longitudinal change in SMM abundances between PTLDS and non-PTLDS patients. Specifically, the abundances of the 72 SMMs selected by elastic net were visualized across all time points using radar plots (Figure 3). Interestingly, the radar plot patterns observed at post-treatment and 1-year follow-up for the PTLDS patients looked similar. In contrast, the radar plot patterns for non-PTLDS patients at these same time points displayed greater differences. This indicated that non-PTLDS patients continued to modify their metabolic response after the end of treatment, but this change was muted in PTLDS patients. An expanded analysis was performed using the SMMs from the time-point biosignatures and the longitudinal changes in radar plot patterns were also more muted in the PTLDS patients than in non-PTLDS patients regardless of the time point at which the biosignature was evaluated (Supplementary Figure 3). The greatest differences in the longitudinal change between the patient groups were observed with the post-treatment time-point biosignature (Supplementary Figure 3B). The large number of SMMs associated with the post-treatment biosignature and the more pronounced differences in the longitudinal radar plot patterns between patient groups for the SMMs inclusive of this time-point biosignature suggest that the metabolic biosignature at the end of treatment might be the most informative for differentiating PTLDS patients from non-PTLDS patients. Overall, these visualizations of the data indicated that longitudinal metabolic responses differed between PTLDS and non-PTLDS patients.
Figure 3.
Analysis of SMM longitudinal behavior in PTLDS and non-PTLDS patients. A, Radar plots using the 72 SMMs selected by elastic net overlaid for all 3 time points. PTLDS patient measurements are depicted on the right, and non-PTLDS patient measurements are depicted on the left. The colors for each time point are orange, green, and purple for baseline, post-treatment, and 1-year follow-up, respectively. B, Diagram of the 12 longitudinal patterns observed for the 72 SMMs selected using elastic net regularization. Higher points indicate a log10 SMM abundance greater than −10; lower points indicate log10 abundance less than −10; missing points indicate a patient sample was not available. C, Heatmaps depicting the proportion of patients who displayed each of the 12 patterns for the 72 SMMs in the PTLDS patient group (left) and the non-PTLDS patient group (right). The columns are ordered from right to left according to the proportion of PTLDS patients (low to high). The SMMs are ordered according to their Rényi diversity groups with lines separating each group; the 34 SMMs with no clear difference in diversity between the groups (top), the 26 SMMs that are clearly more diverse in PTLDS patients (middle), and the 12 SMMs that are clearly more diverse in non-PTLDS patients (bottom). Abbreviations: PTLDS, post-treatment Lyme disease symptoms/syndrome; SMM, small-molecule metabolite.
The radar plots also indicated that within a specific patient group not all SMM abundances change in a consistent longitudinal manner. Theoretically, an SMM could adopt 1 of 27 potential patterns based on whether it had a high, low, or missing abundance at each of the 3 time points. When plots were constructed for the metabolites with the most discriminating potential (elastic net–selected SMMs) only 12 patterns were discovered (Supplementary Figure 3B). The proportions of PTLDS or non-PTLDS patients displaying each trend for each SMM were visualized using heatmaps (Figure 3). Patterns 11, 9, 8, 7, and 6 are more predominant in PTLDS patients, while patterns 10 and 5 are more predominant in non-PTLDS patients. Visual examination of these heatmaps indicated that there was a greater diversity of patterns for each SMM in PTLDS patients as compared with non-PTLDS patients. Using Rényi indices to measure this diversity, 26 features were more diverse in PTLDS patients, 12 features were more diverse in non-PTLDS patients, and there was no difference in diversity between the groups for 34 features. These data demonstrate that PTLDS and non-PTLDS follow different longitudinal trajectories and that these trajectories may be more variable in PTLDS patients than in non-PTLDS patients.
