Abstract
From 2005 to 2018, among 32013 adults with human immunodeficiency virus entering care, median time to antiretroviral therapy (ART) prescription declined from 69 to 6 days, CD4 count at entry into care increased from 300 to 362 cells/μL, and CD4 count at ART prescription increased from 160 to 364 cells/μL.
Keywords: HIV, CD4 count, antiretroviral therapy, universal treatment, treat all
Effective antiretroviral therapy (ART) has considerably reduced morbidity and mortality in people with human immunodeficiency virus (PWH). In the early years of ART, public health agencies recommended that CD4 count be used for timing ART initiation in the absence of an AIDS-defining illness. Due to toxicity, resistance, and adherence concerns with early ART agents, treatment for asymptomatic patients would be deferred until CD4 counts dropped below recommended thresholds. With improvements in potency and tolerability and evidence that supports early ART initiation [1], CD4 count thresholds in treatment guidelines issued by the US Department of Health and Human Services (DHHS) steadily increased from <200 cells/µL in 2001 to <350 cells/µL in 2007 to 350–500 cells/µL in 2009 [2]. In March 2012, the DHHS revised treatment guidelines to recommend therapy for all PWH regardless of CD4 count, commencing the era of “treat all” [2]. The World Health Organization adopted the same recommendation in 2015 [3].
Within the context of these evolving treatment guidelines, we expected to observe reduced time from entry into care to prescription of ART and subsequent increases in CD4 counts at ART prescription. Using observational data from the largest cohort collaboration of PWH in North America, we describe trends in time from entry into care to ART prescription and CD4 count at both milestones between 2005 and 2018.
METHODS
Study Population
The North American AIDS Cohort Collaboration on Research and Design [4] (NA-ACCORD) is a collaboration of more than 20 clinical and interval cohort studies comprising nearly 200 000 PWH in the United States and Canada, and a regional representative of the International Epidemiology Databases to Evaluate AIDS. NA-ACCORD participants have been shown to be demographically similar to PWH in the United State, as captured by the National HIV Surveillance System of the US Centers for Disease Control and Prevention [5].
Participants of clinical cohort studies who attend 2 or more HIV clinic visits within 12 months are eligible for enrollment in the NA-ACCORD. NA-ACCORD participants provide informed consent or contributed data with a waiver of informed consent where approved by local institutional review boards. Clinical data are primarily sourced from point-of-care electronic medical records.
A total of 95 415 adults aged ≥18 years enrolled in the NA-ACCORD between 1 January 2005 and 31 December 2018. Because our focus was the impact of DHHS universal treatment guidelines on PWH engaged in clinical care in the United States, we excluded adults enrolled in study cohorts prior to the cohorts joining the NA-ACCORD [6] (n = 531), studies in Canada (n = 4998), and interval cohort studies (which are not based in clinical practice; n = 5093). Participants with no CD4 count measurement at entry into care (n = 12939), medical records that suggest participants were ART-experienced (history of ART prescription or viral load measurement <500 copies/mL prior to entering care; n = 39253), or previous AIDS diagnosis (n = 588) were also excluded.
The total study population comprised 32 013 participants who entered care with a CD4 count measurement. This sample was used to calculate median CD4 count at entry into care. Of the total sample, 26 555 (83%) were prescribed ART during the study period with a CD4 count measurement at ART prescription. This subsample was used to calculate median CD4 count at ART prescription and time from entry into care to ART prescription.
Outcome Assessment
Time from entry into care to ART prescription was defined as the number of days between the first HIV care visit and the first ART prescription. CD4 count at entry into care (–90/+30 days) and ART prescription (–90/+30 days) were expressed as the number of CD4+ T lymphocytes per microliter of whole blood (cells/µL). For participants who had >1 CD4 count measurement within the 120-day window, we used the measurement that was collected closest to the date of entry into care/ART prescription. If ART was prescribed on the same date as entry into care, we used the same CD4 count measurement for both dates. We defined ART as a treatment regimen that included a protease inhibitor, nonnucleoside reverse transcriptase inhibitor, and/or integrase strand transfer inhibitor.
Statistical Analyses
We calculated median (interquartile range [IQR]) days from entry into care to ART prescription by year of ART prescription. We calculated median (IQR) CD4 count at entry into care by year of entry into care. We calculated median (IQR) CD4 count at ART prescription by year of ART prescription. Additionally, we calculated the above measures stratified by sex, race/ethnicity, injection drug use (IDU), and geographic region of residence.
