Abstract
Background
Systemic inflammation independently predicts future cardiovascular events and is associated with a 2-fold increase in cardiovascular disease (CVD) risk among persons living with human immunodeficiency virus (PLHIV). We examined the association between inflammatory markers, HIV status, and traditional CVD risk factors.
Methods
We conducted a cross-sectional study of Kenyan adults with and without HIV seeking care at Kisumu County Hospital. Using a multiplex immunoassay, we measured interleukin (IL) 1β, IL-6, tumor necrosis factor α (TNF-α), and high-sensitivity C-reactive protein (hsCRP) concentrations. We compared inflammatory marker concentrations by HIV status using the Wilcoxon rank-sum test. Multivariable linear regression was used to evaluate associations between inflammatory biomarkers and HIV status, adjusting for CVD risk factors.
Results
We enrolled 286 PLHIV and 277 HIV-negative participants. Median duration of antiretroviral therapy for PLHIV was 8 years (interquartile range, 4–10) and 96% were virally suppressed. PLHIV had a 51% higher mean IL-6 concentration (P < .001), 39% higher mean IL-1β (P = .005), 40% higher mean TNF-α (P < .001), and 27% higher mean hsCRP (P = .008) compared with HIV-negative participants, independent of CVD risk factors. Male sex, older age, and obesity were associated with higher concentrations of inflammatory markers. Restricting to PLHIV, viral load of ≥1000 copies/mL was associated with higher TNF-α levels (P = .013).
Conclusions
We found higher levels of systemic inflammatory biomarkers among PLHIV who were virally suppressed, and this was independent of traditional CVD risk factors. Further longitudinal analyses to determine whether these inflammatory markers predict future CVD events, and are possible therapeutic targets among PLHIV, are warranted.
Keywords: HIV, inflammation, Kenya, cardiovascular disease risk
Despite achieving viral suppression, people living with HIV (PLHIV) had higher levels of inflammatory markers compared with individuals without HIV, independent of cardiovascular disease risk factors. Further investigations to determine whether inflammatory biomarkers predict cardiovascular events among PLHIV are warranted.
Systemic inflammation has been shown to increase cardiovascular disease (CVD) risk and independently predict future cardiovascular events such as myocardial infarction and stroke [1–5]. In addition, increased levels of biomarkers such as interleukin (IL) 6 and tumor necrosis factor α (TNF-α) have been associated with increased risk of mortality among those with pre-existing CVD [6, 7]. IL-1β has been shown to play a role in the atherosclerosis process and is a future therapeutic target to reduce risk of myocardial infarction or cardiac death [8]. There is evidence that CVD risk remains increased even if there are significant reductions in low-density lipoprotein cholesterol (LDL-C) levels, a marker of CVD risk, after use of lipid-lowering statin therapy [9]. The relative contribution of the inflammatory pathway to this residual CVD risk among both people with and without HIV is unclear [10].
In sub-Saharan Africa (SSA), which accounts for 67% of the global human immunodeficiency virus (HIV) burden, there is growing concern about rising CVD morbidity and its related mortality despite decreasing CVD death rates in high-income countries [11]. Evidence from the United States and Europe suggests that CVD risk among persons living with HIV (PLHIV) is double that of the general population [12–16]. Higher levels of inflammatory biomarkers such as C-reactive protein (CRP) and IL-6 are associated with HIV disease progression [17] and these persist at least up to 1 year following initiation of antiretroviral therapy (ART) [18, 19]. Persistent inflammation may contribute to the increased occurrence of subclinical atherosclerosis, myocardial infarction, and stroke and increased CVD-related mortality among PLHIV [18, 20–22]. In SSA there is a paucity of data regarding the association between inflammation and CVD risk, especially comparing people with and without HIV.
We therefore sought to determine the association between HIV status and inflammatory markers, specifically high-sensitivity CRP (hsCRP), IL-1β, IL-6, and TNF-α. We measured these biomarkers, traditional CVD risk factors, and HIV-specific characteristics among PLHIV and HIV-negative adults in Kisumu, Kenya. We hypothesized that PLHIV would have higher mean inflammatory biomarker levels when compared with HIV-negative study participants.
METHODS
Study Design and Setting
Between September 2017 and May 2018, we conducted a cross-sectional study among 300 women and men with HIV and 300 women and men without HIV from Kisumu County Hospital, a tertiary, public county referral facility located in Western Kenya where HIV prevalence is high (16.3%) [23].
Study Procedures
Participants were eligible if they were at least 30 years of age and lived within a 50-km radius of the hospital. Persons living with HIV had to be engaged in care at the HIV Comprehensive Care Clinic (CCC) and taking ART for at least 6 months. Persons living with HIV who met the inclusion criteria and provided consent were consecutively enrolled by a study nurse from the CCC while HIV-negative participants were recruited from HIV testing points until the sample size was reached. All participants provided written informed consent prior to any study procedures or data collection. Human subjects approval was obtained from the University of Washington Institutional Review Board and locally from the Kenyatta National Hospital/University of Nairobi Ethical and Scientific Review Committee.
Clinical Procedures
Using tablets, study nurses enrolled and interviewed all participants collecting data on sociodemographics, HIV disease status if HIV-positive, and CVD risk factors using the validated World Health Organization STEPS (STEPwise approach to surveillance) questionnaires modified to fit the Kenyan context [24]. Waist and hip circumference, weight, and height were measured to determine waist to hip ratio and body mass index (BMI). Two blood pressure readings on each arm and pulse were measured and averaged. Participants were asked to return the following day after fasting for 8 hours for a blood draw if not already fasting.
