Abstract
Background
Infective endocarditis (IE) is the most feared complication of Staphylococcus aureus bacteremia (SAB). Transesophageal echocardiogram (TEE) is generally recommended for all patients with SAB; however, supporting data for this are limited. We previously developed a scoring system, “PREDICT,” that quantifies the risk of IE and identifies patients who would most benefit most from undergoing TEE. The current prospective investigation aims to validate this score.
Methods
We prospectively screened all consecutive adults (≥18 years) hospitalized with SAB at 3 Mayo Clinic sites between January 2015 and March 2017.
Results
Of 220 patients screened, 199 with SAB met study criteria and were included in the investigation. Of them, 23 (11.6%) patients were diagnosed with definite IE within 12 weeks of initial presentation based on modified Duke’s criteria. Using the previously derived PREDICT model, the day 1 score of ≥4 had a sensitivity of 30.4% and a specificity of 93.8%, whereas a day 5 score of ≤2 had a sensitivity and negative-predictive value of 100%. Additional factors including surgery or invasive procedure in the past 30 days, prosthetic heart valve, and higher number of positive blood culture bottles in the first set of cultures were associated with increased risk of IE independent of the day 5 risk score.
Conclusions
We validated the previously developed PREDICT scoring tools for stratifying risk of IE, and the need for undergoing a TEE, among cases of SAB. We also identified other factors with predictive potential, although larger prospective studies are needed to further evaluate possible enhancements to the current scoring system.
Keywords: Staphylococcus aureus, infective endocarditis, transesophageal echocardiography, bacteremia, bloodstream infection
We validated the previously developed PREDICT scoring system to evaluate the need for transesophageal echocardiogram in Staphylococcus aureus bacteremia. We also attempted to identify variables to improve the performance of this prediction model.
Staphylococcus aureus is a leading cause of community and healthcare-associated bacteremia, which has been associated with significant morbidity and mortality, and can be complicated by metastatic foci of infection, which harbor mortality risks [1]. The population incidence of S. aureus bacteremia (SAB) in the industrialized world ranges from 10 to 45 per 100 000 person-years [2–4]. Infective endocarditis (IE) is one of the most feared complications of SAB. Moreover, this organism has been identified in multiple studies as a risk factor associated with mortality in patients with IE.
The prevalence of IE among patients with SAB has been wide-ranging and this is likely related to the wide-ranging characteristics of cohorts included in investigations. In studies that have been influenced by selection bias due to the exclusion of individuals without transesophageal echocardiogram (TEE), there may have been an overestimation of the rates of IE [5–7]. The true prevalence of IE in SAB, therefore, may be as low as 6–14% [8–12]. Furthermore, the risk of IE may differ based on several factors, including the presence of an underlying prosthetic heart valve or other types of cardiac devices, nosocomial versus community acquisition, and duration of bacteremia.
Until recently, TEE has been generally recommended in patients presenting with SAB, irrespective of the presence or absence of risk factors associated with IE [13–15]. However, TEE is an invasive procedure that can result in complications, albeit uncommon, increase healthcare cost, may not be feasible in some patients due to underlying medical/surgical conditions, and is not readily available in some medical centers. Thus, the development of an individualized risk-based approach to guide utilization of TEE in patients with SAB is warranted [16]. In this regard, several predictors of IE in SAB have already been identified [17–21]. Our group previously published a novel scoring system, “PREDICT” (Predicting Risk of Endocarditis Using a Clinical Tool) that included an individualized risk-scoring system to identify patients with SAB who are more likely to harbor IE and would benefit from TEE (Table 1) [17]. Although the findings of PREDICT were based on a large (N = 678) cohort, patient selection was done retrospectively. Therefore, the current study was designed and prospectively conducted to validate the PREDICT scoring system.
Table 1.
Calculation of PREDICT Score
| CIED | Onset of SAB | |||||||
|---|---|---|---|---|---|---|---|---|
| ICD | PPM | Neither | Community | Healthcare | Nosocomial | Prolonged Bacteremia ≥72 Hours | Total Risk Score | |
| Day 1, points | 2 | 3 | 0 | 2 | 1 | 0 | … | Day 1 score ≥4: perform TEE now; <4: wait until day 5 |
| Day 5, points | 2 | 3 | 0 | 2 | 1 | 0 | 2 | Day 5 score ≥2: Perform TEE; <2: no TEE |
Abbreviations: CIED, cardiovascular implantable electronic device; ICD, implantable cardioverter defibrillator; PPM, permanent pacemaker; PREDICT, Predicting Risk of Endocarditis Using a Clinical Tool; SAB, Staphylococcus aureus bacteremia; TEE, transesophageal echocardiogram.
METHODS
Study Overview and Subjects
PREDICT II was an observational prospective cohort study conducted between January 2015 and March 2017. All consecutive adults (≥18 years) having at least 1 blood culture positive for S. aureus from 3 Mayo Clinic sites (Minnesota, Arizona, and Florida) were screened. Enrolled patients were required to have available follow-up data for 12 weeks after hospital discharge. Patients were identified through review of respective laboratory blood culture results and were then approached for informed consent. Study protocol and consent forms were reviewed and approved by Mayo Clinic Institutional Review Board.
We collected and managed study data using electronic data-capture tools hosted at the Mayo Clinic. REDCap (Research Electronic Data Capture) is a secure, Web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages, and (4) procedures for importing data from external sources [22].
We prospectively collected data including patient demographics, clinical characteristics, and healthcare contact within 90 days preceding hospitalization, including invasive procedures and setting of infection acquisition.
