Abstract
Background
Of new HIV infections in the US, 20% occur among young men who have sex with men (YMSM, ages 13–24), but >50% of YMSM with HIV are unaware of their status. Using Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) data, we projected the clinical benefit and cost-effectiveness of frequent HIV screening among high-risk YMSM from age 15.
Methods
Using a mathematical simulation, we examined 3 screening strategies: Yearly, 6-monthly, and 3-monthly, each in addition to the Status quo (SQ, 0.7–10.3% screened/year, stratified by age). We used published data (YMSM-specific when available) including: HIV incidences (0.91–6.41/100PY); screen acceptance (80%), linkage-to-care/antiretroviral therapy (ART) initiation (76%), HIV transmission (0.3–86.1/100PY, by HIV RNA), monthly ART costs ($2290-$3780), and HIV per-screen costs ($38). Projected outcomes included CD4 count at diagnosis, primary HIV transmissions from ages 15–30, quality-adjusted life expectancy, costs, and incremental cost-effectiveness ratios (ICERs, $/quality-adjusted life-year saved [QALY]; threshold ≤$100 000/QALY).
Results
Compared to SQ, all strategies increased projected CD4 at diagnosis (296 to 477–515 cells/µL) and quality-adjusted life expectancy from age 15 (44.4 to 48.3–48.7 years) among YMSM acquiring HIV. Compared to SQ, all strategies increased discounted lifetime cost for the entire population ($170 800 to $178 100-$185 000/person). Screening 3-monthly was cost-effective (ICER: $4500/QALY) compared to SQ and reduced primary transmissions through age 30 by 40%. Results were most sensitive to transmission rates; excluding the impact of transmissions, screening Yearly was ≤$100 000/QALY (ICER: $70 900/QALY).
Conclusions
For high-risk YMSM in the US, HIV screening 3-monthly compared to less frequent screening will improve clinical outcomes and be cost-effective.
Keywords: Young men who have sex with men, adolescents and young adults, HIV, screening, cost-effectiveness
Among Young Men Who Have Sex With Men at high risk for Human Immunodeficiency Virus in the US, screening should occur more frequently than current recommendations for annual screening.
Prompt HIV diagnosis and treatment improves individual health and reduces onward sexual transmissions [1]. New HIV diagnoses among young men who have sex with men (YMSM) continue to rise, accounting for 1 in 5 new HIV infections in the United States (US) [2]. Yet more than half of YMSM are unaware of their HIV infection [2]. Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) studies 110 and 113 evaluated tenofovir disoproxil fumarate-emtricitabine-based HIV pre-exposure prophylaxis (PrEP) among 15–22-year-olds in the US [3, 4]. ATN 110 reported 4% HIV prevalence at screening among 18–22-year-olds. Despite facilitated access to and adherence support for PrEP use, HIV incidence in ATN 113 was 6/100 person-years among 15–17-year-olds: 10-fold higher than older MSM and 100-fold higher than all US youth [2, 5].
Despite the disproportionate impact of the HIV epidemic on YMSM, there is little evidence to guide how often HIV screening should occur in YMSM [6]. Although noting that some MSM might benefit from more frequent screening, the US Centers for Disease Control and Prevention (CDC) recently found insufficient youth-specific evidence to warrant changing their 2006 recommendation for annual HIV screening among all MSM [6]. We evaluated the clinical benefit and cost-effectiveness of frequent HIV screening strategies for YMSM at high risk of acquiring HIV.
METHODS
Analytic Overview
We used the Cost-Effectiveness of Preventing AIDS Complications (CEPAC) microsimulation model [7] to simulate self-identified, high-risk [3, 4], HIV-uninfected 15-year-old MSM in the US who faced age-stratified risks of HIV infection over their lifetimes (Table 1). High risk was defined based on ATN 110/113 enrollment criteria, including recent history of condomless anal intercourse, sexually transmitted infection, or multiple sexual partners. We modeled the Status quo (SQ), reflecting existing patterns of HIV screening for YMSM (screened at least once, with frequency ranging by age, Table 1 and Supplementary Table 1) [8–11]. We also modeled 3 more frequent HIV screening strategies performed in addition to SQ screening between ages 15–30 years: Yearly, 6-monthly, and 3-monthly.
Table 1.
