Abstract
Background
Human immunodeficiency virus (HIV) infection may accelerate development of aging-related non-AIDS comorbidities (NACMs). The incidence of NACMs is poorly characterized among women living with HIV (WLWH).
Methods
WLWH and HIV-seronegative participants followed in the Women’s Interagency HIV Study (WIHS) through 2009 (when >80% of WLWH used antiretroviral therapy) or onward were included, with outcomes measured through 31 March 2018. Sociodemographics, clinical covariates, and prevalent NACM were determined at enrollment. We used Poisson regression models to determine incident NACM burden (number of NACMs accrued through most recent WIHS visit out of 10 total NACMs assessed) by HIV serostatus and age.
Results
There were 3129 participants (2239 WLWH, 890 HIV seronegative) with 36 589 person-years of follow-up. At enrollment, median age was 37 years, 65% were black, and 47% currently smoked. In fully adjusted analyses, WLWH had a higher incident NACM rate compared with HIV-seronegative women (incidence rate ratio, 1.36 [95% confidence interval (CI), 1.02–1.81]). Incident NACM burden was higher among WLWH vs HIV-seronegative women in most age strata (HIV × age interaction: P = .0438), and women <25 years old had the greatest incidence rate ratio by HIV serostatus at 1.48 (95% CI, 1.19–1.84) compared with those in older age groups. Incident NACM burden was associated with traditional comorbidity risk factors but not HIV-specific indices.
Conclusions
Incident NACM burden was higher among WLWH than HIV-seronegative women. This difference was most dramatic among women aged <25 years, a group for whom routine comorbidity screening is not prioritized. Established non-HIV comorbidity risk factors were significantly associated with incident NACM burden. More data are needed to inform best practices for NACM screening, prevention, and management among WLWH, particularly young women.
Keywords: human immunodeficiency virus, women living with HIV, HIV and aging, non-AIDS comorbidities, comorbidity burden
Among women in the United States, the burden of incident non-AIDS comorbidities was higher for those living with human immunodeficiency virus (HIV) compared with those without HIV, and this difference was greatest for young women, a group not prioritized in routine comorbidity screening.
Due to combination antiretroviral therapy (ART), human immunodeficiency virus (HIV) infection has become a chronic condition for individuals with access to care [1]. Along with increased longevity, persons living with HIV (PLWH) experience a high burden of age-related non-AIDS comorbidities (NACMs) [2–6]. Compared with persons without HIV, NACMs occur disproportionately and prematurely among PLWH [7–9]. Multimorbidity is costly not only to the individual aging with HIV (ie, affected quality of life) [10], but to the healthcare system, leading to higher resource utilization and direct medical costs (ie, $300–$5000 more per patient month for PLWH with comorbidities than for those without) [11].
NACM risk appears to be greater among women living with HIV (WLWH) than men [5, 12, 13]. Biologic and sociobehavioral sex differences have been implicated in HIV acquisition, pathogenesis, reservoir establishment, responses to ART, and curative interventions, and while likely to influence comorbidity development, this remains poorly characterized [14]. Marcus et al demonstrated that NACM occurred 16 years earlier among insured PLWH than HIV-negative matched controls [9]. Differences by sex were found when examining overall and comorbidity-free life expectancy; however, female representation was inadequate (12.3%) [9], as is frequently the case in research involving PLWH [14, 15]. To better understand the role of biologic sex and associated factors in premature NACM accrual among PLWH, comorbidity study, specifically among women, is crucial and has the potential to improve clinical outcomes [16].
We recently evaluated the prevalence of 10 NACMs among >3000 participants in the Women’s Interagency HIV Study (WIHS), the largest prospective United States (US)–based cohort of WLWH and at-risk women without HIV [6]. Virologically suppressed WLWH had a higher mean NACM count than women without HIV overall, and among certain age groups. To understand the longitudinal effects of chronic HIV and age on NACMs among women, we performed a follow-up analysis of NACM incidence and associated factors among WIHS participants.
METHODS
The Women’s Interagency HIV Study
The WIHS is a multicenter prospective US cohort established in 1993 to investigate the progression and sequelae of HIV infection among women. WLWH and at-risk women without HIV enrolled during 4 waves (1994–1995, 2001–2002, 2011–2012, 2013–2015) from 11 cities (Atlanta, Georgia; Birmingham, Alabama; Bronx, New York; Brooklyn, New York; Chapel Hill, North Carolina; Chicago, Illinois; Jackson, Mississippi; Los Angeles, California; San Francisco, California; Miami, Florida; Washington, District of Columbia). Women without HIV were recruited based on being at risk for HIV acquisition (eg, history of sexually transmitted infections, substance use) as previously described [17].
Study visits occurred at 6-month intervals and comprised standardized interviews, physical examinations, and biospecimen collection. Sociodemographics, clinical information including chronic comorbidities, medications, and health behaviors were assessed. Blood testing evaluated kidney and liver function, CD4 count, and HIV viral load. The WIHS protocol has been approved by each site’s institutional review board, and all participants have provided informed consent.
Study Design
We performed a longitudinal assessment of WIHS participants from study enrollment through end of observation to measure incident NACMs and to evaluate the effects of HIV serostatus and age on NACMs over time. As previously described, women followed through 2009 (when >80% of WLWH used ART) or onward were included to focus on the development of age-related NACM in the era of highly effective ART [6]. For this analysis, participants were additionally required to have ≥2 study visits within 2 years of enrollment and ≥1 follow-up study visit after that period to define prevalent (disease present at baseline) and incident (disease occurrence after baseline) NACMs, respectively (Supplementary Figure 1). Age, covariates, and prevalent NACMs were determined at the end of the 2-year baseline period. Incident NACMs were measured through the participant’s last visit or 31 March 2018 if still enrolled.
Outcome Measures
The primary outcome was incident NACM burden, defined as the number of total NACMs per participant that developed over the course of observation. Ten NACMs were evaluated given their age association and significant contribution to morbidity and mortality in the general population and among PLWH: hypertension; dyslipidemia; cardiovascular disease (CVD); diabetes; chronic kidney disease (CKD); liver, bone, and lung disease; psychiatric illness; and non-AIDS cancer. NACMs were rigorously defined (Supplementary Table 1) using up to 3 data sources per comorbidity (self-reported diagnosis or medication use; clinical measurement; and/or laboratory evidence) [6]. For NACMs that may have existed intermittently over time (ie, CKD and depression), data from consecutive study visits were required [6]. NACMs were measured at baseline and at end of observation and were considered incident if present at the latter but not former time point.