Evaluation of Biosignature Small-molecule Metabolites in a Second Patient Cohort
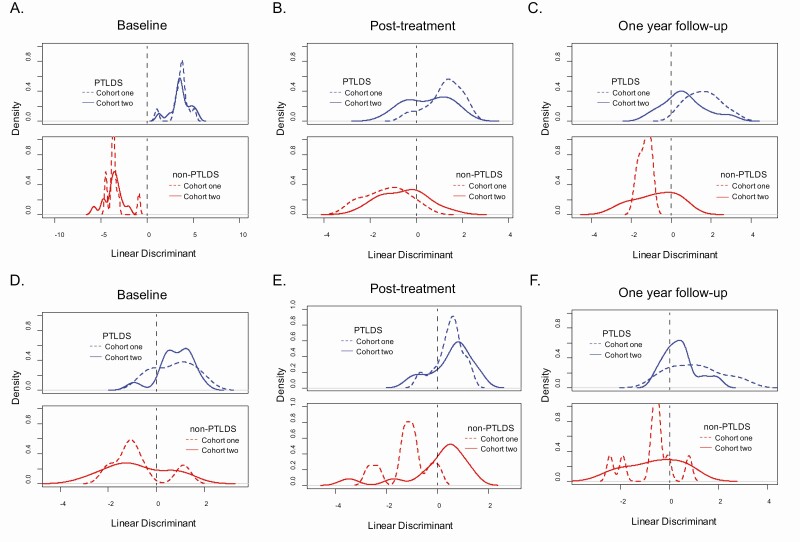
The robustness of the findings in cohort 1 were determined by evaluating the biosignature SMMs in cohort 2 PTLDS (syndrome) patients. A less-stringent abundance fold change of 1.25 was used to determine which SMMs behaved similarly in both cohorts. Based on these criteria, 39 SMMs at baseline, 13 SMMs post-treatment, and 19 SMMs at 1-year follow-up produced consistent abundance changes between PTLDS and non-PTLDS patients in both cohorts (Supplementary Table 4). Focusing on these SMMs, LDA was performed using both patient cohorts (Figure 4A–C). The accuracy for discrimination of PTLDS and non-PTLDS regardless of cohort was 1 (95% CI, .92–1) at baseline, .74 (95% CI, .6–.86) post-treatment, and .83 (95% CI, .69–.93) at 1-year follow-up. Both cohorts had overlapping linear discriminant scores, which fell primarily on different sides of zero for PTLDS or non-PTLDS classifications at all 3 time points, indicating that these sets of features are relatively robust.
Figure 4.
Discrimination of PTLDS and non-PTLDS patients in 2 different cohorts using SMMs that behave similarly. LDA plots of PTLDS (blue) and non-PTLDS (red) in cohort 1 (dashed line) and cohort 2 (solid line). Discrimination at baseline using the 39 SMMs with similar trends in both cohorts (A) and 12 SMMs of those that had putative IDs (D). Discrimination post-treatment using the 13 SMMs with similar trends in both cohorts (B) and 5 SMMs of those that had putative IDs (E). Discrimination at 1-year follow-up using the 19 SMMs with similar trends in both cohorts (C) and 9 SMMs of those that had putative IDs (F). Dashed lines are drawn at 0 to aid in visualization of separation. Abbreviations: ID, identification; LDA, linear discriminant analysis; PTLDS, post-treatment Lyme disease symptoms/syndrome; SMM, small-molecule metabolite.
There were common classes of metabolites with differential abundances in both cohorts, consisting of glycerophospholipids, acylcarnitines, bile acids, and fatty acids (Supplementary Tables 2–4). A second LDA was performed using only SMMs belonging to these common classes of metabolites (Figure 4D–F). The accuracy for discrimination of PTLDS and non-PTLDS regardless of cohort was .78 (95% CI, .64–.89) at baseline, .68 (95% CI, .53–.89) post-treatment, and .74 (95% CI, .58–.86) at 1-year follow-up. With the exception of non-PTLDS patients post-treatment, the linear discriminant scores fell primarily on different sides of zero and had overlapping scores for the same classification in both cohorts at all 3 time points. These findings provided evidence that metabolic pathways can be utilized to develop objective methods for classifying PTLDS (symptoms and syndrome).
Discussion
The major findings of this study were that metabolic differences can be identified between PTLDS and non-PTLDS patients, and there were consistencies in these metabolic changes in 2 separate cohorts of patients. This work provides evidence that an objective measurement can distinguish patients with PTLDS from those who return to health after treatment of acute LD.
Our results were consistent with studies that identified other measurable differences in PTLDS patients. Aucott et al [11] determined that patients with elevated levels of the T-cell chemokine CCL19 at 1 month post-treatment had a higher risk of developing PTLDS by 6 or 12 months post-treatment. Persistent elevated levels of interleukin-23 (IL-23) and C-reactive protein (CRP) after 12 months post-treatment were also observed in PTLDS patients [12, 14]. A single gene (GPR15) was found to be upregulated at baseline in PTLDS patients with respect to patients with resolved LD [16]. Quantification of [11C]DPA-713 (a ligand of the peripheral benzodiazepine receptor) by positron emission tomography demonstrated increased binding in PTLDS patients as compared with healthy controls [33]. The combination of these objective measurements with an SMM biosignature could lead to a robust classifier for PTLDS patients.
