Abstract
Background
Integrase strand transfer inhibitors (InSTIs) are recommended for first-line treatment of persons with human immunodeficiency virus (HIV). We identified risk factors, including baseline minor InSTI resistance mutations, for treatment failure of InSTI-based regimens.
Methods
We studied time-to-treatment failure and time to viral suppression among 1419 drug-naive patients in the Swiss HIV Cohort Study. We performed Cox regression models adjusted for demographic factors, baseline HIV RNA/CD4 cell counts, AIDS-defining events, and the type of InSTI. In 646 patients with a baseline genotypic resistance test of the integrase, we studied the impact of minor integrase resistance mutations.
Results
We observed 121 virological failures during 18 447 person-years of follow-up. A baseline viral load ≥100 000 copies/mL (multivariable hazard ratio [mHR], 2.2; 95% confidence interval [CI], 1.3–3.6) and an AIDS-defining event (mHR, 1.8; 95% CI. 1.1–3.0) were associated with treatment failure. CD4 counts between 200 and 500 cells/µL (mHR, 0.5; 95% CI, .3–.8) and >500 cells/µL (mHR, 0.4; 95% CI, .2–.7) were protective. Time to suppression was shorter in lower viral load strata (mHR, 0.7; 95% CI, .6–.8) and in dolutegravir-based therapy (mHR, 1.2; 95% CI, 1.0–1.4). Minor resistance mutations were found at baseline in 104 of 646 (16%) patients with no effect on treatment outcome.
Conclusions
Factors associated with treatment failure on InSTI-based first-line regimen remained similar to those of older treatments, in particular high viral load and low CD4 counts.
Keywords: HIV, integrase strand transfer inhibitors, drug resistance, minor drug resistance mutations, treatment outcome
Integrase strand transfer inhibitor–based therapies are effective as first-line treatment of persons living with human immunodeficiency virus. Among 1419 patients, we identified a high baseline viral load, low CD4 cell counts, and an AIDS-defining event before treatment initiation as predictors for treatment failure.
Integrase strand transfer inhibitor (InSTI)–based antiretroviral therapies are recommended for first-line treatment of most individuals living with human immunodeficiency virus type 1 (HIV-1) [1]. These potent combinations achieve sustained virological suppression, and treatment failures are rare. Nonetheless, it is important to identify patients with increased risk for therapy failure as it jeopardizes the long-term treatment success and facilitates the emergence of drug resistance.
Failure of potent antiretroviral therapy is associated with several factors [2, 3]. In phase 3 trials, InSTI-based regimens were proven to be at least equally potent as or superior to other antiretroviral regimens [4–7]. The second-generation InSTIs dolutegravir (DTG) and bictegravir (BIC) have a high potency even among individuals with a high viral load or low CD4 count at baseline [5, 8–10]. Phase 3 trials showed that baseline plasma HIV-RNA did not affect DTG-based therapy; for raltegravir, the impact of baseline viral load is discussed controversially [8, 11]. Smaller clinical studies that encompassed drug-naive and treatment-experienced patients suggested that older age [12, 13], lack of adherence [14], origin from a high-prevalence country, injection drug use, and a low CD4 count at baseline [13] increased the risk for failure of InSTI-based therapy.
Another possible reason for the failure of antiretroviral treatment is the presence of pretreatment drug resistance-associated mutations (RAMs), mostly transmitted drug resistance mutations (TDRMs) [2, 15, 16]. Although large studies did not find a correlation between virological failure in drug-naive individuals on InSTIs and the presence of TDRMs [17, 18], some case reports suggest otherwise [19–21]. In European studies, <1% of drug-naive or recently infected individuals had major InSTI mutations [22–26]. However, 2%–17.3% had minor RAMs that often occurred as polymorphisms of the HIV wild type [22–26]. Although they are considered to have little effect on InSTI susceptibility, there is lack of research to which extent they affect InSTI-based treatments [27–29].
Our objective in this study was to identify risk factors for treatment failure of InSTI-based combined antiretroviral treatment (cART) in drug-naive individuals living with HIV from the Swiss HIV Cohort Study (SHCS) and to assess the impact of minor InSTI RAMs on treatment outcome.
METHODS
Study Population and Study Design
We used data from the Swiss HIV Cohorts Study (SHCS) and the SHCS drug resistance database. The SHCS is a nationwide, multicenter, longitudinal study established in 1988. The SHCS population is highly representative as it encompasses 75% of all the patients receiving antiretroviral treatment and 69% of the people with AIDS living in Switzerland. The drug resistance database includes all genotypic resistance tests (GRTs) conducted in Switzerland and is linked to the clinical database [30]. The SHCS continuously enrolls individuals aged ≥18 years and living with HIV independent of the stage and severity of the infection. Data are collected using a structured form at registration and at the semiannual visits. The ethics committees of all participating institutions have approved the SHCS, and written informed consent is obtained from all participants [30, 31].
Patient Selection
We included drug-naive individuals living with HIV from the SHCS who started an InSTI-based antiretroviral treatment between 1 January 2006 and 31 December 2018. If the HIV-1 RNA load was not measured in a patient after treatment start, that patient was excluded. To analyze pretreatment resistance patterns, we identified patients who received a baseline GRT including the integrase using the SHCS drug resistance database.
Definition of Drug Resistance Mutations
Minor and major RAMs were defined based on the International Antiviral Society - United States of America (IAS-USA) recommendations [32] and the Stanford University HIV Drug Resistance Database Version 8.9–1 [33]. The following mutations from the IAS-USA recommendations were included: major mutations: T66I, E92Q, G118R, F121Y, G140R, Y143CHR, S147G, Q148HKR, N155H, and R263K and minor mutations: T66AK, L74M, E92G, T97A, E138AKT, G140ACS, and S153FY.