Independent Variables
For analysis, age at baseline was categorized into 5-year increments from <25 to ≥55 years for all NACMs except for CKD and non-AIDS cancer, where age was collapsed into 10-year increments from <30 to ≥50 years because of the rarity of these conditions.
Statistical Analysis
We compared baseline demographic and clinical characteristics of women by HIV serostatus using χ 2 tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. For each individual NACM, the number and percentage of incident cases, total length of follow-up time (in person-years [PY]), and raw incidence rate (calculated as number of cases/PY × 1000) was computed, both overall and stratified by HIV serostatus. For incident NACM burden, the total number of incident comorbidities, total length of follow-up time (in PY), and raw incidence rate (calculated as total number of comorbidities/PY) was computed, both overall and stratified by HIV serostatus.
For each individual NACM and incident NACM burden, separate Poisson regression models using robust variance estimation were used to generate model-based incidence rate ratios (IRRs) and corresponding 95% confidence intervals (CIs) for independent variables of interest from (1) unadjusted (contained only HIV serostatus) and (2) partially adjusted (additionally included categorized baseline age and HIV × age interaction) models. For the primary outcome of incident NACM burden, a fully adjusted model that controlled for important covariates plus the partially adjusted model terms was utilized. Model-based mean estimates were exponentiated to get the estimated mean incidence rate and 95% CI for each level of the independent variable of interest. Pre-planned contrasts compared HIV serostatus within each age category in the full model. A separate multivariable full model including only WLWH assessed the effect of HIV-specific indices, adjusting for the same covariates, on incident NACM burden. Model fit was assessed through deviance/degrees of freedom goodness-of-fit test and residual plots.
Analyses used SAS version 9.4 software. Significance level was set at α = .05.
RESULTS
Participant Characteristics at Baseline
There were 3129 participants (2239 WLWH, 890 women without HIV) included in the analysis (Supplementary Figure 1) with a total of 36 589 PY of follow-up. At baseline, median age was 37 years and 65% were black (Table 1). Compared with WLWH, women without HIV had significantly higher body mass index (BMI) ≥30 kg/m2 (47% vs 40%, P = .0008) and current use of cigarettes (54% vs 44%), crack/cocaine (15% vs 10%), and alcohol (57% vs 47%) (all P < .0001). WLWH were significantly more likely to use antihypertensive medication (20% vs 16%, P = .0118) and to have prevalent chronic hepatitis C virus infection (12% vs 9%, P = .0051), hepatitis B virus infection (2% vs 1%, P = .0216), and worse kidney function (estimated glomerular filtration rate of 99.3 vs 101.5 mL/minute/1.73 m2, P = .0099) than women without HIV (Table 1). Education level, annual household income, and median depressive symptom score did not significantly differ by HIV serostatus. At baseline, WLWH had a median CD4 count of 484 cells/μL, 69% were on ART, and 45% were virologically suppressed.
Table 1.
Baseline Demographic and Clinical Data of Women Living With or at Risk for Human Immunodeficiency Virus (HIV) Infection at the Time of Enrollment in the Women’s Interagency HIV Studya
| Characteristic | Entire Cohortb (N = 3129) |
HIV Positive (n = 2239) |
HIV Negative (n = 890) |
P Valuec |
|---|---|---|---|---|
| Age, y, median (Q1–Q3) | 37 (31–45) | 38 (32–45) | 36 (28–44) | <.0001 |
| Age group, y | <.0001 | |||
| <25 | 222 (7) | 95 (4) | 127 (14) | |
| 25–29 | 390 (12) | 263 (12) | 127 (14) | |
| 30–34 | 602 (19) | 458 (20) | 144 (16) | |
| 35–39 | 625 (20) | 463 (21) | 162 (18) | |
| 40–44 | 496 (16) | 378 (17) | 118 (13) | |
| 45–49 | 343 (11) | 259 (12) | 84 (9) | |
| 50–54 | 265 (8) | 189 (8) | 76 (9) | |
| ≥55 | 186 (6) | 134 (6) | 52 (6) | |
| Follow-up time, y, median (Q1–Q3) | 14.3 (3–17.2) | 14.3 (3–17.4) | 14.4 (3.2–16.7) | .8321 |
| Race/ethnicity | .1110 | |||
| White, non-Hispanic | 346 (11) | 264 (12) | 82 (9) | |
| Black, non-Hispanic | 2033 (65) | 1438 (64) | 595 (67) | |
| Hispanic | 642 (21) | 465 (21) | 177 (20) | |
| Other | 108 (3) | 72 (3) | 36 (4) | |
| WIHS enrollment wave | <.0001 | |||
| 1994–1995 | 1149 (37) | 862 (39) | 287 (32) | |
| 2001–2002 | 858 (27) | 554 (25) | 304 (34) | |
| 2011–2012 | 333 (11) | 250 (11) | 83 (9) | |
| 2013–2015 | 789 (25) | 573 (26) | 216 (24) | |
| BMI, kg/m2 | .0008 | |||
| <30 | 1779 (58) | 1317 (60) | 462 (53) | |
| ≥30 | 1288 (42) | 882 (40) | 406 (47) | |
| SBP, mm Hg, median (Q1–Q3) | 116 (107–127) | 115 (107–127) | 116 (108–128) | .0742 |