Input Parameters for a Model of Frequent HIV Screening for High-risk Young Men Who Have Sex with Men in the United States
| Population characteristics | Value | Range for sensitivity analysis | Source |
|---|---|---|---|
| Initial age (years) | 15 | 14–30 | [3, 4] |
| Male birth sex (%) | 100 | 100 | |
| HIV prevalence at age <15 years (%) | 0 | 0–6.8 | |
| Annual HIV incidence by age, rate/100PY | |||
| 15–17 years | 6.41 | 0.64–12.82 | [4] |
| 18–22 | 3.29 | 0.33–6.58 | [3] |
| ≥23 | 0.91 | 0.09–1.82 | [12] |
| Current HIV screening practice (annual probability of HIV detection by age) (%) | |||
| 14–15 years | 0.7 | 0.4–3.5 | [8, 9] |
| 16–17 | 1.0 | 0.5–5 | [8, 9] |
| 18–20 | 10.3 | 5.2–51.5 | [9, 10] |
| 21–24 | 8.9 | 4.5–44.5 | [9, 10] |
| 25–29 | 7.6 | 3.8–38.0 | [9, 11] |
| 30–39 | 6.5 | 3.3–32.5 | [9, 11] |
| ≥40 | 5.9 | 3.0–29.5 | [9, 11] |
| Mean CD4 at infection, cells/µL | 667 | 200–800 | [5] |
| Mean HIV RNA at infection, copies/mL | >100 000 | - | [13] |
| Screen characteristics a | |||
| Sensitivity (%) | 99.6 | 50–100 | [14] |
| Specificity (%) | 99.7 | 50–100 | [14] |
| Probability of screen offer and acceptance (%) | 80 | 25–100 | [15, 16] |
| Probability of result return (%) | 97 | 50–100 | [17, 18] |
| Probability of linkage to care (%) | 76 | 25–100 | [19] |
| HIV screening program costs (USD 2018) | |||
| HIV screen | 38.00 | 17.96–71.84 | [20] |
| Completed reactive testb | 76.42 | 36.12–144.46 | [20, 21] |
| Antiretroviral therapy (range, 1 st through 6 th available regimen) | |||
| Efficacy (%)c | 93–81 | - | [22–24] |
| Cost/month (USD 2018)d | 2290–3780 | 0.5–2.0x base case | [25, 26] |
| Loss to follow-up (rate/100PY) | |||
| Adherence >95% | 0.1 | - | [27] |
| Adherence <50% | 84.5 | 41.5–498.6 | [27] |
| Return to care (rate/100PY) | 18.1 | 18.1–100 | [28] |
| Onward transmission (rate/100PY), by disease stage and HIV RNA | |||
| Acute infection, off ARTa | 86.1 | 0–262 | [13, 29, 30] |
| Acute infection, on ARTa | 9.5 | 0–19 | [29–31] |
| >100 000 copies/mL | 16.5 | 0–33 | [29] |
| >10 000–100 000 copies/mL | 14.8 | 0–30 | [29] |
| >3000–10 000 copies/mL | 7.6 | 0–33 | [29] |
| >500–3000 copies/mL | 3.8 | 0–16 | [29] |
| ≤500 copies/mLe | 0.3 | 0–0.6 | [29] |
Abbreviations: ART, antiretroviral therapy; PY, person-year; USD 2018, 2018 US dollars.
a Screen sensitivity and specificity were the same for both the acute and chronic phase. The duration of acute infection is 2 months in the base case (sensitivity analysis range: 0–6 months).
b Includes costs of confirmatory testing and counseling.
c Antiretroviral efficacy is defined as the rate of suppression of HIV RNA <400 copies/mL at 48 weeks.
d ART costs were based on averages for integrase-based regimens: $2290; protease inhibitor-based regimens: $2670; and salvage regimens: $3780.
e Although recent data from adults suggest 0 transmissions occurring from people with HIV with plasma HIV RNA durably suppressed to <50 copies/ml, we lack data to apply the same zero risk to adolescents and young adults, who often have less consistent virologic suppression. We therefore apply a transmission risk of 0.3/100PY to the lowest modeled RNA stratum based on the available data.
Additional details of inputs, including quality-of-life utility weights, may be found in Supplementary Table 2 [32, 33].
We projected mechanisms of HIV detection, care continuum outcomes (proportions diagnosed, linked to care, retained in care, and virologically suppressed), clinical benefits, and costs. We report incremental cost-effectiveness ratios (ICER: difference in cost divided by difference in quality-adjusted life expectancy) for each strategy compared to the next least costly alternative, from a health-care sector perspective (see Supplementary Material). Clinical outcomes and costs are reported undiscounted and discounted at 3%/year, including health and economic benefits attributable to reduced primary transmissions. We defined a strategy as “cost-effective” if its ICER fell below a willingness-to-pay threshold of $100 000/QALY [34]. We report clinical outcomes for the following 4 groups: 1) Initial cohort, excluding primary transmissions from members of this cohort to others; 2) People who acquire HIV through age 30, a subset of the Initial cohort; 3) People who acquire HIV in their lifetimes, a subset of the Initial cohort; 4) Expanded cohort: the Initial cohort and one generation of primary transmissions from members of the Initial cohort to others. In the Expanded cohort, while we modeled HIV incidence, health outcomes, and costs occurring at all ages, we include HIV transmissions arising only from those aged 15–30 years. We report cost-effectiveness outcomes for the Initial and Expanded cohorts.
Model Structure
The CEPAC model is a validated Monte Carlo, state-transition microsimulation model of HIV disease and treatment [7]. Full details of the model, including graphical depictions, are available at https://www.massgeneral.org/medicine/mpec/research/cpac-model. YMSM enter the model at age 15 without HIV and are simulated in monthly cycles through their lifetimes until death.
HIV Disease and Screening
We define HIV incidence as new infections acquired by members of the Initial cohort. People who acquire HIV are assigned user-specified characteristics, including age, CD4 count, and HIV RNA. In the absence of effective antiretroviral therapy (ART), CD4 declines monthly. Each month, modeled patients face risks of opportunistic infection and mortality, determined by current age and CD4 count. HIV diagnosis can occur via 1) SQ of HIV detection (ie, screening and testing currently occurring), 2) testing after developing an opportunistic infection, or 3) the more frequent screening strategy (Yearly/ 6-monthly/3-monthly), which is implemented from ages 15–30. Screen offer and acceptance are assumed to be conditionally independent [35] from previous and subsequent instances; test results are assumed not to impact behavior (ie, no change in condoms used after a negative screen).
Patients who link to care and are prescribed ART experience an initial modeled probability of virologic suppression, and an increase in CD4 count. Those with initial virologic suppression face monthly risks of later virologic failure. At any time (after screening or linkage to care), patients face monthly risks of loss to follow-up; those lost experience a monthly probability of returning to care or return after developing an opportunistic infection.
HIV Transmission
We define primary HIV transmission as one generation of new infections that are transmitted from members of the Initial cohort with HIV to people outside this group (the Expanded cohort). The rate at which a person transmits HIV to others is a function of plasma HIV RNA. HIV RNA levels and thus transmission rates vary by stage of infection (acute and chronic) and response to ART (see Supplementary Material).