RESULTS
Of 32 013 study participants, 26 844 (84%) were men and 5169 (16%) were women; 208 identified as transgender. A total of 14 378 (45%) participants were Black, 10 470 (33%) were White, 5002 (16%) were Hispanic (any race), 694 (2%) were Asian/Pacific Islanders, 140 (0.4%) were Indigenous, 87 (0.3%) were multiracial, and 1242 (4%) were of unknown race/ethnicity. A total of 14 032 (44%) reported male-to-male sexual contact, and 3615 (11%) reported IDU. Median age was 39 years (IQR, 29–49).
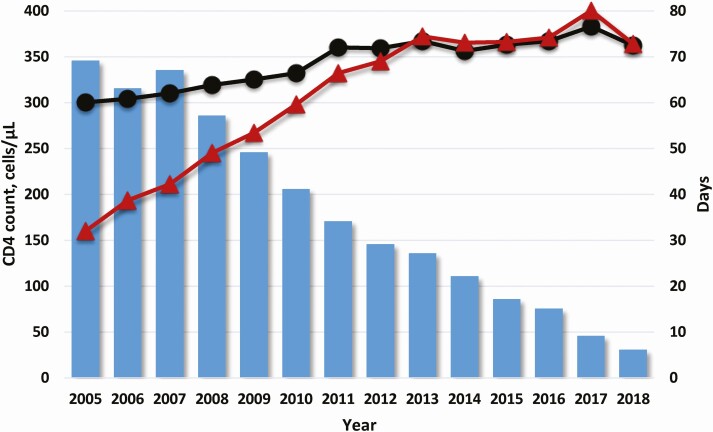
Median time from entry into care to ART prescription declined over the study period (Figure 1, Supplementary Material). Median days from entry into care to ART prescription was 69 (IQR, 20–544) in 2005, 29 (IQR, 11–73) in 2012 (the year DHHS released universal treatment guidelines), and 6 (IQR, 0–16) in 2018. In 2018, 35% of participants who entered HIV care were prescribed ART the same day.
Figure 1.
Median CD4 count at entry into care (black circles) and at antiretroviral therapy (ART) prescription (red triangles) overlaying median days from entry into care to ART prescription (blue bars) by calendar year.
Median CD4 count at entry into care increased from 2005 to 2011 and then remained generally stable through 2018 (Figure 1, Supplementary Material). Median CD4 count at entry into care was 300 cells/µL (IQR, 112–480) in 2005, 359 cells/µL (IQR, 171–542) in 2012, and 362 cells/µL (IQR: 189–534) in 2018.
Median CD4 count at ART prescription increased from 2005 to 2013 and remained generally stable through 2018 (Figure 1, Supplementary Material). Median CD4 count at ART prescription was 160 cells/µL (IQR, 51–291) in 2005, 345 cells/µL (IQR, 177–504) in 2012, and 364 cells/µL (IQR, 187–541) in 2018.
Median time from entry into care to ART prescription declined and median CD4 count at both time points increased over the study period in all subgroups (we note limited numbers of participants living in the Midwest in the latter years of the study period; Supplementary Material). For the majority of the study period, participants of non-White race and living in the South had lower median CD4 counts at entry into care and at ART prescription than participants who were White and living in other regions of the United States, respectively. Median CD4 counts at entry into care and at ART prescription were similar over time by sex and IDU status. Early in the study period, median time to ART prescription was higher in women and participants with a history of IDU than in men and participants with no IDU history, respectively. There were no notable disparities in median time to ART prescription after 2009.
DISCUSSION
Time from entry into HIV care to ART prescription has substantially shortened throughout the modern ART era, reflecting evidence that supports early ART initiation, evolving treatment guidelines, and adoption of “treat all” in clinical practice. In this descriptive study of NA-ACCORD participants, median time from entry into care to ART prescription declined from more than 3 months in 2005 to less than 1 week in 2018.