| DBP, mm Hg, median (Q1–Q3) | 74 (68–80) | 74 (68–81) | 73 (68–80) | .1379 |
| Antihypertensive medication use | 579 (19) | 439 (20) | 140 (16) | .0118 |
| Lipid-lowering medication use | 155 (5) | 108 (5) | 47 (5) | .5966 |
| eGFR, mL/min/1.73 m2 (CKD-EPI), median (Q1–Q3) | 100 (85.4–117) | 99.3 (84.2–116.4) | 101.5 (87.6–118.8) | .0099 |
| CES-D scored, median (Q1–Q3) | 12 (5–22) | 12 (5–23) | 12 (5–22) | .2353 |
| Education | .1848 | |||
| High school or less | 2025 (65) | 1466 (66) | 559 (63) | |
| More than high school | 1099 (35) | 771 (34) | 328 (37) | |
| Income | .7486 | |||
| <$12 000 | 1748 (56) | 1262 (57) | 486 (55) | |
| $12 001–$24 000 | 764 (25) | 544 (24) | 220 (25) | |
| >$24 000 | 591 (19) | 418 (19) | 173 (20) | |
| Insured | 2490 (81) | 1928 (87) | 562 (64) | <.0001 |
| Marital status | 1029 (33) | 741 (33) | 288 (32) | <.0001 |
| Married/partner | 803 (26) | 617 (28) | 186 (21) | |
| Had a partner | 1296 (41) | 881 (39) | 415 (47) | |
| Never married/other | ||||
| Owner of residence | 2293 (73) | 1720 (77) | 573 (64) | <.0001 |
| Cigarette use | <.0001 | |||
| Never | 1077 (35) | 814 (37) | 263 (30) | |
| Current | 1451 (47) | 977 (44) | 474 (54) | |
| Former | 568 (18) | 428 (19) | 140 (16) | |
| Current alcohol use | <.0001 | |||
| None | 1535 (50) | 1158 (52) | 377 (43) | |
| 1–7 drinks/wk | 1202 (39) | 843 (38) | 359 (41) | |
| >7 drinks/wk | 341 (11) | 206 (9) | 135 (16) | |
| Marijuana use | <.0001 | |||
| Never | 1049 (34) | 826 (37) | 223 (25) | |
| Current | 690 (22) | 433 (20) | 257 (29) | |
| Former | 1354 (44) | 957 (43) | 397 (45) | |
| Crack/cocaine use | <.0001 | |||
| Never | 2412 (78) | 1787 (81) | 625 (71) | |
| Current | 350 (11) | 218 (10) | 132 (15) | |
| Former | 333 (11) | 213 (10) | 120 (14) | |
| Opioid use (heroin/methadone) | .0031 | |||
| Never | 2869 (93) | 2078 (94) | 791 (90) | |
| Current | 112 (4) | 71 (3) | 41 (5) | |
| Former | 114 (4) | 69 (3) | 45 (5) | |
| Injection drug use | .0862 | |||
| Never | 2548 (82) | 1819 (82) | 729 (83) | |
| Current | 75 (2) | 47 (2) | 28 (3) | |
| Former | 470 (15) | 350 (16) | 120 (14) | |
| Noninjection drug use | <.0001 | |||
| Never | 864 (28) | 691 (31) | 173 (20) | |
| Current | 863 (28) | 544 (25) | 319 (36) | |
| Former | 1366 (44) | 981 (44) | 385 (44) | |
| Chronic HBV | 64 (2) | 54 (2) | 10 (1) | .0216 |
| Chronic HCV | 345 (11) | 269 (12) | 76 (9) | .0051 |
| CD4 cell count, cells/μL, median (Q1–Q3) | … | 484 (308–698) | … | |
| CD4 nadir, cells/μL, median (Q1–Q3) | … | 280 (159–414) | … | |
| HIV viral load | ||||
| Suppressede | … | 971 (45) | … | |
| 200–999 copies/mL | … | 197 (9) | … | |
| ≥1000 copies/mL | … | 976 (46) | … | |
| ART status | ||||
| No therapy | … | 709 (32) | … | |
| Mono/dual ART | … | 421 (19) | … | |
| Combination ART | … | 1109 (50) | … |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ART, combined antiretroviral therapy; BMI, body mass index; CES-D, Center for Epidemiologic Studies–Depression; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; Q1, first quartile; Q3, third quartile; SBP, systolic blood pressure; WIHS, Women’s Interagency HIV Study.
aColumn percentages may not total 100 due to rounding.
bData missing for BMI (n = 62), SBP (n = 2), DBP (n = 2), CES-D (n = 1), CD4 count (n = 82), and CD4 nadir (n = 82).
cχ 2 test performed for categorical variables and Wilcoxon rank-sum test for continuous variables.
dRange 0–60, threshold for depressive symptoms ≥16.
eHIV viral load <200 copies/mL and/or less than the lower limit of quantification of the assay.
Prevalent and Incident NACM Burden
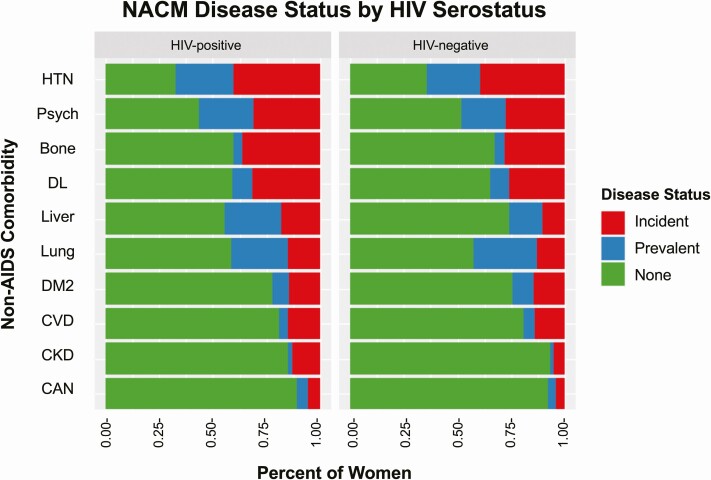
Figure 1 shows the distribution of women with prevalent NACMs at baseline and incident NACMs at the end of follow-up by HIV serostatus. Of 10 NACMs evaluated, mean NACM burden at baseline was higher among WLWH than women without HIV (1.4 vs 1.2, P = .0063), though only prevalent liver disease (26% vs 16%, P < .0001) and psychiatric illness (26% vs 21%, P = .0028) differed by HIV serostatus (Supplementary Table 2). The unadjusted incident NACM rate by HIV serostatus and age is shown in Supplementary Figure 2.
Figure 1.
Distribution of women with prevalent (blue, disease present at baseline), incident (red, disease occurrence after baseline), or neither prevalent nor incident (green) non-AIDS comorbidities over the course of observation in the Women’s Interagency HIV Study, stratified by human immunodeficiency virus serostatus. Abbreviations: Bone, bone disease; CAN, non-AIDS cancer; CKD, chronic kidney disease; CVD, cardiovascular disease; DL, dyslipidemia; DM2, type 2 diabetes mellitus; HIV, human immunodeficiency virus; HTN, hypertension; Liver, liver disease; Lung, lung disease; NACM, non-AIDS comorbidity; Psych, psychiatric illness.