Laboratory Procedures
Blood samples were collected at least 8 hours after fasting for quantification of lipids (total cholesterol, high-density lipoprotein cholesterol [HDL-C], LDL-C, triglycerides), glucose, and inflammatory markers including hsCRP, IL-1β, IL-6, and TNF-α as well as CD4 and viral load for PLHIV. Blood samples were processed to obtain serum and stored at the Kenya Medical Research Institute (KEMRI) laboratory at −80°C. Absolute CD4+ T-cell count and HIV-1 RNA viral load testing were performed at the KEMRI laboratory in Kisumu. HIV RNA viral load values below 50 copies/mL were classified as undetectable. Absolute CD4 cell counts were measured using flow cytometry. Samples for lipids, glucose, and inflammatory markers were batched and shipped for testing to the University of Washington, Seattle. Serum lipids, glucose, and hsCRP tests were performed at the University of Washington Research Testing Services using an automated Beckman Coulter, Inc. Brea, CA AU5812. All samples were tested in duplicate according to the manufacturer’s protocols.
Cytokine Assays
Serum samples for IL-1β, IL-6, and TNF-α were analyzed using Mesoscale Discovery, Rockville, MD (MSD) VPlex Proinflammatory Panel 1 (human kit). Samples with a coefficient of variation greater than 30% were rerun and the duplicate with the lower coefficient of variation was averaged for the analyses. If a biomarker level was below the lower limit of detection for the assay, the lower limit was used as the biomarker value. Lower limit of detection concentrations were 0.2 mg/L for hsCRP, 0.01 pg/mL for IL-1β, 0.05 pg/mL for IL-6, and 0.01 pg/mL for TNF-α.
Primary Outcomes and Dependent Variables
The primary outcomes were inflammatory biomarker concentrations: hsCRP, IL-1β, IL-6, and TNF-α. HIV status was the exposure. Multivariable models were adjusted for age, gender, smoking status, blood pressure, BMI, hypertension, diabetes, and lipids determined a priori and supported by the literature. We assessed the presence of effect modification by metabolic syndrome and high atherosclerotic CVD risk (ASCVD) [25]. Metabolic syndrome was defined by the 2009 Consensus Criteria as any 3 of the following: (1) abdominal obesity (waist circumference of >88 cm for women and >94 cm for men), (2) triglycerides 150 mg/dL or greater, (3) HDL-C less than 50 mg/dL for women and less than 40 mg/dL for men, (4) blood pressure greater than 130/85 mmHg, and (5) fasting plasma glucose of 100 mg/dL or greater [26]. For each participant without prior history of myocardial infarction or stroke, we calculated their 10-year ASCVD risk score using the Pooled Cohort Equation as outlined in the 2019 American College of Cardiology/American Heart Association Guideline on the Primary Prevention of Cardiovascular Disease [25, 27].
Statistical Analysis
Continuous variables were summarized using means and standard deviations for normally distributed data and by medians and interquartile range (IQR) for non–normally distributed variables. We compared people with and without HIV using a t test for normally distributed continuous variables, Wilcoxon rank-sum test for non–normally distributed continuous variables, and chi-square test for categorical variables. We restricted our analysis to hsCRP values of 10 mg/L or less as hsCRP values greater than 10 mg/L are most consistent with a transient or acute-phase response as would commonly occur with acute infections common in this setting. Due to the cross-sectional nature of the study, we were unable to repeat measurements after 2 weeks to rule out transient acute inflammation.
We compared inflammatory markers by HIV status using the Wilcoxon rank-sum test. We created separate multivariable linear regression models to evaluate the association between (log-transformed) IL-1β, IL-6, TNF-α, and hsCRP and HIV status and adjusted for CVD risk factors including age, sex, smoking, alcohol use, diet (at least 5 servings of fruits and vegetables per day), physical activity (at least 150 minutes of moderate activity or 75 minutes of vigorous activity per week), BMI, hypertension and diabetes, and lipids. Subgroup analyses for metabolic syndrome and high ASCVD risk (score >7.5%) were carried out to assess whether associations of each biomarker with HIV status were consistent across the subgroups by including an interaction term between HIV status and the subgroup variable. In a separate model including only PLHIV, we included nadir CD4 count, viral suppression, and ART duration as covariates.
We report the exponentiated B-coefficients and their calculated 95% confidence intervals (CIs) representing the fold increase/decrease in biomarker level. We used a significance (α) level of 0.05. All analyses were conducted using Stata version 14.0 (StataCorp, College Station, TX).
RESULTS
Participant Characteristics
Of the 600 eligible participants, complete data were available for 563 participants (94%): 286 PLHIV and 277 HIV-negative participants. The median age was 45 years (IQR, 40–54 years) for PLHIV and 40 years (IQR, 31–54 years) for HIV-negative participants. Persons living with HIV were older (P < .001), less educated (P < .001), had a lower BMI (P < .001), and had consumed less alcohol in the past 12 months (P = .007) as compared with HIV-negative participants (Table 1). The prevalences of metabolic syndrome, hypertension, and abdominal obesity were significantly lower among PLHIV as compared with those without HIV (Table 1). There were no significant differences in smoking, diet, physical activity, triglycerides, HDL-C, and fasting glucose levels by HIV status.
Table 1.