Data Acquisition and Definitions
Staphylococcus aureus bacteremia was classified as nosocomial, healthcare associated, or as community acquired, as previously described by Friedman et al [23]. Prolonged bacteremia was defined as positive blood cultures 72 hours or more after the first positive blood culture result. Only patients who met the modified Duke’s criteria for definite IE were included. Echocardiographic evidence of IE was defined as presence of an oscillating intracardiac mass, perivalvular abscess, new valvular regurgitation, or new dehiscence of a prosthetic valve [24].
Statistical Methods
To validate the performance of the previously derived prediction models in the current dataset, logistic regression models were used to predict the risk of IE from the simple point-based summary indexes, separately for day 1 and 5 scores. The models were then refitted by including all the separate constituent variables in place of the single risk score, and the results of both model formulations are presented for the sake of completeness. Model performance was evaluated without refitting the model coefficients to the new data but rather using “frozen” (ie, previously derived) regression coefficients from the original model fits, so as to avoid any unfair advantage. Statistical indexes, such as the c-statistic (equivalently, area under the receiver operating characteristic curve) and Nagelkerke’s generalized R2 index, were used to validate each model’s predictive discrimination. Model calibration was assessed graphically with smooth calibration curves, and formally with the Cox calibration test (a test for zero intercept given that the slope is fixed to be 1). The calibration curves were estimated using a locally weighted scatterplot smoother (lowess) to relate the model-predicted probabilities of IE (x axis) to the observed outcomes of whether or not IE actually occurred (y axis). For reference, the 45° line of identity representing ideal model calibration is displayed along with symbols for subgroup proportions when grouping the predictions into quintiles (5 equally sized groups); deviations from the reference line indicate bias in the model predictions. Tests for calibration involved deriving the predicted log odds of IE from the multivariable logistic model (formulated from the previous study and applied to the current sample), then fitting a second logistic model with only this linear predictor as an offset variable, and finally testing the intercept term for statistical significance. We also explored the possibility of updating risk scores with additional information available at the time of SAB diagnosis. Because the effective sample size available (ie, number of IE cases) did not permit a full multivariable inspection of new potential risk factors, each factor was gauged for predictive potential in separately fitted logistic models that adjusted for risk by using the patient’s day 5 risk score as a covariate. A likelihood ratio test was constructed from the model to test the adjusted association between that variable and the IE outcome (ie, test for incremental value beyond the risk score itself). All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Study Population
A total of 220 patients were admitted with SAB during the study period from January 2015 to March 2017. Thirteen patients were excluded as they declined to participate in the study; 5 were excluded because they died or were discharged prior to consent; and 3 were pediatric cases. After exclusions, 199 adult patients with SAB (41.7% community onset, 44.2% healthcare associated, and 14.1% nosocomial) who met study criteria were included in the final analysis. Baseline characteristics are summarized in Table 2. The majority of patients were male (67.3%) and the median age was 63.9 years (interquartile range [IQR], 50.1–72.0 years). A cardiovascular implantable electronic device (CIED) was present in 31 (15.5%) patients, of whom 13 had permanent pacemakers (PPMs) and 18 had implantable cardioverter defibrillators (ICDs). Only 5.5% patients had a prosthetic heart valve. Fifteen percent of S. aureus isolates were methicillin resistant (MRSA).
Table 2.
Patient Cohort of Pertinent Clinical Features and an Assessment of Predictive Performance Beyond the Day 5 Risk Score
| Variable | n | % (n) Missing | IE (n = 23) | No IE (n = 176) | Unadjusted P Valuea | D5 Risk Score–adjusted P Valueb |
|---|---|---|---|---|---|---|
| Age at admission,c years | 199 | 0% | 67.6 (51.4, 82.3) | 63.4 (50.0, 71.5) | .452c | .785 |
| Male | 199 | 0% | 69.6% (16) | 67.0% (118) | .809 | .898 |
| Caucasian | 199 | 0% | 95.7% (22) | 85.2% (150) | .170 | .207 |
| Diabetes mellitus | 197 | 1.0% (2) | 13.0% (3) | 33.3% (58) | .048 | .188 |
| Device | 199 | 0% | ||||
| Neither | 56.5% (13) | 88.1% (155) | ||||