Model Inputs
HIV Disease and Screening
Age-stratified risks of incident HIV infection were derived from ATN 110/113 and published sources (Table 1). All screening strategies used a fourth-generation HIV immunoassay (sensitivity/specificity: 99.6/99.7%) [14]. Age-stratified SQ screening rates were derived from survey studies [8, 10, 11] and calibrated to CDC data on stage of disease at diagnosis [9]; this resulted in a 0.7–10.3% annual probability of detection, varying by age (Supplementary Table 1). In the frequent screening strategies, we assigned an 80% conditionally independent, combined probability of being offered and accepting HIV screening at each opportunity [15, 16], 97% probability of receiving a result [17, 18], and 76% probability of linkage to care and ART receipt after a positive screen, based on YMSM-specific data when available [19]. For the annual, 6-monthly, and 3-monthly strategies this translates to 78%, 95%, and 99% annual probabilities of receiving a screening program test result, respectively, which are constant across age groups. Screening costs were $38.00 per screen, plus an additional $76.42 per completed reactive screen reflecting costs of confirmatory testing and counseling [20, 21]. ART costs ranged from $2290–$3780/month, depending on regimen (Table 1 footnote) [25, 26].
HIV Transmission
Transmission rates were 0.3–86.1 transmissions/100 person-years, varying by HIV RNA levels, with the highest transmission rates for those with acute infection and not yet taking ART (Table 1) [29–31]. Costs associated with averted transmissions included clinical care, laboratory monitoring, and ART (see Supplementary Material).
Sensitivity Analyses and Additional Analyses
To understand the robustness of our findings in the face of uncertainty in underlying data and assumptions, we undertook 1-way sensitivity analyses (varying single parameters through plausible ranges noted in Table 1) and multiway sensitivity analyses (varying the most influential parameters together) [36]. Additional analyses included scenarios in which: people enter the model at older ages, because a screening policy may be implemented starting at ages older than 15; 20–50% of the population proves “hard-to-reach,” refusing screening despite additional $20/screen to offer screening; cost-effectiveness outcomes are examined over a 15-year horizon (vs lifetime in the base case); HIV screening intervals are as frequent as monthly; the age distribution of transmissions averted varies; and, given that youth may attach different values to preference-based health-state utilities than adults [37, 38] we report ICERs in $/year-of-life saved (YLS).
Additional details of methods (Supplementary Tables 1 and 2) and sensitivity analyses are provided in the Supplementary Materials.
RESULTS
Clinical Outcomes for Initial Cohort
Among the Initial cohort, the median age of acquiring HIV was 22 years (IQR: 17–47); in this cohort, all frequent screening strategies increased undiscounted life expectancy compared to SQ (54.32–54.54 vs 52.06 quality-adjusted years) by 2.26–2.48 quality-adjusted years (Figure 1). Among people who acquired HIV (Supplementary Table 3), 3-monthly led to the shortest time spent with undiagnosed HIV (23.65 months, 40.48 months less than SQ), the highest CD4 count at diagnosis (515 cells/µL, an increase of 219 cells/µL over SQ), and the longest undiscounted quality-adjusted life expectancy (48.69 quality-adjusted years, an increase of 4.28 quality-adjusted years over SQ).
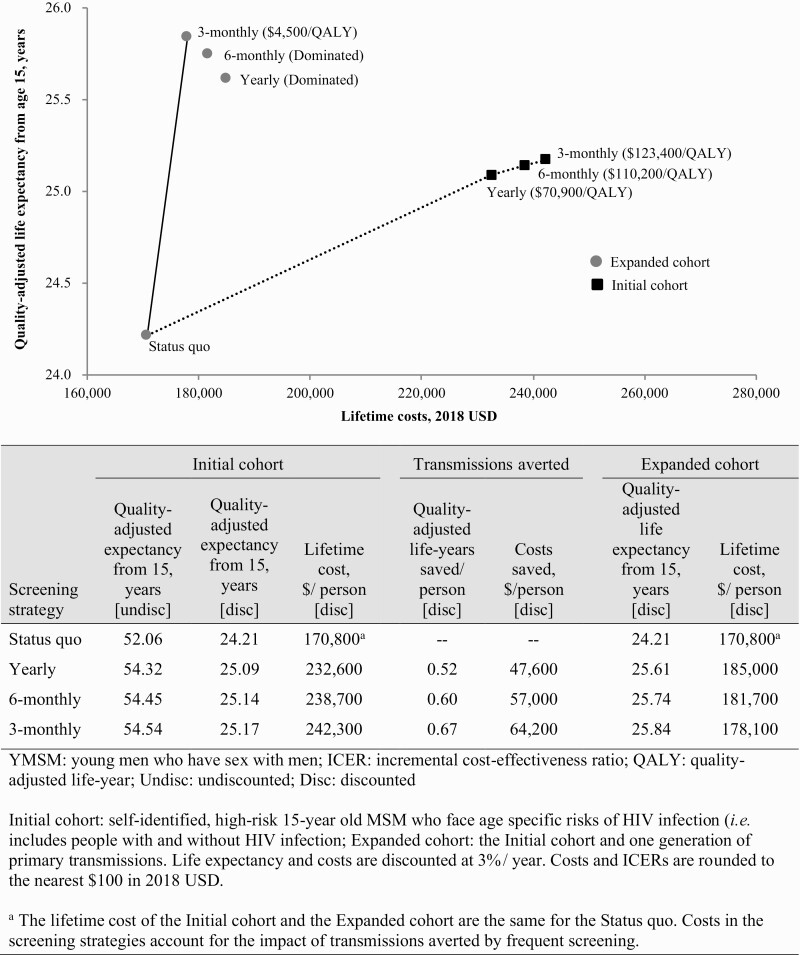
Figure 1.