The following RAMs from the HIV Drug Resistance Database with a HIVdb score ≥30 were also defined as major mutations: E92V, Y143AGK, Q146P, V151L, and N155S. Mutations with a penalty score ≥10 and <30 were in addition to the IAS-USA recommendations included as minor mutations: H51Y, L74FI, E95K, P142T, Q148N, V151I, N155D, E157Q, G163KR, S230R, and D232N.
Outcome
Our primary end points were time to viral suppression and time to virological failure. The follow-up time was defined as the period from the start of the InSTI-based regimen until the end of InSTI therapy. Data were censored at the last visit, the end of InSTI-based therapy, or at the patient’s death. Data were not censored when the patient changed from one InSTI to another or when nucleoside reverse-transcriptase inhibitor background ART was modified or adapted.
Time to viral suppression was defined as the time from treatment start to the first viral load <50 HIV-1 RNA copies/mL. Virological failure was defined as follows: 2 consecutive RNA values >50 copies/mL after at least 180 days of continuous treatment, 1 value >50 copies/mL after 180 days of treatment followed by treatment change to another drug class, or no viral suppression <50 copies/mL after more than 180 days of treatment.
Statistical Analyses
We used Stata/SE version 15.1 for statistical analyses. We performed univariable and multivariable Cox regressions to identify the effect of baseline characteristics on time to viral suppression and time to virological failure. The following factors were considered: age at therapy start, ethnicity, transmission risk group, HIV-1 RNA load, CD4 cell count, history of an AIDS-defining event at or before treatment start, the type of InSTI administered, and the presence of InSTI RAMs. Another factor included was the financial independence of the individual. Patients whose salary generated more than 50% of their income were considered more financially independent than those who predominantly relied on other sources for their income, such as unemployment benefits. In the multivariable model, factors with a P value <.1 in the univariable model and previously described risk factors for treatment outcome (age at treatment start, ethnicity, transmission risk group, the type of InSTI) were included. Continuous variables were categorized if likelihood ratio tests showed significant departure from linearity. Levels of self-reported adherence between patients who experienced virological failure and those without treatment failure were compared using the Pearson χ 2 test. Self-reported adherence is assessed every 6 months; the data closest to the treatment failure or censoring was chosen [34]. We tested the proportional hazard assumption by calculating Schönfeld residuals and by use of graphical procedures. No violations of the proportionality hazard assumption were detected. The level of significance was considered at P value <.05. To determine whether our results differed by the administered InSTI, we performed additional analyses where we stratified by the type of InSTI. Additionally, we studied the subgroup of patients with a baseline viral load >100 000 HIV-1 RNA copies/mL in detail.
RESULTS
Study Population
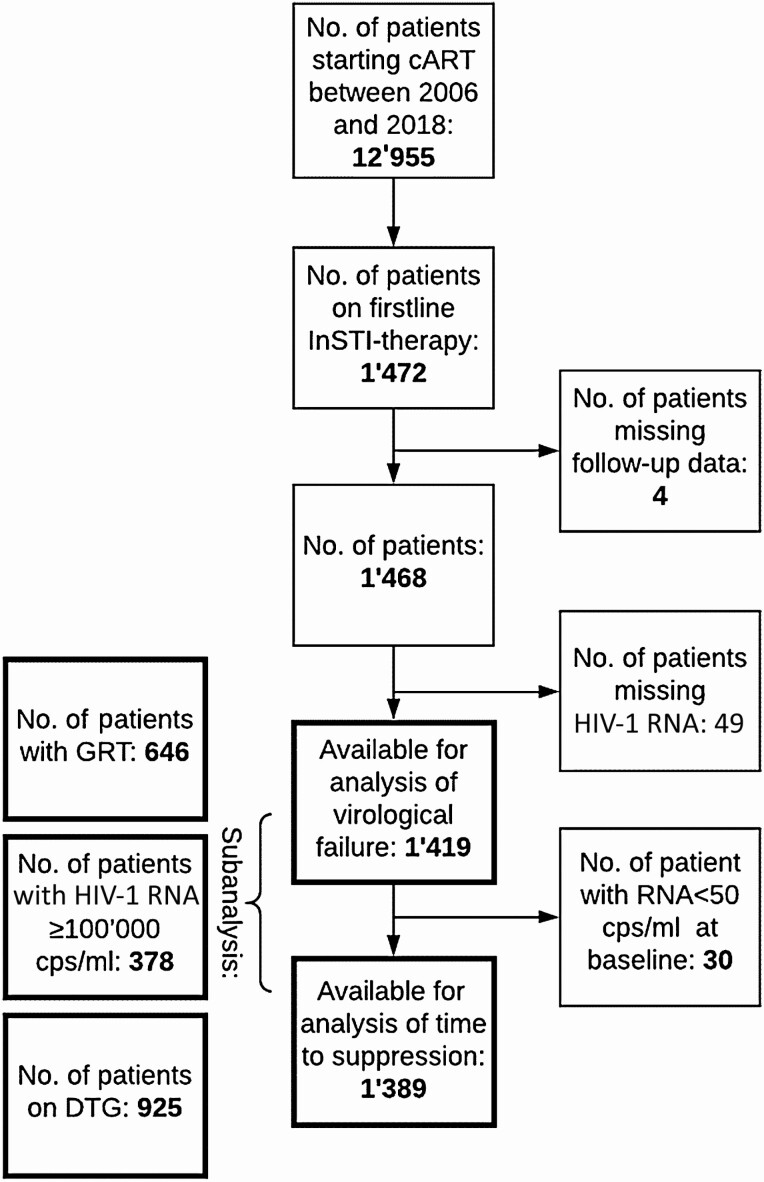
We identified 1472 drug-naive individuals living with HIV-1 who started an InSTI-based cART (Figure 1). We excluded 4 (0.3%) patients, as follow-up data were not available and 49 (3%) patients because of missing HIV-1 RNA values. Thus, 1419 of 1472 (96%) patients were included for study time to virological failure and 1389 (94%) for study time to viral suppression. The InSTI most often administered was DTG (n = 925, 65%) followed by EVG (n = 281, 20%) and RGV (n = 213, 15%). None of the participants received BIC, which was introduced in Switzerland in 2018. Table 1 shows the baseline characteristics of our study population. Of the 1419 individuals in our study, 646 (45%) had a baseline GRT including the integrase performed and 378 (27%) had a baseline viral load ≥100 000 HIV-1 RNA copies/mL.
