Abstract
Quantification of hepatitis B virus (HBV) DNA and hepatitis C virus (HCV) RNA often is performed in specimens that have been frozen and thawed more than once. To ensure optimal therapeutic and prognostic value, it is important to establish whether viral load measurements are affected by repeated freeze-thaw (FT) cycles. We therefore evaluated the effect of multiple FT cycles on HBV DNA and HCV RNA quantification by testing serum specimens subjected to one (baseline), two, four, and eight FT cycles with the appropriate Chiron Quantiplex assay. Linear regression analysis showed minor increases of 1.7% per FT cycle for both HBV DNA and HCV RNA. The rise in HCV RNA levels was more pronounced among low-concentration samples, since further analysis revealed an increase of 3.2% per FT cycle among samples with 0.2 to 3.86 Meq of HCV RNA per ml. Given that the coefficient of variation for the Quantiplex assays is generally 10 to 15%, the minor increases in HBV DNA and HCV RNA levels with progressive FT cycles for the specimens tested were recognized only because analysis of variance revealed a statistically significant trend (P < 0.05). Due to the minor statistical trend, the clinical impact for individual patient specimens is likely to be limited, but it may deserve further study. In conclusion, the concentration of HBV DNA and HCV RNA in serum specimens subjected to up to eight short-term FT cycles was stable.
Assessment of the serum or plasma viral load, a measure of viral replication, has become a valuable part of the clinician’s armamentarium for managing patients with chronic hepatitis B or C (9, 13, 14). The concentration of hepatitis C virus (HCV) RNA and hepatitis B virus (HBV) DNA in serum is used both to identify patients that are more likely to respond to treatment and to monitor treatment and follow-up. In practice, serum specimens may be frozen after collection, and a single serum specimen may be thawed and refrozen numerous times for analysis. To ensure optimal prognostic and therapeutic value of viral nucleic acid measurements, it is necessary to understand the stability of HCV RNA and HBV DNA in specimens subjected to freeze-thaw (FT) conditions.
Researchers planning retrospective studies employing frozen specimens obtained from serum banks must know not only the effects of long-term frozen storage but also the impact of multiple FT cycles on the stability of viral nucleic acid in serum. For example, if multiple FT cycles affect the integrity of HCV RNA and HBV DNA in serum, then the results of retrospective studies performed on specimens subjected to multiple cycles may be compromised. On the other hand, if multiple FT cycles have no impact on HCV RNA and HBV DNA stability, there may be less need to aliquot serum when it is first collected, thereby saving valuable laboratory time and resources. Earlier studies have examined the effect of frozen storage on the integrity of HCV RNA (3, 6, 7, 15, 17) and HBV DNA (10) in serum. Some studies have included an analysis of the stability of HCV RNA in specimens subjected to multiple FT cycles (4, 6, 7, 12, 15, 17); however, these studies were limited by the small number of samples tested, the use of nonstandardized assays, the lack of a clinically relevant end point, and/or the lack of rigorous statistical analysis. No data have been published to date on the integrity of HBV DNA in serum specimens subjected to multiple FT cycles. Clearly, an accurate assessment of the impact of multiple FT cycles on HCV RNA and HBV DNA stability would be helpful for the appropriate interpretation of results from studies involving specimens subjected to multiple FT cycles.
In this study, we determined the impact of multiple FT cycles on the quantification of HCV RNA and HBV DNA in serum. The Quantiplex HCV RNA 2.0 and HBV DNA 1.0 assays (Chiron Diagnostics Corporation, Walpole, Mass.), based on branched-DNA (bDNA) technology, were chosen for this analysis because of their wide dynamic ranges (nearly 4 log10) and their ability to detect small (two- to threefold) changes in viral load. Serum specimens were chosen to cover a large portion of the dynamic range of both assays. Results were analyzed by a scattergram and linear regression by using sufficient numbers of specimens to ensure that the statistical power of our analysis could reliably detect changes in HCV RNA and HBV DNA concentrations.
MATERIALS AND METHODS
Specimens.
