BACKGROUND:
The optimum timing for temporary ileostomy closure after low anterior resection is still open.
OBJECTIVE:
This trial aimed to compare early (2 wk) versus late (12 wk) stoma closure.
DESIGN:
The study included 2 parallel groups in a multicenter, randomized controlled clinical trial.
SETTINGS:
The study was conducted at 3 Swiss hospitals.
PATIENTS:
Patients undergoing low anterior resection and temporary ileostomy for cancer were included.
INTERVENTIONS:
Patients were randomly allocated to early or late stoma closure. Before closure, colonic anastomosis was examined for integrity.
MAIN OUTCOME MEASURES:
The primary efficacy outcome was the Gastrointestinal Quality of Life Index 6 weeks after resection. Secondary end points included safety (morbidity), feasibility, and quality of life 4 months after low anterior resection.
RESULTS:
The trial was stopped for safety concerns after 71 patients were randomly assigned to early closure (37 patients) or late closure (34 patients). There were comparable baseline data between the groups. No difference in quality of life occurred 6 weeks (mean Gastrointestinal Quality of Life Index: 99.8 vs 106.0; p = 0.139) and 4 months (108.6 vs 107.1; p = 0.904) after index surgery. Intraoperative tendency of oozing (visual analog scale: 35.8 vs 19.3; p = 0.011), adhesions (visual analog scale: 61.3 vs 46.2; p = 0.034), leak of colonic anastomosis (19% vs 0%; p = 0.012), leak of colonic or ileal anastomosis (24% vs 0%; p = 0.002), and reintervention (16% vs 0%; p = 0.026) were significantly higher after early closure. The concept of early closure failed in 10 patients (27% vs 0% in the late closure group (95% CI for the difference, 9.4%–44.4%)).
LIMITATIONS:
The trial was prematurely stopped because of safety issues. The aimed group size was not reached.
CONCLUSIONS:
Early stoma closure does not provide better quality of life up to 4 months after low anterior resection but is afflicted with significantly adverse feasibility and higher morbidity when compared with late closure. See Video Abstract at http://links.lww.com/DCR/B665.
CIERRE DE LA ILEOSTOMÍA TEMPORAL: 2 VERSUS 12 SEMANAS POSTERIOR A LA RESECCIÓN RECTAL POR CÁNCER: UNA ADVERTENCIA DE UN ESTUDIO MULTICÉNTRICO CONTROLADO RANDOMIZADO PROSPECTIVO
ANTECEDENTES:
El momento óptimo para el cierre temporal de la ileostomía posterior a la resección anterior baja es aun controversial.
OBJETIVO:
Este estudio tuvo como objetivo comparar el cierre del estoma temprano (2 semanas) versus tardío (12 semanas).
DISEÑO:
Estudio clínico controlado, randomizado, multicéntrico, de dos grupos paralelos.
ENTORNO CLINICO:
El estudio se llevó a cabo en 3 hospitales suizos.
PACIENTES:
Se incluyeron pacientes sometidos a resección anterior baja e ileostomía temporal por cáncer.
INTERVENCIONES:
Los pacientes fueron asignados aleatoriamente al cierre del estoma temprano o tardío. Antes del cierre, se examinó la integridad de la anastomosis colónica.
PRINCIPALES MEDIDAS DE VALORACION:
El principal resultado de eficacia fue el Índice de Calidad de Vida Gastrointestinal 6 semanas después de la resección. Los criterios secundarios incluyeron la seguridad (morbilidad), factibilidad y calidad de vida 4 meses posterior a la resección anterior baja.
RESULTADOS:
El estudio se detuvo por motivos de seguridad después de que 71 pacientes fueron asignados aleatoriamente a cierre temprano (37 pacientes) o cierre tardío (34 pacientes). Hubo datos de referencia comparables entre los grupos. No se produjeron diferencias en la calidad de vida 6 semanas (índice de calidad de vida gastrointestinal, media 99,8 vs. 106; p = 0,139) y 4 meses (108,6 vs 107,1, p = 0,904) después de la cirugía inicial. Tendencia intraoperatoria de supuración (escala analógica visual 35,8 vs 19,3, p = 0,011), adherencias (escala analógica visual 61,3 vs 46,2, p = 0,034), fuga de anastomosis colónica (19% vs 0%, p = 0,012), fuga de anastomosis colónica o ileal (24% vs 0%, p = 0,002) y reintervención (16% vs 0%, p = 0,026) fueron significativamente mayores después del cierre temprano. El concepto de cierre temprano fracasó en 10 pacientes (27% vs ninguno en el grupo de cierre tardío (intervalo de confianza del 95% para la diferencia: 9,4% a 44,4%)).
LIMITACIONES:
El estudio se detuvo prematuramente debido a problemas de seguridad. No se alcanzó el tamaño del grupo previsto.