| PPM | 17.4% (4) | 5.1% (9) | ||||
| ICD | 26.1% (6) | 6.8% (12) | ||||
| SAB onset | 199 | 0% | ||||
| Community | 65.2% (15) | 38.6% (68) | ||||
| Healthcare | 34.8% (8) | 45.5% (80) | ||||
| Nosocomial | 0.0% (0) | 15.9% (28) | ||||
| Central IV catheter prior to SAB | 199 | 0% | 21.7% (5) | 25.0% (44) | .733 | .122 |
| Hemodialysis | 198 | 0.5% (1) | 13.0% (3) | 11.4% (20) | .820 | .531 |
| Malignancy | 196 | 1.5% (3) | 13.0% (3) | 22.5% (39) | .297 | .693 |
| Immunocompromised (>30 days) | 199 | 0% | 8.7% (2) | 26.1% (46) | .066 | .223 |
| Solid-organ transplant | 196 | 1.5% (3) | 0.0% (0) | 5.2% (9) | .275 | .201 |
| HCST | 199 | 0% | 0.0% (0) | 4.5% (8) | .600d | .193 |
| Cardiomyopathy | 198 | 0.5% (1) | 39.1% (9) | 21.1% (37) | .055 | .880 |
| Cerebrovascular accident | 194 | 2.5% (5) | 17.4% (4) | 7.0% (12) | .090 | .056 |
| BMI,c kg/m2 | 198 | 0.5% (1) | 26.5 (23.2, 29.1) | 28.5 (24.6, 33.0) | .075c | .093 |
| Intravenous drug use | 195 | 2.0% (4) | 0.0% (0) | 1.7% (3) | >.999d | .672 |
| Immobilization | 199 | 0% | 4.3% (1) | 5.1% (9) | .874 | .798 |
| Surgery 30 days prior to SAB | 199 | 0% | 0.0% (0) | 19.9% (35) | .018 | .040 |
| Prosthetic valve | 199 | 0% | 21.7% (5) | 3.4% (6) | <.001 | .002 |
| CRT | 196 | 1.5% (3) | 8.7% (2) | 0.6% (1) | .037d | .147 |
| VAD | 199 | 0% | 8.7% (2) | 4.5% (8) | .392 | .649 |
| Prosthetic joint | 198 | 0.5% (1) | 8.7% (2) | 10.3% (18) | .812 | .310 |
| Residence at time of SAB = home | 195 | 2.0% (4) | 100.0% (23) | 81.4% (140) | .024 | .009 |
| Received IV infusion/wound care in prior 30 days | 198 | 0.5% (1) | 13.0% (3) | 40.0% (70) | .012 | .135 |
| Hospitalized for ≥2 days in prior 90 days | 195 | 2.0% (4) | 17.4% (4) | 43.0% (74) | .018 | .152 |
| MSSA | 199 | 0% | 82.6% (19) | 70.5% (124) | .223 | .237 |
| Duration of symptomsa | 199 | 0% | 3.0 (2.0, 7.0) | 2.0 (1.0, 5.0) | .146c | .375 |
| Level of care on admission = ICU | 199 | 0% | 30.4% (7) | 17.0% (30) | .121 | .307 |
| Fever on presentation | 199 | 0% | 73.9% (17) | 60.8% (107) | .222 | .165 |
| Heart failure symptoms on presentation | 197 | 1.0% (2) | 21.7% (5) | 4.0% (7) | <.001 | .001 |
| SIRS | 198 | 0.5% (1) | 78.3% (18) | 72.6% (127) | .562 | .374 |
| Unknown source of SAB | 199 | 0% | 47.8% (11) | 19.3% (34) | .002 | .159 |
| Hours to first positive blood culturec | 199 | 0% | 11.0 (8.0, 14.0) | 15.0 (11.5, 18.0) | <.001c | .002 |
| Percentage of bottles positive | 199 | 0% | .006 | .022 | ||
| ≤50% | 4.3% (1) | 36.4% (64) | ||||
| 51–99% | 17.4% (4) | 16.5% (29) | ||||
| 100% | 78.3% (18) | 47.2% (83) |
Abbreviations: BMI, body mass index; CRT, cardiac resynchronization therapy; D5, day 5; HCST, hematopoietic stem cell transplantation; ICD, implantable cardioverter defibrillator; ICU, intensive care unit; IE, infective endocarditis; IV, intravenous; MSSA, methicillin-susceptible Staphylococcus aureus; PPM, permanent pacemaker; SAB, Staphylococcus aureus bacteremia; SIRS, systemic inflammatory response syndrome; VAD, ventricular assist device.
aUnadjusted P values are from tests of association (Wilcoxon rank-sum test, Pearson chi-square test, or Fisher’s exact test as appropriate).
bD5 risk-adjusted P values are from the likelihood ratio test in logistic regression models with D5 risk score as a continuous covariate, which are used to assess the adjusted association between that variable and the IE outcome (ie, to assess incremental value beyond the risk score).
cContinuous variables are reported as median (25th, 75th percentiles) and are tested for unadjusted association with IE status using the Wilcoxon rank-sum test.
dThe P value from Fisher’s exact test was used when an expected cell frequency was <2.
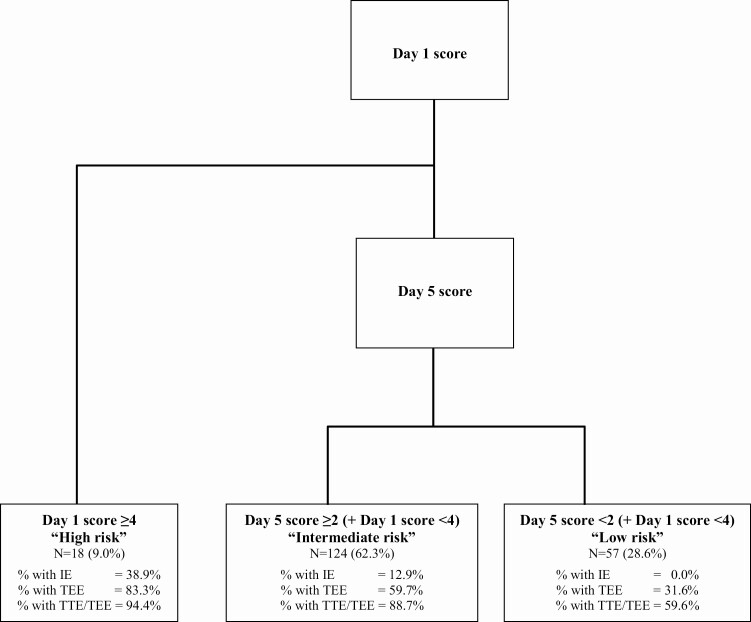
Overall, 53.8% of patients underwent TEE within 12 weeks of SAB diagnosis. Median time to obtain TEE was 3.0 (IQR, 1.0–4.0) days in those with IE versus 4.0 (IQR, 3.0–6.0) days in those without IE (P = .003). Nuclear imaging studies were conducted in 24 (12%) patients, including tagged white blood cell (WBC) scan in 15 and 18F-fluorodeoxyglucose positron emission tomography–computed tomography (18F-FDG PET/CT) scan in 9 individuals (Supplementary Table 1). Cardiac and/or extracardiac foci of infection were identified in 17 (71%) of these patients. A total of 23 patients (11.6%) fulfilled the modified Duke’s criteria for definite IE, of whom 18 (78.2%) had native valve IE and 5 (21.7%) had prosthetic valve IE. None of the patients with nosocomial SAB had IE, whereas 15 of 83 patients (18.1%) with community-acquired SAB had IE. In our cohort, 18 (9.0%) individuals had a day 1 score of 4 or higher, while 124 (62.3%), and 57 (28.6%) individuals had a lower day 1 score followed by a day 5 score of 2 or higher and less than 2, respectively (Figure 1).