Figure 1.
Flow diagram of study inclusion.
Abbreviations: cART, combined antiretroviral therapy; DTG, dolutegravir; GRT, genotypic resistance test; HIV, human immunodeficiency virus; InSTI, integrase strand transfer inhibitor.
Table 1.
Baseline Characteristics of the Study Population
| Baseline Characteristic | All Patients, n = 1419 | Patients With a Genotypic Resistance Test, n = 646 | No Minor InSTI Mutation, n = 542 | ≥1 Minor InSTI Mutation, n = 104 |
|---|---|---|---|---|
| Median (IQR) age at start of combined antiretroviral treatment, years | 39 (31–49) | 38 (30–49) | 38 (30–49) | 37 (31–47.5) |
| Sex (%) | ||||
| Male | 1176 (82.9) | 539 (83.4) | 457 (84.3) | 82 (78.9) |
| Female | 243 (17.1) | 107 (16.6) | 85 (15.7) | 22 (21.2) |
| Ethnicity (%) | ||||
| White | 1096 (77.2) | 508 (78.6) | 425 (78.4) | 83 (79.8) |
| Black | 168 (11.8) | 63 (9.8) | 47 (8.7) | 16 (15.4) |
| Other | 155 (10.9) | 75 (11.6) | 70 (12.9) | 5 (4.8) |
| Transmission category (%) | ||||
| Men who have sex with men | 842 (59.4) | 402 (62.2) | 335 (61.8) | 67 (64.4) |
| Heterosexual males | 241 (17.0) | 99 (15.3) | 89 (16.4) | 10 (9.6) |
| Heterosexual females | 195 (13.7) | 91 (14.1) | 71 (13.1) | 20 (19.2) |
| Intravenous drug users | 59 (4.2) | 22 (3.3) | 17 (3.1) | 4 (3.9) |
| Other | 82 (5.8)) | 33 (5.1) | 30 (5.5) | 3 (2.9) |
| Subtype (%) | ||||
| B | 364 (25.7) | 364 (56.4) | 312 (57.6) | 52 (50.0) |
| non-B | 253 (17.8) | 253 (39.2) | 204 (37.6) | 49 (47.1) |
| not available | 802 (56.5) | 29 (4.5) | 26 (4.8) | 4 (3.9) |
| HIV-1 RNA (%),copies/mL | ||||
| <10 000 | 437 (30.8) | 194 (30.0) | 161 (29.7) | 33 (31.7) |
| 10 000–99 999 | 604 (42.6) | 260 (40.3) | 218 (40.2) | 42 (40.4) |
| ≥100 000 | 378 (26.6) | 192 (29.7) | 163 (30.1) | 29 (27.9) |
| Log median (IQR) HIV-1 RNA copies/mL | 4.5 (3.5–5.1) | 4.5 (3.7–5.2) | 4.5 (3.7–5.2) | 4.5 (3.3–5.1) |
| CD4 cell count (%),cells/µL | ||||
| <200 | 281 (19.8) | 135 (20.9) | 106 (19.6) | 29 (27.9) |
| 200–500 | 724 (51.0) | 306 (47.4) | 267 (49.3) | 39 (37.5) |
| >500 | 414 (29.2) | 205 (31.7) | 169 (31.2) | 36 (34.6) |
| Median (IQR) CD4 cells/µL | 381 (226–549) | 391 (230–551) | 391 (238–552) | 386 (186–545) |
| AIDS-defining event at baseline (%) | 125 (8.8) | 43 (6.7) | 35 (6.5) | 8 (7.7) |
| InSTI administered (%) | ||||
| RGV | 213 (15.0) | 67 (10.4) | 54 (10.0) | 13 (12.5) |
| EVG | 281 (19.8) | 124 (19.2) | 108 (19.9) | 16 (15.4) |
| DTG | 925 (65.2) | 455 (70.4) | 380 (70.1) | 75 (72.1) |
| Antiretroviral treatment combinations (%) | ||||
| 3TC+ABC+DTG | 460 (32.4) | 227 (35.1) | 198 (36.5) | 29 (27.9) |
| DTG+ETC+TDF | 259 (18.3) | 150 (23.2) | 120 (22.1) | 30 (28.9) |
| DTG+ETC+TAF | 143 (10.8) | 42 (6.5) | 34 (6.3) | 8 (7.7) |
| COB+ETC+EVG+TAF | 130 (9.2) | 53 (8.2) | 47 (8.7) | 6 (5.8) |
| COB+ETC+EVG+TDF | 123 (8.7) | 59 (9.1) | 50 (9.2) | 9 (8.7) |
| ETC+RGV+TDF | 126 (8.9) | 37 (5.7) | 30 (5.5) | 7 (6.7) |
| Other drug combinations | 178 (11.8) | 78 (12.1) | 63 (11.6) | 15 (14.4) |
Abbreviations: 3TC, lamivudine; ABC, abacavir; COB, cobicistat; DTG, dolutegravir; ETC, emtricitabine; EVG, elvitegravir; HIV, human immunodeficiency virus; InSTI, integrase strand transfer inhibitor; IQR, interquartile range; RGV, raltegravir; TAF, tenofovir alafenamide; TDF, tenofovir.