In partially adjusted models, incident NACM burden was greater in WLWH compared with women without HIV (0.19/PY vs 0.16/PY; IRR 1.21 [95% CI, 1.13–1.29]) (Table 2). The incidence was higher among WLWH than women without HIV for CKD (IRR, 3.14 [95% CI, 1.80–5.49]), liver disease (IRR, 2.56 [95% CI, 1.85–3.54]), psychiatric illness (IRR, 1.38 [95% CI, 1.02–1.86]), dyslipidemia (IRR 1.36 [95% CI, 1.14–1.62]), and bone disease (IRR, 1.35 [95% CI, 1.14–1.58]). However, incident hypertension, diabetes, CVD, lung disease, and non-AIDS cancer did not differ significantly by HIV serostatus (Table 2). Supplementary Table 3 shows the incidence of individual NACM and incident NACM burden stratified by HIV serostatus and age (for incident burden, HIV × age interaction: P = .0063).
Table 2.
Incidence Rates of Non-AIDS Comorbidities in Women by Human Immunodeficiency Virus Serostatus
| Cases/Total Population (%), Person-Time, and Rate per 1000 PYs | Unadjusted IRR (95% CI)a | Partially Adjusted IRR (95% CI)a | |||
|---|---|---|---|---|---|
| Comorbidity | Total | HIV Positive | HIV Negative | HIV Positive vs HIV Negative | HIV Positive vs HIV Negative |
| Hypertension | 1.07 (.95–1.22) | 0.91 (.78–1.06) | |||
| Incident cases | 1260/2314 (54) | 909/1643 (55) | 351/671 (53) | ||
| Person-time, y | 19 119 | 13 513 | 5606 | ||
| Incident rate | 65.90 | 67.27 | 62.61 | ||
| Psychiatric illness | 1.35 (1.16–1.57) | 1.38 (1.02–1.86) | |||
| Incident cases | 940/2372 (40) | 694/1665 (42) | 246/707 (35) | ||
| Person-time, y | 20 458 | 13 831 | 6626 | ||
| Incident rate | 45.95 | 50.18 | 37.12 | ||
| Bone disease | 1.37 (1.20–1.56) | 1.35 (1.14–1.58) | |||
| Incident cases | 1057/2986 (35) | 807/2137 (38) | 250/849 (29) | ||
| Person-time, y | 29 281 | 20 561 | 8720 | ||
| Incident rate | 36.10 | 39.25 | 28.67 | ||
| Dyslipidemia | 1.34 (1.17–1.54) | 1.36 (1.14–1.62) | |||
| Incident cases | 938/2840 (33) | 707/2030 (35) | 231/810 (29) | ||
| Person-time, y | 27 703 | 19 253 | 8450 | ||
| Incident rate | 33.86 | 36.72 | 27.34 | ||
| Liver disease | 2.48 (1.98–3.11) | 2.56 (1.85–3.54) | |||
| Incident cases | 498/2399 (21) | 406/1647 (25) | 92/752 (12) | ||
| Person-time, y | 22 294 | 14 274 | 8020 | ||
| Incident rate | 22.34 | 28.44 | 11.47 | ||
| Lung disease | 1.15 (.93–1.42) | 1.03 (.77–1.38) | |||
| Incident cases | 461/2288 (20) | 343/1658 (21) | 118/630 (19) | ||
| Person-time, y | 23 199 | 16 627 | 6572 | ||
| Incident rate | 19.87 | 20.63 | 17.96 | ||
| Type 2 diabetes mellitus | 0.97 (.79–1.19) | 1.06 (.80–1.41) | |||
| Incident cases | 460/2877 (16) | 328/2072 (16) | 132/805 (16) | ||
| Person-time, y | 30 704 | 22 069 | 8635 | ||
| Incident rate | 14.98 | 14.86 | 15.29 | ||
| Cardiovascular disease | 1.07 (.88–1.31) | 1.06 (.83–1.36) | |||
| Incident cases | 467/2995 (16) | 340/2149 (16) | 127/846 (15) | ||
| Person-time, y | 32 691 | 23 342 | 9349 | ||
| Incident rate | 14.29 | 14.57 | 13.58 | ||
| Chronic kidney disease | 2.56 (1.89–3.46) | 3.14 (1.80–5.49)b | |||
| Incident cases | 333/3063 (11) | 286/2185 (13) | 47/878 (5) | ||
| Person-time, y | 34 108 | 24 019 | 10 089 | ||
| Incident rate | 9.76 | 11.91 | 4.66 | ||
| Cancer, non-AIDS | 1.51 (1.05–2.17) | 1.39 (.89–2.18)b | |||
| Incident cases | 167/2983 (6) | 131/2125 (6) | 36/858 (4) | ||
| Person-time, y | 33 698 | 23 821 | 9877 | ||
| Incident rate | 4.96 | 5.50 | 3.65 | ||
| Incident NACM burden/PY | 1.22 (1.15–1.29) | 1.21 (1.13–1.29) | |||
| Incident count | 6581 | 4951 | 1630 | ||
| Person-time, y | 36 589 | 26 131 | 10 458 | ||
| Incident rate | 0.1799 | 0.1895 | 0.1559 | ||
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; IRR, incidence rate ratio; NACM, non-AIDS comorbidity; PY, person-years.
aPoisson regression analysis performed using robust variance estimation to generate IRRs of each NACM and incident NACM burden using unadjusted (HIV serostatus) or partially adjusted (HIV serostatus, baseline age group, HIV × age interaction) models.
bAge at baseline was categorized into 5-year increments from <25 to ≥55 years for all NACMs except for chronic kidney disease and non-AIDS cancer, which were rare occurrences and thus age-collapsed into 10-year increments from <30 to ≥50 years.