Figure 1.
PREDICT II scores, rates of infective endocarditis, and echocardiogram use in the study population. Abbreviations: IE, infective endocarditis; PREDICT, Predicting Risk of Endocarditis Using a Clinical Tool; TEE, transesophageal echocardiogram; TTE, transthoracic echocardiogram.
Validation of the PREDICT Model
The clinical prediction models for identifying risk of IE at day 1 and day 5 following an SAB diagnosis were previously developed based on a retrospective study (PREDICT) design from a cohort of 678 patients (of whom 12.5% had IE) [17]. The risk scores included the following variables: onset of SAB, presence of CIED, and prolonged bacteremia (day 5 only). The complete list of study variables is presented in Table 2. For the current cohort, the median risk score was 2 for both days 1 (IQR, 1–2) and 5 (IQR, 1–4), with values for the 23 patients with IE ranging from 1 to 5 for day 1 and from 2 to 7 for day 5.
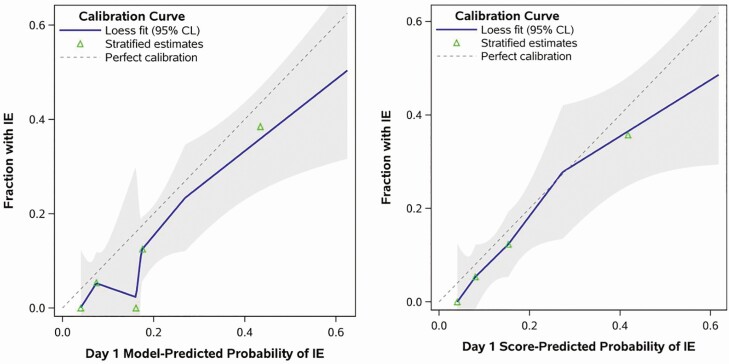
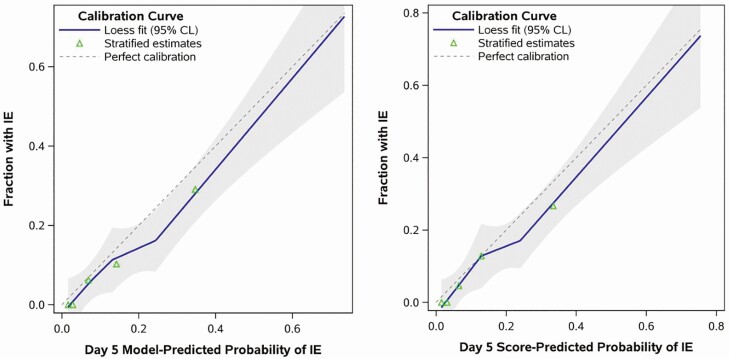
To evaluate model performance, logistic regression using the original model coefficients for the summary day 1 and day 5 risk scores was applied to the current data. The results demonstrated good discrimination in predicting IE, with both models stratifying patient risk at least as well in the validation dataset (eg, c-statistic = 0.754 and 0.824 for day 1 and 5, respectively) as they did in the original study (c-statistic = 0.720 and 0.794). Model discrimination statistics compared similarly when all the constituent risk factors were included as separate variables in the model rather than the summary risk score (Supplementary Table 2). Test performance statistics for all possible cutoffs of day 1 and day 5 prediction tools are reported in Table 3. A day 1 score of 4 or higher demonstrated a sensitivity of 30.4% and specificity of 93.8% compared with the original PREDICT model where the sensitivity and specificity were 21.2% and 95.6%, respectively. The sensitivity and specificity of a day 5 score of 2 or higher were 100% and 32.4%, which were generally comparable to the previous study with a sensitivity and specificity of 94% and 41.1%, respectively [17]. In addition, the published models appeared to be well calibrated to the validation cohort for both day 1 and day 5 versions, judging by the close proximity of the calibration curves to the hypothetical perfect calibration line (Figures 2 and 3). There was also no detectable evidence of miscalibration via statistical testing, based on Cox’s calibration test of zero intercept (day 1: P = .099; day 5: P = .160).
Table 3.
