Abstract
Background
Intracellular tenofovir diphosphate (TFV-DP) concentration in dried blood spots (DBSs) is used to monitor cumulative pre-exposure prophylaxis (PrEP) adherence. We evaluated TFV-DP in DBSs following daily oral PrEP (emtricitabine 200 mg/tenofovir diphosphate 300 mg) among pregnant and postpartum adolescent girls and young women (AGYW).
Methods
Directly observed PrEP was administered for 12 weeks in a pregnancy (14–24 weeks’ gestation, n = 20) and postpartum (6–12 weeks postpartum, n = 20) group of AGYW aged 16–24 years in sub-Saharan Africa. Weekly DBS TFV-DP was measured by validated liquid chromatography–tandem mass spectrometry assay. Week 12 TFV-DP distributions were compared between groups with Wilcoxon test. Population pharmacokinetic models were fit to estimate steady-state concentrations and create benchmarks for adherence categories. Baseline correlates of TFV-DP were evaluated.
Results
Median age was 20 (IQR, 19–22) years. Of 3360 doses, 3352 (>99%) were directly observed. TFV-DP median (IQR) half-life was 10 (7–12) days in pregnancy and 17 (14–21) days postpartum, with steady state achieved by 5 and 8 weeks, respectively. Observed median (IQR) steady-state TFV-DP was 965 fmol/punch (691–1166) in pregnancy versus 1406 fmol/punch (1053–1859) postpartum (P = .006). Modeled median steady-state TFV-DP was 881 fmol/punch (667–1105) in pregnancy versus 1438 fmol/punch (1178–1919) postpartum. In pooled analysis, baseline creatinine clearance was associated with observed TFV-DP concentrations.
Conclusions
TFV-DP in African AGYW was approximately one-third lower in pregnancy than postpartum. These Population-specific benchmarks can be used to guide PrEP adherence support in pregnant/postpartum African women.
Clinical Trials Registration
Keywords: intracellular TFV-DP, PrEP in pregnancy, adolescence
Concentrations of tenofovir diphosphate in dried blood spots were approximately one-third lower during pregnancy than postpartum after 12 weeks of directly observed dosing among African adolescent girls and young women on daily pre-exposure prophylaxis. We recommend population-specific benchmarks for adherence support.
Pregnancy and the postpartum period represent important windows of elevated human immunodeficiency virus (HIV) risk for women [1, 2] and this has important implications for both mothers and infants in generalized HIV epidemics [3, 4]. When taken as prescribed, daily oral pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) is highly effective for reducing HIV acquisition among men who have sex with men (MSM) and HIV-serodiscordant couples [5]. PrEP use during pregnancy and breastfeeding appears feasible, safe, and acceptable [6] and a growing number of national HIV programs now recommend PrEP for women at elevated HIV risk during these critical periods [7].
PrEP efficacy correlates directly with FTC/TDF adherence [5]. Because of the limitations of self-reported measures, tenofovir diphosphate (TFV-DP; an intracellular metabolite of tenofovir)—measured in red blood cells (RBCs) using dried blood spots (DBSs)—is used as a biological marker of adherence [8–13] integrated into adherence support strategies [14, 15]. Tenofovir diphosphate concentrations in DBSs characterize cumulative exposure over the prior 6–8 weeks [11, 16, 17], analogous to glycated hemoglobin (HbA1c) for cumulative glucose exposure. DBS assays report drug concentrations in femtomoles measured in a 3mm disc punched from the dried blood on the card. Additionally, in studies of MSM, TFV-DP concentrations in DBSs were shown to predict PrEP efficacy [18, 19]. In iPrEX Open Label Extension of the iPrEX study (OLE), for example, HIV incidence was 4.7 per 100 person-years when TFV-DP was not detected, 2.3 per 100 person-years at concentrations less than 350 fmol/punch, and 0.6 per 100 person-years at 350–699 fmol/punch, and no new infections were observed when TFV-DP concentrations were 700 fmol/punch or greater.
Given limited prior research in this population, TFV-DP concentrations in DBSs have not been rigorously evaluated in women, most notably during pregnancy and postpartum, periods of profound physiological change [20]. Further, women continuing PrEP during pregnancy had reductions in plasma tenofovir concentrations (58%) and TFV-DP concentrations in DBSs (27%) [9]. Similarly, plasma tenofovir area-under-the-concentration-time curves (AUC) were approximately 20% lower during pregnancy compared with nonpregnant women in the context of treatment for HIV [21] and hepatitis B [22], likely due to increased renal clearance [23]. To account for physiological changes that may influence drug concentrations, tailored TFV-DP in DBS adherence benchmarks are needed in pregnant and postpartum women.