The classes of metabolites identified in this study are similar to those described in patients with chronic fatigue syndrome (CFS). Similar to our findings, changes in glycerophospholipid, aromatic and branched-chain amino acid, carnitine, bile acid, fatty acid, and sphingolipid metabolism were described in studies involving patients with CFS [34–36]. These common pathways could be indicative of the similar symptoms or perhaps overlapping pathophysiologies for both syndromes. A comparison of metabolic profiles between patients with CFS and PTLDS would provide more clarity on the relationship between these 2 syndromes.
Some classes of metabolites described in this study are concurrent with those previously identified as being altered in patients with early LD [37]. The specific alterations in these pathways between PTLDS and non-PTLDS patients could be due to differential immune responses at baseline or sustained inflammation in PTLDS patients post-treatment or at 1-year follow-up [10–14]. Specifically, decreased levels of acylcarnitines were observed in patients with early LD [37] and levels of 10 acylcarnitine metabolites were lower in non-PTLDS at baseline and/or post-treatment and also lower in PTLDS patients at 1-year follow-up. This trend was also observed in cohort 2, with lower levels of isovalerylcarnitine and propionylcarnitine in non-PTLDS patients at baseline and post-treatment, respectively, as well as lower levels of acetylcarnitine in PTLDS patients at 1-year follow-up (Supplementary Tables 2–4). As decreased acylcarnitine levels are associated with fatigue and cognitive complaints, the sustained lower levels in PTLDS patients at 1 year could be associated with residual symptoms [38, 39]. The current results do not rule out the possibility of PTLDS being attributed to inefficient clearance of spirochetes [8, 9]. Future longitudinal metabolomics analyses of a large cohort of non-PTLDS patients, similar to the transcriptomic studies performed previously [16, 40], could improve understanding of how metabolic alterations of early LD resolve in non-PTLDS patients and provide a road map to understand nontypical metabolic resolution in PTLDS patients.
A limitation of this study is the small sample sizes within the cohorts. Previous studies have demonstrated a broad distribution among PTLDS and non-PTLDS patients and that capturing the true mean of these patient populations can be hindered by small sample sizes [11, 14, 16]. Additionally, the statistical analyses were performed without adjusting for multiple comparisons. This was done to broaden the list of biosignature SMMs with the understanding that some might be false positives. The discriminant analyses used can be subject to overfitting and were not tested further due to the small sample sizes. Missing values were also present in the data due to either absence of a peak or presence of a peak below the limit of detection. Missing values are common in LC-MS data and they can influence data interpretation, especially in studies with smaller sample sizes [41]. The unblinded design of this study is another limitation. The metabolic profiles might also be dependent on specific differences within cohorts, such as enrollment criteria, the use of prescription or over-the-counter medications, or particular symptom(s) that persist. Thus, additional studies with larger sample sizes that include other chronic conditions such as CFS are needed to validate our findings. However, the ability to distinguish PTLDS patients from 2 separate cohorts provides strong support that the use of objective metabolic signatures will be of value in the understanding of PTLDS.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Amy Fleshman (Centers for Disease Control and Prevention, Fort Collins) for performing immunoglobulin (Ig) M and IgG immunoblots on cohort 1 patient samples. We thank Kristofor Webb (Colorado State University, Fort Collins) for support in the operation of LC-MS instrumentation. The mass spectrometry data are available at the National Institutes of Health Common Fund’s National Metabolomics Data Repository (NMDR) website, the Metabolomics Workbench (https://www.metabolomicsworkbench.org), where it has been assigned project ID PR000954 and study ID ST001391. The data can be accessed directly via the project DOI: 10.21228/M8CM3N.
Financial support. This project was supported by the Centers for Disease Control and Prevention and in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the Centers for Disease Control and Prevention; the National Institute of Allergy and Infectious Diseases grants R01 AI141656 and R33 AI100228; and the National Institute of Arthritis and Musculoskeletal and Skin Diseases grant P30 AR070254.