Test Performance of Day 1 and Day 5 Prediction Tools
| Cutoff j | % (n) With Score ≥ j | Sensitivity (95% CI), % | Specificity (95% CI), % | PPV (95% CI), % | NPV (95% CI), % |
|---|---|---|---|---|---|
| Day 1 score ≥ j | |||||
| 1 | 88.4 (176) | 100.0 (85.2 to 100.0) | 13.1 (8.5 to 19.0) | 13.1 (8.5 to 19.0) | 100.0 (85.2 to 100.0) |
| 2 | 50.8 (101) | 82.6 (61.2 to 95.0) | 53.4 (45.8 to 60.9) | 18.8 (11.7 to 27.8) | 95.9 (89.9 to 98.9) |
| 3 | 14.1 (28) | 43.5 (23.2 to 65.5) | 89.8 (84.3 to 93.8) | 35.7 (18.6 to 55.9) | 92.4 (87.4 to 95.9) |
| 4 | 9.0 (18) | 30.4 (13.2 to 52.9) | 93.8 (89.1 to 96.8) | 38.9 (17.3 to 64.3) | 91.2 (86.0 to 94.9) |
| 5 | 3.0 (6) | 13.0 (2.8 to 33.6) | 98.3 (95.1 to 99.6) | 50.0 (11.8 to 88.2) | 89.6 (84.4 to 93.6) |
| Day 5 score ≥ j | |||||
| 1 | 92.0 (183) | 100.0 (85.2 to 100.0) | 9.1 (5.3 to 14.3) | 12.6 (8.1 to 18.3) | 100.0 (79.4 to 100.0) |
| 2 | 71.4 (142) | 100.0 (85.2 to 100.0) | 32.4 (25.5 to 39.8) | 16.2 (10.6 to 23.3) | 100.0 (93.7 to 100.0) |
| 3 | 49.7 (99) | 91.3 (72.0 to 98.9) | 55.7 (48.0 to 63.2) | 21.2 (13.6 to 30.6) | 98.0 (93.0 to 99.8) |
| 4 | 30.2 (60) | 69.6 (47.1 to 86.8) | 75.0 (67.9 to 81.2) | 26.7 (16.1 to 39.7) | 95.0 (89.9 to 98.0) |
| 5 | 9.0 (18) | 39.1 (19.7 to 61.5) | 94.9 (90.5 to 97.6) | 50.0 (26.0 to 74.0) | 92.3 (87.4 to 95.7) |
| 6 | 5.5 (11) | 30.4 (13.2 to 52.9) | 97.7 (94.3 to 99.4) | 63.6 (30.8 to 89.1) | 91.5 (86.5 to 95.1) |
| 7 | 2.0 (4) | 13.0 (2.8 to 33.6) | 99.4 (96.9 to 100.0) | 75.0 (19.4 to 99.4) | 89.7 (84.6 to 93.6) |
Abbreviations: CI, confidence interval; NPV, negative-predictive value; PPV, positive-predictive value.
Figure 2.
Calibration of the day 1 prediction tool. The figure shows the calibration curves for predicting the risk of IE using the published day 1 scoring system. Predictions were generated from logistic regression in 2 ways, by modeling both of the constituent risk factors for the day 1 score (1) as 2 separate variables (“Model-Predicted” results in the left panel) and (2) as a single summary index (“Score-Predicted” results in the right panel). Calibration is judged by the Loess-estimated calibration curve (solid line) and its proximity to the reference line representing perfect calibration (dashed line). Shaded regions are 95% confidence limits; symbols depict observed proportions with IE for 5 equally sized subgroups. Abbreviations: CL, confidence limit; IE, infective endocarditis.
Figure 3.
Calibration of the day 5 prediction tool. The figure shows the calibration curves for predicting risk of IE using the published day 5 scoring system. Predictions were generated from logistic regression in 2 ways, by modeling all of the constituent risk factors for the day 5 score (1) as 3 separate variables (“Model-Predicted” results in the left panel) and (2) as a single summary index (“Score-Predicted” results in the right panel). Calibration is judged by the Loess-estimated calibration curve (solid line) and its proximity to the reference line representing perfect calibration (dashed line). Shaded regions are 95% confidence limits; symbols depict observed proportions with IE for 5 equally sized subgroups. Abbreviations: CL, confidence limit; IE, infective endocarditis.
Using data available at the time of SAB diagnosis above, we explored the possibility that updating the existing day 5 prediction model with additional predictors would improve the original scores’ predictions. However, the low number of IE cases did not permit a full multivariable inspection of candidate risk factors and thus these results, yielded from separately fitted logistic models with the day 5 risk score as an adjusting covariate, should be interpreted with caution and considered hypothesis generating.
Of the potential risk factors examined, recent surgery, presence of a prosthetic heart valve, community-acquired SAB, heart failure, shorter time to positivity of blood culture bottles, and higher number of positive blood culture bottles in the first set of cultures were associated with having IE (risk-adjusted P < .05) independent of the day 5 risk score (Table 2).
DISCUSSION
In the current investigation (PREDICT II), we validate the previously developed scoring system to predict the risk of IE and determine the need for TEE in patients presenting with SAB. Results from our prospective analysis of the PREDICT score were similar to those observed in the previously published retrospective analysis [17]. In the current population, day 1 scores of 4 or higher had a high specificity of 94%, whereas the day 5 score of 2 had a high negative-predictive value of 100%. In addition, other variables (recent surgery, presence of a prosthetic heart valve, heart failure symptoms, shorter time to positive blood cultures for S. aureus, and increased percentage of bottles positive on first culture) were identified as having predictive potential, which suggests improvements to the current tool’s ability to predict underlying IE are possible. However, due to the small effective sample size in the study population, we were not able to conduct a more rigorous multivariable analysis to further evaluate the predictive value of these additional variables. Future studies, however, are needed to further score and validate these potential predictors.