The pharmacokinetic (PK) component of International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) 2009 sought to establish TFV-DP concentrations in DBSs in pregnancy and postpartum over 12 weeks of directly observed daily oral PrEP. We succeeded in developing TFV-DP–based benchmarks to guide future adherence support interventions.
METHODS
Study Design and Participants
The PK component enrolled peripartum adolescent and young women from Malawi, South Africa, Uganda, and Zimbabwe. Briefly, we recruited women from maternal and child health services for 2 separate groups: pregnancy and postpartum. Women in the pregnancy group enrolled between 14 and 24 weeks’ gestation based on best obstetrical estimate incorporating prior obstetric ultrasound [24]. The postpartum group enrolled 6–12 weeks following delivery. Additional eligibility criteria included age 16–24 years, confirmed HIV-negative, agreement to take daily oral PrEP under direct observation for 12 weeks, no social circumstances that would interfere with study participation, and no clinical or laboratory evidence of underlying comorbidities. All women were provided with information about the study, including the risks and benefits of participation. Written informed consent (or assent with parental consent, per local regulations) was obtained prior to initiating study procedures.
Study Procedures
At screening, we collected basic demographic and medical information, measuring height, weight, hematocrit, and estimated creatinine clearance (CrCl). Participants agreed to take daily oral co-formulated PrEP (FTC 200 mg/TDF 300 mg) under direct observation for 84 consecutive days. Following instruction in pill-swallowing technique, participants developed dynamic individual plans for direct observation, including in-person visits at the clinic, home, or alternate community sites. Real-time video documentation of pill ingestion was offered as an alternate mode of documentation when in-person observation was not feasible.
Participants completed 12 once-weekly visits to collect DBS specimens via venipuncture and medical data, including weight. Support for sexual health promotion and PrEP use was provided using adapted Integrated Next Step Counseling [25]. Whole blood (50 μL/spot) was pipetted onto Whatman 903 Protein Saver Cards (Fisher Scientific, Fairlawn, NJ, USA), dried at room temperature, stored in plastic bags with a desiccant at −80oC, then shipped for centralized testing. Safety monitoring reports included grade 3 or higher adverse events using the Division of AIDS (DAIDS) toxicity tables [26] and adverse pregnancy outcomes: low birth weight (<2500 g), preterm delivery (<37 weeks’ gestation), spontaneous abortion (fetal loss <20 weeks’ gestation), stillbirth (fetal loss ≥20 weeks’ gestation), and small for gestational age (<10th percentile by the INTERGROWTH-21st scale).
Pharmacokinetic Testing
Extractions from a 50-μL DBS were tested for TFV-DP using a validated high-performance liquid chromatography–tandem mass spectrometry (LC-MS/MS) method at the University of Cape Town, South Africa (Supplementary Material) [18]. The lower limit of quantification for TFV-DP was 16.6 fmol/3-mm punch. A correction factor was applied for the TFV-DP per 50 μL to convert to fmol/3-mm punch, as these units have been used in previous studies.
Sample Size Considerations
A target sample size of at least 15 women per group was consistent with similar studies of directly observed PrEP that estimated steady-state concentrations of TFV-DP, providing adequate precision for outcome estimates [16]. We increased the sample size to 20 participants per group to account for potential attrition. This study was not powered to assess safety or efficacy.
Statistical Analysis
We summarized and compared demographic and clinical characteristics at enrollment between the pregnancy and postpartum groups. We used Fisher’s exact test to estimate differences in proportions and t tests or Wilcoxon rank-sum tests (as appropriate) for continuous measures.
For each group, we plotted median and interquartile range (IQR) TFV-DP concentrations for each week and fit a Loess curve and 95% confidence interval (CI) to the concentration-time data. The plateau in the concentration-time curve was assumed to represent steady-state. Tenofovir diphosphate at week 12 (steady-state) was analyzed as median, IQR, mean, standard deviation (SD), and 95% CI in the pregnancy and postpartum groups. The Wilcoxon rank-sum test was used to evaluate differences in the distribution of 12-week concentrations between groups. Additionally, a nonlinear mixed-effects population PK model was fit to the TFV-DP data using a 1-compartment constant input model using Phoenix (Certara, Princeton, NJ). The model was used to summarize PK parameters for both groups and to assess the influence of demographic covariates on the PK parameters with the groups pooled (Supplementary Material).