Characteristics of 598 Study Participants Stratified by HIV Status
| Variable | Total (N = 564) |
HIV-Positive (n = 287) | HIV-Negative (n = 277) | P |
|---|---|---|---|---|
| 1. Sociodemographic characteristics | ||||
| Age categories, n (%) | <.001 | |||
| <40 years | 201 (35.6) | 68 (23.7) | 133 (48.0) | |
| 40–49 years | 162 (28.7) | 106 (36.9) | 56 (20.2) | |
| 50–59 years | 128 (22.7) | 83 (28.9) | 45 (16.2) | |
| ≥60 years | 73 (12.9) | 30 (10.5) | 43 (15.5) | |
| Sex (female) | 299 (50.0) | 144 (50.2) | 138 (49.8) | .93 |
| Marital status, n (%) | .002 | |||
| Single | 38 (6.4) | 15 (5.2) | 23 (8.3) | |
| Currently married | 414 (73.4) | 205 (68.3) | 235 (78.9) | |
| Separated/widowed/divorced | 112 (19.8) | 80 (26.7) | 40 (13.4) | |
| Education, n (%) | <.001 | |||
| Less than primary school completed | 78 (13.8) | 39 (13.6) | 39 (14.1) | |
| Primary school completed | 211 (37.4) | 124 (43.2) | 87 (31.4) | |
| Secondary school completed | 183 (32.4) | 95 (33.1) | 88 (31.8) | |
| More than secondary school completed | 92 (16.3) | 29 (10.1) | 63 (22.7) | |
| 2. Characteristics of persons living with HIV | ||||
| Nadir CD4 count,a cells/mm3 | … | 365 (213 571) | … | |
| Time since diagnosis, years | … | 9 (5, 11) | … | |
| Regimen, n (%) | ||||
| First-line regimen (non-PI) | … | 248 (86.4) | … | |
| Second-line regimen (PI) | … | 38 (13.2) | … | |
| Third-line regimen (PI) | … | 1 (0.3) | … | |
| ART duration, years | … | 8 (4, 10) | … | |
| Current CD4 count,c cells/mm3 | … | 512 (364, 666) | … | |
| Viral loadc (copies/mL), n (%) | ||||
| Undetectable (<50) | … | 229 (79.8) | … | |
| Low-level viremia (50–1000) | … | 46 (16.0) | … | |
| Viremic (>1000) | … | 13 (4.2) | … | |
| 3. Traditional risk factors | ||||
| BMI categories (kg/m2), n (%) | <.001 | |||
| Underweight (<18.5) | 51 (9.0) | 32 (11.1) | 19 (6.9) | |
| Normal weight (18.5–24.9) | 319 (56.6) | 177 (61.7) | 142 (51.3) | |
| Overweight (25–29.9) | 119 (21.1) | 54 (18.8) | 65 (23.5) | |
| Obese (>30) | 75 (13.3) | 24 (8.4) | 51 (18.4) | |
| Smoking, n (%) | .17 | |||
| Never smoked | 494 (87.6) | 249 (88.4) | 245 (88.4) | |
| Ever smoked but stopped | 43 (7.6) | 27 (9.4) | 16 (5.8) | |
| Current smoker | 27 (4.8) | 11 (3.8) | 16 (5.8) | |
| Alcohol use in past 12 months | 105 (18.6) | 41 (14.3) | 64 (23.1) | .007 |
| Recommended healthy diet | 31 (5.5) | 12 (4.2) | 19 (6.9) | .16 |
| Recommended physical activity | 134 (95) | 79 (95.0) | 55 (95) | .92 |
| Waist circumference, mean (SD), cm | ||||
| Female | 84.6 (13.0) | 82.3 (12.6) | 87.0 (13.1) | <.002 |
| Male | 82.0 (11.0) | 81.2 (9.8) | 82.9 (12.2) | .22 |
| 4. Components of metabolic syndrome criteria,d n (%) | ||||
| Elevated blood pressure (≥135/85 mmHg) | 161 (28.5) | 63 (22.0) | 98 (35.4) | <.001 |
| Abdominal obesity (waist circumference >88 cm for women and >94 cm for men) | 130 (23.0) | 54 (18.8) | 76 (27.4) | .01 |
| Low HDL-C (<50 mg/dL for women and <40 mg/dL for men) | 167 (29.6) | 75 (26.1) | 92 (33.2) | .06 |
| Elevated triglycerides (≥150 mg/dL) | 48 (8.5) | 30 (10.5) | 18 (6.5) | .09 |
| Elevated fasting plasma glucose (≥100 mg/dL) | 28 (5.0) | 10 (3.5) | 18 (6.5) | .09 |
| Metabolic syndrome | 50 (9.0) | 18 (6.3) | 32 (11.6) | .027 |
Data are presented as n (%) or median (IQR) unless otherwise indicated.
Abbreviations: BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; HIV, human immunodeficiency virus; IQR, interquartile range; PI, protease inhibitor.
aEighteen participants were missing nadir CD4 count data.
cTwo participants were missing CD4 results and viral load result.
dExcludes 34 participants without blood sample data.
Among PLHIV, the current median CD4 count was 512 cells/mm3 (IQR, 364–666 cells/mm3) and the median duration on ART was 8 years (IQR, 4–10 years). The majority (86%) of PLHIV were on a first-line regimen (2 nucleoside reverse transcriptase inhibitors [NRTIs] plus 1 non-NRTI) with only 13% on a protease inhibitor (PI)–based regimen (2 NRTIs plus PI). Eighty percent (229/287) of PLHIV had an undetectable HIV RNA level (<50 copies/mL), 16% (46/287) had low-level viremia (HIV RNA, 50–1000 copies/mL) and 4% (12/287) had high-level viremia (HIV RNA concentration ≥1000 copies/mL) (Table 1).