Previously validated scores in SAB cases include VIRSTA, which utilizes IE predictors such as cerebral or extra-cerebral emboli, vertebral osteomyelitis, and persistent bacteremia [18]. However, waiting to establish persistent SAB can ultimately delay securing an IE diagnosis. Early diagnosis of endocarditis in high-risk patients may impact the choice of antimicrobial therapy (addition of rifampin and an aminoglycoside in prosthetic valve IE) and need for early surgical intervention (if myocardial abscess or other indications for surgical intervention are observed on TEE) or device extraction and therefore outcomes. The PREDICT score is simple and can be used at day 1 of SAB to identify high-risk patients without requiring further imaging or laboratory evaluations. Other previously published prediction models have been selective by including only MRSA bacteremia or nosocomial SAB [20, 25].
PREDICT scores divide patients into low-risk (day 1 and 5 score <2), intermediate-risk (day 1 score <4, day 5 score ≥2), and high-risk (day 1 score ≥4) groups. In our cohort, there were 57 (28.6%) patients with day 1 and day 5 scores of less than 2 and none of these patients had underlying IE. These findings are consistent with prior publications. In a study by Buitron et al [25], for example, no cases of IE were seen in those with MRSA bacteremia and absence of the identified risk factors; similar findings were reported by Heriot et al [21]. Moreover, Kaasch and colleagues [20] confirmed that patients with SAB without IE were less likely to have identifiable IE risk factors (prolonged bacteremia, presence of an intracardiac device, hemodialysis dependency, osteomyelitis, or spinal infection).
Among the 18 patients in our cohort classified as high risk with a day 1 score of 4 or higher, 7 (38.9%) had IE. In the intermediate-risk category, 16 of 124 patients had IE (12.9%). Based on these findings, TEE may be avoided in those with low risk as the negative-predictive value of a day 1 and day 5 score less than 2 is 100%, whereas TEE should be done early in the high-risk group as it may impact immediate IE management. For the intermediate-risk group, there is a need to identify more risk factors to further stratify this population, as the decision to obtain a TEE is not as certain as it is in high- or low-risk groups. Based on risk-adjusted analyses of association, we identified additional variables with predictive potential that may be considered in decision making in the intermediate-risk group. However, larger studies are needed to incorporate these into the scoring system. The timing of TEE may also not be as urgent in this group as compared with that for the high-risk group.
Current guidelines recommend ruling out IE by TEE in all cases of catheter-associated SABs, regardless of the presence or absence of IE risk factors, and to treat for 4 to 6 weeks if TEE has not been performed despite absence of clinical signs or risk factors for IE [26]. These guidelines are based on earlier studies that reported a relatively high prevalence of IE in patients with SAB [5–7]. However, these studies had significant limitations related to selection and referral biases as only a minority of patients had TEE based on physician discretion.
In our study, surgery or an invasive procedure in the prior 30 days was identified as a risk factor associated with the development of IE. Postoperative wounds have previously been identified as risk factors associated with SAB [27, 28], in addition to the isolation of S. aureus from a surgical wound [29]. The presence of a prosthetic heart valve has been associated with increased risk of IE and this is likely due to the tendency of S. aureus to form biofilm and attach to prosthetic material surfaces. In one study of 51 patients with SAB with prosthetic valves, endocarditis was detected in up to 51% of patients [30]. The presence of heart failure was also a significant predictor of IE. Heart failure may be due to pre-existing valvulopathy, which predisposes to IE, or as a complication of IE. Shorter time-to-bacterial growth for the first blood culture and higher number of positive blood culture bottles were both associated with a higher risk of IE in our study. Previous studies have shown similar associations [31]. This is likely due to high bacterial burden, which may be a risk factor for IE or, alternatively, a result of IE. Shorter time-to-culture positivity has also been an independent predictor of mortality in SAB [31]. Intravenous drug use (IDU) was not a significant risk factor associated with IE in our cohort, but only 3 patients described this as a potential risk factor. In a retrospective validation study of the PREDICT score in 257 patients with SAB, however, IDU was a risk factor for IE and was added to the PREDICT score, resulting in improved sensitivities of 42.1% and 97% and specificities of 88.6% and 32% at day 1 and day 5 [32].
Prediction scores cannot and should not replace clinical judgement. Patients with SAB and new-onset heart failure or clinical evidence of IE should undergo TEE regardless of their score. Transesophageal echocardiogram gives an accurate visualization of cardiac valves and surrounding structures, and studies have clearly shown the superiority of TEE over transthoracic echocardiography [33–35]. In addition, TEE may result in lower odds of in-hospital mortality in patients with SAB [36]. It is, however, an invasive procedure that carries the risk of esophageal perforation and complication of anesthesia. Also, it may be contraindicated in those with esophageal masses, stricture, or severe thrombocytopenia. In these situations, an alternative anatomic imaging modality, such as cardiac computed tomography angiography (CTA), or functional imaging, such as 18F-FDG PET/CT may be more appropriate [37].
Primary limitations of our analysis include its observational design and a relatively small sample size. In addition, approximately half of the patients did not undergo TEE. We tried to minimize selection bias by including consecutive patients who were hospitalized with SAB. Our institutions are tertiary referral centers, and therefore referral bias may also be present. We did not exclude patients without TEE; therefore, it can be argued that, in some patients, IE diagnosis may have been missed. However, lack of relapse of SAB during the 12-week follow-up period without antimicrobial therapy for IE suggests that these patients were unlikely to have underlying IE or were cured with less than recommended antimicrobial regimens. Thus, due to the wide-ranging duration of antimicrobial therapy in low-risk patients within our study cohort, we cannot conclude if a shorter duration (2 weeks) of antimicrobial therapy is adequate for cure, specifically if a TEE is deferred.