CONCLUSIÓN:
El cierre temprano del estoma no proporciona una mejor calidad de vida hasta 4 meses posterior a una resección anterior baja, esto se ve afectado por efectos adversos significativos durante su realización y una mayor morbilidad en comparación con el cierre tardío. Consulte Video Resumen en http://links.lww.com/DCR/B665.
Keywords: Closure, Complications, Low anterior resection, Protective ileostomy, Quality of life
See Editorial on page 1303.
Creation of a diverting stoma is an established method to protect low colorectal or coloanal anastomosis (LA) after rectal cancer surgery.1 Numerous studies proved a decrease of clinically relevant anastomotic leaks and the need for interventional or surgical revisions when a protective stoma was created.1–6 Because of several advantages like being a relatively simple surgical procedure, comfortable stoma care for the patient, less stoma prolapses, and reduced risk of compromising the colonic blood supply,7,8 loop ileostomy is the preferred method by the majority compared with loop colostomy.
Regardless of the positive effect on the healing of LA, stoma presence may be a big burden for the patient. Several studies demonstrated a negative effect on the patients’ quality of life (QoL).9–12 Therefore, the concept of an early stoma closure (EC), that is, ≈2 weeks after rectal resection, has been proposed13–15 as an alternative to late stoma closure (LC) after 12 weeks.16 Several randomized controlled studies7,17,18 report promising results for the concept of EC after low anterior resection (LAR) in selected patients, whereas another study had to be prematurely terminated because of safety after EC.19 The aim of the present prospective randomized controlled multicenter trial was to evaluate this early time point of protective stoma closure after LAR with regard to patient QoL, general and intraoperative feasibility, and safety (morbidity and mortality) in comparison with LC.
PATIENTS AND METHODS
Patients undergoing LAR between November 2007 and March 2014 for rectal cancer in 3 surgical departments were eligible for participation. The study was approved by the local ethic committee (Ethikkommission beider Basel Reference No. 266/07) and registered at www.clinicaltrials.gov (Registration No. NCT02609451).
Inclusion and Exclusion Criteria
Inclusion criteria were age >18 years, planned anastomosis at ≤5 cm from the anal verge with consecutive fecal diversion via loop ileostomy, and obtained informed consent. Exclusion criteria were pregnancy, allergy to contrast agent, limited contractual capability, and abdominopelvic or severe nonsurgical complications.
Randomization and Surgical Procedure
If criteria were met 5 to 8 days after open LAR, patients were randomly assigned to the group of EC (2 wk) or LC (12 wk) in case of uneventful postoperative course. The randomization was controlled by the coordinating centre (Hospital of Liestal) and was achieved with a computerized random number table with a repetition cycle of 5. The randomization codes were kept hidden within consecutively numbered envelopes and were hence blinded to patients and investigators. Shortly before stoma closure (median = 1 d), the low anastomosis was investigated by palpation, contrast enema via stoma, and in hazardous situations by additional proctoscopy. The time point of randomization before this control of the anastomosis was chosen to avoid selection bias. According to an intention-to-treat principle, data of patients from the EC group, who presented with incomplete integrity of the anastomosis, remained assigned to the EC group. In these cases, the stoma closure was performed when the anastomosis exhibited to be healed.
Intravenous antibiotic prophylaxis was administered 30 to 60 minutes before stoma closure. All of the closure procedures were performed under general anesthesia by peristomal skin excision, mobilization of the everted ileum, and short segmental resection of each, the afferent and efferent stomal limb, before reconnection. End-to-end anastomosis was performed by a double layer running suture (PDS II (polydioxanone) Suture 5-0, Johnson & Johnson Medical Limited, Livingston, United Kingdom) or side-to-side by stapler (PROXIMATE Linear Cutter 75 mm, ETHICON Endo-Surgery (Europe) GmbH Johnson & Johnson Company, Norderstedt, Germany), depending on the surgeon’s decision. The small gap in the meso was closed in all of the patients. The fascia was closed using an absorbable running suture (PDS II loop, strength 2, Johnson & Johnson Medical Limited), and skin was closed over a subcutaneous drain using nonabsorbable monofilm sutures 3-0 or 4-0 in interrupted vertical backstitch technique.
Preoperative and Intraoperative Assessments
The patients’ preoperative QoL data were assessed using the Gastrointestinal Quality of Life Index (GQLI)20,21 and the European Organization for Research and Treatment of Cancer–Quality of Life Questionnaire–Core 30 (EORTC-QLQ-C30) questionnaire22 before LAR.
All issues of intraoperative feasibility were assessed within a standardized protocol. The blood loss, tendency of oozing, difference of the 2 bowel limb diameters and adhesions, both the epifascial (parastomal) and subfascial (intra-abdominal) were each estimated by the surgeon by means of visual analog scales. Postoperatively, the period of necessary parenteral fluid administration, first defecation, time until full oral intake, and total length of hospital stay composed of both surgeries and early surgical complications were recorded.