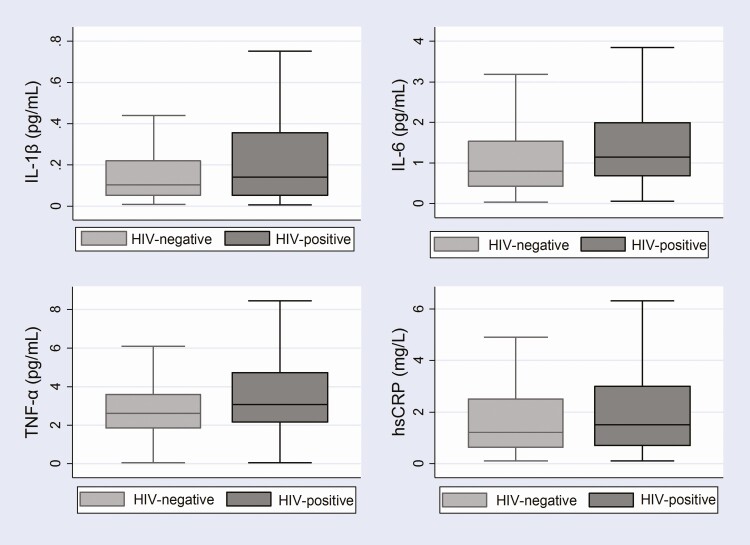
Overall, the median (IQR) IL-1β, IL-6, TNF-α, and hsCRP levels were 0.11 pg/mL (0.05–0.30), 0.94 pg/mL (0.50–1.83), 2.78 pg/mL (1.96–4.02), and 1.4 mg/L (0.6– 2.8), respectively. Median IL-1β, IL-6, TNF-α, and hsCRP levels were higher among PLHIV compared with HIV-negative participants (Figure 1). Persons living with HIV had a significantly higher median IL-1β (0.14 pg/mL vs 0.10 pg/mL, P = .019), IL-6 (1.15 pg/mL vs 0.80 pg/mL, P < .001), TNF-α (3.06 pg/mL vs 2.61 pg/mL, P < .001), and hsCRP (1.5 mg/L vs 1.2 mg/L, P = .052) level compared with HIV-negative participants (Figure 1).
Figure 1. .
Boxplots of median and interquartile ranges of serum biomarker levels comparing HIV-positive and HIV-negative participants. Abbreviations: HIV, human immunodeficiency virus; hsCRP, high-sensitivity C-reactive protein; IL-1β, interleukin 1 beta; IL-6, interleukin 6; TNF-α, tumor necrosis factor α.
IL-6 showed the greatest mean difference among the biomarkers examined comparing PLHIV and HIV-negative participants. After adjusting for age, sex, smoking, alcohol use, diet, physical activity, BMI, hypertension, diabetes, and lipids, PLHIV had a 51% higher mean IL-6 level (P < .001), a 39% higher mean IL-1β level (P = .005), a 40% higher mean TNF-α level (P < .001), and a 27% higher mean hsCRP level (P = .008) compared with HIV-negative participants (Table 2).
Table 2.
Exponentiated B-Coefficient Estimates and Confidence Intervals for the Association Between Biomarkers and HIV Status
| Unadjusted | Adjusteda | |||||
|---|---|---|---|---|---|---|
| Variable | Exponentiated β-Coefficient | 95% CI | P | Exponentiated β-Coefficient | 95% CI | P |
| IL-1β (pg/mL) | 1.38 | 1.06, 1.59 | .010 | 1.39 | 1.10, 1.73 | .005 |
| IL-6 (pg/mL) | 1.46 | 1.21, 1.75 | <.001 | 1.51 | 1.23, 1.84 | <.001 |
| TNF-α (pg/mL) | 1.45 | 1.22, 1.70 | <.001 | 1.40 | 1.16, 1.67 | <.001 |
| hsCRP (mg/L) | 1.22 | 1.02, 1.45 | .027 | 1.27 | 1.06, 1.52 | .008 |
Exponentiated B-coefficients represent the fold increase in mean level of the inflammatory biomarkers comparing HIV-positive and HIV-negative participants. For example, 1.39 is interpreted as PLHIV having a 39% higher mean IL-1 level as compared with HIV-negative participants (P = .005).
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; hsCRP, high-sensitivity C-reactive protein; IL, interleukin; PLHIV, persons living with HIV; TNF-α, tumor necrosis factor α.
aAdjusted for age, sex, smoking, alcohol use, diet, physical activity, body mass index, hypertension and diabetes, and lipids.
Stratification by Metabolic Syndrome and High Atherosclerotic Cardiovascular Disease Risk
We previously reported that the prevalence of metabolic syndrome and calculated ASCVD risk among these study participants was lower among PLHIV compared with HIV-negative participants [25]. However, in this analysis, the differences in biomarker levels between those with and without metabolic syndrome/high ASCVD risk in PLHIV were not significantly different from similar differences in those without HIV. We therefore observed no significant interaction between HIV status and metabolic syndrome or ASCVD and the inflammatory markers.
Factors Associated With IL-1β, IL-6, TNF-α, and hsCRP
The demographic and CVD risk factors found to be independently associated with higher IL-1β levels were male sex and obesity (BMI >30 kg/m2) (Table 3). A significantly higher IL-6 level was associated with older age (>50 years), male sex, and lower HDL-C (Table 4). While a significantly higher TNF-α was associated with older age (>60 years) and male sex, TNF-α levels were significantly lower among participants with diabetes (Table 5). Older age (>50 years) and being overweight or obese and a current smoker were associated with higher hsCRP (Table 6).