Potential conflicts of interest. G. P. W. reports receiving research grants from National Institutes of Health (NIH)/Immunetics, Inc; Institute for Systems Biology; Rarecyte, Inc; NIH/Tufts; Colorado State University/NIH; and Quidel Corporation. He owns equity in Abbott/AbbVie, has been an expert witness in malpractice cases involving babesiosis and Lyme disease, has lectured for various medical centers, and is an unpaid board member of the American Lyme Disease Foundation; he is an employee of New York Medical College. J. T. B., C. R. M., and G. P. W. report a US patent application, “High Sensitivity Method for Early Lyme Disease Detection” (application no. 15/046 204; patent issued 2 June 2020 [US Patent No. 10 669 567 B2]), and a US provisional patent application, “Use of Metabolic Biosignatures for Differentiation of Early Lyme Disease from Southern Tick–Associated Rash Illness (STARI)” (application no. 62/277 252). J. N. A. serves on the Scientific Advisory Board for Bay Area Lyme Foundation and has been issued a patent for “Elevated CCL19 after completion of therapy for acute Lyme disease identifies patients at risk for development of post-treatment Lyme disease syndrome who will benefit from further antibiotic therapy” [US Patent No. 10 481 165]. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Steere AC, Strle F, Wormser GP, et al. . Lyme borreliosis. Nat Rev Dis Primers 2016; 2:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pritt BS, Mead PS, Johnson DKH, et al. . Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis 2016; 16:556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fallon BA, Zubcevik N, Bennett C, et al. . The general symptom questionnaire-30 (GSQ-30): a brief measure of multi-system symptom burden in Lyme disease. Front Med (Lausanne) 2019; 6:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wormser GP, McKenna D, Karmen CL, et al. . Prospective evaluation of the frequency and severity of symptoms in Lyme disease patients with erythema migrans compared with matched controls at baseline, 6 months, and 12 months. Clin Infect Dis 2020. doi: 10.1093/cid/ciz1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weitzner E, McKenna D, Nowakowski J, et al. . Long-term assessment of post-treatment symptoms in patients with culture-confirmed early Lyme disease. Clin Infect Dis 2015; 61:1800–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wormser GP, Dattwyler RJ, Shapiro ED, et al. . The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43:1089–134. [DOI] [PubMed] [Google Scholar]
- 7.Bechtold KT, Rebman AW, Crowder LA, Johnson-Greene D, Aucott JN. Standardized symptom measurement of individuals with early Lyme disease over time. Arch Clin Neuropsychol 2017; 32:129–41. [DOI] [PubMed] [Google Scholar]
- 8.Crossland NA, Alvarez X, Embers ME. Late disseminated Lyme disease: associated pathology and spirochete persistence posttreatment in rhesus macaques. Am J Pathol 2018; 188:672–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bockenstedt LK, Gonzalez DG, Haberman AM, Belperron AA. Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. J Clin Invest 2012; 122:2652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandra A, Wormser GP, Klempner MS, et al. . Anti-neural antibody reactivity in patients with a history of Lyme borreliosis and persistent symptoms. Brain Behav Immun 2010; 24:1018–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aucott JN, Soloski MJ, Rebman AW, et al. . CCL19 as a chemokine risk factor for posttreatment Lyme disease syndrome: a prospective clinical cohort study. Clin Vaccine Immunol 2016; 23:757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strle K, Stupica D, Drouin EE, Steere AC, Strle F. Elevated levels of IL-23 in a subset of patients with post-Lyme disease symptoms following erythema migrans. Clin Infect Dis 2014; 58:372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sjöwall J, Fryland L, Nordberg M, et al. . Decreased Th1-type inflammatory cytokine expression in the skin is associated with persisting symptoms after treatment of erythema migrans. PLoS One 2011; 6:e18220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uhde M, Ajamian M, Li X, Wormser GP, Marques A, Alaedini A. Expression of C-reactive protein and serum amyloid A in early to late manifestations of Lyme disease. Clin Infect Dis 2016; 63:1399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uhde M, Indart A, Fallon BA, et al. . C-reactive protein response in patients with post-treatment Lyme disease symptoms versus those with myalgic encephalomyelitis/chronic fatigue syndrome. Clin Infect Dis 2018; 67:1309–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouquet J, Soloski MJ, Swei A, et al. . Longitudinal transcriptome analysis reveals a sustained