Efficiency frontiers: Cost-effectiveness outcomes, Initial and Expanded cohorts. The Initial cohort consists of self-identified, high-risk 15-year-old men who have sex with men who face age-specific risks of HIV infection (ie, people with and without HIV infection). The Expanded cohort consists of the Initial cohort and one generation of primary transmissions. The vertical axis shows discounted life expectancy, and the horizontal axis shows discounted per-person lifetime cost (USD 2018). Incremental cost-effectiveness ratios (ICERs) are shown next to the strategies which lie on the efficiency frontier (in parentheses, rounded to $100). Strategies below the line represent dominated strategies, or a less efficient use of resources. Cost-effectiveness outcomes including the impact of 15 years of transmissions are shown in circles (Expanded cohort) and outcomes excluding the impact of transmissions (Initial cohort) are shown in squares. Including the impact of transmissions, the ICER of 3-monthly screening remained ≤$100 000/QALY ($4500/QALY); excluding transmissions, the ICER of Yearly screening was ≤$100 000/QALY ($70 900/QALY). Comparing 3-monthly to the Status quo, the discounted gain in quality-adjusted life-years and costs saved per person attributable to averted transmissions was 0.67 quality-adjusted years and $64 200, respectively. Additional details of methods may be found in the Supplementary Materials.
Mechanisms of HIV Detection and Care Continuum Outcomes: People Acquiring HIV over a 15-year Horizon
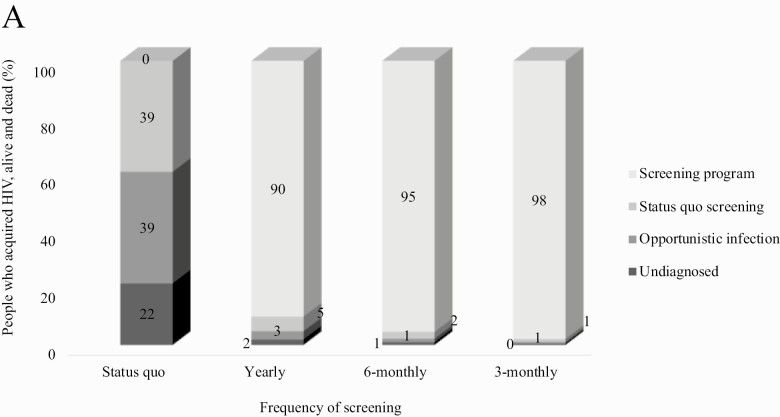
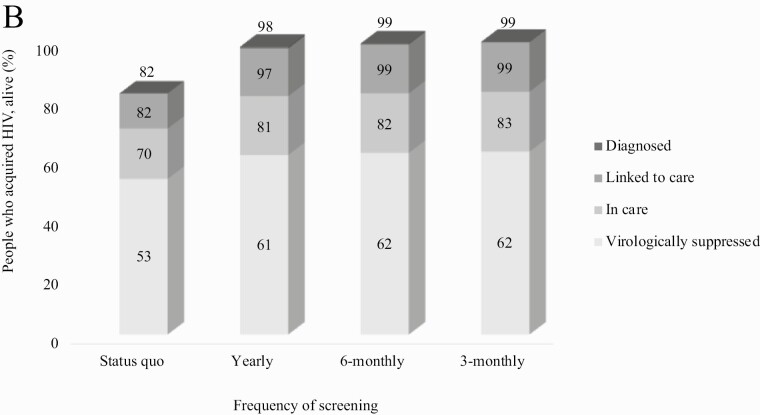
Over a 15-year horizon, compared to SQ, all more frequent screening strategies reduced the proportion of people with HIV detected via opportunistic infection (39% vs ≤3%) and the proportion not detected (22% vs ≤2%) through age 30 (Figure 2A). Frequent screening also increased the proportion diagnosed through age 30 from 82% (SQ) to 99% (3-monthly) and increased virologic suppression from 53% (SQ) to 62% (3-monthly; Figure 2B).
Figure 2A.
Mechanisms of HIV detection for people who acquire HIV through age 30. This figure includes all people in the model simulation who ever acquired HIV through age 30, regardless of the age of diagnosis. Status quo screening reflects existing patterns of HIV screening. More frequent screening strategies were performed in addition to Status quo screening. All more frequent screening strategies identified a substantial proportion of YMSM (90%–98%). 3-monthly and 6-monthly screening resulted in the lowest proportions undetected (0%–1%) and diagnosed via opportunistic infection (1%).
Figure 2B.
Cross-sectional HIV care continuum outcomes for people acquiring HIV through age 30. Among people alive with HIV through age 30, the proportion diagnosed with HIV, linked to care, retained in care, and virologically suppressed are shown, assessed cross-sectionally at age 30 + 11 months. In the Status quo, we did not estimate a proportion diagnosed but unlinked to care. 3-Monthly screening led to the most favorable care continuum outcomes at age 30 + 11 months: 99% diagnosed, and 99% linked to care, 83% retained in care, and 62% virologically suppressed.
Transmissions
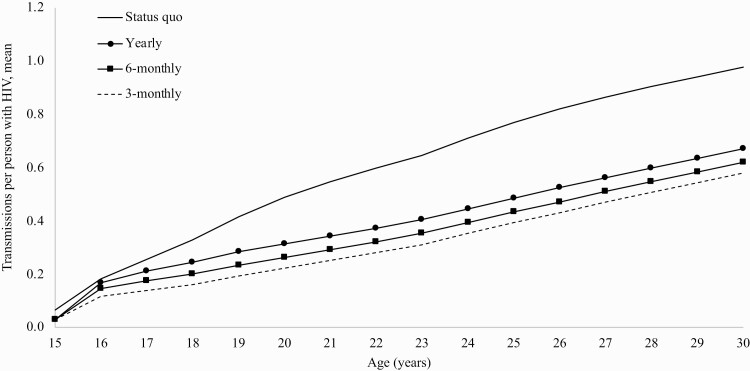
With SQ, the rate of transmissions attributable to the Initial cohort through age 30 was 2.3/100 person-years (PY), with peak transmissions from youth aged 17.9 years (2.9/100PY, Supplementary Table 4). Considering only a denominator of people with HIV over a 15-year horizon, the rate of primary transmissions was 12.6/100PY. Comparing 3-monthly to SQ, there was a 40% reduction in the mean cumulative number of primary transmissions at age 30 (0.58 vs 0.98/person with HIV; Figure 3).