End Points and Follow-up
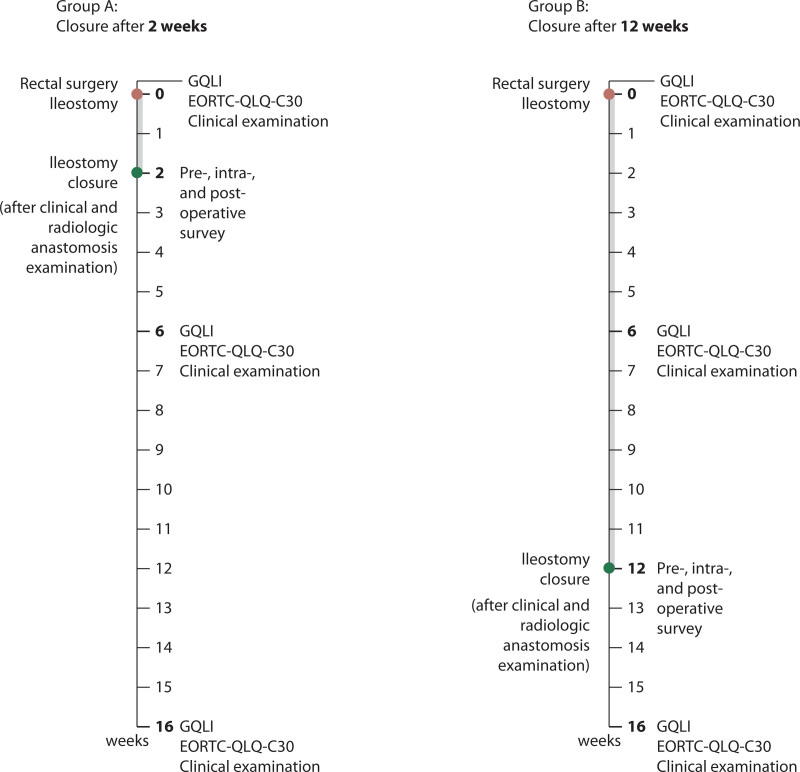
Follow-up took place 6 weeks and 4 months after LAR in the hospitals where the patient’s operation was performed. Here, QoL and late postoperative complications were assessed, and clinical examination was performed. The schedules for both groups are depicted in Figure 1.
FIGURE 1.
Schedule of surgical treatments and clinical investigations. GQLI = Gastrointestinal Quality of Life Index; EORTC-QLQ-C30: European Organization for Research and Treatment of Cancer–Quality of Life Questionnaire–Core 30.
The primary end point was QoL, assessed by the GQLI questionnaire (0–144 points), 6 weeks after LAR, that is, ≈4 weeks after stoma closure in the EC group and ≈6 weeks before stoma closure in the LC group. Secondary end points were the same QoL 6 weeks after LAR but assessed by the EORTC-QLQ-C30 questionnaire (score 0–100) and QoL 4 months after LAR, assessed by GQLI and EORTC-QLQ-C30 questionnaire; additional secondary end points were intraoperative feasibility (operation time, blood loss, tendency of oozing, parastomal and intra-abdominal adhesions, difference in diameters between the 2 stoma limbs), postoperative recovery (need of parenteral fluids, first defecation, full oral intake, length of hospital stay), morbidity and safety, and the general feasibility based on the check of the colonic anastomosis.
Biometrical and Statistical Analysis
The targeted size of the study groups was set on the goal to detect a 10-point difference in mean GQLI score with an estimated SD of 15. Aimed group size was 48 patients each to achieve a power of 0.9 and a defined α-error of 0.05. For calculation we used a 2-sided, 2-sample t test assuming equal variances of the control and the intervention group. Data are expressed through median (range). To evaluate statistical significance, Mann–Whitney U test (continuous data, eg, QoL, factors determining intraoperative feasibility) or Fisher exact test (categorical data, eg, morbidity or reoperation rate) were used. The calculation of the lower and upper limits of the 95% CI for the difference between 2 independent proportions (ie, 27% vs 0% for early vs late failure) was based on the Wilson score procedure with a correction for continuity.23 All p values were 2-sided, and p values <0.05 were considered significant. For our primary outcome, QoL using GQLI, we performed repeated-measures analyses (ANOVA). Graph Pad Prism 6.07 for Windows Software (GraphPad Software Inc, La Jolla, CA) was used for calculations.