Table 3.
Unadjusted and Adjusted Multivariable Linear Regression of Factors Associated With IL-1β
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| β-Coefficient (95% CI) | P | β-Coefficient (95% CI) | P | |
| HIV status | .27 (.06, .47) | .010 | .30 (.07, .52) | .011 |
| Age (years) | ||||
| 30–39 | Reference | Reference | ||
| 40–49 | .24 (−.11, .49) | .061 | .10 (−.17, .37) | .473 |
| 50–59 | .03 (−.25, .31) | .837 | −.11 (−.42, .20) | .487 |
| ≥60 | .04 (−.29, .38) | .796 | −.06 (−.42, .30) | .746 |
| Sex | ||||
| Female | Reference | Reference | ||
| Male | .32 (.13, .53) | .002 | .37 (.13, .60) | .002 |
| BMI (kg/m2) | ||||
| Normal (18–24) | Reference | Reference | ||
| Underweight (<18) | −.16 (−.52, .20) | .379 | −.26 (−.61, .11) | .175 |
| Overweight (25–29) | −.27 (−.53, −.01) | .039 | −.10 (−.37, .17) | .469 |
| Obese (≥30) | .12 (−.18, .42) | .429 | .36 (.04, .69) | .030 |
| Current smoker | .18 (−.18, .42) | .459 | .08 (−.41, .57) | .741 |
| Alcohol use in past 12 months | .13 (−.13, .39) | .322 | .01 (−.28, .28) | .984 |
| Hypertension | −.13 (−.38, .12) | .306 | .11 (−.14, .38) | .373 |
| Diabetes | .01 (−.46, .48) | .964 | .03 (−.44, .51) | .887 |
| LDL cholesterol >130 mg/dL | −.34 (−.64, −.05) | .024 | −.42 (−.96, .11) | .120 |
| HDL cholesterol <50 mg/dL for males and <40 mg/dL for females | −.15 (−.37, .71) | .183 | −.08 (−.32, .16) | .526 |
| Total cholesterol >200 mg/dL | −.21 (−.48, .07) | .139 | .04 (−.46, .54) | .871 |
| Triglycerides >150 mg/dL | −.01 (−.38, .36) | .969 | −.13 (−.52, .27) | .524 |
Abbreviations: BMI, body mass index; CI, confidence interval; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; IL-1β, interleukin 1β; LDL, low-density lipoprotein.
Table 4.
Unadjusted and Adjusted Multivariable Linear Regression of Factors Associated With IL-6
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| β-Coefficient (95% CI) | P | β-Coefficient (95% CI) | P | |
| HIV status | .38 (.19, .56) | <.001 | .41 (.21, .61) | <.001 |
| Age (years) | ||||
| 30–39 | Reference | Reference | ||
| 40–49 | .33 (.10, .56) | .005 | .17 (−.06, .42) | .163 |
| 50–59 | .48 (.23, .73) | <.001 | .28 (−.01, .55) | .051 |
| ≥60 | .71 (.41, 1.01) | <.001 | .59 (.27, .92) | <.001 |
| Sex | ||||
| Female | Reference | Reference | ||
| Male | .36 (.18, .55) | <.001 | .34 (.13, .55) | .001 |
| BMI (kg/m2) | ||||
| Normal (18–24) | Reference | Reference | ||
| Underweight (<18) | −.16 (−.50, .18) | .355 | −.22 (−.55, .10) | .183 |
| Overweight (25–29) | −.24 (−.50, −.18) | .048 | −.10 (.35, .14) | .406 |
| Obese (≥30) | .10 (−.19, .38) | .505 | .27 (−.04, .58) | .083 |
| Current smoker | .35 (−.09, .79) | .117 | .19 (−.26, .63) | .410 |
| Alcohol use in past 12 months | .17 (−.07, .42) | .162 | .17 (−.87, .42) | .198 |
| Hypertension | −.15 (−.07, .38) | .194 | .08 (−.15, .31) | .501 |
| Diabetes | −.31 (−.74, .12) | .158 | −.33 (−.75, .10) | .129 |
| LDL cholesterol >130 mg/dL | −.13 (−.15, −.41) | .355 | −.01 (−.49, .46) | .962 |
| HDL cholesterol <50 mg/dL for males and <40 mg/dL for females | −.05 (−.16, .25) | .660 | .24 (−.02, .45) | .031 |
| Total cholesterol >200 mg/dL | .16 (−.08, .42) | .191 | .08 (−.36, .52) | .729 |
| Triglycerides >150 mg/dL | .21 (−.13, .55) | .225 | −.01 (−.36, .34) | .973 |
Abbreviations: BMI, body mass index; CI, confidence interval; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; IL-6, interleukin 6; LDL, low-density lipoprotein.
Table 5.