Figure 3.
Mean primary transmissions per person among people who acquire HIV through age 30. Among people who acquire HIV by age 30, the graph presents the mean number of cumulative primary HIV transmissions by each year of age for each strategy. Comparing 3-monthly screening to Status quo, there was a 40% reduction (0.58 vs 0.98) in the mean number of primary transmissions at age 30. 3-Monthly screening led to the fewest projected transmissions.
Cost-effectiveness Results: Expanded Cohort
Among the Expanded cohort, SQ led to the lowest projected HIV-related healthcare costs, with lifetime discounted costs of $170 800/person (Figure 1, circles, Supplementary Table 5). Lifetime population HIV-related costs were greatest for Yearly: $185 000/person. Due to the impact of transmissions, the Yearly and 6-monthly strategies were both more costly and less effective than 3-monthly (strongly dominated; Figure 1). SQ was both less costly and less effective than any of the frequent screening strategies; the ICER for 3-monthly compared to SQ was $4500/QALY. Considering only the Initial cohort (excluding the impact of transmissions; squares), the ICER for Yearly compared to SQ was $70 900/QALY.
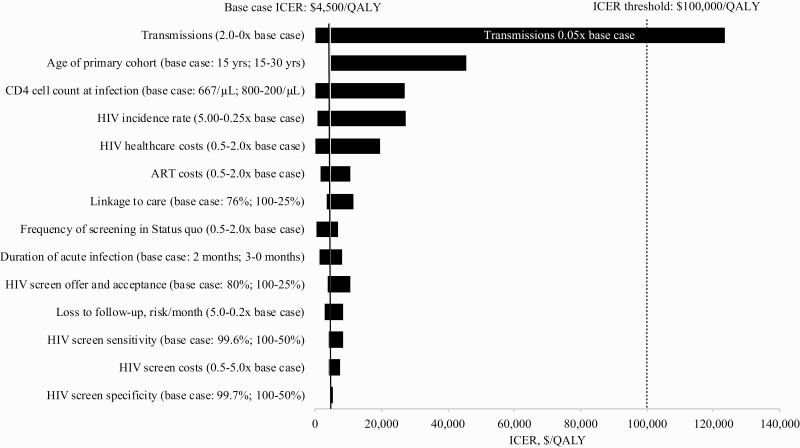
Sensitivity and Additional Analyses
In one-way sensitivity analyses, ICERs for 3-monthly versus 6-monthly remained ≤$100 000/QALY despite wide ranges in linkage to care, screen offer and acceptance, screen test characteristics, and HIV care, ART, and screen costs (Figure 4). Up to a total cost per screen of $760/screen, the ICER of 3-monthly remained ≤$100 000/QALY (Supplementary Table 6). Rates of HIV transmission had the greatest impact on the ICER; however, only if the rate of HIV transmission was <0.05 times the base case rate did the ICER of 3-monthly exceed $100 000/QALY (Figure 4). In two-way sensitivity analyses, we varied transmission rates simultaneously with other parameters: In birth cohorts older than the base case (ie, model start at ages 18–28, when HIV incidence is lower), the ICER of 3-monthly remained ≤$100 000/QALY, except at ages >22 years and with transmissions less than or equal to half the base case (Supplementary Figure 1). When transmission rates were doubled and incidence rates were one quarter the base case, 3-monthly became cost-saving (Supplementary Figure 2). If there was no increased transmission risk during acute infection above chronic HIV infection, the ICER of 3-monthly remained ≤ $100 000/QALY even if subsequent transmission rates through chronic infection were one-twentieth base case values (Supplementary Figure 3).
Figure 4.
Sensitivity analyses: Incremental cost-effectiveness ratio of 3-monthly compared to the next least costly strategy, Expanded cohort. On the vertical axis, parameters and the ranges over which they are varied are shown. Incremental cost-effectiveness ratios (ICERs) for the comparison of 3-monthly vs 6-monthly are shown on the horizontal axis, in $/quality-adjusted life year (QALY). The range of ICERs for each varied parameter is indicated by the horizontal bars. Longer horizontal bars indicate parameters to which the model results are more sensitive. The black vertical line indicates the ICER for 3-monthly compared to 6-monthly in the base case ($4500/QALY). The vertical line indicates the cost-effectiveness threshold for this analysis, $100 000/QALY, and the white text within the bar indicates parameter values at which the threshold is crossed. 3-monthly compared to 6-monthly exceeds $100 000/QALY only if transmission rates are set to ≤0.05 times their base case values. Abbreviation: ART, antiretroviral therapy.
In the “hard-to-reach” scenario analysis, even when 50% of the population refused any screen despite an additional $20/screen to offer screening, the ICER of 3-monthly remained ≤$100 000/ QALY (Supplementary Table 7). When even more frequent screening was examined, screening monthly was ≤$100 000/QALY (ICER: $2300/QALY); screening 2-, 3-, 4-, 5-, or 6-monthly was more costly and less effective than monthly screening (Supplementary Table 8). When cost-effectiveness outcomes were calculated over a 15-year horizon through age 30, the ICER of 3-monthly remained ≤ $100 000/ QALY ($11 700/QALY, Supplementary Table 9). Scenarios varying the age of people transmitted to in the Expanded cohort (Supplementary Table 10) or examining the potential impact of future, lower cost generic ART did not impact results; the ICER of 3-monthly remained ≤$100 000/QALY. Without quality-of-life utility weights, the ICER for 3-monthly versus 6-monthly was $4900/YLS (Supplementary Table 11).