RESULTS
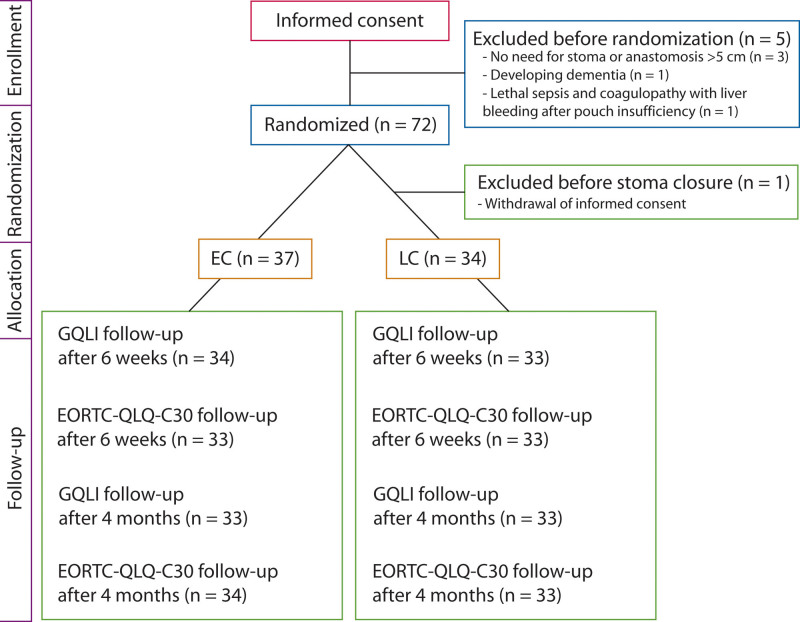
Informed consent was obtained from 77 patients, and 72 patients were randomly assigned. After secondary withdrawal of consent by 1 patient, 71 patients were available for final analysis, whereas few patients could not participate in every survey (Fig. 2). Patient characteristics are outlined in Table 1. There were no significant differences in patient and disease characteristics between the 2 groups. The median interval between LAR and stoma closure was 15 days (range, 10–134 d) in EC group and 89 days (range, 76–128 d) in the LC group. In 4 (11%) of 37 patients in the EC group, EC was not feasible, that is, it was contraindicated because of disturbed healing of the low colonic anastomosis, whereas in all patients of the LC group, stoma closure was safe at the scheduled time 12 weeks after LAR. No death occurred in either group during the study period.
FIGURE 2.
CONSORT flow diagram. EC = early closure (closure of protective ileostomy 2 wk after low anterior resection); LC = late closure (closure of protective ileostomy 12 wk after low anterior resection); GQLI = Gastrointestinal Quality of Life Index; EORTC-QLQ-C30: European Organization for Research and Treatment of Cancer–Quality of Life Questionnaire–Core 30.
TABLE 1.
Patient, disease, and treatment characteristics
| Patient baseline data | Early closure (n = 37) | Late closure (n = 34) |
|---|---|---|
| Men, n (%) | 21 (57) | 26 (76) |
| Median age (range), y | 67 (41–88) | 67 (48–87) |
| ASA classification, median (range) | 2 (1–3) | 2 (2–3) |
| 1, n (%) | 1 (3) | 0 (0) |
| 2, n (%) | 25 (68) | 24 (71) |
| 3, n (%) | 11 (29) | 10 (29) |
| 4, n (%) | 0 (0) | 0 (0) |
| Diabetes mellitus, n (%) | 5 (14) | 4 (12) |
| BMI, median (range), kg/m2 | 25.4 (18.2–33.3) | 25.2 (16.5–36.0) |
| Cortisone medication, n (%) | 1 (3) | 1 (3) |
| Previous abdominal operation(s) before LAR, n (%) | 12 (32) | 10 (29) |
| Disease characteristics | ||
| UICC postoperative stage of rectal cancer, n (%) | ||
| 0 | 4 (11) | 4 (12) |
| I | 11 (30) | 14 (41) |
| II | 9 (24) | 5 (15) |
| III | 11 (30) | 7 (20) |
| IV | 2 (5) | 4 (12) |
| Neoadjuvant therapy (chemotherapy and radiotherapy), n (%) | 13 (35) | 12 (35) |
| Adjuvant chemotherapy, n (%) | 17 (46) | 13 (38) |
| Treatment characteristics | ||
| Adjuvant chemotherapy in progress during ileostomy closure, n (%) | 0 (0) | 11 (32) |
| Time of stoma closure after primary surgery in days, median (range) | 15 (10–134) | 89 (76–128) |
| Colorectal/coloanal anastomosis | ||
| Median distance from anal verge (range), cm | 3 (1–5) | 2.75 (1–5) |
| Handsewn transanal, n (%) | 4 (11) | 3 (9) |
| Stapled, n (%) | 33 (89) | 31 (91) |
| Transverse coloplasty (Bern pouch), n (%) | 32 (86) | 27 (77) |
| Side-to-end, n (%) | 4 (11) | 5 (14) |
| End-to-end, n (%) | 1 (3) | 1 (3) |
| J-pouch, n (%) | 0 (0) | 1 (3) |
| Ileum anastomosis (stoma closure) | ||
| Stapled side-to-side anastomosis, n (%) | 2 | 0 |
| Handsewn end-to-end anastomosis, n (%) | 35 | 34 |
LAR = low anterior resection; UICC = International Union Against Cancer.