Unadjusted and Adjusted Multivariable Linear Regression of Factors Associated With TNF-α
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| β-Coefficient (95% CI) | P | β-Coefficient (95% CI) | P | |
| HIV status | .37 (.20, .53) | <.001 | .34 (.16, .52) | <.001 |
| Age (years) | ||||
| 30–39 | Reference | Reference | ||
| 40–49 | .33 (.12, .54) | .002 | .22 (−.01, .45) | .052 |
| 50–59 | .38 (.15, .60) | .001 | .23 (−.02, .48) | .073 |
| ≥60 | .50 (−.23, .77) | <.001 | .42 (.13, .71) | .005 |
| Sex | ||||
| Female | Reference | Reference | ||
| Male | .31 (.14, .48) | <.001 | .27 (.08, −.46) | .005 |
| BMI (kg/m2) | ||||
| Normal (18–24) | Reference | Reference | ||
| Underweight (<18) | −.19 (−.50, .11) | .210 | −.26 (−.55, .04) | .088 |
| Overweight (25–29) | −.18 (−.39, .04) | .119 | −.05 (−.27, .17) | .671 |
| Obese (≥30) | −.19 (−.45, .07) | .144 | −.06 (−.34, .22) | .646 |
| Current smoker | .13 (−.26, .52) | .506 | −.02 (−.42, .39) | .927 |
| Alcohol use in past 12 months | .12 (−.10, .33) | .289 | .12 (−.11, .35) | .306 |
| Hypertension | .03 (−.17, .24) | .745 | .10 (−.11, .31) | .873 |
| Diabetes | −.37 (−.76, .02) | .063 | −.43 (−.84, −.03) | .045 |
| LDL cholesterol >130 mg/dL | .02 (−.23, .27) | .894 | −.20 (−.63, .23) | .359 |
| HDL cholesterol <50 mg/dL for males and <40 mg/dL for females | .01 (−.18, .19) | .938 | .16 (−.04, .35) | .110 |
| Total cholesterol >200 mg/dL | .11 (−.11, .34) | .326 | .20 (−.20, .60) | .319 |
| Triglycerides >150 mg/dL | .15 (−.15, .45) | .336 | −.02 (−.34, .30) | .893 |
Abbreviations: BMI, body mass index; CI, confidence interval; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; LDL, low-density lipoprotein; TNF-α, tumor necrosis factor α.
Table 6.
Unadjusted and Adjusted Multivariable Linear Regression of Factors Associated With hsCRP
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| β-Coefficient (95% CI) | P | β-Coefficient (95% CI) | P | |
| HIV status | .20 (.02, .37) | .027 | .24 (.06, .42) | .008 |
| Age (years) | ||||
| 30–39 | Reference | Reference | ||
| 40–49 | .28 (.07, .49) | .010 | .15 (−.06, .37) | .161 |
| 50–59 | .54 (.32, .77) | <.001 | .42 (.18, .67) | .001 |
| ≥60 | .57 (.28, .85) | <.001 | .59 (.30, .88) | <.001 |
| Sex | ||||
| Female | Reference | Reference | ||
| Male | −.21 (−.38, −.03) | −.06 (−.25, −.13) | .523 | |
| BMI (kg/m2) | ||||
| Normal (18–24) | Reference | Reference | ||
| Underweight (<18) | −.14 (−.44, .16) | .361 | −.19 (−.48, .11) | .221 |
| Overweight (25–29) | .46 (.25, .67) | <.001 | .43 (.21, .64) | <.001 |
| Obese (≥30) | .84 (.58, 1.11) | <.001 | .83 (.56, 1.11) | <.001 |
| Current smoker | .02 (−.19, .60) | .318 | .39 (.01, .78) | .050 |
| Alcohol use in past 12 months | −.28 (−.50, −.58) | .014 | −.17 (−.39, .05) | .131 |
| Hypertension | .29 (.07, .50) | .009 | −.02 (−.22, .18) | .857 |
| Diabetes | .08 (−.33, .48) | .713 | −.04 (−.41, .34) | .843 |
| LDL cholesterol >130 mg/dL | .28 (.02, .53) | .034 | −.07 (−.48, .34) | .733 |
| HDL cholesterol <50 mg/dL for males and <40 mg/dL for females | .28 (.08, .47) | .005 | .17 (−.03, .37) | .089 |
| Total cholesterol >200 mg/dL | .22 (−.01, .45) | .057 | .06 (−.30, .44) | .727 |
| Triglycerides >150 mg/dL | .46 (.12, .30) | .003 | .19 (−.12, .50) | .228 |
Abbreviations: BMI, body mass index; CI, confidence interval; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; hsCRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein.
In a model restricting the analysis to participants who are PLHIV, we added HIV-specific factors, including ART regimen, nadir CD4 count, current CD4 count, viral load, and duration of ART use in addition to the CVD risk factors. Persons living with HIV who had a viral load of 1000 or more copies/mL had a 114% higher TNF-α level as compared with those who had an undetectable viral load (P = .013). Apart from TNF-α, there was no association between the HIV-specific risk factors and the other biomarkers.
DISCUSSION
In this study, we found a higher level of inflammatory markers (IL-1β, IL-6, TNF-α, and hsCRP) among PLHIV, the majority of whom were virally suppressed, compared with HIV-negative participants. These higher concentrations among PLHIV persisted even after adjusting for traditional risk factors for CVD, including dyslipidemia, obesity, diabetes, and smoking. Our findings are among the first to examine the relationships between inflammation, HIV, and CVD risk factors in SSA and are consistent with some but not all of the literature from Europe, North America, and SSA [18, 28–32]. In addition, we demonstrated that TNF-α concentrations were even greater among PLHIV who were not virally suppressed relative to PLHIV with optimal viral suppression. Those PLHIV not achieving viral suppression (HIV RNA concentrations ≥1000 copies/mL) had twice the concentration of TNF-α compared with those PLHIV with an undetectable viral load. Higher levels of biomarkers among those not virally suppressed may indicate residual inflammation as a result of persistent viremia or a low pretreatment CD4 count [33]. This is consistent with other studies that have shown that high viral load concentrations are correlated with high levels of inflammation and increased CVD risk [33].