Sera positive for either HBV DNA or HCV RNA were obtained from routine clinical samples. All sera were separated from the clot within 4 h of collection. A total of 21 HBV DNA-positive specimens and 21 HCV RNA-positive specimens were tested. The viral nucleic acid levels in the specimens tested were consistent with the distribution normally observed in positive clinical samples. HBV DNA concentrations at baseline ranged from 3.69 to 5,100 Meq/ml (0.57 to 3.7 log10 Meq/ml), with a geometric mean of 262 Meq/ml (2.42 log10 Meq/ml). HCV RNA concentrations at baseline ranged from 0.2 to 57.41 Meq/ml (−0.70 to 1.76 log10 Meq/ml), with a geometric mean of 5.26 Meq/ml (0.72 log10 Meq/ml). All specimens had been previously frozen and thawed only once, and the first FT cycle served as the specimen baseline. Specimens were aliquoted and subjected to additional FT cycles, up to eight total. For each FT cycle, specimens were frozen at −70°C (±2°C) for a minimum of 2 h and then were thawed in a temperature-controlled water bath at 25°C (±1°C) for 1 h. After thawing, specimens were centrifuged at 14,000 rpm for 1 min in an Eppendorf Microfuge (catalog no. S4156) to collect any condensate.
HBV DNA quantification.
The Quantiplex HBV DNA 1.0 assay, based on bDNA technology, was used according to the manufacturer’s instructions. This assay has been shown to be sensitive, specific, and linear over a nearly 4 log10 quantification range (1, 8). The HBV 1.0 bDNA assay demonstrates inter- and intrarun coefficients of variation of 10 to 15% and has been shown to reproducibly detect twofold changes in HBV DNA levels (8). All specimens were tested in duplicate, and the quantity of HBV DNA in each specimen was determined from a standard curve run in parallel for each assay. Results were expressed in megaequivalents per milliliter, with 1 Meq defined as the amount of HBV DNA that generates a level of light emission equivalent to that of 106 copies of an HBV DNA standard.
HCV RNA quantification.
The Quantiplex HCV RNA 2.0 assay (Chiron Diagnostics Corporation) was used according to the manufacturer’s instructions. This assay, which is based on bDNA technology, has been shown to be highly reproducible, sensitive, specific, and linear over a nearly 4 log10 quantification range (5). The HCV 2.0 bDNA assay demonstrates inter- and intrarun coefficients of variation of 10 to 15% and can reproducibly detect approximately threefold changes in HCV RNA levels. In addition, the HCV 2.0 bDNA assay reliably measures RNA from all six major HCV genotypes (5). All specimens and controls were tested in duplicate, and the quantity of HCV RNA in each specimen was determined from a standard curve run in parallel for each assay. Results were expressed in megaequivalents per milliliter, with 1 Meq defined as the amount of HCV RNA that generates a level of light emission equivalent to that of 106 copies of an HCV RNA standard (2).
Statistical methods.
The raw data were evaluated by examining the mean levels of HBV DNA and HCV RNA after one (baseline), two, four, and eight FT cycles for each patient. All assay data were natural log transformed prior to analysis, and changes in viral nucleic acid concentration were evaluated by scattergram and linear regression analysis as described previously (10, 16). Sufficient numbers of specimens were assessed to achieve a relevant 95% confidence interval or its equivalent.
RESULTS
HBV DNA FT stability.
The stability of HBV DNA in serum was evaluated by scattergram and linear regression analysis. In addition, the number of samples showing a ≥20% change in HBV DNA levels after two, four, and eight FT cycles was determined. Results from all 21 HBV DNA-positive samples were considered together in these analyses.
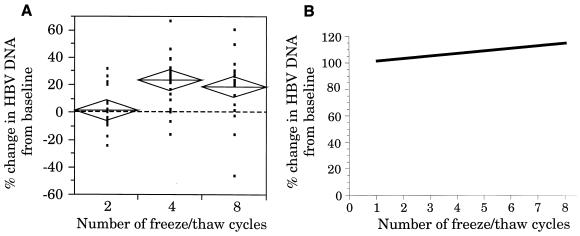
Figure 1A shows the scattergram analysis of the variations in HBV DNA levels for all specimens after two, four, and eight FT cycles compared to the baseline level. This scattergram shows that mean HBV DNA levels increased with progressive FT cycles. In addition, at least 18 of the 21 individual specimens showed a slight increase in HBV DNA concentration after four and eight FT cycles.
FIG. 1.
(A) Scattergram showing the percent change in HBV DNA levels in serum specimens exposed to two, four, and eight FT cycles compared to the baseline level. The change in HBV DNA concentration for each specimen is depicted as a data point, with the percent change from the baseline plotted on the y axis and the number of FT cycles on the x axis. The height of the diamonds overlaying the data points represents the 95% confidence interval; the diamond width represents the group sample size; and the line across each diamond represents the mean. The dotted horizontal line at 0% represents no change in HBV DNA levels and was derived from the baseline mean for each sample. (B) Linear regression analysis of the changes in HBV DNA levels in serum specimens subjected to up to eight FT cycles.