Intraoperative Technical Feasibility, Safety, and General Feasibility
Intraoperative feasibility and postoperative recovery data are given in Table 2. Postoperative minor and major complications rates (including postoperative anastomotic leakages) are given in Table 3.
TABLE 2.
Intraoperative feasibility and postoperative recovery
| Intraoperative results | EC (n = 37), median (range) | LC (n = 34), median (range) | p |
|---|---|---|---|
| Operation time, min | 130 (60–240) | 110 (60–257) | 0.197 |
| Blood loss, mL | 14 (5–150) | 9 (5 -100) | 0.780 |
| Tendency of oozing (VAS) | 28 (4–78) | 14.5 (5–60) | 0.011 |
| Epifascial (parastomal) adhesions (VAS) | 67 (3–99) | 47.5 (4–88) | 0.034 |
| Subfascial (intra-abdominal) adhesions (VAS) | 31 (0–100) | 39 (0–81) | 0.569 |
| Difference in bowel limb diameter (VAS) | 28 (0–83) | 33 (3–84) | 0.097 |
| Postoperative recovery in days | |||
| End of intravenous fluid administration | 3 (1–35) | 2 (1–25) | 0.992 |
| Time until first defecation | 2 (1–5) | 2 (0–4) | 0.190 |
| Time until full oral intake | 4 (1–26) | 4 (2–10) | 0.772 |
| Total length of hospital stays | 28 (17–77) | 27 (17–87) | 0.211 |
VAS = visual analog scale; EC = early closure; LC = late closure.
TABLE 3.
Postoperative complications after stoma closure
| Variable | Early closure (n = 37), n (%) | Late closure (n = 34), n (%) | p |
|---|---|---|---|
| Minor complications (Clavien–Dindo grade I–II) | |||
| Temporary intestinal obstruction | 4 (11) | 5 (14) | 0.73 |
| Diarrhea | 2 (5) | 2 (6) | 1.00 |
| High-output stoma with rehospitalization attributed to acute renal insufficiency | 0 (0) | 2 (6) | 0.49 |
| Wound complications | 2 (5) | 2 (6) | 0.49 |
| Urinary tract infection | 2 (5) | 0 (0) | 0.49 |
| Pneumonia | 0 (0) | 1 (3) | 0.48 |
| Venous catheter site infection | 1 (3) | 0 (0) | 0.49 |
| Pneumothorax | 1 (3) | 0 (0) | 0.49 |
| Total of minor complications | 12 (32) | 12 (35) | 1.00 |
| Major complications (Clavien–Dindo grade ≥III) | |||
| Leakage of large-bowel anastomosis after stoma closure | 3 (8) | 0 (0) | 0.240 |
| Leakage of ileal anastomosis | 2 (5) | 0 (0) | 0.494 |
| Wound infection at stoma closure site | 1 (3) | 0 (0) | 1.000 |
| Total of major complications (reoperation/reintervention) | 6 (16) | 0 (0) | 0.026 |
| Readmission | 3 (8) | 3 (9) | 1.000 |
| Overall morbidity after stoma closure | 18 (49) | 10 (29) | 0.145 |
| Stoma closure failure rate | 10a (27) | 0 (0) | 0.001 |
Data include 4 patients with a disturbed healing of the low colorectal or coloanal anastomosis in the examination before planned stoma closure and 6 patients with a major complication after stoma closure.
In total, stoma closure failed in 10 patients in the EC group (27%); as mentioned before, in 4 symptom-free patients, stoma closure was not feasible, because an anastomotic leakage has been detected before scheduled closure (radiologically in 3 cases, by palpation with an inconspicuous antegrade contrast enema in 1 case). In 1 of the symptom-free patients, fecal discharge from the intra-abdominal drainage appeared 1 day after LAR. In a surgical revision, no anastomotic leakage of the LA was detected, so the patient was not excluded and remained in the allocated group, that is, EC. However, 2 weeks later after uneventful recovery, an antegrade contrast enema showed an anastomotic leakage of the LA. Consecutively, the stoma closure was postponed.
Three patients had a leakage of the LA after stoma closure in the EC group. One patient developed a rectovaginal fistula, which was occluded 2 weeks after stoma closure. Two patients showed systemic infection signs 6 and 24 days after stoma closure. Both patients could be managed by draining a presacral abscess and antibiotics.
Two patients developed a leak of the ileum anastomosis with abscess formation at the stoma closure site, occurring 8 and 15 days after stoma closure. Both patients needed operative revision. Another patient received surgical revision attributed to subcutaneous abscess formation.
It seems that diabetes mellitus could have had an influence on the development of anastomotic leakage, because 4 of the 9 patients with diabetes developed an insufficiency (p = 0.039, risk ratio = 5.2 (95% CI, 1.375–19.42)). However, with a low prevalence of diabetes among our study groups, as well as of the outcome, a multivariable analysis within the scope of our small sample size was inauspicious. Therefore, we only have a hint of the possible influence. Other factors (age (p = 0.385), sex (p = 0.41), BMI (p = 0.488), and neoadjuvant concomitant chemoradiotherapy (p = 1.000)) did not seem to have significant influence.