We observed a slight increase in median CD4 count at entry into HIV care over the study period, indicating modest progress toward earlier diagnosis and referral to care, which is critical for reaching 90–90–90 targets and ending the HIV epidemic [7, 8]. A significant proportion of participants entered into care with CD4 counts <350 cells/µL throughout the study period, which suggests continued challenges with HIV screening and may have implications for policy and programming that promote expanded testing and efficient linkage to care. We observed a more apparent increase in median CD4 count at ART prescription over the study period, which was expected with changing treatment guidelines. In our study population, the gap between median CD4 count at entry into care and at ART prescription had been narrowing since 2005, and there was little difference between median CD4 counts at the 2 time points after 2012, likely due to implementation of universal treatment initiation practices [9].
We note that this study used data collected during the course of clinical care and not primarily for research purposes. We observed a lower number of eligible study participants who entered care and received ART prescriptions in the latter years of the study period than in earlier years, likely due to data reporting lags, though we do not expect that this impacted our overall findings. Additionally, due to NA-ACCORD eligibility criteria, participants of this study were restricted to adults engaged in HIV care. Compared with all people diagnosed with HIV in the United States, the NA-ACCORD enrolls a higher proportion of men and participants of White race. Women and people of color are more likely to face structural barriers to HIV care [10–12], which may impact the generalizability of our findings.
These findings indicate progress toward prompt diagnosis, referral to care, and ART prescription in PWH in the United States between 2005 and 2018. Further increases in median CD4 count at both entry into care and at ART prescription may be possible with expanded implementation of effective test-and-treat strategies, particularly those that focus on reaching women, racial and ethnic minorities, and other marginalized populations disproportionately affected by HIV.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments.North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Collaborating Cohorts and Representatives: AIDS Clinical Trials Group Longitudinal Linked Randomized Trials: Constance A. Benson and Ronald J. Bosch. AIDS Link to the IntraVenous Experience: Gregory D. Kirk Emory-Grady. HIV Clinical Cohort: Vincent Marconi and Jonathan Colasanti. Fenway Health HIV Cohort: Kenneth H. Mayer and Chris Grasso. HAART Observational Medical Evaluation and Research: Robert S. Hogg, P. Richard Harrigan, Julio S. G. Montaner, Benita Yip, Julia Zhu, Kate Salters, and Karyn Gabler. HIV Outpatient Study: Kate Buchacz and Jun Li. HIV Research Network: Kelly A Gebo and Richard D. Moore. Johns Hopkins HIV Clinical Cohort: Richard D. Moore and John T. Carey. Special Immunology Unit Patient Care and Research Database, Case Western Reserve University: Benigno Rodriguez. Kaiser Permanente Mid-Atlantic States: Michael A. Horberg. Kaiser Permanente Northern California: Michael J. Silverberg. Longitudinal Study of Ocular Complications of AIDS: Jennifer E. Thorne. Multicenter AIDS Cohort Study/Women’s Interagency HIV Study (MACS/WIHS) Combined Cohort Study: Todd Brown, Phyllis Tien, and Gypsyamber D’Souza. Maple Leaf Medical Clinic: Frederic Crouzat, Mona Loutfy, Graham Smith, and Meenakshi Gupta. McGill University Health Centre, Chronic Viral Illness Service Cohort: Marina B. Klein. Multicenter Hemophilia Cohort Study–II: Charles Rabkin. Ontario HIV Treatment Network Cohort Study: Abigail Kroch, Ann Burchell, Adrian Betts, and Joanne Lindsay. Parkland/UT Southwestern Cohort: Ank Nijhawan. Retrovirus Research Center, Universidad Central del Caribe, Bayamon Puerto Rico: Robert F. Hunter-Mellado and Angel M. Mayor. Southern Alberta Clinic Cohort: M. John Gill. Study of the Consequences of the Protease Inhibitor Era: Jeffrey N. Martin. Study to Understand the Natural History of HIV/AIDS in the Era of Effective Therapy: Jun Li and John T. Brooks. University of Alabama at Birmingham 1917 Clinic Cohort: Michael S. Saag, Michael J. Mugavero, and James Willig. University of California at San Diego: Laura Bamford and Maile Karris. University of North Carolina at Chapel Hill HIV Clinic Cohort: Joseph J. Eron and Sonia Napravnik. University of Washington HIV Cohort: Mari M. Kitahata and Heidi M. Crane. Vanderbilt Comprehensive Care Clinic HIV Cohort: Timothy R. Sterling, David Haas, Peter Rebeiro, and Megan Turner. Veterans Aging Cohort Study: Lesley Park and Amy Justice.