DISCUSSION
Current CDC guidelines recommend annual HIV screening for MSM and acknowledge that those at higher risk of infection may benefit from more frequent HIV screening [6]. Using a mathematical simulation model, we projected the value of more frequent HIV screening strategies, added to current screening practice, in 15-year-old YMSM in the US who self-report as being at high risk for HIV infection. We had four key findings. First, 3-monthly screening markedly increased life expectancy. Among people who acquired HIV, the projected life expectancy gain from 3-monthly screening was 4.28 quality-adjusted years compared to SQ (48.69 vs 44.41 quality-adjusted years, from age 15, Supplementary Table 3). When considering the entire population of high risk YMSM (both with and without HIV), 3-monthly screening increased life expectancy by 2.48 quality-adjusted years compared to SQ (54.54 vs 52.06). Screening 3-monthly also offered the best value for money, with an ICER of $4500/QALY compared to SQ, accounting for the life expectancy gained and costs averted due to reduced onward transmissions. If primary transmissions were excluded—limiting the clinical and economic benefits only to those accrued by the Initial cohort—the ICER of 3-monthly screening rose to $123 400/QALY (Figure 1). In this scenario, Yearly screening remained ≤$100 000/QALY (ICER $70 900/QALY).
Second, this analysis highlights opportunities for improved implementation of the current annual screening recommendations. If the current CDC guidelines for annual screening could be met among YMSM, we projected important gains compared to SQ in HIV care continuum outcomes, such as the proportion diagnosed (98% versus 82%) and the proportion virologically suppressed (61% versus 53%) by age 30. Implementing HIV screening for high-risk YMSM, similar to other sexual health interventions such as HPV vaccinations, however, relies on health care providers’ accurate assessment of sexual histories and patients’ disclosure. This may be difficult in practice: among CDC-funded programs serving youth in 2015, YMSM received only 28% of HIV tests despite comprising 83% of new HIV diagnoses in the US [39].
Third, the cost-effectiveness of the evaluated HIV screening strategies depends on reported high HIV incidence among the youngest high-risk MSM. In ATN studies 110/113, HIV incidence in the youngest MSM (ages 15–17) was twice that of older MSM (ages 18–22): 6.4 vs 3.3/100PY [3, 4]. Most participants lived at home and were enrolled in school [3, 4]; although incidence rates were high in ATN 110/113, this population may not represent the highest risk group of YMSM. While current HIV incidence rates are unknown for key subgroups (including by race/ethnicity, socio-economic status, or geographic location), our HIV incidence inputs and transmission rate outputs are similar to published reports for sexual minority males [1, 2, 40], and our conclusions remained robust to wide variations in these parameters.
Fourth, the cost-effectiveness of a specific frequent screening interval depends on onward HIV transmissions. Excluding transmissions, the ICER of Yearly screening was ≤$100 000/QALY. Including transmissions, screening very frequently generated the lowest ICERS (monthly, ICER: $2300/QALY; Supplementary Table 8). Because this finding depends on assumptions about transmissibility during acute infection, for which data are limited (see Supplementary Material), as well as the costs and life expectancy associated with those infections, aligning HIV screening guidelines with current CDC PrEP guidelines—which recommend 3-monthly HIV screening—may also be favorable from both patients’ and clinical providers’ perspectives. In practice, because healthy youth may interact infrequently with traditional healthcare sites, such as scheduled clinic visits, we interpret our results to suggest that self-identified high-risk youth should be offered HIV screening at virtually any opportunity when they present to care. Even if 3-monthly screening is costly ($760/screen), investments in innovative, effective screening approaches (eg, venue-based screening or mobile units [41]) are likely to provide excellent value for money (Supplementary Table 6).
This analysis has important limitations. First, we assumed constant, conditionally independent rates of screen offer and acceptance across serial screenings; in the base case, we also assumed that a change in policy could be implemented without substantial change in per-screen cost. However, in sensitivity analyses, accounting for increased costs incurred during efforts to reach a portion of the population who refuse testing, the ICER of 3-monthly versus 6-monthly remained ≤$100 000/QALY. Second, we modeled only newly-infected YMSM; we did not account for the added benefit of detecting people with undiagnosed HIV nor those who have fallen out of HIV care at the start of a screening program; this would increase the clinical benefit of all screening programs. Third, we derived HIV incidence rates from PrEP studies, but there are several factors that might lead to higher or lower HIV incidence rates and resulting onward transmissions over time. For example, community-level improvements in prevention, screening and treatment might reduce onward HIV transmissions at later ages over time (raising the ICERs of all screening strategies; Figure 4), whereas accounting for the chain effect of averting additional generations of HIV transmissions would lower the ICERs. Our policy conclusions, however, remained robust if transmission risks among YMSM were even 0.05 times of those in the base case, or if cost-effectiveness outcomes were considered only over a 15-year time horizon. This analysis will provide a foundation for future analyses examining the incremental benefit of PrEP in addition to screening in this population.
Our findings expand to YMSM similar conclusions from simulation model-based analyses of adult high-risk MSM. Three studies found 3-monthly screening to be cost-effective (range: cost-saving to $45 000/QALY) [42–44]. A fourth study found that screening more often than annually was not cost-effective in all adult MSM [45]; our results would be expected to differ from this last study because we modeled a younger, higher-risk population as well as routine use of newer fourth generation immunoassays, and recent recommendations to start ART for all people with HIV.
Our updated findings should inform new CDC recommendations for more frequent screening in self-identified high-risk YMSM. For high-risk young men who have sex with men in the US, HIV screening every 3 months compared to less frequent screening will improve clinical outcomes and be cost-effective.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. All authors contributed substantively to this article in the following ways: study design (A. M. N., A. L. C.), data analysis (A. M. N., A. J. B., A. L. C.), interpretation of results (all authors), drafting the manuscript (A. M. N., A. J. B., A. L. C.), critical revision of the manuscript (all authors), and final approval of submitted version (all authors).