Time to Virological Failure
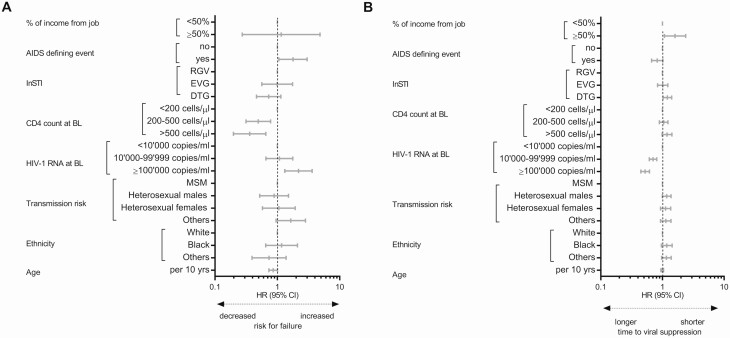
During the 18 447 person-years of follow-up, we observed 121 virological failures. Twenty-three of 121 patients had a viral load >1000 HIV-1 RNA copies/mL at the time of virological failure. Nine of 121 patients did not reach viral suppression within 180 days, and all others failed treatment after having achieved viral suppression. Figure 2 and Supplementary Table 1 summarize the results of the multivariable analysis of time to virological failure. A hazard ratio (HR) >1 implies more virological failures in the analyzed group compared with the reference group.
Figure 2.
Multivariable Cox regression. Predictors of virological failure (A) and time to viral suppression (B) among drug-naive individuals living with HIV (A: n = 1419, B: n = 1389). Abbreviations: BL, baseline; CI, confidence interval; DTG, dolutegravir; EVG, elvitegravir; HIV, human immunodeficiency virus; mHR, multivariable hazard ratio; InSTI, integrase strand transfer inhibitor; MSM, men having sex with men; RGV, raltegravir.
Among patients with treatment failure, a report of missing at least 1 dose of ART in the past month was more frequent (9 of 121 [7.4%] vs 41 of 1298 [3.6%], P exact = 0.049) than among nonfailing patients.
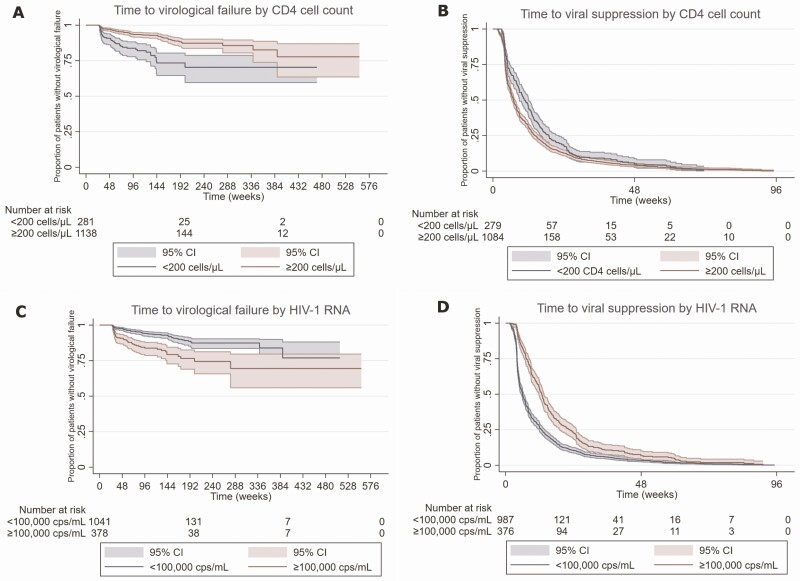
A CD4 cell count at baseline >200 cells/µL was associated with fewer failures (<200 cells/µL: reference, 200–500/µL: multivariable HR [mHR], 0.5; 95% confidence interval [CI], .3–.8 and >500/µL: mHR, 0.4; 95% CI, .2–.7; Figure 3). An HIV-1 RNA load ≥100 000 copies/mL was associated with failures (mHR, 2.2; 95% CI, 1.3–3.6) compared with a viral load <10 000 copies/mL (Figure 3). In addition, patients who experienced an AIDS-defining event had an increased chance for failure (mHR, 1.8; 95% CI, 1.1–3.0). The 2 most common AIDS-defining events were pneumocystis pneumonia and esophageal candidiasis, which occurred in 45 (3.2%) and 29 (2.0%) of 1419 patients, respectively.
Figure 3.
Kaplan-Meier curves with time to virological failure and time to suppression comparing patients by the CD4 cell count (A and B) and HIV type 1 RNA copies/mL (C and D) at baseline. Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus.