Studies examining the relationships between HIV, inflammation, and ART in SSA are limited and have reported heterogeneous results. A multicountry study including sites in Kenya, Nigeria, South Africa, Uganda, and Zambia reported higher levels of CRP but no differences in IL-6 levels comparing PLHIV on ART with individuals without HIV [30]. A study in South Africa comparing individuals without and with HIV who were ART naive and those on ART found that mean TNF-α levels were significantly increased pre-ART among those with opportunistic infections and TNF-α remained elevated 1 year post-ART. However, IL-6 levels were comparable in treated patients without opportunistic coinfection compared with those without HIV [31]. The Monitoring of Early Treatment Adherence Study recruited participants from Uganda and South Africa who were starting ART in late-stage disease and found that individuals starting ART had higher levels of IL-6 pretreatment but found no difference in levels of biomarkers comparing those initiating treatment with earlier- or late-stage disease 1 year after treatment initiation [32]. In summary, ART appears to lower inflammatory markers, but whether low-grade inflammation persists compared with HIV-negative individuals remains a question. In our cross-sectional study in Kenya, low-grade inflammation appears to persist despite ART. The heterogeneity of results across studies may reflect context-specific differences as well as different underlying pathophysiology for each biomarker, as suggested by Siedner et al [32].
Consistent with previously published literature, we found an increase in hsCRP, IL-6, and TNF-α levels with increasing age, especially above the age of 50 years. This is thought to occur due to natural aging resulting in a persistent low-grade inflammation, a process termed “inflammaging” [34]. Males in our study had higher mean IL-1β, IL-6, and TNF-α concentrations compared with females. This has been reported in previous studies and may be explained by the downregulation of these inflammatory marker genes by estrogen [35, 36]. We also report that higher IL-6 levels were associated with lower HDL-C, and higher levels of hsCRP and IL-1β were noted among obese individuals. Obesity has been linked to low-grade inflammation as adipose tissue releases cytokines that trigger the production of CRP [37]. In addition, current smoking was associated with increased levels of TNF-α. Smoking cessation and obesity interventions have been shown to decrease CRP levels, and thus may provide an opportunity for reducing inflammation and CVD risk in both persons with and without HIV [38].
Contrary to our expectation, there was no significant interaction between HIV and metabolic syndrome. Higher levels of inflammation have been seen in cardiometabolic conditions such as metabolic syndrome and hypertension [39], but the data have not been consistent. This may be explained by genetic factors or the fact that participants in our study were relatively healthy PLHIV but would need further investigation.
The strengths of this study are that we included participants both with and without HIV. The participants with HIV were on highly active ART, the majority having achieved viral suppression, and are thus representative of the current ART program in Kenya [40]. We also measured several inflammatory markers. The limitations of this study include the cross-sectional design, which limits inferences of causality. While we found significant differences in age, BMI, and components of metabolic syndrome comparing participants with and without HIV, we did adjust for these variables in our analysis. We did not measure the inflammatory markers over time to observe their trajectory. In addition, we lack data regarding CVD endpoints such as myocardial infarction or stroke to test how these biomarkers predict these events. Further longitudinal studies in SSA prospectively measuring and following inflammatory markers and CVD events will further our understanding of the relationship between inflammation and CVD risk in PLHIV and could justify intervention studies targeting inflammation for CVD risk reduction.
Conclusions
Greater systemic inflammation was seen among PLHIV compared with HIV-negative individuals, independent of traditional CVD risk factors. Persistent inflammation among PLHIV, even those with optimal viral suppression, is a potential mechanism contributing to increased CVD risk among PLHIV. Periodic measurement of inflammatory markers may be useful for risk stratification and predicting CVD events over time, ultimately helping to identify those PLHIV at highest risk for CVD morbidity and mortality. Research is also needed to determine whether interventions that target both the metabolic and inflammatory pathways reduce CVD risk in PLHIV in SSA.
Notes
Author contributions. C. F., S. T. P., and S. J. M. developed and implemented the CVD study protocol. S. J. M., J. P. H., S. T. P., and C. F. designed this analysis. S. J. P., J. W., A. O., P. M. M., and S. J. M. coordinated data collection. S. J. M. and S. J. P. analyzed the data, and S. J. M. drafted the manuscript. All authors contributed to editing of the manuscript and approved submission of the final draft for publication.
Disclaimer. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the views of the Government of Kenya or the National Institutes of Health.
Financial support. This work was supported by grants from the US National Institutes of Health (grant number R21TW010459) and the Fogarty International Center (grant number D43 TW009580).