Acknowledgments. The authors gratefully acknowledge Taige Hou, who assisted with programming, and the Cost-Effectiveness of Preventing AIDS Complications (CEPAC) research team in the Medical Practice Evaluation Center at Massachusetts General Hospital for providing feedback on study design and interpretation.
Financial support. The Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) 110 and 113 studies were supported from the National Institutes of Health [U01 HD040533 and U01 HD040474 to SGH, RJL and CMW] through the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with supplemental funding from the National Institute on Drug Abuse and the National Institute of Mental Health. Additional support for this research included: Eleanor and Miles Shore Scholars in Medicine Fellowship [to AMN]; the Harvard University Center for AIDS Research [P30AI060354 to AMN]; the Eunice Kennedy Shriver National Institute for Child Health and Human Development [K08 HD094638 to AMN, R01 HD079214 to ALC]; the International Maternal Pediatric AIDS Clinical Trials Network Early Investigator Award [UM1AI068632 to AMN]; the National Institute of Allergy and Infectious Diseases [NIAID T32 AI007433 to AMN, R01 AI42006 to KAF; R37 AI093269 to RPW]; the UCLA Center for HIV Identification, Prevention, and Treatment Services (CHIIPTS) National Institute of Mental Health grant [P30MH58107 to RJL]; the UCLA Center for AIDS Research (CFAR) grant [5P30AI028697 to RJL]; and the UCLA Clinical Translational Science Institute (CTSI) grant [UL1TR001881 to RJL]; the National Institute on Drug Abuse [R01 DA015612 to ADP]; the National Institute of Mental Health [R01MH105203 to ADP]; and the Steve and Deborah Gorlin MGH Research Scholars Award [to RPW].
Potential conflicts of interest. A. M. N., A. J. B. B., S. G. H., J. H. A. F., K. A. F., R. P. W., S. C. R., P. K., A. D. P., M. C. W., C. M. W., and A. L. C. have no conflicts of interest or financial disclosures. R. J. L. has been a consultant to and received fees from Gilead, Merck, and Roche. The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Li Z, Purcell DW, Sansom SL, Hayes D, Hall HI. Vital Signs: HIV transmission along the continuum of care - United States, 2016. MMWR Morb Mortal Wkly Rep 2019; 68:267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2010–2016. HIV Surveill Suppl Rep 2019; 24. Available at: http://www.cdc.gov/. Accessed 7 February 2020. [Google Scholar]
- 3.Hosek SG, Rudy B, Landovitz R, et al. ; Adolescent Trials Network (ATN) for HIVAIDS Interventions . An HIV preexposure prophylaxis demonstration project and safety study for young MSM. JAIDS 2017; 74:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hosek SG, Landovitz RJ, Kapogiannis B, et al. . Safety and feasibility of antiretroviral preexposure prophylaxis for adolescent men who have sex with men aged 15 to 17 years in the United States. JAMA Pediatr 2017; 171:1063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neilan AM, Dunville R, Ocfemia MCB, et al. . The optimal age for screening adolescents and young adults without identified risk factors for HIV. J Adolesc Health 2018; 62:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiNenno EA, Prejean J, Irwin K, et al. . Recommendations for HIV screening of gay, bisexual, and other men who have sex with men - United States, 2017. MMWR Morb Mortal Wkly Rep 2017; 66:830–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cost-effectiveness of preventing AIDS complications model. Available at: https://www.massgeneral.org/medicine/mpec/research/cpac-model. Accessed 7 February 2020.
- 8.Phillips G 2nd, Ybarra ML, Prescott TL, Parsons JT, Mustanski B. Low rates of human immunodeficiency virus testing among adolescent gay, bisexual, and queer men. J Adolesc Health 2015; 57:407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas—2013. HIV Surveill Suppl Rep 2015; 20. Available at: https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html./ Accessed 7 February 2020. [Google Scholar]
- 10.Sumartojo E, Lyles C, Choi K, et al. ; City Study Team . Prevalence and correlates of HIV testing in a multi-site sample of young men who have sex with men. AIDS Care 2008; 20:1–14. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez T, Zlotorzynska M, Sineath C, Kahle E, Sullivan P. The annual American men’s internet survey of behaviors of men who have sex with men in the United States: 2014 Key indicators report. JMIR Public Health Surveill 2016; 2:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molina JM, Capitant C, Spire B, et al. ; ANRS IPERGAY Study Group . On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015; 373:2237–46. [DOI] [PubMed] [Google Scholar]
- 13.Robb ML, Eller LA, Kibuuka H, et al. ; RV 217 Study Team . Prospective study of acute hIV-1 infection in adults in East Africa and Thailand. N Engl J Med 2016; 374:2120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention and Association of Public Health Laboratories. Laboratory testing for the diagnosis of HIV infection: Updated recommendations. Available at: 10.15620/cdc.23447. Published 27 June 2014. Accessed 7 February 2020. [DOI]
- 15.Schechter-Perkins EM, Koppelman E, Mitchell PM, Morgan JR, Kutzen R, Drainoni ML. Characteristics of patients who accept and decline ED rapid HIV testing. Am J Emerg Med 2014; 32:1109–12. [DOI] [PubMed] [Google Scholar]
- 16.Calderon Y, Chou K, Cowan E, et al. . Analysis of HIV testing acceptance and risk factors of an adolescent cohort using emergency department-based multimedia HIV testing and counseling. Sex Transm Dis 2013; 40:624–8. [DOI] [PubMed] [Google Scholar]
- 17.Martin EG, Paltiel AD, Walensky RP, Schackman BR. Expanded HIV screening in the United States: what will it cost government discretionary and entitlement programs? A budget impact analysis. Value Health 2010; 13:893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lally MA, van den Berg JJ, Westfall AO, et al. ; Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) . HIV Continuum of Care for Youth in the United States. JAIDS 2018; 77:110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall HI, Frazier EL, Rhodes P, et al. . Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med 2013; 173:1337–44. [DOI] [PubMed] [Google Scholar]
- 20.Eggman AA, Feaster DJ, Leff JA, et al. . The cost of implementing rapid HIV testing in sexually transmitted disease clinics in the United States. Sex Transm Dis 2014; 41:545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schackman BR, Eggman AA, Leff JA, et al. . Costs of expanded rapid HIV testing in four emergency departments. Public Health Rep 2016; 131(Suppl 1):71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walmsley SL, Antela A, Clumeck N, et al. ; SINGLE Investigators . Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369:1807–18. [DOI] [PubMed] [Google Scholar]
- 23.Cahn P, Fourie J, Grinsztejn B, et al. . Week 48 analysis of once-daily vs. twice-daily darunavir/ritonavir in treatment-experienced HIV-1-infected patients. AIDS 2011; 25:929–39. [DOI] [PubMed] [Google Scholar]
- 24.Yazdanpanah Y, Fagard C, Descamps D, et al. ; ANRS 139 TRIO Trial Group . High rate of virologic suppression with raltegravir plus etravirine and darunavir/ritonavir among treatment-experienced patients infected with multidrug-resistant HIV: results of the ANRS 139 TRIO trial. Clin Infect Dis 2009; 49:1441–9. [DOI] [PubMed] [Google Scholar]
- 25.Thomson Healthcare. Average Wholesale Price: Red Book. Available at: http://sites.truvenhealth.com/redbook/index.html. Accessed 7 February 2020.