A subanalysis showed that the results were comparable when the data were censored at the change of any substance in the treatment regimen, not only at the end of InSTI-based therapy (Supplementary Table 2). The results were similar when the Cox regression analysis was restricted to patients on DTG (Supplementary Table 3). Baseline HIV-1 RNA ≥100 000 copies/mL was associated with virological failure (mHR, 2.2; 95% CI, 1.1–4.4), while a CD4 count >200 cells/µL was protective (200–500 cells/µL: mHR, 0.4; 95% CI, .2–.8 and >500 cells/µL: mHR, 0.4; 95% CI, .2–.8).
In the subanalysis that included patients with a baseline viral load ≥100 000 copies/mL (Supplementary Table 4), only the CD4 count at baseline affected treatment outcome. Patients with at least 200 CD4 cells/µL had a lower chance for failure than those with <200 cells/µL (200–500 cells/µL: mHR, 0.3; 95% CI, .2–.6 and >500 cells/µL: mHR, 0.2; 95% CI, .05–.6).
Time to Viral Suppression
Median (interquartile range) time to viral suppression was 50 days (29–107), and the median time between 2 HIV-1 RNA measurements in the first year was 10.4 weeks (8.5–13.0). Figure 2 and Supplementary Table 1 show the results of the analysis for time to viral suppression. An HR >1 implies a shorter time to viral suppression in the analyzed group compared with the reference group.
A viral load ≥10 000 copies/mL at baseline was associated with longer time to suppression compared with a viral load <10 000 copies/mL (10 000–99 999 copies/mL: mHR, 0.7; 95% CI, .6–.8 and ≥100 000 copies/mL: mHR, 0.5; 95% CI, .4–.6; Figure 3). Patients on a first-line therapy with DTG (mHR, 1.2; 95% CI, 1.0–1.4) and financially independent patients had a shorter time to viral suppression (mHR, 1.6; 95% CI, 1.1–2.4).
Among patients with an HIV-1 RNA load ≥100 000 copies/mL at baseline, time to viral suppression was shorter with a baseline CD4 count >500 copies/µL (mHR, 1.5; 95% CI, 1.0–2.2). Time to suppression was also shorter under a first-line therapy with DTG (mHR, 1.7; 95% CI, 1.2–2.3) than under therapy with other InSTIs.
In the subanalysis that included only patients on DTG, time to viral suppression was increased in individuals with a viral load ≥10 000 copies/mL (10 000–99 999 copies/mL: mHR, 0.8; 95% CI, .7–.9 and ≥100 000 copies/mL: mHR, 0.6; 95% CI, .4–.7) and was decreased in financially independent patients (mHR, 1.7; 95% CI, 1.1–2.6).
Across the analyses, other demographic factors and the mode of transmission were not significantly associated with the virologic outcome.
Impact of InSTI Resistance Associated With Minor Mutations at Baseline
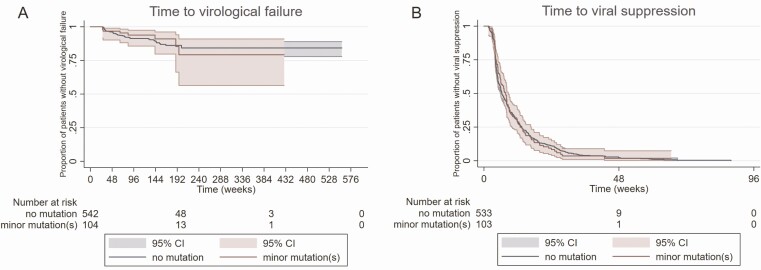
Among 646 patients with a pretreatment GRT, no one had major mutations. We detected minor mutations in 104 (16%) patients. The most common mutations were L74I (n = 65, 8.6%), V151I (n = 14, 1.9%), and E157Q (n = 14, 1.6%). All other RAMs were present in <1.6% of the cases (see Supplementary Table 5). The highest prevalence of L74I was found among subtype A (14 of 24 patients, 41.2%) and subtype G (5 of 12 patients, 41.7%) infections. L74I occurred among 30 of 364 (8.2%) of subtype B infections. We did not observe an effect of the presence of minor InSTI RAMs on both therapeutic outcomes studied (time to failure: mHR, 0.9; 95% CI, .4–1.9 and time to suppression: mHR, 1.0; 95% CI, .8–1.2; Figure 4). Most of the other risk factors found to correlate with the outcome in the primary analysis affected the therapeutic outcome in the subgroup (Supplementary Table 6).
Figure 4.
Kaplan-Meier curves with time to virological failure and time to suppression comparing patients with and without minor integrase strand transfer inhibitor resistance-associated mutations. Abbreviations: CI, confidence interval.
DISCUSSION
To our knowledge, this is the first observational study to analyze the risk factors for failing InSTI-based therapy in drug-naive individuals living with HIV-1, including minor integrase RAMs.
In general, response to InSTI-based first-line treatment of drug-naive patients was excellent. Nevertheless, a high viral load and/or a low CD4 count at baseline were associated with more treatment failures and shorter time to suppression. Among patients who presented with a baseline viral load ≥10 000 copies/mL, DTG therapy showed a superior activity in decreasing the time to viral suppression than other InSTIs studied. The superiority of DTG over first-generation InSTIs and other antiretroviral drugs in the treatment of drug-naive patients with a high viral load has been shown in various randomized, controlled studies [4–7]. However, contrary to the findings in those trials, high viral load/low CD4 count at baseline also jeopardized treatment success among participants on DTG in our study. These findings are in line with the New Antiretroviral and Monitoring Strategies in HIV-Infected Adults in Low-Income Countries (NAMSAL) and ADVANCE trials, which found evidence that treatment success while on DTG is impaired among patients with a baseline viral >100 000 copies/mL [35, 36]. Transmitted and acquired nonnucleoside reverse-transcriptase inhibitor drug resistances are important drivers to change to DTG in resource-limited settings [37]. DTG-based regimens are highly potent and cost-effective treatment options, although weight gain, in particular, in women of African origin under DTG even more aggravated with TAF-based regimens was described [32, 36]. Nevertheless, taken together in resource-limited settings where frequent RNA monitoring is difficult, a first-line therapy with DTG might be safer and more reliable in patients who present with high baseline viral loads.