To develop benchmarks for TFV-DP in DBSs to guide adherence interpretations, similar to other studies [11, 12, 15], we first created the 7 doses/week benchmark using the 25th percentile in observed TFV-DP concentrations at 12 weeks. Assuming linear dose proportionality of TFV-DP concentrations, as demonstrated in other trials [16], we divided the 7 doses/week benchmark by 7 to estimate the TFV-DP concentration produced by each dose per week. We then estimated TFV-DP benchmarks for 2 additional categories: 2–6 doses/week and <2 doses/week. For the second approach, we created benchmarks using simulations of TFV-DP via the PK model. The simulations included 1, 2, 3, 4, 5, 6, and 7 doses/week, on average, for each group. A receiver operating characteristic (ROC) curve analysis was conducted for each group to identify TFV-DP cutoffs that best characterized the same 3 adherence categories.
Ethical Considerations
The protocol was approved by in-country institutional review boards and ethics committees and US institutional review boards affiliated with the enrolling sites. The trial protocol is registered in ClinicalTrials.gov (NCT03386578) and can be found at https://www.impaactnetwork.org/studies/IMPAACT2009.asp.
RESULTS
From March to June 2019, 20 pregnant and 20 postpartum women were enrolled. Baseline characteristics for each study group are summarized in Table 1. Of 3360 possible PrEP doses, 3352 (99.6%) were directly observed: 3335 in-person, 13 by real-time video, and 4 by time-stamped video. Eight doses were not directly observed; 3 women each took 1 dose that was not directly observed and the remaining 5 doses were missed by 3 women (1, 1, and 3 doses). The 12 weekly visits were completed by all 20 pregnant participants. One postpartum participant missed her final visit and DBS specimen collection; however, given that her concentrations were already at steady-state, we carried forward her week 11 TFV-DP concentration and included all participants in our final analysis.
Table 1.
Maternal Baseline Characteristics for Pharmacokinetic Component
| Characteristic | Pregnancy Group (n = 20) | Postpartum Group (n = 20) | Total (N = 40) | Group Difference (P Value) |
|---|---|---|---|---|
| Age, y | ||||
| Median (Q1, Q3) | 20.0 (19.5, 22.5) | 20.0 (19.0, 22.0) | 20.0 (19.0, 22.0) | .38a |
| Race, n (%) | ||||
| Black African | 20 (100) | 20 (100) | 40 (100) | |
| Baseline weeksb | ||||
| Median (Q1, Q3) | 18.0 (15.0, 20.0) | 7.00 (7.00, 9.00) | 11.5 (7.00, 18.0) | |
| Minimum, maximum | 11.0, 23.0 | 6.00, 12.0 | 6.00, 23.0 | |
| Weight, kg | ||||
| Median (Q1, Q3) | 58.9 (56.1, 65.1) | 55.0 (50.8, 62.0) | 56.8 (54.2, 65.1) | .07a |
| Minimum, maximum | 49.4, 68.8 | 46.6, 75.2 | 46.6, 75.2 | |
| BMI, kg/m2 | ||||
| Median (Q1, Q3) | 24.3 (22.7, 25.6) | 22.0 (20.7, 24.5) | 23.1 (21.6, 24.9) | .012a |
| Minimum, maximum | 21.4, 29.3 | 18.4, 30.9 | 18.4, 30.9 | |
| Hematocrit, % | ||||
| Median (Q1, Q3) | 34.9 (33.0, 37.1) | 40.8 (39.1, 41.7) | 38.2 (34.9, 40.8) | <.001a |
| Minimum, maximum | 29.7, 39.6 | 36.1, 45.0 | 29.7, 45.0 | |
| Creatinine clearance, mL/minute | ||||
| Median (Q1, Q3) | 180 (158, 188) | 120 (116, 135) | 152 (120, 181) | <.001a |
| Minimum, maximum | 132, 216 | 89.4, 183 | 89.4, 216 | |
| Country, n (%) | ||||
| Malawi | 0 (0) | 7 (35) | 7 (18) | .004c |
| South Africa | 4 (20) | 0 (0) | 4 (10) | |
| Uganda | 8 (40) | 4 (20) | 12 (30) | |
| Zimbabwe | 8 (40) | 9 (45) | 17 (43) |
Abbreviations: BMI, body mass index; Q1, first quartile; Q3, third quartile.
aWilcoxon test.
bBaseline weeks are “gestational age (weeks)” for the pregnancy group and baseline “weeks postpartum” for the postpartum group.
cFisher’s exact test.