Fully Adjusted Incident NACM Burden by HIV and Age
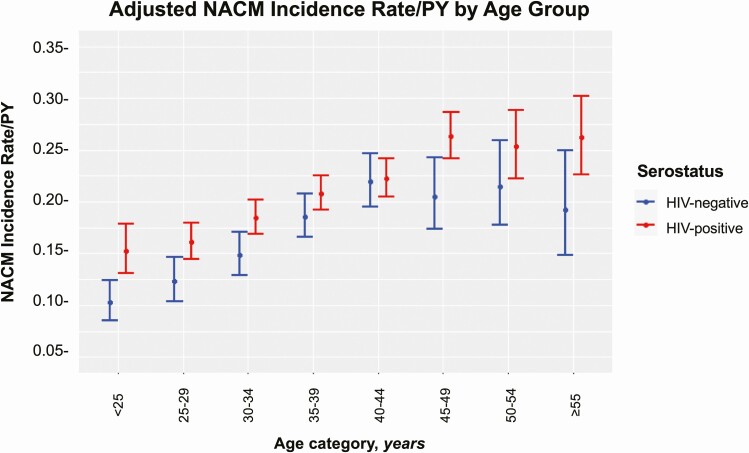
In fully adjusted models controlling for race, BMI, education, income, marital status, own residence, and current use of cigarettes, alcohol, and crack/cocaine (in addition to HIV, baseline age group, and HIV × age), WLWH had a significantly higher incident NACM rate compared with women without HIV (IRR, 1.36 [95% CI, 1.02–1.81]). The incident NACM burden was significantly higher among WLWH compared with women without HIV in most age strata (Figure 2; HIV × age interaction: P = .0438). Women aged <25 years had the greatest IRR at 1.48 (95% CI, 1.19–1.84) vs those aged 25–29 (IRR, 1.31 [95% CI, 1.09–1.57]), 30–34 (IRR, 1.25 [95% CI, 1.09–1.43]), 35–39 (IRR, 1.12 [95% CI, 1.003–1.25]), 40–44 (IRR, 1.01 [95% CI, .90–1.14]), 45–49 (IRR, 1.28 [95% CI, 1.08–1.53]), 50–54 (IRR, 1.18 [95% CI, .95–1.46]), and ≥55 years (IRR, 1.36 [95% CI, 1.02–1.81]).
Figure 2.
Incident non-AIDS comorbidity (NACM) burden per person-year (PY) by human immunodeficiency virus (HIV) serostatus and baseline age in 5-year increments. In addition to HIV serostatus, baseline age group, and HIV × age interaction, the adjusted model included the following characteristics assessed during the enrollment period: race, body mass index, household income, residence, marital status, education, smoking history, current alcohol use, history of crack/cocaine use, enrollment wave in the Women’s Interagency HIV Study.
Factors Associated With Incident NACM Burden
In fully adjusted models controlling for the aforementioned baseline covariates, the estimated incident NACM rate was significantly associated with traditional comorbidity risk factors identified in the general population. We observed higher incident NACM rates among women of white race, who had a BMI ≥30 kg/m2, household income <$24 000, did not have their own residence, and reported current use of cigarettes or crack/cocaine (Table 3). Education status and current alcohol use were not associated with incident NACM burden. In an adjusted model including only WLWH (controlling for the aforementioned covariates and HIV-specific indices), enrollment CD4 count, CD4 nadir, ART status, and viral load were not significantly associated with the estimated incident NACM rate (Supplementary Table 4).
Table 3.
Multivariable Analysis of Risk Factors at Study Enrollment Associated With the Incident Burden of Non-AIDS Comorbidities in Women Living With or at Risk for Human Immunodeficiency Virus Infection
| Risk Factor | Estimated Incident NACM Rate (95% CI) | IRR (95% CI) | P Valuea |
|---|---|---|---|
| HIV serostatusb | |||
| Positive | 0.21 (.20–.22) | 1.36 (1.02–1.81) | <.0001 |
| Negative | 0.17 (.16–.18) | Ref | |
| Age group, yb | |||
| ≥55 | 0.22 (.19–.26) | 1.71 (1.41–2.09) | <.0001 |
| 50–54 | 0.23 (.21–.26) | 1.66 (1.38–2.00) | |
| 45–49 | 0.23 (.21–.26) | 1.72 (1.47–2.02) | |
| 40–44 | 0.22 (.20–.24) | 1.46 (1.25–1.70) | |
| 35–39 | 0.20 (.18–.21) | 1.36 (1.17–1.58) | |
| 30–34 | 0.17 (.15–.18) | 1.21 (1.04–1.41) | |
| 25–29 | 0.14 (.13–.16) | 1.06 (.90–1.25) | |
| <25 | 0.13 (.11–.14) | Ref | |
| BMI, kg/m2 | |||
| ≥30 | 0.20 (.19–.22) | 1.15 (1.09–1.21) | <.0001 |
| <30 | 0.18 (.16–.19) | Ref | |
| Race | |||
| Non-Hispanic black | 0.17 (.16–.18) | 0.83 (.78–.89) | <.0001 |
| Hispanic | 0.19 (.17–.20) | 0.89 (.83–.97) | |
| Other non-Hispanic | 0.19 (.16–.22) | 0.91 (.78–1.05) | |
| White | 0.21 (.19–.22) | Ref | |
| Education | |||
| High school or less | 0.19 (.18–.20) | 1.01 (.96–1.07) | .6504 |
| More than high school | 0.19 (.17–.20) | Ref | |
| Income | |||
| <$12 000 | 0.20 (.19–.22) | 1.12 (1.05–1.20) | .0002 |
| $12 001–$24 000 | 0.18 (.17–.20) | 1.02 (.95–1.10) | |
| >$24 000 | 0.18 (.17–.19) | Ref | |
| Marital status | |||
| Had a partner | 0.19 (.18–.20) | 1.01 (.96–1.08) | .1680 |
| Never partner/other | 0.18 (.17–.20) | 0.96 (.91–1.01) | |
| Married/partner | 0.19 (.17–.20) | Ref | |
| Own residence | |||
| No | 0.20 (.18–.21) | 1.08 (1.02–1.14) | .0067 |
| Yes | 0.18 (.17–.19) | Ref | |
| Cigarette use | |||
| Current | 0.21 (.19–.22) | 1.19 (1.12–1.27) | <.0001 |
| Former | 0.19 (.17–.20) | 1.09 (1.02–1.17) | |
| Never | 0.17 (.16–.19) | Ref | |
| Current alcohol use | |||
| >7 drinks/wk | 0.19 (.17–.20) | 0.96 (.88–1.04) | .1457 |
| 1–7 drinks/wk | 0.18 (.17–.20) | 0.95 (.90–1.00) | |
| None | 0.19 (.18–.21) | Ref | |
| Crack/cocaine use | |||
| Current | 0.20 (.18–.21) | 1.10 (1.02–1.19) | .0310 |
| Former | 0.19 (.17–.21) | 1.06 (.99–1.14) | |
| Never | 0.18 (.17–.19) | Ref |
Abbreviations: BMI, body mass index; CI, confidence interval; HIV, human immunodeficiency virus; IRR, incidence rate ratio; NACM, non-AIDS comorbidity; Ref, reference value.
aAdjusted linear regression with all covariates listed included in the model plus Women’s Interagency HIV Study enrollment wave and HIV × age interaction (P = .0438 for the interaction term).
bAdjusted for HIV serostatus × age interaction.