The presence of minor InSTI RAMs at baseline was not associated with worse outcome. Many of the minor RAMs we detected were present as polymorphisms even before InSTIs were introduced into the clinical routine in Europe [38]. L74I and V151I are polymorphic mutations. L74I was most common among subtype A and G infections [39]. E157Q is a common polymorphic mutation. Other large, randomized, controlled trials also found that InSTIs are effective among patients who carry E157Q mutant viruses [35]. All the other mutations we found, including T97A, are known to decrease InSTI susceptibility in combination with other mutations [40], which were not present in our patients. Hence, although pretreatment of minor InSTI RAMs is common among drug-naive individuals living with HIV-1 in Switzerland, it is reassuring that their presence does not affect treatment outcome.
Across all analyses, time to viral suppression was shorter if patients were financially independent. There was a trend that suggests that older age at treatment start also decreased the risk for failure and the time to suppression. These findings might be explained by better adherence in patients with more favorable social conditions and in older patients [41]. In the absence of RAMs, nonadherence to therapy has been shown to be the most common reason for treatment failure [3]. The proportion of patients who reported decreased adherence in our study was also significantly higher in the group that experienced failure. These results show that disparities that arise from demographic and economic factors in conjunction with presumably lower adherence remain relevant even in a cohort that is subject to regular follow-up, is based in a high-income country with universal healthcare access, and for participants being treated with the most potent drug classes.
Although the SHCS is highly representative and a considerable number of drug-naive participants had an integrase resistance test available, the number of treatment failures was small, which may impair the study’s statistical power. We used a cutoff of 50 copies of RNA/mL to define a virological failure; the number of events was too small for multivariable analyses when we chose a cutoff of 200 or 500 RNA copies/mL. Furthermore, we had predominantly male White participants, which limited the generalization of these findings to a more diverse group.
CONCLUSIONS
Many of the risk factors commonly associated with therapeutic failure such as the severity of immunodeficiency, stage of the disease, and financial situation, were still relevant despite the potency of InSTIs. The chance for virological failure was consistently associated with the baseline viral load and the CD4 count, even in patients on DTG.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Members of the Swiss HIV Cohort Study: Aebi-Popp K, Anagnostopoulos A, Battegay M, Bernasconi E, Böni J, Braun DL, Bucher HC, Calmy A, Cavassini M, Ciuffi A, Dollenmaier G, Egger M, Elzi L, Fehr J, Fellay J, Furrer H, Fux CA, Gònthard HF (president of the Swiss HIV Cohort Study [SHCS]), Haerry D (deputy of the “Positive Council”), Hasse B, Hirsch HH, Hoffmann M, Hösli I, Huber M, Kahlert CR (chair of the Mother & Child Substudy), Kaiser L, Keiser O, Klimkait T, Kouyos RD, Kovari H, Ledergerber B, Martinetti G, Martinez de Tejada B, Marzolini C, Metzner KJ, Mòller N, Nicca D, Paioni P, Pantaleo G, Perreau M, Rauch A (chair of the Scientific Board), Rudin C, Scherrer AU (head of Data Center), Schmid P, Speck R, Stöckle M (chair of the Clinical and Laboratory Committee), Tarr P, Trkola A, Vernazza P, Wandeler G, Weber R, Yerly S.
Author contributions. A. P., A. U. S., and H. F. G. conceived and designed the study. A. P. and A. U. S. performed the analysis. A. U. S., S. Y., M. P., M. S., H. F., A. C., M. C., E. B., and H. F. G. collected and contributed data. A. P., A. U. S., and H. F. G. wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgments. The authors thank the patients who participate in the SHCS; the physicians and study nurses for excellent patient care; the resistance laboratories for high-quality genotyping drug resistance testing; SmartGene (Zug, Switzerland) for technical support; Alexandra Scherrer, Susanne Wild, and Anna Traytel from the SHCS data center for data management; and Danièle Perraudin and Mirjam Minichiello for administration.
Financial support. This study was financed within the framework of the SHCS, supported by the Swiss National Science Foundation (grant 177499, grant 179 571 to H. F. G.), by the SHCS Research Foundation, and by the Yvonne Jacob Foundation (to H. F. G.). The data are gathered by the 5 Swiss university hospitals, 2 Cantonal hospitals, 15 affiliated hospitals, and 36 private physicians (listed in http://www.shcs.ch/180-health-care-providers).