- 26.Levinson D.Medicaid drug price comparisons: average manufacturer price to published prices. Department of Health and Human Services, 2005. Available at: https://www.oig.hhs.gov/oei/reports/oei-05-05-00240.pdf. Accessed 7 February 2020. [Google Scholar]
- 27.Fleishman JA, Yehia BR, Moore RD, Korthuis PT, Gebo KA; HIV Research Network . Establishment, retention, and loss to follow-up in outpatient HIV care. JAIDS 2012; 60:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helleberg M, Engsig FN, Kronborg G, et al. . Retention in a public healthcare system with free access to treatment: a Danish nationwide HIV cohort study. AIDS 2012; 26:741–8. [DOI] [PubMed] [Google Scholar]
- 29.Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 2009; 23:1397–404. [DOI] [PubMed] [Google Scholar]
- 30.Wawer MJ, Gray RH, Sewankambo NK, et al. . Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis 2005; 191:1403–9. [DOI] [PubMed] [Google Scholar]
- 31.Kroon EDMB, Phanuphak N, Shattock AJ, et al. . Acute HIV infection detection and immediate treatment estimated to reduce transmission by 89% among men who have sex with men in Bangkok. J Int AIDS Soc 2017; 20:21708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schackman BR, Goldie SJ, Freedberg KA, Losina E, Brazier J, Weinstein MC. Comparison of health state utilities using community and patient preference weights derived from a survey of patients with HIV/AIDS. Med Decis Making 2002; 22:27–38. [DOI] [PubMed] [Google Scholar]
- 33.Paltiel AD, Scharfstein JA, Seage GR 3rd, et al. . A Monte Carlo simulation of advanced HIV disease: application to prevention of CMV infection. Med Decis Making 1998; 18:S93–105. [DOI] [PubMed] [Google Scholar]
- 34.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014; 371:796–7. [DOI] [PubMed] [Google Scholar]
- 35.Sox H, Higgins M, Owens D.. Using Bayes’ theorem to interpret a sequence of tests. Med Decis Making. 2nd ed. Chichester, West Sussex, UK: John Wiley & Sons, Ltd, 2013:84–7. [Google Scholar]
- 36.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD; ISPOR-SMDM Modeling Good Research Practices Task Force . Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–6. Value Health 2012; 15:835–42. [DOI] [PubMed] [Google Scholar]
- 37.Ratcliffe J, Chen G, Stevens K, et al. . Valuing child health utility 9D health states with young adults: insights from a time trade off study. Appl Health Econ Health Policy 2015; 13:485–92. [DOI] [PubMed] [Google Scholar]
- 38.Ratcliffe J, Huynh E, Stevens K, Brazier J, Sawyer M, Flynn T. Nothing about us without us? A comparison of adolescent and adult health-state values for the child health utility-9D using profile case best-worst scaling. Health Econ 2016; 25:486–96. [DOI] [PubMed] [Google Scholar]
- 39.Stein R, Song W, Marano M, Patel H, Rao S, Morris E. HIV testing, linkage to HIV medical care, and interviews for partner services among youths - 61 health department jurisdictions, United States, Puerto Rico, and the U.S. Virgin Islands, 2015. MMWR Morb Mortal Wkly Rep 2017; 66:629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balaji AB, An Q, Smith JC, et al. . High HIV incidence and prevalence and associated factors among adolescent sexual minority males, 3 cities, 2015. Clin Infect Dis 2017; 66:936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frye V, Wilton L, Hirshfield S, et al. ; All About Me Study Team . Preferences for HIV test characteristics among young, Black Men Who Have Sex With Men (MSM) and transgender women: Implications for consistent HIV testing. PLoS One 2018; 13:e0192936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lucas A, Armbruster B. The cost-effectiveness of expanded HIV screening in the United States. AIDS 2013; 27:795–801. [DOI] [PubMed] [Google Scholar]
- 43.Hutchinson AB, Farnham PG, Sansom SL, Yaylali E, Mermin JH. Cost-effectiveness of frequent HIV testing of high-risk populations in the United States. JAIDS 2016; 71:323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cipriano LE, Zaric GS, Holodniy M, Bendavid E, Owens DK, Brandeau ML. Cost effectiveness of screening strategies for early identification of HIV and HCV infection in injection drug users. PLoS One 2012; 7:e45176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Long EF. HIV screening via fourth-generation immunoassay or nucleic acid amplification test in the United States: a cost-effectiveness analysis. PLoS One 2011; 6:e27625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.