Observed Pharmacokinetics of Tenofovir Diphosphate
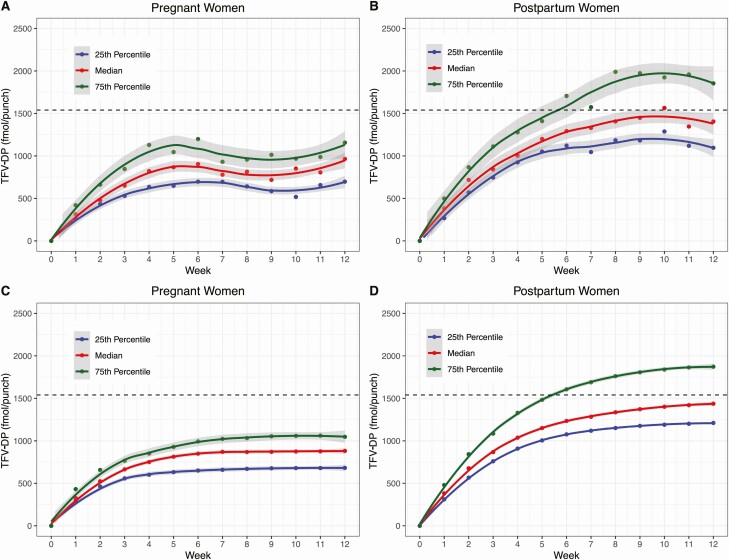
Weekly concentrations accumulated to steady-state at approximately week 5 in the pregnancy group (Figure 1A) and week 8 in the postpartum group (Figure 1B). Table 2 summarizes the distribution of observed TFV-DP concentrations (fmol/punch) in DBSs collected at week 12. Median concentrations were 31% lower in the pregnancy group (965; IQR: 691, 1166) than in the postpartum group (1406; IQR: 1053, 1859; P < .05).
Figure 1.
Observed and fitted TFV-DP concentrations in pregnant and postpartum women by study week (A and B, observed; C and D, modeled). Dashed line = USA median; gray shading represents the 95% confidence interval for Loess curve. Abbreviation: TFV-DP, tenofovir diphosphate.
Table 2.
Summary of Tenofovir Diphosphate Concentrations at Steady-State
| Statistic | Pregnancy Group | Postpartum Group | P |
|---|---|---|---|
| N | 20 | 20 | |
| Mean fmol/punch | 964 | 1713 | .0205a |
| SD | 345 | 1295 | |
| 95% CI | (802, 1125) | (1107, 2319) | |
| Median fmol/punch | 965 | 1406 | .0064b |
| Q1 | 691 | 1053 | |
| Q3 | 1166 | 1859 | |
| Minimum fmol/punch | 419 | 624 | |
| Maximum fmol/punch | 1618 | 6696 |
Steady-state was defined as week 12 for all but 1 participant; for 1 participant in the postpartum group who missed the week 12 visit, values from the week 11 visit were used. Abbreviations: CI, confidence interval; Q1, first quartile; Q3, third quartile; SD, standard deviation.
at test.
bWilcoxon test.
Pharmacokinetic Model
The model-fitted concentrations (Figure 1C and 1D) were similar to the observed concentrations. One postpartum participant had outlier measurements at week 11 (8743 fmol/punch) and week 12 (6696 fmol/punch), which were severalfold higher than the next highest concentrations. These data were retained in all analyses. The fitted week 12 median TFV-DP was 881 fmol/punch (IQR: 667, 1105) in pregnancy versus 1438 fmol/punch (IQR: 1178, 1919) postpartum, a 39% difference. Tenofovir diphosphate accumulated with a median half-life of 10 days (IQR: 7.4, 12.4) in pregnancy and 17 days (IQR: 13.7, 21.2) postpartum, consistent with steady-state being achieved by 5 and 8 weeks, respectively (Figure 1). In analyses combining both groups, age was not associated with clearance. Weight and hematocrit reduced the objective function but did not reduce interindividual variability on clearance. Baseline CrCl reduced the objective function (44.67 to 19.92) and reduced interindividual variability (49% to 34%) on clearance; thus, baseline CrCl was associated with lower steady-state TFV-DP. For every 1-mL/min difference in baseline CrCl there was a 0.96% (95% CI, 0.65%, 1.26%) difference in TFV-DP in the opposite direction.