The linear regression analysis of the HBV DNA-positive specimens is shown in Fig. 1B. This analysis showed a slight increase of 1.7% in HBV DNA concentration from the baseline per FT cycle. This increase in HBV DNA concentration with progressive FT cycles was statistically significant (P < 0.05).
An evaluation of samples showing a ≥20% change in HBV DNA concentration is shown in Table 1. Whereas only 19% of the samples showed a ≥20% increase in HBV DNA concentration after two FT cycles, 66.7% of the samples showed a ≥20% increase in HBV DNA concentration after four and eight FT cycles. By comparison, less than 5% of the samples showed a ≥20% decrease in HBV DNA concentration after four and eight FT cycles.
TABLE 1.
Evaluation of samples showing a ≥20% change in viral load with progressive FT cycles
| Type of sample and no. of FT cyclesa | No. of samples (%) with:
|
|
|---|---|---|
| ≥20% increase | ≥20% decrease | |
| HBV DNA positive | ||
| 2 | 4 (19.0) | 1 (4.8) |
| 4 | 14 (66.7) | 0 (0) |
| 8 | 14 (66.7) | 1 (4.8) |
| HCV RNA positive | ||
| 2 | 4 (19.0) | 4 (19.0) |
| 4 | 3 (14.2) | 4 (19.0) |
| 8 | 5 (23.8) | 1 (4.8) |
A total of 21 HBV DNA-positive specimens and 21 HCV RNA-positive specimens were tested for each FT cycle.
HCV RNA FT stability.
A total of 21 HCV RNA-positive specimens were tested. Since a natural division was apparent in the HCV RNA-positive samples, they were divided into two groups for further statistical analysis: 10 low-concentration samples (0.2 to 3.86 Meq/ml; −0.70 to 0.59 log10 Meq/ml) and 11 high-concentration samples (9.32 to 57.41 Meq/ml; 0.97 to 1.76 log10 Meq/ml).
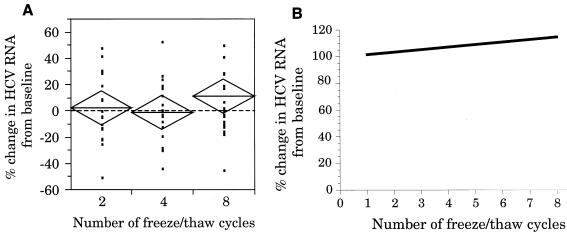
The scattergram analysis of the variations in HCV RNA levels for all specimens after two, four, and eight FT cycles, compared to the baseline level, is shown in Fig. 2A. This scattergram shows a slight increase in mean HCV RNA levels after eight FT cycles. The scattergram analysis of the low-concentration samples also showed an increase in mean HCV RNA levels after eight FT cycles (data not shown). In addition, eight of the ten low-concentration samples showed a slight increase in HCV RNA concentration after eight FT cycles. No increase in mean HCV RNA levels was observed in the scattergram of high-concentration samples (data not shown).
FIG. 2.
(A) Scattergram showing the percent change in HCV RNA levels in serum specimens exposed to two, four, and eight FT cycles compared to the baseline level. The mean diamond analysis of the data is as described in the legend to Fig. 1. (B) Linear regression analysis of the changes in HCV RNA levels in serum specimens subjected to up to eight FT cycles.
Linear regression analysis of all HCV RNA-positive specimens is shown in Fig. 2B. This analysis showed an overall 1.7% increase in HCV RNA concentration per FT cycle (P = 0.04). Additional linear regression analysis of the low- and high-concentration groups revealed a 3.2% increase per FT cycle for the low-concentration group (P = 0.0002) and a 0.02% increase for the high-concentration group (P = 0.13).
Table 1 includes the evaluation of samples showing a ≥20% change in HCV RNA concentration. This analysis shows that after two, four, and eight FT cycles, fewer than 25% of the samples showed a ≥20% increase and fewer than 20% of the samples showed a ≥20% decrease in HCV RNA concentration.