Our study was largely an internal validation cohort and future studies to validate this tool in an external cohort are required with the inclusion of underrepresented risk groups such as those with a history of IDU. We did not evaluate the diagnostic role of other imaging modalities (CTA, magnetic resonance imaging, or 18F-FDG PET/CT) that have been evaluated in recent years in the diagnosis of IE and its complications. To date, there are no head-to-head studies comparing these new diagnostic modalities to TEE in SAB populations. Future prediction models, however, should include these imaging modalities, which offer some advantages to TEE, particularly in selected cases of prosthetic valve evaluation and identification of extracardiac foci of involvement secondary to IE.
Overall, we were able to validate the previously derived PREDICT score in this prospective cohort study (PREDICT II). We have also identified additional candidate variables that may be incorporated in future prediction models to improve the specificity of scoring systems. These scoring systems, however, do not replace clinical judgement in individual patient management.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Financial support: This work was supported by CTSA (grant number UL1 TR002377) from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH).
Potential conflicts of interest. M. R. S. reports research funds from TYRX Inc and Medtronic for prior research unrelated to this study administered according to a sponsored research agreement between Mayo Clinic and the study sponsor, which prospectively defined the scope of the research effort and corresponding budget; and honoraria/consulting fees from Medtronic Inc and Aziyo Biologics Inc, unrelated to this project. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Shorr AF, Tabak YP, Killian AD, Gupta V, Liu LZ, Kollef MH. Healthcare-associated bloodstream infection: a distinct entity? Insights from a large U.S. database. Crit Care Med 2006; 34:2588–95. [DOI] [PubMed] [Google Scholar]
- 2.Laupland KB, Lyytikäinen O, Søgaard M, et al. ; International Bacteremia Surveillance Collaborative . The changing epidemiology of Staphylococcus aureus bloodstream infection: a multinational population-based surveillance study. Clin Microbiol Infect 2013; 19:465–71. [DOI] [PubMed] [Google Scholar]
- 3.El Atrouni WI, Knoll BM, Lahr BD, Eckel-Passow JE, Sia IG, Baddour LM. Temporal trends in the incidence of Staphylococcus aureus bacteremia in Olmsted County, Minnesota, 1998 to 2005: a population-based study. Clin Infect Dis 2009; 49:e130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhee Y, Aroutcheva A, Hota B, Weinstein RA, Popovich KJ. Evolving epidemiology of Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol 2015; 36:1417–22. [DOI] [PubMed] [Google Scholar]
- 5.Abraham J, Mansour C, Veledar E, Khan B, Lerakis S. Staphylococcus aureus bacteremia and endocarditis: the Grady Memorial Hospital experience with methicillin-sensitive S aureus and methicillin-resistant S aureus bacteremia. Am Heart J 2004; 147:536–9. [DOI] [PubMed] [Google Scholar]
- 6.Wilson R, Hamburger M. Fifteen years’ experience with staphylococcus septicemia in a large city hospital; analysis of fifty-five cases in the Cincinnati General Hospital 1940 to 1954. Am J Med 1957; 22:437–57. [DOI] [PubMed] [Google Scholar]
- 7.Fowler VG Jr, Li J, Corey GR, et al. . Role of echocardiography in evaluation of patients with Staphylococcus aureus bacteremia: experience in 103 patients. J Am Coll Cardiol 1997; 30:1072–8. [DOI] [PubMed] [Google Scholar]
- 8.Mylotte JM, McDermott C, Spooner JA. Prospective study of 114 consecutive episodes of Staphylococcus aureus bacteremia. Rev Infect Dis 1987; 9:891–907. [DOI] [PubMed] [Google Scholar]
- 9.Chang FY, MacDonald BB, Peacock JE Jr, et al. . A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 2003; 82:322–32. [DOI] [PubMed] [Google Scholar]
- 10.Nickerson EK, Hongsuwan M, Limmathurotsakul D, et al. . Staphylococcus aureus bacteraemia in a tropical setting: patient outcome and impact of antibiotic resistance. PLoS One 2009; 4:e4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler VG Jr, Olsen MK, Corey GR, et al. . Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003; 163:2066–72. [DOI] [PubMed] [Google Scholar]
- 12.Oestergaard LB, Schmiegelow MD, Bruun NE, et al. . The associations between socioeconomic status and risk of Staphylococcus aureus bacteremia and subsequent endocarditis—a Danish nationwide cohort study. BMC Infect Dis 2017; 17:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Castro S, Cartoni D, d’Amati G, et al. . Diagnostic accuracy of transthoracic and multiplane transesophageal echocardiography for valvular perforation in acute infective endocarditis: correlation with anatomic findings. Clin Infect Dis 2000; 30:825–6. [DOI] [PubMed] [Google Scholar]
- 14.Holden E, Bashir A, Das I, et al. . Staphylococcus aureus bacteraemia in a UK tertiary referral centre: a “transoesophageal echocardiogram for all” policy. J Antimicrob Chemother 2014; 69:1960–5. [DOI] [PubMed] [Google Scholar]