The number of patients receiving neoadjuvant therapy was similar in both groups (39.4% vs 40.0%). Therefore, the higher leak rate of LA in the EC versus LC group (7 vs 0) cannot been attributed to different numbers in neoadjuvant treatment.
Quality of Life
GQLI QoL data (subdivided into the following scales: GI symptoms, emotion, physical function, social function, medical treatment) are given in Table 4. Also, repeated-measures analyses were performed to test whether change from baseline in GQLI score is different by treatment group, but there was no significance. EORTC-QLQ-C30 QoL data showed no significant difference in any of the 15 scales (global health status, functional scales, symptom scales, each with additional subdivisions) at any survey time point (data not shown).
TABLE 4.
Quality of life measured by the GQLI
| QoL measured by GQLI | Early closure (n = 37), median (range) | Late closure (n = 34), median (range) | p |
|---|---|---|---|
| Preoperatively | 109.5 (39–143) | 117.5 (69–142) | 0.258 |
| 6 weeks after LAR | 97.0 (55–130) | 108.0 (59–132) | 0.139 |
| 4 months after LAR | 106.0 (75–143) | 109.0 (75–134) | 0.904 |
QoL = quality of life; GQLI: Gastrointestinal Quality of Life (maximum score: 144 points, higher scores mean better GI health-related quality of life); LAR = low anterior resection.
DISCUSSION
This prospective, randomized controlled multicenter trial investigated the effect of 2 different closure times of a protective ileostomy after LAR on intraoperative technical and general feasibility, safety, and patient QoL. After an interim analysis attributed to cumulating cases of leakage of the ileum anastomosis and the low colorectal/coloanal anastomosis in the EC group, the study was stopped ahead of schedule, that is, before the aimed size of 48 patients per group was reached. Nevertheless, data from a total of 71 patients were gained, hence reaching a power of 0.83 concerning the primary end point. Secondary end points yielded significant results despite smaller group sizes. Because EC failed in a total of 10 patients and patients undergoing early closure did not benefit from higher QoL, a closure as early as 2 weeks after LAR is not recommended. By early randomization, selection bias was minimized to make a statement regarding general feasibility of the EC concept. Furthermore, the time point of EC differs from some of the other randomized controlled trials (RCTs), providing additional scientific value.
Intraoperative Feasibility
Technical difficulties during surgery (ie, tendency of oozing and epifascial adhesions) appeared significantly more frequently in the EC group, impairing the intraoperative technical feasibility at this early time point. Because a sufficient healing of the LA has been assumed ≈7 days after LAR,24 and intra-abdominal and parastomal adhesions are likely to start developing from the 14th postoperative day on,13 the closure time point for the EC group was chosen with regard to the highest possible safety for the LA. Moreover, the median time for detection of an anastomotic leakage was 12 days in a Swedish study, with 40% of the leakages being diagnosed between postoperative days 11 and 20, which indicates that stoma closure as early as on day 10 after LAR13 may be too early and may increase the risk for LA leakage.25,26 Because adhesions attenuate in the course of the further healing process, this issue may be of interest when alternative later closure times (eg, 8, 10, or 12 weeks) are evaluated.
Safety and General Feasibility on Schedule
Further to the impaired intraoperative feasibility, the impracticality of a scheduled stoma closure after 2 weeks in the EC group failed in a significant number of patients. This was because of incomplete integrity of the LA detected before stoma closure (4 patients), as well as anastomotic leakage of the LA after EC (3 patients). Furthermore, 3 patients in the EC group developed other major surgical complications not related to the LA (Table 3). This led to a significantly higher need for surgical or radiologic reinterventions, compromising patient recovery and safety in the EC group. This is in contrast to the findings of other comparable RCTs,7,17,27 which described similar complication and reoperation rates for both, EC and LC group, although patients from the EC group showed significantly more wound complications.7 It has to be outlined that their patients were carefully selected and were only randomized after uneventful postoperative course and after satisfactory control of the LA (by means of contrast enema, CT scan and rectoscopy). Even if an early ileostomy closure might be feasible and safe in selected patients, a benefit regarding low anterior resection syndrome (LARS)28 or QoL27 could not be shown.
A recently published randomized controlled study19 was also prematurely terminated because of a dramatically higher morbidity after early (30 d after LAR) ileostomy closure. This supports the results of the present study that an early ileostomy closure bears an unnecessary risk for the patient.