differential gene expression signature in patients treated for acute Lyme disease. mBio 2016; 7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pegalajar-Jurado A, Fitzgerald BL, Islam MN, et al. . Identification of urine metabolites as biomarkers of early Lyme disease. Sci Rep 2018; 8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molins CR, Ashton LV, Wormser GP, et al. . Development of a metabolic biosignature for detection of early Lyme disease. Clin Infect Dis 2015; 60:1767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molins CR, Ashton LV, Wormser GP, et al. . Metabolic differentiation of early Lyme disease from southern tick-associated rash illness (STARI). Sci Transl Med 2017; 9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerstholt M, Vrijmoeth H, Lachmandas E, et al. . Role of glutathione metabolism in host defense against Borrelia burgdorferi infection. Proc Natl Acad Sci USA 2018; 115:E2320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glader O, Puljula E, Jokioja J, Karonen M, Sinkkonen J, Hytönen J. NMR metabolome of Borrelia burgdorferi in vitro and in vivo in mice. Sci Rep 2019; 9:8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aucott JN, Rebman AW, Crowder LA, Kortte KB. Post-treatment Lyme disease syndrome symptomatology and the impact on life functioning: is there something here? Qual Life Res 2013; 22:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2020. Available at: https://www.R-project.org/. [Google Scholar]
- 24.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw 2010; 33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhn M. caret: classification and regression training: R package version 6.0–85. 2020. Available at: https://CRAN.R-project.org/package=caret.
- 26.Wickham H.ggplot2: elegant graphics for data analysis. New York: Springer, 2016. [Google Scholar]
- 27.Rényi A. On measures of entropy and information. Maths Stat Prob 1961; 1:547–61. [Google Scholar]
- 28.Tothmeresz B. Comparison of different methods for diversity ordering. J Veg Sci 1995; 6:283–90. [Google Scholar]
- 29.Venables WN, Ripley BD.. Modern applied statistics with S. 4th ed. New York: Springer, 2002. [Google Scholar]
- 30.Wishart DS, Jewison T, Guo AC, et al. . HMDB 3.0–the human metabolome database in 2013. Nucleic Acids Res 2013; 41:D801–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schymanski EL, Jeon J, Gulde R, et al. . Identifying small molecules via high resolution mass spectrometry: communicating confidence. Environ Sci Technol 2014; 48:2097–8. [DOI] [PubMed] [Google Scholar]
- 32.Li SZ, Park Y, Duraisingham S, et al. . Predicting network activity from high throughput metabolomics. PLoS Comput Biol 2013; 9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coughlin JM, Yang T, Rebman AW, et al. . Imaging glial activation in patients with post-treatment Lyme disease symptoms: a pilot study using [11C]DPA-713 PET. J Neuroinflammation 2018; 15:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naviaux RK, Naviaux JC, Li K, et al. . Metabolic features of chronic fatigue syndrome. Proc Natl Acad Sci USA 2016; 113:E5472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Germain A, Ruppert D, Levine SM, Hanson MR. Metabolic profiling of a myalgic encephalomyelitis/chronic fatigue syndrome discovery cohort reveals disturbances in fatty acid and lipid metabolism. Mol Biosyst 2017; 13:371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagy-Szakal D, Barupal DK, Lee B, et al. . Insights into myalgic encephalomyelitis/chronic fatigue syndrome phenotypes through comprehensive metabolomics. Sci Rep 2018; 8:10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitzgerald BL, Molins CR, Islam MN, et al. . Host metabolic response in early Lyme disease. J Proteome Res 2020; 19:610–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cristofano A, Sapere N, La Marca G, et al. . Serum levels of acyl-carnitines along the continuum from normal to Alzheimer’s dementia. PLoS One 2016; 11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reuter SE, Evans AM. Long-chain acylcarnitine deficiency in patients with chronic fatigue syndrome: potential involvement of altered carnitine palmitoyltransferase-I activity. J Intern Med 2011; 270:76–84. [DOI] [PubMed] [Google Scholar]
- 40.Petzke MM, Volyanskyy K, Mao Y, et al. . Global transcriptome analysis identifies a diagnostic signature for early disseminated Lyme disease and its resolution. mBio 2020; 11. doi: 10.1128/mBio.00047-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Do KT, Wahl S, Raffler J, et al. . Characterization of missing values in untargeted MS-based metabolomics data and evaluation of missing data handling strategies. Metabolomics 2018; 14:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.