DISCUSSION
There is limited published literature on the FT stability of HBV DNA and HCV RNA. In many cases, studies were performed with insufficient numbers of specimens and/or poorly reproducible assays (6, 7, 12, 15, 17). Reliable assessment of the effects of multiple FT cycles on viral load measurements requires the following: (i) a quantitative assay capable of generating a reproducible relationship between viral load and the assay’s output signal, (ii) testing of sufficient numbers of specimens (such that changes in viral load measurements due to FT cycling can be distinguished from inter- and intra-assay variability), (iii) appropriate statistical analysis of the data, and (iv) a descriptive end point that reflects a clinically relevant change. In this study, we have applied all of these criteria in evaluating the stability of HBV DNA and HCV RNA in serum specimens subjected to multiple FT cycles—the standardized and highly reproducible Quantiplex assays were used to measure viral load; sufficient numbers of specimens were assessed to achieve a relevant 95% confidence interval or its equivalent; both scattergram and linear regression analyses were used to evaluate the data; and, consistent with our earlier studies on HBV DNA and HCV RNA stability (10, 11), we arbitrarily chose a ≥20% change in viral load as an indication of clinical relevance.
Our results showed that HBV DNA and HCV RNA concentrations were reasonably stable in specimens exposed to up to eight FT cycles. No significant loss in viral levels was observed—fewer than 5% of the HBV DNA-positive samples and fewer than 20% of the HCV RNA-positive samples showed a ≥20% decrease from the baseline values after eight FT cycles. In fact, linear regression analysis showed slight increases of 1.7% per FT cycle for both HBV DNA and HCV RNA. The rise in HCV RNA levels was more pronounced among low-concentration samples, since further analysis revealed an increase of 3.2% per FT cycle among samples with lower concentrations of HCV RNA (0.2 to 3.86 Meq/ml). Slight trends toward increasing HBV DNA and HCV RNA concentrations were also shown by scattergram analysis.
Although the coefficient of variation for the Quantiplex assays is generally 10 to 15%, the increases in HBV DNA and HCV RNA levels with progressive FT cycles for the specimens tested were statistically significant since analysis of variance yielded P values of less than 0.05. Increases in HCV RNA levels in specimens stored at low temperatures have been observed in other studies (7, 11, 15). While we have no definitive explanation for this phenomenon, the measured increases in viral load does not appear to be due to evaporative loss, since all the tubes were sealed. Given that the statistical trends observed in this study were minor, the clinical impact for individual patient specimens is likely to be limited, but it may deserve further investigation.
The finding that HBV DNA and HCV RNA levels in serum specimens subjected to repeated FT cycles were reasonably stable may have practical implications for both prospective and retrospective studies. For example, specimens tested for HBV DNA and HCV RNA need not have multiple aliquots for reliable repeat clinical testing, and results of HBV DNA or HCV RNA assays performed on specimens exposed to up to eight short-term FT cycles may be regarded as valid. While the results of this study demonstrate the effect of multiple FT cycles on viral load levels in the dynamic ranges of the Quantiplex HBV and HCV assays, it is possible that specimens with viral concentrations outside these ranges may not behave in the same manner. Also, the time intervals between FT cycles and the duration of frozen storage in this study were relatively short. It may be that HBV DNA and HCV RNA stability differs in specimens exposed to longer intervals between FT cycles and extended frozen storage.
In summary, we have evaluated the stability of HBV DNA and HCV RNA in clinical specimens subjected to up to eight FT cycles using standardized and highly reproducible bDNA assays. Our data support the conclusion that HBV DNA and HCV RNA in separated serum are stable for at least eight FT cycles.
ACKNOWLEDGMENTS
We thank Kristina Whitfield for graphics and Linda Wuestehube for writing and editorial assistance.
This study was supported by Chiron Corporation and by The Toronto Hospital and Toronto Medical Laboratories.