DISCUSSION
In this large, multicenter US-based prospective observational cohort of women with and without HIV that included >36 000 PY of follow-up, we found that WLWH had a significantly higher burden of incident NACMs than at-risk women without HIV. The difference in incident NACM rates by HIV serostatus was greatest among young women and in particular those aged <25 years, a group for whom routine comorbidity screening recommendations are not prioritized [18, 19]. Traditional, but not HIV-specific, comorbidity risk factors were significantly associated with incident NACM burden among WLWH. These findings have broad-ranging implications for HIV care models and research priorities, and argue for additional study of NACM pathogenesis among WLWH specifically, and for sex-stratified comorbidity screening and prevention strategy development among PLWH to mitigate the elevated NACM risk in this population.
We previously showed in a cross-sectional analysis of the WIHS that the burden of prevalent NACM was significantly higher among WLWH than women without HIV overall and in certain age groups [6]. The current longitudinal study builds on those data by illustrating that women, regardless of HIV serostatus, began accruing comorbidities early in life (ie, as young as in their 20s), that incident NACM burden was higher among WLWH, and that the impact of living with HIV on comorbidity development may be most significant among young women. These findings substantiate that WLWH are susceptible to “premature” multimorbidity, as suggested by other male-predominant cohorts examining NACMs among PLWH [2, 3, 7], and that comorbidity risk assessment and intervention should optimally begin for women in their childbearing years.
The greatest difference in incident comorbidity burden by HIV serostatus occurred among women <25 years old. In comparison, among 39 000 PLWH and 387 785 HIV-negative adults insured through Kaiser Permanente, comorbidity-free life expectancy (assessed 2014–2016) at age 21 was 14.5 and 30.9 years, respectively [9]. Our data, among women specifically, revealed a difference in NACM incidence by HIV serostatus that commenced at least a decade earlier. This dramatic disparity in age at risk for comorbidity onset among the cohorts is likely due to differences in participant sociodemographics. While WIHS participants are predominantly urban women of color with a high prevalence of obesity, substance use, and poverty [17], participants from other multisite cohorts examining multimorbidity among PLWH primarily comprise white men with stable access to care and higher income [2, 3, 9]. Additional studies are needed to investigate the interactions of sex, race, access to care, and other social determinants of health on mediating comorbidity development among PLWH [20].
Notably, women without HIV in our study also began accruing NACM as early as in their third decade of life. In a recent multicohort, multiethnic analysis of 32 833 participants (>50% female), women compared with men exhibited a significantly steeper increase in blood pressure trajectory that began as early as in their 20s and continued throughout their life [19]. It is possible that young women, regardless of HIV serostatus, may be particularly vulnerable to comorbidity incidence and progression [18, 19]. Along with female-specific biology, complex socioeconomic, environmental, and structural factors can affect physiology and coalesce to increase the risk of several comorbidities, and even premature mortality, among women compared with men [21].
Our data highlight the need to prioritize WLWH, particularly young women, for early NACM screening to identify those at highest risk of amassing comorbidities and to offer timely, targeted risk-modification interventions. Since PLWH experience multimorbidity at least a decade earlier than peers without HIV, age-anchored clinical guidance on comorbidity screening for the general adult population is inappropriate for use among PLWH. Such guidance misses the opportunity for early NACM detection among PLWH who may have decades of comorbidity-free life to preserve [10]. Furthermore, prior reports indicate that WLWH experience higher NACM burden and more severe disease than men [5, 12]; thus, sex attributes may differentially affect comorbidity onset and progression among PLWH compared with the general population. Primary care guidelines for PLWH [22] should consider more comprehensive and sex-stratified recommendations for comorbidity screening and prevention as data evolve on differential biologic risks and associated lifestyle factors driving NACM burden among WLWH vs men.
Current risk assessment tools developed in the general population, such as for CVD and bone fracture risk, underperform among PLWH [23, 24], thus failing to identify substantial numbers of WLWH who may benefit from earlier interventions to mitigate premature NACM onset. Novel comorbidity screening and prevention tools that take into account HIV- and female-specific pathogenic processes are needed [25] and should be integrated into comprehensive strategies focused on aggressive modification of traditional comorbidity risk factors as well as HIV disease control. Corroborating prior studies [6, 26], incident NACM burden among WLWH was associated with elevated BMI, current substance use, and certain sociodemographics, but not with HIV-related factors. This underscores the important contribution of social determinants of health in driving comorbidity burden among WLWH, as also found for women without HIV, and argues for increased attention and resources dedicated to improving women’s health systematically, especially for those from high-risk communities [27].
The early occurrence of multimorbidity among WLWH is likely multifactorial, related to a higher prevalence of traditional comorbidity risk factors and viral coinfections compared with the general population, the type and duration of ART exposure, and HIV-associated chronic inflammation and immune activation hastening the natural aging process [11]. Elevations in inflammatory biomarkers (ie, high-sensitivity C-reactive protein, interleukin 6, D-dimer) have been associated with NACM events and all-cause mortality among PLWH on suppressive ART [28, 29]. While only 45% of WLWH in this analysis were virologically suppressed at baseline, the vast majority (>80%) were suppressed by end of observation. Measures of longitudinal HIV viremia have been associated with incident myocardial infarction and mortality among male-predominant cohorts of PLWH [30, 31]. However, the effect of cumulative viremia on incident comorbidity burden and its relationship to chronic inflammation and immune activation warrants additional investigation.