Adherence Benchmarks for Tenofovir Diphosphate
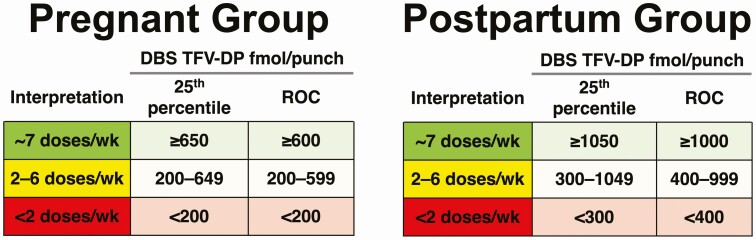
Figure 2 shows adherence interpretations using the 25th percentile approach and ROC analysis approach for each group. The observed 25th percentile TFV-DP concentrations for 7 doses/week were rounded down to the nearest 50 fmol/punch (691 to >650 and 1053 to >1050). Because dose proportionality was assumed, we estimated that the concentration corresponding to 1 dose/week in the pregnancy group was 650/7 = 93 fmol/punch. Therefore, the lower value was set at 200 fmol/punch for less than 2 doses/week. The same approach was used for the postpartum group. Estimates based on the 25th percentile approach and the ROC approach were similar.
Figure 2.
Proposed adherence thresholds for study groups. Abbreviations: DBS, dried blood spot; ROC, receiver operating characteristic curve; TFV-DP, tenofovir diphosphate.
Safety
Daily oral PrEP was well tolerated by participants in both groups. Eight grade 3 or higher adverse events were observed among 4 pregnant women; 1 participant experienced 1 grade 4 event (placenta accreta) and 4 grade 3 events (disseminated intravascular coagulation, peritoneal hemorrhage, intrauterine fetal death, and endometritis); 1 participant had a grade 3 event (decreased CrCl, transient); another 2 participants had 2 grade 3 events (spontaneous abortion and failed induction of labor). Due to the circumstances surrounding these events, and based on site investigators’ extensive clinical experience managing pregnant women and other adults receiving FTC/TDF for antiretroviral therapy or PrEP, all were deemed not related to study treatment; upon review of all available information, the protocol team confirmed agreement with each assessment. No grade 3 or higher events were reported for postpartum maternal participants.
For the pregnancy group, 2 infants each had 1 grade 3 adverse event (increased blood creatinine, neonatal respiratory distress). One grade 3 infant adverse event was observed in the postpartum group (increased blood creatinine). These transient events were all coded as not related to treatment following site and protocol team review.
Of the 20 pregnancies, 5 (25%) had an adverse pregnancy outcome. One spontaneous abortion and 1 stillbirth were observed and deemed not related to the study treatment (see above). Of the 18 pregnancies resulting in live births, there was 1 (6%) preterm delivery at 36 weeks’ gestation and 2 children (11%) were born small for gestational age. No new HIV infections were observed during follow-up.
DISCUSSION
Among adolescent girls and young women in sub-Saharan Africa—in the setting of near perfect PrEP adherence—we found that TFV-DP concentrations in DBSs were approximately one-third lower in pregnancy than postpartum. Tenofovir diphosphate levels in DBSs observed in pregnancy also appeared lower than those reported in analogous studies of men and nonpregnant, nonlactating women in the United States [16]. The implication of these findings for PrEP efficacy is unknown, as data about the adherence-efficacy relationship during pregnancy remain limited. As HIV programs integrate PrEP into maternal–child health settings [4, 27, 28], additional research is needed. The adherence benchmarks established in this study can guide future adherence-efficacy studies in this population.
The lowered TFV-DP concentrations in DBSs observed during pregnancy are consistent with existing medical literature [23]. An important limitation of these past studies was limited assessment of adherence, which is especially needed for TFV-DP in DBSs as it captures adherence over weeks of dosing. Our study addressed this problem by incorporating direct observation of daily PrEP. With only 8 of 3360 (0.2%) doses not directly observed, this study provides a high level of confidence about PrEP adherence over the 12-week follow-up period.
The implication of these lowered TFV-DP concentrations for PrEP efficacy remains unclear. Existing data suggest that PrEP is effective across a range of TFV-DP concentrations. In the iPrEX OLE study, for example, only 1 of 28 men newly diagnosed with HIV had TFV-DP levels greater than 350 fmol/punch at the seroconversion visit, and the observed concentration (611 fmol/punch) was in the range of 2–3 doses/week on average [11]. In HPTN 082 (Evaluation of Daily Oral PrEP as a Primary Prevention Strategy for Young African Women: A Vanguard Study), all 4 women who acquired HIV had TFV-DP concentrations less than 350 fmol/punch [15]. In the Pre-exposure prophylaxis (PrEP) Implementation for Young Women and Adolescents in Kenya (PrIYA) study, which provided PrEP to pregnant and postpartum women, the single HIV seroconversion documented was in a woman with TFV-DP concentration below the limit of quantitation [29]. In these studies, PrEP was provided as part of broader HIV-prevention services, so it is difficult to accurately attribute its independent protective effect. Because TFV-DP levels in DBSs measure the cumulative average exposure over the past 6–8 weeks, it is also difficult to assess temporal changes in PrEP adherence within that window. Nevertheless, all HIV infections occurred in individuals with very low TFV-DP concentrations, suggesting that higher adherence and drug levels confer protection against HIV during this period of heightened risk.