Interestingly, 3 patients with leakage of the LA after EC were asymptomatic before stoma closure and, more importantly, showed no radiologic signs of leakage in contrast enema. These findings are in accordance with Alves et al,7 who described 3 patients experiencing rectovaginal fistula formation after normal-considered contrast enemas, requiring, once more, surgical revision with loop ileostomy. Therefore, the specificity and the diagnostic validity of this examination are questioned, as other authors have already done.25,29 In a retrospective analysis, only 2 of 5 asymptomatic leakages of a LA could be detected via contrast enema before stoma closure.25 Thus, the value of contrast enema in this context is hazardous. Additional studies might require an analysis of the specificity and sensitivity of the contrast enema for detection of anastomotic pathologies, for example, by a routinized contrast enema 2 weeks after LAR and shortly before ileostomy closure.30 Furthermore, although the difference is not significant, the only 2 leakages of the ileum anastomosis appeared in the EC group, probably promoted by the poorer intraoperative feasibility in the EC group.
Risk Factors
Although almost a third of the LC group had adjuvant chemotherapy after LAR and before stoma closure, more major complications appeared; therefore, when considering a 2- to 3-week chemotherapy-free interval, ongoing chemotherapy is no contraindication for stoma closure and above all not a reason to postpone it. Neoadjuvant therapy also has no influence on major complications according to our data, because the number of patients receiving neoadjuvant therapy was similar in both groups, but major complications appeared only in the EC group. Furthermore, only 2 of the 7 patients experiencing a leaking LA in the EC group underwent neoadjuvant therapy.
Quality of Life
It could be shown that a protective ileostomy does not have an adverse effect on QoL as often stated before.10,11 In the present study, a comparable QoL (GQLI) score could be assessed at 2 survey times, that is, 6 weeks and 4 months after LAR for the EC and LC groups. These results are in accordance with the comparable prospective RCTs on this topic, showing no difference in QoL 12 months after LAR.7,27 Moreover, this conclusion is double-checked by the use of a second QoL questionnaire (EORTC-QLQ-C30) as part of the present study. The reasons for these QoL data remain speculative but definitely appear not to depend much on the presence or absence of a stoma.
CONCLUSION
Closure of ileostomy at 2 weeks does not provide better QoL 6 weeks and 4 months after low rectal resection but is afflicted with significantly adverse feasibility and higher morbidity when compared with closure at 12 weeks. Closure of ileostomy 2 weeks after rectal resection cannot be recommended.
ACKNOWLEDGMENTS
Special thanks are given to Rok Dolanc, M.D., for help in establishing the study protocol; Ueli Güller, M.D., for biometrical discussion and calculation; Annemarie Susanne Dittrich, M.D., for data managing; and the participating hospitals Liestal, Fribourg, and Rheinfelden for providing the facilities to perform the present trial.
Footnotes
Funding/Support: None reported.
Financial Disclosure: None reported.
Presented at the annual congress of the Swiss Surgical Society, Bern, Switzerland, May 20 to 22, 2015, and awarded the Felix Largiadèr prize for the best paper in visceral surgery.
REFERENCES
- 1.Ulrich AB, Seiler C, Rahbari N, Weitz J, Büchler MW. Diverting stoma after low anterior resection: more arguments in favor. Dis Colon Rectum. 2009;52:412–418. [DOI] [PubMed] [Google Scholar]
- 2.Marusch F, Koch A, Schmidt U, et al. Value of a protective stoma in low anterior resections for rectal cancer. Dis Colon Rectum. 2002;45:1164–1171. [DOI] [PubMed] [Google Scholar]
- 3.Matthiessen P, Hallböök O, Rutegård J, Simert G, Sjödahl R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg. 2007;246:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peeters KC, Tollenaar RA, Marijnen CA, et al. Dutch Colorectal Cancer Group. Risk factors for anastomotic failure after total mesorectal excision of rectal cancer. Br J Surg. 2005;92:211–216. [DOI] [PubMed] [Google Scholar]
- 5.Pérez Domínguez L, García Martínez MT, Cáceres Alvarado N, Toscano Novella A, Higuero Grosso AP, Casal Núñez JE. Morbidity and mortality of temporary diverting ileostomies in rectal cancer surgery. Cir Esp. 2014;92:604–608. [DOI] [PubMed] [Google Scholar]
- 6.Hanna MH, Vinci A, Pigazzi A. Diverting ileostomy in colorectal surgery: when is it necessary? Langenbecks Arch Surg. 2015;400:145–152. [DOI] [PubMed] [Google Scholar]