Female-specific anatomic, chromosomal, immunologic, hormonal, and lifestyle factors likely interplay in a complex fashion to expedite aging and comorbidity incidence among WLWH [14, 32]. “Immunoaging,” the natural waning of immunity occurring with advanced age, is accelerated by HIV [33]. Among PLWH, despite ART-induced virologic suppression, immunoaging is attributed to persistent systemic inflammation as well as ongoing T-cell activation (from residual HIV replication, chronic viral coinfections, and translocated gut microbial products) and is associated with dysfunctional immunometabolism, dysregulated coagulation, and inflammatory vasculopathy [34–36]. Such mechanisms have been implicated in contributing to early NACM accrual among PLWH, which may be exacerbated by estrogen insufficiency among WLWH [32]. A hallmark of natural aging in women, hypoestrogenism leads to a proinflammatory state, thereby compounding the systemic inflammation of chronic HIV [32, 37]. While premenopausal status in the general population is protective against the development of several NACMs (eg, CVD and osteoporosis) [38, 39], this biologic benefit may be attenuated among WLWH who may experience menopause earlier and more severely [40].
This study warrants mention of limitations. First, some of the NACMs relied on self-report due to lack of available objective measures for confirmation, such as tissue pathology or imaging results [6]. This could have resulted in underestimation of incident NACM burden. Second, given the study objective to describe NACM accumulation over the life course of women, time-varying factors, such as the onset of menopause or obesity, were not evaluated. Third, we were not able to assess the longitudinal effects of using different antiretroviral classes, considering the effects of ART switching and nonadherence. Finally, nor were we able to describe the relationship between time-updated HIV viremia and incident NACM given the scope of this analysis.
In conclusion, this study is the first of its scale to comprehensively examine incident age-stratified comorbidity burden, and associated risk factors, among WLWH and at-risk women without HIV. The rate of NACM accrual was high for all women, though higher for WLWH, and associated with traditional comorbidity risk factors including social determinants of health. Strikingly, comorbidity incidence among women began in the third decade of life, suggesting high susceptibility to “premature aging” and supporting the need for earlier, more aggressive NACM risk assessment and interventions for young WLWH and at-risk women that could be integrated into a broader women’s health agenda during reproductive age. Implementation science, including innovative HIV- and female-specific clinical risk assessment and risk-reducing tools, tailored to the needs of young WLWH, will be paramount to address the synergy of HIV and premature multimorbidity, fueled by underlying social determinants, in this high-risk population.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the Women’s Interagency HIV Study (WIHS) participants who contributed time and data to this study; the WIHS for its support and data utilization; and the WIHS site co-investigators for serving as site liaisons for data collaboration.
Disclaimer. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Financial support. Data in this manuscript were collected by the WIHS, now the MACS/WIHS Combined Cohort Study (MWCCS). MWCCS (Principal Investigators): Atlanta Clinical Research Site (CRS) (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange, and Elizabeth Golub), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Chicago-Northwestern CRS (Steven Wolinsky), U01-HL146240; Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Los Angeles CRS (Roger Detels), U01-HL146333; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208; UAB-MS CRS (Mirjam-Colette Kempf, Jodie Dionne Odom, and Deborah Konkle-Parker), U01-HL146192; and University of North Carolina CRS (Adaora Adimora), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute, with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Human Genome Research Institute, National Institute on Aging, National Institute of Dental and Craniofacial Research, National Institute of Allergy and Infectious Diseases, National Institute of Neurological Disorders and Stroke, National Institute of Mental Health, National Institute on Drug Abuse, National Institute of Nursing Research, National Cancer Institute, National Institute on Alcohol Abuse and Alcoholism, National Institute on Deafness and Other Communication Disorders, and National Institute of Diabetes and Digestive and Kidney Diseases. MWCCS data collection is also supported by UL1-TR000004 (University of California, San Francisco Clinical & Translational Science Alliance [CTSA]), P30-AI-050409 (Emory Center for AIDS Research [CFAR]), P30-AI-050410 (University of North Carolina CFAR), and P30-AI-027767 (University of Alabama at Birmingham CFAR). This work was also supported by the Emory Specialized Center of Research Excellence (SCORE) on Sex Differences (grant number U54AG062334 to I. O.). L. F. C. is also supported by the National Center for Advancing Translational Sciences of the NIH (award numbers UL1TR002378 and KL2-TL1TR002381).
Potential conflicts of interest. P. C. T. reports grants from NIH, during the conduct of the study; and grants from Merck, outside the submitted work. F. J. P. reports personal fees from Gilead Sciences, Janssen, ViiV, and Merck, outside the submitted work. S. N. reports research funding from AbbVie and Gilead; has received consulting fees from Vir, Theratechnologies and BioMarin; and has served on event adjudication committees for BMS/PRA and FHI360. A. A. A. reports personal fees from Merck and personal fees and grants from Gilead, outside the submitted work. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Marcus JL, Chao CR, Leyden WA, et al. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr 2016; 73:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallant J, Hsue PY, Shreay S, Meyer N. Comorbidities among US patients with prevalent HIV infection—a trend analysis. J Infect Dis 2017; 216:1525–33. [DOI] [PubMed] [Google Scholar]
- 3.Wong C, Gange SJ, Moore RD, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) . Multimorbidity among persons living with human immunodeficiency virus in the United States. Clin Infect Dis 2018; 66:1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiels MS, Islam JY, Rosenberg PS, Hall HI, Jacobson E, Engels EA. Projected cancer incidence rates and burden of incident cancer cases in HIV-infected adults in the United States through 2030. Ann Intern Med 2018; 168:866–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palella FJ, Hart R, Armon C, et al. ; HIV Outpatient Study (HOPS) . Non-AIDS comorbidity burden differs by sex, race, and insurance type in aging adults in HIV care. AIDS 2019; 33:2327–35. [DOI] [PubMed] [Google Scholar]
- 6.Collins LF, Sheth AN, Christina Mehta C, et al. The prevalence and burden of non-AIDS comorbidities among women living with or at-risk for HIV infection in the United States. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; 53:1120–6. [DOI] [PubMed] [Google Scholar]