Since higher TFV-DP concentrations are associated with greater levels of protection—and lowered concentrations were observed in pregnancy in our study—our findings highlight the importance of strict adherence to PrEP during this period. Our proposed adherence benchmark for 7 doses/week, on average, was 650 fmol/punch, which in MSM corresponds to an adherence level of 4 doses/week (ie, 700 fmol/punch) [17]. Similarly, the median steady-state concentration for 7 doses/week observed during pregnancy (965 fmol/punch) was also substantially lower than for similar studies of men and nonpregnant, nonlactating women (1540 fmol/punch) in the United States [16].
Using these data, we developed DBS TFV-DP adherence benchmarks for pregnancy and postpartum women taking oral PrEP. These benchmarks will inform the second phase of IMPAACT 2009, in which participants receive drug concentration–guided counseling to support adherence after choosing oral PrEP for HIV prevention. Given the paucity of adherence metrics for PrEP in women, however, this may also provide a valuable framework for ongoing work in the field. These benchmarks were similar whether using 25th percentiles of observed data or ROC curves on modeled data (Figure 2); however, we recommend the use of 25th percentiles as a more conservative approach. Such benchmarks may help characterize adherence behaviors in longitudinal studies and provide more granularity compared with qualitative interpretations (eg, detectable vs undetectable) of TFV-DP concentrations. When integrated into clinical care, DBS TFV-DP concentrations should be used to initiate nonjudgmental conversations about challenges, and solutions, and not interpreted as absolute indicators of adherence.
In our study of possible TFV-DP correlates, baseline CrCl best explained variability in TFV-DP. Hematocrit and weight also reduced the objective function but did not explain interindividual variability as well as baseline CrCl. All variables are biologically plausible covariates for TFV-DP, but all were also different between the pregnant and nonpregnant groups, creating redundancies that make it difficult to attribute cause and effect in these analyses. Nevertheless, assessments of baseline CrCl may assist in the interpretation of TFV-DP values that are borderline between 2 adherence categories.
Alongside the study’s many strengths, we acknowledge important limitations. First, while measurement of intracellular TFV-DP via DBSs holds practical advantages for adherence monitoring [16, 17] and has been used in numerous settings, RBCs are not the target site for tenofovir drug action. Measurement of tenofovir in other compartments during pregnancy and postpartum, including in cervicovaginal fluid, and peripheral blood mononuclear cells (PBMCs), is needed to enhance our understanding of PrEP exposure during these periods. Second, our validated TFV-DP assay is dependent on RBC concentration and the physiologic change observed in pregnancy will result in dilution of RBCs and therefore TFV-DP. These concentrations also represent an averaging of TFV-DP over the course of the second and third trimesters of pregnancy. The study was not designed to obtain separate estimates of median TFV-DP levels for each trimester because of the duration of follow-up needed to reach steady-state and the unpredictable timing of delivery. Third, we focused on tenofovir as the primary agent of interest consistent with other studies; however, tenofovir in PrEP is paired with FTC, which was not analyzed in this study. The PKs of FTC require further evaluation, but it is plausible that this second agent provides some HIV protection, even if tenofovir and tenofovir metabolite concentrations are low. Fourth, we recruited separate participants in the pregnancy and postpartum periods. A study that performed PK evaluations in the same participants for both time periods could have reduced interindividual variability. However, such an approach would present important practical challenges (eg, a lengthy overall observation period, an intensive daily follow-up, need for an intermediary “washout” window) that may have compromised study implementation. Finally, there is emerging evidence that other formulations for PrEP, including emtricitabine/tenofovir alafenamide, are efficacious; however, these regimens have not been studied in women. These TFV-DP adherence benchmarks require further validation when other agents are used. This study did not attempt to assess the safety or efficacy of TDF/FTC when used for HIV prevention in pregnancy.