- 7.Alves A, Panis Y, Lelong B, Dousset B, Benoist S, Vicaut E. Randomized clinical trial of early versus delayed temporary stoma closure after proctectomy. Br J Surg. 2008;95:693–698. [DOI] [PubMed] [Google Scholar]
- 8.Güenaga KF, Lustosa SA, Saad SS, Saconato H, Matos D. Ileostomy or colostomy for temporary decompression of colorectal anastomosis. Cochrane Database Syst Rev. 2007;(1):CD004647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ihnát P, Guňková P, Peteja M, Vávra P, Pelikán A, Zonča P. Diverting ileostomy in laparoscopic rectal cancer surgery: high price of protection. Surg Endosc. 2016;30:4809–4816. [DOI] [PubMed] [Google Scholar]
- 10.O’Leary DP, Fide CJ, Foy C, Lucarotti ME. Quality of life after low anterior resection with total mesorectal excision and temporary loop ileostomy for rectal carcinoma. Br J Surg. 2001;88:1216–1220. [DOI] [PubMed] [Google Scholar]
- 11.Tsunoda A, Tsunoda Y, Narita K, Watanabe M, Nakao K, Kusano M. Quality of life after low anterior resection and temporary loop ileostomy. Dis Colon Rectum. 2008;51:218–222. [DOI] [PubMed] [Google Scholar]
- 12.Gooszen AW, Geelkerken RH, Hermans J, Lagaay MB, Gooszen HG. Quality of life with a temporary stoma: ileostomy vs. colostomy. Dis Colon Rectum. 2000;43:650–655. [DOI] [PubMed] [Google Scholar]
- 13.Menegaux F, Jordi-Galais P, Turrin N, Chigot JP. Closure of small bowel stomas on postoperative day 10. Eur J Surg. 2002;168:713–715. [DOI] [PubMed] [Google Scholar]
- 14.Bakx R, Busch OR, van Geldere D, Bemelman WA, Slors JF, van Lanschot JJ. Feasibility of early closure of loop ileostomies: a pilot study. Dis Colon Rectum. 2003;46:1680–1684. [DOI] [PubMed] [Google Scholar]
- 15.Hindenburg T, Rosenberg J. Closing a temporary ileostomy within two weeks. Dan Med Bull. 2010;57:A4157. [PubMed] [Google Scholar]
- 16.Thalheimer A, Bueter M, Kortuem M, Thiede A, Meyer D. Morbidity of temporary loop ileostomy in patients with colorectal cancer. Dis Colon Rectum. 2006;49:1011–1017. [DOI] [PubMed] [Google Scholar]
- 17.Lasithiotakis K, Aghahoseini A, Alexander D. Is early reversal of defunctioning ileostomy a shorter, easier and less expensive operation? World J Surg. 2016;40:1737–1740. [DOI] [PubMed] [Google Scholar]
- 18.Danielsen AK, Park J, Jansen JE, et al. Early closure of a temporary ileostomy in patients with rectal cancer: a multicenter randomized controlled trial. Ann Surg. 2017;265:284–290. [DOI] [PubMed] [Google Scholar]
- 19.Bausys A, Kuliavas J, Dulskas A, et al. Early versus standard closure of temporary ileostomy in patients with rectal cancer: a randomized controlled trial. J Surg Oncol. 2019;120:294–299. [DOI] [PubMed] [Google Scholar]
- 20.Eypasch E, Wood-Dauphinée S, Williams JI, Ure B, Neugebauer E, Troidl H. The Gastrointestinal Quality of Life Index: a clinical index for measuring patient status in gastroenterologic surgery [in German]. Chirurg. 1993;64:264–274. [PubMed] [Google Scholar]
- 21.Eypasch E, Williams JI, Wood-Dauphinee S, et al. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg. 1995;82:216–222. [DOI] [PubMed] [Google Scholar]
- 22.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 23.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17:873–890. [DOI] [PubMed] [Google Scholar]
- 24.Rolandelli R, Roslyn JJ. Surgical management and treatment of sepsis associated with gastrointestinal fistulas. Surg Clin North Am. 1996;76:1111–1122. [DOI] [PubMed] [Google Scholar]
- 25.Larsson A, Lindmark G, Syk I, Buchwald P. Water soluble contrast enema examination of the integrity of the rectal anastomosis prior to loop ileostomy reversal may be superfluous. Int J Colorectal Dis. 2015;30:381–384. [DOI] [PubMed] [Google Scholar]
- 26.Jörgren F, Johansson R, Damber L, Lindmark G. Anastomotic leakage after surgery for rectal cancer: a risk factor for local recurrence, distant metastasis and reduced cancer-specific survival? Colorectal Dis. 2011;13:272–283. [DOI] [PubMed] [Google Scholar]
- 27.Park J, Danielsen AK, Angenete E, et al. Quality of life in a randomized trial of early closure of temporary ileostomy after rectal resection for cancer (EASY trial). Br J Surg. 2018;105:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keane C, Park J, Öberg S, et al. Functional outcomes from a randomized trial of early closure of temporary ileostomy after rectal excision for cancer. Br J Surg. 2019;106:645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalady MF, Mantyh CR, Petrofski J, Ludwig KA. Routine contrast imaging of low pelvic anastomosis prior to closure of defunctioning ileostomy: is it necessary? J Gastrointest Surg. 2008;12:1227–1231. [DOI] [PubMed] [Google Scholar]
- 30.Karsten BJ, King JB, Kumar RR. Role of water-soluble enema before takedown of diverting ileostomy for low pelvic anastomosis. Am Surg. 2009;75:941–944. [PubMed] [Google Scholar]