REFERENCES
- 1.Butterworth L-A, Prior S L, Buda P J, Faoagali J L, Cooksley G. Comparison of four methods for quantitative measurement of hepatitis B viral DNA. J Hepatol. 1996;24:686–691. doi: 10.1016/s0168-8278(96)80264-9. [DOI] [PubMed] [Google Scholar]
- 2.Collins M L, Zayati C, Detmer J J, Daly B, Kolberg J A, Cha T A, Irvine B D, Tucker J, Urdea M S. Preparation and characterization of RNA standards for use in quantitative branched DNA hybridization assays. Anal Biochem. 1995;226:120–129. doi: 10.1006/abio.1995.1199. [DOI] [PubMed] [Google Scholar]
- 3.Damen M, Sillekens P, Sjerps M, Melsert R, Frantzen I, Reesink H W, Lelie P N, Cuypers H T M. Stability of hepatitis C virus RNA during specimen handling and storage prior to NASBA amplification. J Virol Methods. 1998;72:175–184. doi: 10.1016/s0166-0934(98)00024-x. [DOI] [PubMed] [Google Scholar]
- 4.Davis G L, Lau J Y, Urdea M S, Neuwald P D, Wilber J C, Lindsay K, Perrillo R P, Albrecht J. Quantitative detection of hepatitis C virus RNA with a solid-phase signal amplification method: definition of optimal conditions for specimen collection and clinical application in interferon-treated patients. Hepatology. 1994;19:1337–1341. [PubMed] [Google Scholar]
- 5.Detmer J, Lagier R, Flynn J, Zayati C, Kolberg J, Collins M, Urdea M, Sánchez-Pescador R. Accurate quantification of hepatitis C virus (HCV) RNA from all HCV genotypes by using branched-DNA technology. J Clin Microbiol. 1996;34:901–907. doi: 10.1128/jcm.34.4.901-907.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fong T-L, Charboneau F, Valinluck B, Govindarajan S. The stability of serum hepatitis C viral RNA in various handling and storage conditions. Arch Pathol Lab Med. 1993;117:150–151. [PubMed] [Google Scholar]
- 7.Halfon P, Khiri H, Gerolami V, Bourliere M, Feryn J M, Reynier P, Gauthier A, Cartouzou G. Impact of various handling and storage conditions on quantitative detection of hepatitis C virus RNA. J Hepatol. 1996;25:307–311. doi: 10.1016/s0168-8278(96)80116-4. [DOI] [PubMed] [Google Scholar]
- 8.Hendricks D A, Stowe B S, Hoo B S, Kolberg J, Irvine B S, Neuwald P D, Urdea M S, Perillo R P. Quantitation of HBV DNA in human serum using a branched DNA (bDNA) signal amplification assay. Am J Clin Pathol. 1995;104:537–546. doi: 10.1093/ajcp/104.5.537. [DOI] [PubMed] [Google Scholar]
- 9.Hoofnagle J H, DiBisceglie A M. The treatment of chronic viral hepatitis. N Engl J Med. 1997;336:347–356. doi: 10.1056/NEJM199701303360507. [DOI] [PubMed] [Google Scholar]
- 10.Krajden M, Comanor L, Rifkin O, Grigoriew A, Minor J M, Kapke G F. Assessment of hepatitis B virus DNA stability in serum by the Chiron Quantiplex branched-DNA assay. J Clin Microbiol. 1998;36:382–386. doi: 10.1128/jcm.36.2.382-386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krajden, M., J. M. Minor, J. Zhao, O. Rifkin, and L. Comanor. Submitted for publication.
- 12.Lin H J, Shi N, Mizokami M, Hollinger F B. Polymerase chain reaction assay for hepatitis C virus RNA using a single tube for reverse transcription and serial rounds of amplification with nested primer pairs. J Med Virol. 1992;38:220–225. doi: 10.1002/jmv.1890380312. [DOI] [PubMed] [Google Scholar]
- 13.Neumann A U, Lam N P, Dahari H, Gretch D R, Wiley T E, Layden T J, Perelson A S. Hepatitis C viral dynamics in vivo and the efficiency of interferon-alpha therapy. Science. 1998;282:103–107. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- 14.Nowak M A, Bonhoeffer S, Hill A M, Boehme R, Thomas H C, McDade H. Viral dynamics in hepatitis B virus infection. Proc Natl Acad Sci USA. 1996;93:4398–4402. doi: 10.1073/pnas.93.9.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quan C M, Krajden M, Zhao J, Chan A W. High-performance liquid chromatography to assess the effect of serum storage conditions on the detection of hepatitis C virus by the polymerase chain reaction. J Virol Methods. 1992;43:299–308. doi: 10.1016/0166-0934(93)90148-k. [DOI] [PubMed] [Google Scholar]
- 16.Sall J, Lehman A. JMP start statistics. A guide to statistics and data analysis using JMP and JMP IN® software. Hockessin, Del: SAS Institute and Duxbury Press; 1993. [Google Scholar]
- 17.Wang J-T, Wang T-H, Sheu J-C, Lin S-M, Lin J-T, Chen D-S. Effects of anticoagulants and storage of blood samples on efficacy of the polymerase chain reaction assay for hepatitis C virus. J Clin Microbiol. 1992;30:750–753. doi: 10.1128/jcm.30.3.750-753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]