Potential conflicts of interest. J. P. H. reports grants from the National Institutes of Health, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997; 336:973–9. [DOI] [PubMed] [Google Scholar]
- 2.Danesh J, Wheeler JG, Hirschfield GM, et al. . C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004; 350:1387–97. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 2002; 347:1557–65. [DOI] [PubMed] [Google Scholar]
- 4.Vos AG, Idris NS, Barth RE, Klipstein-Grobusch K, Grobbee DE. Pro-inflammatory markers in relation to cardiovascular disease in HIV infection: a systematic review. PLoS One 2016; 11:e0147484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaptoge S, Di Angelantonio E, Lowe G, et al. . C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010; 375:132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuomisto K, Jousilahti P, Sundvall J, Pajunen P, Salomaa V. C-reactive protein, interleukin-6 and tumor necrosis factor alpha as predictors of incident coronary and cardiovascular events and total mortality: a population-based, prospective study. Thromb Haemost 2006; 95:511–8. [DOI] [PubMed] [Google Scholar]
- 7.Lowe G, Woodward M, Hillis G, et al. . Circulating inflammatory markers and the risk of vascular complications and mortality in people with type 2 diabetes and cardiovascular disease or risk factors: the ADVANCE study. Diabetes 2014; 63:1115–23. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377:1119–31. [DOI] [PubMed] [Google Scholar]
- 9.Murphy SA, Cannon CP, Wiviott SD, McCabe CH, Braunwald E. Reduction in recurrent cardiovascular events with intensive lipid-lowering statin therapy compared with moderate lipid-lowering statin therapy after acute coronary syndromes from the PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction 22) trial. J Am Coll Cardiol 2009; 54:2358–62. [DOI] [PubMed] [Google Scholar]
- 10.Aday AW, Ridker PM. Targeting residual inflammatory risk: a shifting paradigm for atherosclerotic disease. Front Cardiovasc Med 2019; 6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roth GA, Johnson C, Abajobir A, et al. . Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 2017; 70:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah ASV, Stelzle D, Lee KK, et al. . Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation 2018; 138:1100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Currier JS, Taylor A, Boyd F, et al. . Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr 2003; 33:506–12. [DOI] [PubMed] [Google Scholar]
- 14.Lang S, Mary-Krause M, Cotte L, et al. ; French Hospital Database on HIV-ANRS CO4 . Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS 2010; 24:1228–30. [DOI] [PubMed] [Google Scholar]
- 15.Obel N, Thomsen HF, Kronborg G, et al. . Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis 2007; 44:1625–31. [DOI] [PubMed] [Google Scholar]
- 16.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boulware DR, Hullsiek KH, Puronen CE, et al. ; INSIGHT Study Group . Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis 2011; 203:1637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tenorio AR, Zheng Y, Bosch RJ, et al. . Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210: 1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wada NI, Jacobson LP, Margolick JB, et al. . The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr 2009; 51:268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nordell AD, McKenna M, Borges ÁH, Duprez D, Neuhaus J, Neaton JD; INSIGHT SMART, ESPRIT Study Groups; SILCAAT Scientific Committee . Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc 2014; 3:e000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross AC, Rizk N, O’Riordan MA, et al. . Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis 2009; 49:1119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ministry of Health. Kenya HIV Estimates Report. Nairobi, Kenya: Ministry of Health, 2018. [Google Scholar]
- 24.World Health Organization. The WHO STEPwise approach to chronic disease risk factor surveillance (STEPS). Available at: http://www.who.int/ncds/surveillance/steps/STEPS_Instrument_v2.1.pdf. Accessed 1 May 2017.
- 25.Masyuko SJ, Page ST, Kinuthia J, et al. . Metabolic syndrome and 10-year cardiovascular risk among HIV-positive and HIV-negative adults: a cross-sectional study. Medicine (Baltimore) 2020; 99:e20845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alberti KGMM, Eckel Robert H, Grundy Scott M, et al. . Harmonizing the metabolic syndrome. Circulation 2009; 120: 1640–5. [DOI] [PubMed] [Google Scholar]
- 27.Arnett DK, Blumenthal RS, Albert MA, et al. . 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019; 140:e596–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neuhaus J, Jacobs DR Jr, Baker JV, et al. . Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 2010; 201:1788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eastburn A, Scherzer R, Zolopa AR, et al. . Association of low level viremia with inflammation and mortality in HIV-infected adults. PLoS One 2011; 6:e26320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroeze S, Wit FW, Rossouw TM, et al. . Plasma biomarkers of human immunodeficiency virus-related systemic inflammation and immune activation in sub-Saharan Africa before and during suppressive antiretroviral therapy. J Infect Dis 2019; 220:1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassol E, Malfeld S, Mahasha P, et al. . Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J Infect Dis 2010; 202:723–33. [DOI] [PubMed] [Google Scholar]
- 32.Siedner MJ, Bwana MB, Asiimwe S, et al. ; META Study Investigators . Timing of antiretroviral therapy and systemic inflammation in sub-Saharan Africa: results from the META longitudinal cohort study. J Infect Dis 2019; 220:1172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghislain M, Bastard JP, Meyer L, et al. ; ANRS-COPANA Cohort Study Group . Late antiretroviral therapy (ART) initiation is associated with long-term persistence of systemic inflammation and metabolic abnormalities. PLoS One 2015; 10:e0144317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babu H, Ambikan AT, Gabriel EE, et al. . Systemic inflammation and the increased risk of inflamm-aging and age-associated diseases in people living with HIV on long term suppressive antiretroviral therapy. Front Immunol 2019; 10:1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med 2000; 51:245–70. [DOI] [PubMed] [Google Scholar]
- 36.An J, Ribeiro RC, Webb P, et al. . Estradiol repression of tumor necrosis factor-alpha transcription requires estrogen receptor activation function-2 and is enhanced by coactivators. Proc Natl Acad Sci U S A 1999; 96:15161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev 2013; 14:232–44. [DOI] [PubMed] [Google Scholar]
- 38.Feinstein MJ, Hsue PY, Benjamin LA, et al. . Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation 2019; 140:e98–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lacerda HR, Falcão Mda C, de Albuquerque VM, et al. . Association of inflammatory cytokines and endothelial adhesion molecules with immunological, virological, and cardiometabolic disease in HIV-infected individuals. J Interferon Cytokine Res 2014; 34:385–93. [DOI] [PubMed] [Google Scholar]
- 40.National AIDS and STI Control Programme (NASCOP). Preliminary KENPHIA 2018 report. Nairobi, Kenya: NASCOP, 2020. [Google Scholar]