NA-ACCORD Study Administration, Executive Committee: Richard D. Moore, Keri N. Althoff, Stephen J. Gange, Mari M. Kitahata, Jennifer S. Lee, Michael S. Saag, Michael A. Horberg, Marina B. Klein, Rosemary G. McKaig, and Aimee M. Freeman. Administrative Core: Richard D. Moore, Keri N. Althoff, and Aimee M. Freeman.
Data Management Core: Mari M. Kitahata, Stephen E. Van Rompaey, Heidi M. Crane, Liz Morton, Justin McReynolds, and William B. Lober.
Epidemiology and Biostatistics Core: Stephen J. Gange, Jennifer S. Lee, Brenna Hogan, Bin You, Elizabeth Humes, Lucas Gerace, Cameron Stewart, and Sally Coburn.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH) or the US Centers for Disease Control and Prevention (CDC).
Financial support. This work was supported by National Institutes of Health (NIH) grants U01AI069918, F31AI124794, F31DA037788, G12MD007583, K01AI093197, K01AI131895, K23EY013707, K24AI065298, K24AI118591, K24DA000432, KL2TR000421, N01CP01004, N02CP055504, N02CP91027, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050409, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01DA011602, R01DA012568, R01 AG053100, R24AI067039, R34DA045592, U01AA013566, U01AA020790, U01AI038855, U01AI038858, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01DA03629, U01DA036935, U10EY008057, U10EY008052, U10EY008067, U01HL146192, U01HL146193, U01HL146194, U01HL146201, U01HL146202, U01HL146203, U01HL146204, U01HL146205, U01HL146208, U01HL146240, U01HL146241, U01HL146242, U01HL146245, U01HL146333, U24AA020794,U54MD007587, UL1RR024131, UL1TR000004, UL1TR000083, UL1TR002378, Z01CP010214, and Z01CP010176; contracts CDC-200-2006-18797 and CDC-200-2015-63931 from the Centers for Disease Control and Prevention (CDC); contract 90047713 from the Agency for Healthcare Research and Quality, USA; contract 90051652 from the Health Resources and Services Administration, USA; the Grady Health System; grants CBR-86906, CBR-94036, HCP-97105, and TGF-96118 from the Canadian Institutes of Health Research; and the Ontario Ministry of Health and Long Term Care, and the Government of Alberta. Additional support was provided by the National Institute of Allergy and Infectious Diseases; National Cancer Institute; National Heart, Lung, and Blood Institute; Eunice Kennedy Shriver National Institute of Child Health and Human Development; National Human Genome Research Institute; National Institute for Mental Health; National Institute on Drug Abuse; National Institute on Aging; National Institute of Dental and Craniofacial Research; National Institute of Neurological Disorders and Stroke; National Institute of Nursing Research; National Institute on Alcohol Abuse and Alcoholism; National Institute on Deafness and Other Communication Disorders; and National Institute of Diabetes and Digestive and Kidney Diseases.
Potential conflicts of interest. J. S. L. reports grants paid to John Hopkins from NIH during the conduct of the study. E. A. H. reports grants paid to John Hopkins from NIH during the conduct of the study. B. C. H. reports grants paid to John Hopkins from NIH during the conduct of the study. M. J. G. reports grants from NIH to the University of Calgary (subgrant from Johns Hopkins) during the conduct of the study and has received personal fees while serving as an ad hoc member of HIV national advisory boards to Merck, Gilead Sciences, and Viiv Healthcare (all outside of this work). J. J. E. Jr. receives personal fees as a consultant to Merck, Viiv Healthcare, and Gilead Sciences; receives personal fees from Janssen; and is an investigator on clinical research projects funded by contracts to the University of North Carolina at Chapel Hill from Gilead Sciences, Viiv Healthcare, and Janssen (all outside of this work). T. R. S. reports grants from NIH (NA-ACCORD) during the conduct of the study. V. D. L. reports grants paid to their institution (CIHR-PJT 148595, CIHR-PJT 156147) outside the submitted work. M. J. S. has received research funding from Gilead Sciences outside this work. K. N. A. has received consulting fees from the All of Us Study (NIH) and TrioHealth Inc (serves on scientific advisory board) and has received personal fees as a consultant for coronavirus disease 2018 communication to MedIQ (all outside of this work). All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