Potential conflicts of interest. H. F. G. has received unrestricted research grants from Gilead Sciences and Roche; fees for data and safety monitoring board membership from Merck; consulting/advisory board membership fees from Gilead Sciences, Merck, and ViiV Healthcare; and grants from SystemsX and the National Institutes of Health. The institution of H. F. has received educational grants form Gilead Sciences, ViiV, MSD, Janssen, AbbVie, and Sandoz. The institution of M. C. reports research grants from Gilead and ViiV outside the submitted work. A. C. reports unrestricted education grants from ViiV, MSD, AbbVie, Gilead, and BMS outside the submitted work and research grants from MSD outside the submitted work. E. B. reports fees to his institution for their participation to advisory boards and travel grants from Gilead Sciences, MSD, ViiV Healthcare, Pfizer, AbbVie, and Sandoz outside the submitted work. M. S. reports grants from MSD and Gilead and fees paid to his institution for advisory board participation to AbbVie, MSD, Gilead, Mepha, Sandoz, and ViiV Healthcare. All remaining authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Swiss HIV Cohort Study:
K Aebi-Popp, A Anagnostopoulos, M Battegay, E Bernasconi, J Böni, D L Braun, H C Bucher, A Calmy, M Cavassini, A Ciuffi, G Dollenmaier, M Egger, L Elzi, J Fehr, J Fellay, H Furrer, C A Fux, H F Günthard, D Haerry, B Hasse, H H Hirsch, M Hoffmann, I Hösli, M Huber, C R Kahlert, L Kaiser, O Keiser, T Klimkait, R D Kouyos, H Kovari, B Ledergerber, G Martinetti, B Martinez de Tejada, C Marzolini, K J Metzner, N Müller, D Nicca, P Paioni, G Pantaleo, M Perreau, A Rauch, C Rudin, A U Scherrer, P Schmid, R Speck, M Stöckle, P Tarr, A Trkola, P Vernazza, G Wandeler, R Weber, and S Yerly
References
- 1.Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society–USA Panel. JAMA 2018; 320:379–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wittkop L, Günthard HF, de Wolf F, et al. ; EuroCoord-CHAIN Study Group . Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): a European multicohort study. Lancet Infect Dis 2011; 11:363–71. [DOI] [PubMed] [Google Scholar]
- 3.McCluskey SM, Siedner MJ, Marconi VC. Management of virologic failure and HIV drug resistance. Infect Dis Clin North Am 2019; 33:707–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Abbraccio M, Busto A, De Marco M, Figoni M, Maddaloni A, Abrescia N. Efficacy and tolerability of integrase inhibitors in antiretroviral-naive patients. AIDS Rev 2015; 17:171–85. [PubMed] [Google Scholar]
- 5.Clotet B, Feinberg J, van Lunzen J, et al. ; ING114915 Study Team . Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 2014; 383:2222–31. [DOI] [PubMed] [Google Scholar]
- 6.Orrell C, Hagins DP, Belonosova E, et al. ; ARIA Study Team . Fixed-dose combination dolutegravir, abacavir, and lamivudine versus ritonavir-boosted atazanavir plus tenofovir disoproxil fumarate and emtricitabine in previously untreated women with HIV-1 infection (ARIA): week 48 results from a randomised, open-label, non-inferiority, phase 3b study. Lancet HIV 2017; 4:e536–46. [DOI] [PubMed] [Google Scholar]
- 7.van Lunzen J, Maggiolo F, Arribas JR, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect Dis 2012; 12:111–8. [DOI] [PubMed] [Google Scholar]
- 8.Raffi F, Rachlis A, Stellbrink HJ, et al. , SPRING-2 Study Group . Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet 2013; 381:735–43. [DOI] [PubMed] [Google Scholar]
- 9.Sax PE, DeJesus E, Mills A, et al. ; GS-US-236-0102 study team . Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet 2012; 379:2439–48. [DOI] [PubMed] [Google Scholar]
- 10.Walmsley SL, Antela A, Clumeck N, et al. ; SINGLE Investigators . Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369:1807–18. [DOI] [PubMed] [Google Scholar]
- 11.Lennox JL, DeJesus E, Lazzarin A, et al. ; STARTMRK Investigators . Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet 2009; 374:796–806. [DOI] [PubMed] [Google Scholar]
- 12.Mata-Marín JA, Smeke AE, Rodriguez MR, et al. Effectiveness and risk factors for virological outcome of raltegravir-based therapy for treatment-experienced HIV-infected patients. Drugs R D 2017; 17:225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leierer G, Rieger A, Steuer A, et al. Difference in factors associated with low-level viraemia and virological failure: results from the Austrian HIV Cohort Study. J Int AIDS Soc 2014; 17:19667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gras G, Schneider MP, Cavassini M, et al. Patterns of adherence to raltegravir-based regimens and the risk of virological failure among HIV-infected patients: the RALTECAPS Cohort Study. J Acquir Immune Defic Syndr 2012; 61:265–9. [DOI] [PubMed] [Google Scholar]
- 15.Bansi L, Geretti AM, Dunn D, et al. ; UK Collaborative Group on HIV Drug Resistance and UK Collaborative HIV Cohort Study . Impact of transmitted drug-resistance on treatment selection and outcome of first-line highly active antiretroviral therapy (HAART). J Acquir Immune Defic Syndr 2010; 53:633–9. [PubMed] [Google Scholar]
- 16.Chaix ML, Desquilbet L, Descamps D, et al. ; French PRIMO Cohort Study Group (ANRS CO 06); French ANRS AC11 Resistance Study Group . Response to HAART in French patients with resistant HIV-1 treated at primary infection: ANRS Resistance Network. Antivir Ther 2007; 12:1305–10. [PubMed] [Google Scholar]