In summary, in the setting of directly observed PrEP administration, we show that TFV-DP concentrations were approximately one-third lower during pregnancy compared with postpartum. Additional research is needed to understand tenofovir levels at pharmacologically active sites, cervicovaginal fluid, and PBMCs, and to further characterize the relationship between PrEP adherence and efficacy. Nevertheless, when risk of incident HIV infection during pregnancy and breastfeeding is high, we recommend the continued scale-up of such services and provide tailored TFV-DP benchmarks to support PrEP adherence.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The IMPAACT 2009 protocol team acknowledges the extraordinary commitment and courage of the study participants. The authors thank the communities that supported this research and the site teams that implemented the study with such rigor. The authors gratefully acknowledge the contributions of the site investigators and staff who conducted the IMPAACT 2009 study.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT). Overall support for IMPAACT was provided by the National Institute of Allergy and Infectious Diseases with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health, all components of the National Institutes of Health (award numbers UM1AI068632 to the IMPAACT Leadership and Operations Center, UM1AI068616 to the IMPAACT Statistical and Data Management Center, and UM1AI106716 to the IMPAACT Laboratory Center; and by NICHD contract number HHSN275201800001I). Pharmaceutical support was provided by Gilead Sciences, Inc.
Potential conflicts of interest. P. L. A. reports grants and personal fees from Gilead Sciences, outside the submitted work. J. F. R. reports employment, stockholding, and other fees from Gilead Sciences, outside the submitted work. B. H. C. reports grants from Gilead Foundation (support for global health fellows), outside the submitted work. L. S.-C. reports grants to her institution from the National Institutes of Health, outside the submitted work. B. J. reports grants from National Institute of Allergy and Infectious Diseases/Division of AIDS, during the conduct of the study. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
IMPAACT 2009 Team. Zimbabwe—University of Zimbabwe Clinical Trials Research Centre, Seke North: Vongai Chanaiwa, MSc Epi; Suzen Maonera, MSN; Lucia Mungate, DNA. Malawi—College of Medicine–Johns Hopkins Research Project, Blantyre: Sharon Kunkanga Mambiya, MBBS; Abigail Mnemba, Dip. Nursing and Midwifery; Flora Chithila, BSc. Uganda—Baylor College of Medicine Children’s Foundation, Kampala: Phionah Nakabuye, BCP; Muzamil Nsibuka Kisekka, B. Pharma; Victoria Ndyanabangi, MBChB; Uganda–Makerere University–Johns Hopkins University, Kampala: Brenda Gati Mirembe, MBChB, MSc Epi; Phionah Kibalama Ssemambo, MBChB, MPH; Annette Miwanda Ssekasi, URN. South Africa—Wits Reproductive Health and HIV Institute, Shandukani: Elizea Horne, MBChB; Siphokazi Sibisi, BPharm; Janet Grab, BPharm.
Contributor Information
IMPAACT 2009 Team:
Vongai Chanaiwa, Suzen Maonera, Lucia Mungate, Sharon Kunkanga Mambiya, Abigail Mnemba, Flora Chithila, Phionah Nakabuye, Muzamil Nsibuka Kisekka, Victoria Ndyanabangi, Brenda Gati Mirembe, Phionah Kibalama Ssemambo, Annette Miwanda Ssekasi, Elizea Horne, Siphokazi Sibisi, and Janet Grab
References
- 1.Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS Med 2014; 11:e1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graybill LA, Kasaro M, Freeborn K, et al. . Incident HIV among pregnant and breast-feeding women in sub-Saharan Africa: a systematic review and meta-analysis. AIDS 2020; 34:761–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tippett Barr BA, van Lettow M, van Oosterhout JJ, et al. . National estimates and risk factors associated with early mother-to-child transmission of HIV after implementation of option B+: a cross-sectional analysis. Lancet HIV 2018; 5:e688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UNICEF; UNAIDS; World Health Organization. Key considerations for programming and prioritization. Going the “Last Mile” to EMTCT: a road map for ending the HIV epidemic in children. New York: UNICEF; 2020. Available at: http://www.childrenandaids.org/sites/default/files/2019-11/191118%20-%201-EMTCT%20Brief%20v5%20-%20FINAL%20-%20WEB.pdf. Accessed 16 February 2020. [Google Scholar]
- 5.Fonner VA, Dalglish SL, Kennedy CE, et al. . Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS 2016; 30:1973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joseph Davey DL, Pintye J, Baeten JM, et al. ; PrEP in Pregnancy Working Group . Emerging evidence from a systematic review of safety of pre-exposure prophylaxis for pregnant and postpartum women: where are we now and where are we heading? J Int AIDS Soc 2020; 23:e25426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies N, Heffron R. Global and national guidance for the use of pre-exposure prophylaxis during peri-conception, pregnancy and breastfeeding. Sex Health 2018; 15:501–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumenthal J, Pasipanodya EC, Jain S, et al. . Comparing self-report pre-exposure prophylaxis adherence questions to pharmacologic measures of recent and cumulative pre-exposure prophylaxis exposure. Front Pharmacol 2019; 10:721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pyra M, Anderson PL, Hendrix CW, et al. ; Partners Demonstration Study Team . Tenofovir and tenofovir-diphosphate concentrations during pregnancy among HIV-uninfected women using oral preexposure prophylaxis. AIDS 2018; 32:1891–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montgomery MC, Oldenburg CE, Nunn AS, et al. . Adherence to pre-exposure prophylaxis for HIV prevention in a clinical setting. PLoS One 2016; 11:e0157742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant RM, Anderson PL, McMahan V, et al. ; iPrEx Study Team . Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14:820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pyra M, Anderson P, Haberer JE, et al. . Tenofovir-diphosphate as a marker of HIV pre-exposure prophylaxis use among East African men and women. Front Pharmacol 2019; 10:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu AY, Cohen SE, Vittinghoff E, et al. . Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med 2016; 176:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blumenthal J.Results from a PrEP demonstration project for at-risk cisgender women in the US. PrEP in cisgender women; Conference on Retroviruses and Opportunistic Infections, Boston, MA; 2020. Available at: http://www.croiconference.org/sessions/results-prep-demonstration-project-risk-cisgender-women-us. Accessed 1 April 2020. [Google Scholar]
- 15.Celum CL.PrEP adherence and effect of drug level feedback among young African Women in HPTN 082. Mexico City, Mexico. 2019. Available at: http://programme.ias2019.org/Abstract/Abstract/2328. Accessed 1 April 2020. [Google Scholar]
- 16.Anderson PL, Liu AY, Castillo-Mancilla JR, et al. . Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother 2018; 62:e01710–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castillo-Mancilla JR, Zheng JH, Rower JE, et al. . Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses 2013; 29:384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng JH, Rower C, McAllister K, et al. . Application of an intracellular assay for determination of tenofovir-diphosphate and emtricitabine-triphosphate from erythrocytes using dried blood spots. J Pharm Biomed Anal 2016; 122:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson PL, Glidden DV, Liu A, et al. ; iPrEx Study Team . Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4:151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pariente G, Leibson T, Carls A, Adams-Webber T, Ito S, Koren G. Pregnancy-associated changes in pharmacokinetics: a systematic review. PLoS Med 2016; 13:e1002160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Best BM, Burchett S, Li H, et al. ; International Maternal Pediatric and Adolescent AIDS Clinical Trials (IMPAACT) P1026s Team . Pharmacokinetics of tenofovir during pregnancy and postpartum. HIV Med 2015; 16:502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cressey TR, Harrison L, Achalapong J, et al. . Tenofovir exposure during pregnancy and postpartum in women receiving tenofovir disoproxil fumarate for the prevention of mother-to-child transmission of hepatitis B virus. Antimicrob Agents Chemother 2018; 62:e01686-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bierhoff M, Smolders EJ, Tarning J, et al. . Pharmacokinetics of oral tenofovir disoproxil fumarate in pregnancy and lactation: a systematic review. Antivir Ther 2019; 24:529–40. [DOI] [PubMed] [Google Scholar]
- 24.Committee on Obstetric Practice American Institute of Ultrasound in Medicine Society for Maternal–Fetal Medicine. Committee opinion no 700: methods for estimating the due date. Obstet Gynecol 2017; 129:e150–4. doi: 10.1097/AOG.0000000000002046. [DOI] [PubMed] [Google Scholar]
- 25.Amico KR, Miller J, Balthazar C, et al. . Integrated next step counseling (iNSC) for sexual health and PrEP use among young men who have sex with men: implementation and observations from ATN110/113. AIDS Behav 2019; 23:1812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Division of AIDS (DAIDS). Table for grading the severity of adult and pediatric adverse events, corrected version 2.1.2017. Available at: https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf. Accessed 16 February 2020.
- 27.Pintye J, Kinuthia J, Roberts DA, et al. . Brief report: integration of PrEP services into routine antenatal and postnatal care: experiences from an implementation program in Western Kenya. J Acquir Immune Defic Syndr 2018; 79:590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. WHO technical brief: preventing HIV during pregnancy and breastfeeding in the context of pre-exposure prophylaxis (PrEP).2017. Available at: https://apps.who.int/iris/bitstream/handle/10665/255866/WHO-HIV-2017.09-eng.pdf;jsessionid=A77182FCE270B18DEFEE3034DD1453DE?sequence=1. Accessed 16 February 2020.
- 29.Pintye J, Kinuthia J, Abuna F, et al. . Frequency and predictors of tenofovir-diphosphate detection among young Kenyan women in a real-world pre-exposure prophylaxis implementation program. Clin Infect Dis 2020; 71:e509–12. doi: 10.1093/cid/ciaa181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.