North American AIDS Cohort Collaboration on Research and Design:
Constance A Benson, Ronald J Bosch, Gregory D Kirk Emory-Grady, Kenneth H Mayer, Chris Grasso, Robert S Hogg, P Richard Harrigan, Julio S G Montaner, Benita Yip, Julia Zhu, Kate Salters, Karyn Gabler, Kate Buchacz, Jun Li, Kelly A Gebo, Richard D Moore Johns, Richard D Moore, John T Carey, Benigno Rodriguez, Michael A Horberg, Michael J Silverberg, Jennifer E Thorne, Todd Brown, Phyllis Tien, Gypsyamber D’Souza, Frederic Crouzat, Mona Loutfy, Graham Smith, Meenakshi Gupta, Marina B Klein, Charles Rabkin, Abigail Kroch, Ann Burchell, Adrian Betts, Joanne Lindsay, Ank Nijhawan, Robert F Hunter-Mellado, Angel M Mayor, M John Gill, Jeffrey N Martin, Jun Li, John T Brooks, Michael S Saag, Michael J Mugavero, James Willig, Laura Bamford, Maile Karris, Joseph J Eron, Sonia Napravnik, Mari M Kitahata, Heidi M Crane, Timothy R Sterling, David Haas, Peter Rebeiro, Megan Turner, Lesley Park, Amy Justice, Richard D Moore, Keri N Althoff, Stephen J Gange, Mari M Kitahata, Jennifer S Lee, Michael S Saag, Michael A Horberg, Marina B Klein, Rosemary G McKaig, Aimee M Freeman, Richard D Moore, Keri N Althoff, Aimee M Freeman, Mari M Kitahata, Stephen E Van Rompaey, Heidi M Crane, Liz Morton, Justin McReynolds, William B Lober, Stephen J Gange, Jennifer S Lee, Brenna Hogan, Bin You, Elizabeth Humes, Lucas Gerace, Cameron Stewart, and Sally Coburn
References
- 1.Kitahata MM, Gange SJ, Abraham AG, et al. ; NA-ACCORD Investigators . Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med 2009; 360:1815–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panel of Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at https://aidsinfo.nih.gov/guidelines/archive/adult-and-adolescent-guidelines/. Accessed 29 July 2020.
- 3.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV;2015. Available at https://apps.who.int/iris/handle/10665/186275. Accessed 31 July 2020.
- 4.Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Int J Epidemiol 2007; 36:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Althoff KN, Buchacz K, Hall HI, et al. U.S. trends in antiretroviral therapy use, HIV RNA plasma viral loads, and CD4 T-lymphocyte cell counts among HIV-infected persons, 2000 to 2008. Ann Intern Med 2012; 157:325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Althoff KN, Wong C, Hogan B, et al. ; North American AIDS Cohort Collaboration on Research and Design . Mind the gap: observation windows to define periods of event ascertainment as a quality control method for longitudinal electronic health record data. Ann Epidemiol 2019; 33:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joint United Nations Programme on HIV/AIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Joint United Nations Programme on HIV/AIDS (UNAIDS); 2014. Available at https://www.unaids.org/en/resources/documents/2017/90-90-90. Accessed 31 July 2020. [PubMed]
- 8.Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP.. Ending the HIV epidemic: a plan for the United States. JAMA 2019; 321:844–5. [DOI] [PubMed] [Google Scholar]
- 9.Brazier E, Maruri F, Duda SN, et al. ; IeDEA Consortium . Implementation of “Treat-all” at adult HIV care and treatment sites in the Global IeDEA Consortium: results from the site assessment survey. J Int AIDS Soc 2019; 22:e25331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aziz M, Smith KY. Challenges and successes in linking HIV-infected women to care in the United States. Clin Infect Dis 2011; 52 Suppl 2:S231–7. [DOI] [PubMed] [Google Scholar]
- 11.Freeman R, Gwadz MV, Silverman E, et al. Critical race theory as a tool for understanding poor engagement along the HIV care continuum among African American/Black and Hispanic persons living with HIV in the United States: a qualitative exploration. Int J Equity Health 2017; 16:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Messer LC, Quinlivan EB, Parnell H, et al. Barriers and facilitators to testing, treatment entry, and engagement in care by HIV-positive women of color. AIDS Patient Care STDS 2013; 27:398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.