- 17.Abram ME, Ram RR, Margot NA, et al. Lack of impact of pre-existing T97A HIV-1 integrase mutation on integrase strand transfer inhibitor resistance and treatment outcome. PLoS One 2017; 12:e0172206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulkarni R, Hodder SL, Cao H, Chang S, Miller MD, White KL. Week 48 resistance analysis of elvitegravir/cobicistat/emtricitabine/tenofovir DF versus atazanavir + ritonavir + emtricitabine/tenofovir DF in HIV-1 infected women (WAVES Study GS-US-236-0128). HIV Clin Trials 2017; 18:164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young B, Fransen S, Greenberg KS, et al. Transmission of integrase strand-transfer inhibitor multidrug-resistant HIV-1: case report and response to raltegravir-containing antiretroviral therapy. Antivir Ther 2011; 16:253–6. [DOI] [PubMed] [Google Scholar]
- 20.Boyd SD, Maldarelli F, Sereti I, et al. Transmitted raltegravir resistance in an HIV-1 CRF_AG-infected patient. Antivir Ther 2011; 16:257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGee KS, Okeke NL, Hurt CB, McKellar MS. Canary in the coal mine? Transmitted mutations conferring resistance to all integrase strand transfer inhibitors in a treatment-naive patient. Open Forum Infect Dis 2018; 5:ofy294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ambrosioni J, Nicolás D, Manzardo C, et al. Integrase strand-transfer inhibitor polymorphic and accessory resistance substitutions in patients with acute/recent HIV infection. J Antimicrob Chemother 2017; 72:205–9. [DOI] [PubMed] [Google Scholar]
- 23.Zoufaly A, Kraft C, Schmidbauer C, Puchhammer-Stoeckl E. Prevalence of integrase inhibitor resistance mutations in Austrian patients recently diagnosed with HIV from 2008 to 2013. Infection 2017; 45:165–70. [DOI] [PubMed] [Google Scholar]
- 24.Tostevin A, White E, Dunn D, et al. ; UK HIV Drug Resistance Database . Recent trends and patterns in HIV-1 transmitted drug resistance in the United Kingdom. HIV Med 2017; 18:204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scherrer AU, Yang WL, Kouyos RD, et al. ; Swiss HIV Cohort Study . Successful prevention of transmission of integrase resistance in the Swiss HIV Cohort Study. J Infect Dis 2016; 214:399–402. [DOI] [PubMed] [Google Scholar]
- 26.Gunthard HF, Calvez V, Paredes R, et al. Human immunodeficiency virus drug resistance: 2018 recommendations of the International Antiviral Society–USA Panel. Clin Infect Dis 2019; 68:177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhee SY, Grant PM, Tzou PL, et al. A systematic review of the genetic mechanisms of dolutegravir resistance. J Antimicrob Chemother 2019; 74:3135–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charpentier C, Malet I, Andre-Garnier E, et al. Phenotypic analysis of HIV-1 E157Q integrase polymorphism and impact on virological outcome in patients initiating an integrase inhibitor-based regimen. J Antimicrob Chemother 2018; 73:1039–44. [DOI] [PubMed] [Google Scholar]
- 29.Charpentier C, Descamps D. Resistance to HIV integrase inhibitors: about R263K and E157Q mutations. Viruses 2018; 10:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoeni-Affolter F, Ledergerber B, Rickenbach M, et al. ; Swiss HIV Cohort Study . Cohort profile: the Swiss HIV Cohort Study. Int J Epidemiol 2010; 39:1179–89. [DOI] [PubMed] [Google Scholar]
- 31.Abela IA, Scherrer AU, Böni J, et al. ; Swiss HIV Cohort Study . Emergence of drug resistance in the Swiss HIV Cohort Study under potent antiretroviral therapy is observed in socially disadvantaged patients. Clin Infect Dis 2020; 70:297–303. [DOI] [PubMed] [Google Scholar]
- 32.Phillips AN, Bansi-Matharu L, Venter F, et al. Updated assessment of risks and benefits of dolutegravir versus efavirenz in new antiretroviral treatment initiators in sub-Saharan Africa: modelling to inform treatment guidelines. Lancet HIV 2020; 7:e193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhee SY, Gonzales MJ, Kantor R, Betts BJ, Ravela J, and Shafer RW. Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucleic Acids Res 2003; 31:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glass TR, Sterne JA, Schneider MP, et al. ; Swiss HIV Cohort Study . Self-reported nonadherence to antiretroviral therapy as a predictor of viral failure and mortality. AIDS 2015; 29:2195–200. [DOI] [PubMed] [Google Scholar]
- 35.Kouanfack C, Mpoudi-Etame M, Omgba Bassega P, et al. ; NAMSAL ANRS 12313 Study Group . Dolutegravir-based or low-dose efavirenz-based regimen for the treatment of HIV-1. N Engl J Med 2019; 381:816–26. [DOI] [PubMed] [Google Scholar]
- 36.Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019; 381:803–15. [DOI] [PubMed] [Google Scholar]
- 37.Kumlien J, Grönberg G, Nilsson B, Månsson O, Zopf D, Lundblad A. Structural and immunochemical analysis of three alpha-limit dextrin oligosaccharides. Arch Biochem Biophys 1989; 269:678–89. [DOI] [PubMed] [Google Scholar]
- 38.Casadellà M, van Ham PM, Noguera-Julian M, et al. ; SPREAD Programme . Primary resistance to integrase strand-transfer inhibitors in Europe. J Antimicrob Chemother 2015; 70:2885–8. [DOI] [PubMed] [Google Scholar]
- 39.El Bouzidi K, Kemp SA, Datir RP, et al. High prevalence of integrase mutation L74I in West African HIV-1 subtypes prior to integrase inhibitor treatment. J Antimicrob Chemother 2020; 75:1575–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fransen S, Gupta S, Danovich R, et al. Loss of raltegravir susceptibility by human immunodeficiency virus type 1 is conferred via multiple nonoverlapping genetic pathways. J Virol 2009; 83:11440–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hines DM, Ding Y, Wade RL, Beaubrun A, Cohen JP. Treatment adherence and persistence among HIV-1 patients newly starting treatment. Patient Prefer Adherence 2019; 13:1927–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.