- 8.Schouten J, Wit FW, Stolte IG, et al. ; AGEhIV Cohort Study Group . Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis 2014; 59:1787–97. [DOI] [PubMed] [Google Scholar]
- 9.Marcus JL, Leyden WA, Alexeeff SE, et al. Comparison of overall and comorbidity-free life expectancy between insured adults with and without HIV infection, 2000-2016. JAMA Netw Open 2020; 3:e207954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins LF, Armstrong WS. What it means to age with HIV infection: years gained are not comorbidity free. JAMA Netw Open 2020; 3:e208023. [DOI] [PubMed] [Google Scholar]
- 11.Lerner AM, Eisinger RW, Fauci AS. Comorbidities in persons with HIV: the lingering challenge. JAMA 2019; doi: 10.1001/jama.2019.19775. [DOI] [PubMed] [Google Scholar]
- 12.Chow FC, Regan S, Zanni MV, et al. Elevated ischemic stroke risk among women living with HIV infection. AIDS 2018; 32:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frazier EL, Sutton MY, Tie Y, Fagan J, Fanfair RN. Differences by sex in cardiovascular comorbid conditions among older adults (aged 50–64 or ≥65 years) receiving care for human immunodeficiency virus. Clin Infect Dis 2019; 69:2091–100. [DOI] [PubMed] [Google Scholar]
- 14.Scully EP. Sex differences in HIV infection. Curr HIV/AIDS Rep 2018; 15:136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandhi M, Smeaton LM, Vernon C, et al. ; Womenʼs Health Inter-Network Scientific Committee (WHISC) . Low rate of sex-specific analyses in presentations at the Conference on Retroviruses and Opportunistic Infections (CROI) meeting, 2018: room to improve. J Acquir Immune Defic Syndr 2019; 81:e158–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartz D, Chitnis T, Kaiser UB, et al. Clinical advances in sex- and gender-informed medicine to improve the health of all: a review. JAMA Intern Med 2020; 180:574–83. [DOI] [PubMed] [Google Scholar]
- 17.Adimora AA, Ramirez C, Benning L, et al. Cohort profile: the Women’s Interagency HIV Study (WIHS). Int J Epidemiol 2018; 47:393–394i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arora S, Stouffer GA, Kucharska-Newton AM, et al. Twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction. Circulation 2019; 139:1047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji H, Kim A, Ebinger JE, et al. Sex differences in blood pressure trajectories over the life course. JAMA Cardiol 2020; 5:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silverstein M, Hsu HE, Bell A. Addressing social determinants to improve population health: the balance between clinical care and public health. JAMA 2019; doi: 10.1001/jama.2019.18055. [DOI] [PubMed] [Google Scholar]
- 21.Heise L, Greene ME, Opper N, et al. ; Gender Equality, Norms, and Health Steering Committee . Gender inequality and restrictive gender norms: framing the challenges to health. Lancet 2019; 393:2440–54. [DOI] [PubMed] [Google Scholar]
- 22.Thompson MA, Horberg MA, Agwu AL, et al. Primary care guidance for persons with human immunodeficiency virus: 2020 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa1391. [DOI] [PubMed] [Google Scholar]
- 23.Kapelios CJ, Argyris AA, Protogerou AD, et al. Progression of subclinical vascular damage in people living with HIV is not predicted by current cardiovascular risk scores: a prospective 3-year study. J Acquir Immune Defic Syndr 2020; 83:504–12. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Sharma A, Shi Q, et al. Improved fracture prediction using different fracture risk assessment tool adjustments in HIV-infected women. AIDS 2018; 32:1699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moran CA, Sheth AN, Mehta CC, et al. The association of C-reactive protein with subclinical cardiovascular disease in HIV-infected and HIV-uninfected women. AIDS 2018; 32:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Althoff KN, Gebo KA, Moore RD, et al. ; North American AIDS Cohort Collaboration on Research and Design . Contributions of traditional and HIV-related risk factors on non-AIDS-defining cancer, myocardial infarction, and end-stage liver and renal diseases in adults with HIV in the USA and Canada: a collaboration of cohort studies. Lancet HIV 2019; 6:e93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davidson KW, Kemper AR, Doubeni CA, et al. Developing primary care-based recommendations for social determinants of health: methods of the U.S. Preventive Services Task Force. Ann Intern Med 2020; 173:461–7. [DOI] [PubMed] [Google Scholar]
- 28.Angelidou K, Hunt PW, Landay AL, et al. Changes in inflammation but not in T-cell activation precede non-AIDS-defining events in a case-control study of patients on long-term antiretroviral therapy. J Infect Dis 2018; 218:239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tien PC, Choi AI, Zolopa AR, et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr 2010; 55:316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salinas JL, Rentsch C, Marconi VC, et al. Baseline, time-updated, and cumulative HIV care metrics for predicting acute myocardial infarction and all-cause mortality. Clin Infect Dis 2016; 63:1423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang R, Haberlen SA, Palella FJ Jr, et al. Viremia copy-years and mortality among combination antiretroviral therapy-initiating HIV-positive individuals: how much viral load history is enough? AIDS 2018; 32:2547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raghavan A, Rimmelin DE, Fitch KV, Zanni MV. Sex differences in select non-communicable HIV-associated comorbidities: exploring the role of systemic immune activation/inflammation. Curr HIV/AIDS Rep 2017; 14:220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Appay V, Sauce D. Assessing immune aging in HIV-infected patients. Virulence 2017; 8:529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39:633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butterfield TR, Landay AL, Anzinger JJ. Dysfunctional immunometabolism in HIV infection: contributing factors and implications for age-related comorbid diseases. Curr HIV/AIDS Rep 2020; 17:125–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duffau P, Wittkop L, Lazaro E, et al. ; ANRS CO3 Aquitaine Cohort Study Group . Association of immune-activation and senescence markers with non-AIDS-defining comorbidities in HIV-suppressed patients. AIDS 2015; 29:2099–108. [DOI] [PubMed] [Google Scholar]
- 37.Hewagama A, Patel D, Yarlagadda S, Strickland FM, Richardson BC. Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes Immun 2009; 10:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S, Eghbali M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ 2017; 8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manolagas SC, O’Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol 2013; 9:699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tariq S, Anderson J, Burns F, Delpech V, Gilson R, Sabin C. The menopause transition in women living with HIV: current evidence and future avenues of research. J Virus Erad 2016; 2:114–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.