- 15.Incani A, Hair C, Purnell P, et al. . Staphylococcus aureus bacteraemia: evaluation of the role of transoesophageal echocardiography in identifying clinically unsuspected endocarditis. Eur J Clin Microbiol Infect Dis 2013; 32:1003–8. [DOI] [PubMed] [Google Scholar]
- 16.Daniel WG, Erbel R, Kasper W, et al. . Safety of transesophageal echocardiography: a multicenter survey of 10 419 examinations. Circulation 1991; 83:817–21. [DOI] [PubMed] [Google Scholar]
- 17.Palraj BR, Baddour LM, Hess EP, et al. . Predicting Risk of Endocarditis Using a Clinical Tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2015; 61:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tubiana S, Duval X, Alla F, et al. ; VIRSTA/AEPEI Study Group . The VIRSTA score, a prediction score to estimate risk of infective endocarditis and determine priority for echocardiography in patients with Staphylococcus aureus bacteremia. J Infect 2016; 72:544–53. [DOI] [PubMed] [Google Scholar]
- 19.Bai AD, Agarwal A, Steinberg M, et al. . Clinical predictors and clinical prediction rules to estimate initial patient risk for infective endocarditis in Staphylococcus aureus bacteraemia: a systematic review and meta-analysis. Clin Microbiol Infect 2017; 23:900–6. [DOI] [PubMed] [Google Scholar]
- 20.Kaasch AJ, Fowler VG Jr, Rieg S, et al. . Use of a simple criteria set for guiding echocardiography in nosocomial Staphylococcus aureus bacteremia. Clin Infect Dis 2011; 53:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heriot G, Yeoh J, Street A, Ratnam I. Echocardiography has minimal yield and may not be warranted in Staphylococcus aureus bacteremia without clinical risk factors for endocarditis. Eur J Clin Microbiol Infect Dis 2015; 34:1231–6. [DOI] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman ND, Kaye KS, Stout JE, et al. . Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 2002; 137:791–7. [DOI] [PubMed] [Google Scholar]
- 24.Baddour LM, Wilson WR, Bayer AS, et al. ; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; Council on Cardiovascular Surgery and Anesthesia; Stroke Council . Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132:1435–86. [DOI] [PubMed] [Google Scholar]
- 25.Buitron de la Vega P, Tandon P, Qureshi W, et al. . Simplified risk stratification criteria for identification of patients with MRSA bacteremia at low risk of infective endocarditis: implications for avoiding routine transesophageal echocardiography in MRSA bacteremia. Eur J Clin Microbiol Infect Dis 2016; 35:261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mermel LA, Allon M, Bouza E, et al. . Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49:1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen AG, Wachmann CH, Espersen F, Scheibel J, Skinhøj P, Frimodt-Møller N. Treatment and outcome of Staphylococcus aureus bacteremia: a prospective study of 278 cases. Arch Intern Med 2002; 162:25–32. [DOI] [PubMed] [Google Scholar]
- 28.Lautenschlager S, Herzog C, Zimmerli W. Course and outcome of bacteremia due to Staphylococcus aureus: evaluation of different clinical case definitions. Clin Infect Dis 1993; 16:567–73. [DOI] [PubMed] [Google Scholar]
- 29.Petti CA, Sanders LL, Trivette SL, Briggs J, Sexton DJ. Postoperative bacteremia secondary to surgical site infection. Clin Infect Dis 2002; 34:305–8. [DOI] [PubMed] [Google Scholar]
- 30.El-Ahdab F, Benjamin DK Jr, Wang A, et al. . Risk of endocarditis among patients with prosthetic valves and Staphylococcus aureus bacteremia. Am J Med 2005; 118:225–9. [DOI] [PubMed] [Google Scholar]
- 31.Siméon S, Le Moing V, Tubiana S, et al. ; VIRSTA/AEPEI Study Group . Time to blood culture positivity: an independent predictor of infective endocarditis and mortality in patients with Staphylococcus aureus bacteraemia. Clin Microbiol Infect 2019; 25:481–8. [DOI] [PubMed] [Google Scholar]
- 32.Longobardo L, Klemm S, Cook M, et al. . Risk assessment for infected endocarditis in Staphylococcus aureus bacteremia patients: when is transesophageal echocardiography needed? Eur Heart J Acute Cardiovasc Care 2019; 8: 476–84. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro SM, Young E, De Guzman S, et al. . Transesophageal echocardiography in diagnosis of infective endocarditis. Chest 1994; 105:377–82. [DOI] [PubMed] [Google Scholar]
- 34.Erbel R, Rohmann S, Drexler M, et al. . Improved diagnostic value of echocardiography in patients with infective endocarditis by transoesophageal approach: a prospective study. Eur Heart J 1988; 9:43–53. [PubMed] [Google Scholar]
- 35.Shively BK, Gurule FT, Roldan CA, Leggett JH, Schiller NB. Diagnostic value of transesophageal compared with transthoracic echocardiography in infective endocarditis. J Am Coll Cardiol 1991; 18:391–7. [DOI] [PubMed] [Google Scholar]
- 36.Urja P, Walters RW, Vivekanandan R, et al. . Trends in the use of echocardiography in patients with Staphylococcus aureus bacteremia: an analysis using the Nationwide Inpatient Sample data. Echocardiography 2019; 36:1625–32. [DOI] [PubMed] [Google Scholar]
- 37.Mahmood M, Kendi AT, Ajmal S, et al. . Meta-analysis of 18F-FDG PET/CT in the diagnosis of infective endocarditis. J Nucl Cardiol 2019; 26:922–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.