Abstract
Background
Candida auris is an emerging multidrug-resistant yeast that contaminates healthcare environments causing healthcare-associated outbreaks. The mechanisms facilitating contamination are not established.
Methods
C. auris was quantified in residents’ bilateral axillary/inguinal composite skin swabs and environmental samples during a point-prevalence survey at a ventilator-capable skilled-nursing facility (vSNF A) with documented high colonization prevalence. Environmental samples were collected from all doorknobs, windowsills and handrails of each bed in 12 rooms. C. auris concentrations were measured using culture and C. auris-specific quantitative polymerase chain reaction (qPCR) The relationship between C. auris concentrations in residents’ swabs and associated environmental samples were evaluated using Kendall’s tau-b (τ b) correlation coefficient.
Results
C. auris was detected in 70/100 tested environmental samples and 31/57 tested resident skin swabs. The mean C. auris concentration in skin swabs was 1.22 × 105 cells/mL by culture and 1.08 × 106 cells/mL by qPCR. C. auris was detected on all handrails of beds occupied by colonized residents, as well as 10/24 doorknobs and 9/12 windowsills. A positive correlation was identified between the concentrations of C. auris in skin swabs and associated handrail samples based on culture (τ b = 0.54, P = .0004) and qPCR (τ b = 0.66, P = 3.83e−6). Two uncolonized residents resided in beds contaminated with C. auris.
Conclusions
Colonized residents can have high C. auris burdens on their skin, which was positively related with contamination of their surrounding healthcare environment. These findings underscore the importance of hand hygiene, transmission-based precautions, and particularly environmental disinfection in preventing spread in healthcare facilities.
Keywords: Candida auris, transmission, infection control, fungi, HAI
Candidaauris causes healthcare-associated outbreaks that are difficult to control. Environmental contamination is common in these settings. At a ventilator-capable skilled-nursing facility, we found environmental contamination was positively related to the colonization burdens on associated residents’ skin.
Candida auris is an emerging pathogenic yeast of increasing global concern [1, 2]. Like other pathogenic Candida, C. auris can cause life-threatening invasive infections with high mortality rates [3, 4]. C. auris can colonize the skin, which increases risk for developing a blood stream infection [5]. Treatment options are limited due to drug-resistance, as many isolates are resistant to at least 1 but often 2 and sometimes all 3 classes of antifungals [6]. The public-health impact of C. auris is further amplified by its ability to cause persistent outbreaks in healthcare settings, which is uncharacteristic of other pathogenic yeasts [4, 7–12]. In the United States, C. auris has been problematic in long-term acute-care hospitals (LTACHs) and ventilator-capable skilled nursing facilities (vSNFs), which provide high-acuity care for medically complicated and vulnerable populations over extended periods [4]. C. auris has spread among vSNFs and LTACHs in the same patient-sharing networks, facilitating the expansion of C. auris within and across geographical regions [4, 13, 14]. C. auris continues to spread on a global scale and cases have now been documented in over 30 countries [15]. Whole-genome sequence-based strain typing has found all isolates characterized to date fit within just 5 highly clonal lineages, highlighting the central role transmission has played in the public health impact of this novel pathogen [11, 15, 16].
Transmission is driven in part by contamination of the healthcare environment and medical equipment, where C. auris can remain viable for weeks [17]. Disinfecting these surfaces is difficult due to the extensive nature of contamination and practical challenges inherent to the vSNF and LTACH settings such as frequency of multi-occupancy rooms. There is a need to further develop environmental control strategies for this emergent pathogen. The shedding of viable C. auris cells from colonized patients has been suggested to facilitate environmental contamination, although data directly demonstrating this association is not available [17–19]. Improving our understanding of how environmental contamination occurs can help inform infection control strategies. Here we assess the relationship between the C. auris colonization burden on resident’s skin and environmental contamination at a vSNF with high C. auris prevalence.
METHODS
Settings
Samples were collected in a 70-bed ventilator-capable unit of a 300-bed SNF (vSNF A) in Chicago, Illinois, USA, in October 2018. The first C. auris colonization case at this facility was identified in March 2017 during a point-prevalence survey (PPS) that was performed using culture. Six subsequent PPSs occurring during March 2017– September 2018 were also performed using culture and documented a rise in C. auris colonization prevalence on the ventilator-capable floor, reaching 71% [13]. At the time of sampling, this facility was a participant in a heightened infection prevention and control (IPC) program designed to control the spread of multidrug-resistant organisms (MDROs) through a bundle of interventions that included cohorting residents colonized by the same MDRO, increased alcohol-based hand rub availability, dedicating a full time environmental service staff member to disinfecting the vSNF unit with a sporicidal agent, and daily bathing of residents with 2% chlorhexidine gluconate (CHG) wipes [13]. At the time of this study, the C. auris colonization status of many residents was already determined from the previous PPS. These previous results were taken into consideration when describing the distribution of C. auris in the facility.
Sample Collection
Screening of residents for C. auris was performed as part of the ongoing surveillance and IPC efforts by Chicago Department of Public Health. Bilateral axillary/inguinal composite skin swabs were collected from residents on vSNF A using a single BD Eswab in 1 mL of liquid AMIES Medium (#220245, BD Diagnostics). Residents were screened regardless of whether they had previously been positive for C. auris. Residual material from these samples was used to quantify C. auris colonization burdens as approved by Centers for Disease Control and Prevention’s (CDC’s) human subjects internal review board.
On the same day, environmental samples were collected from 12 rooms from the following surfaces: the windowsills, the inside and outward facing doorknobs, and the left and right handrails of each bed. Prior colonization data were referenced when rooms were selected such that at least 1 room without a known C. auris colonized resident was included. Samples were collected from defined surface areas using 3MTM Cellulose Sponge-Sticks with neutralizing buffer (3M Healthcare, St. Paul, MN). The quantity of C. auris recovered was normalized by dividing the number of cells detected by the surface area sampled and expressed as cells/100 cm2. The time when the surfaces were last cleaned and disinfected was unknown. Both resident and environmental samples were stored at 4°C and tested within 72 hours after collection as described below.
Sample Processing
All patient samples were processed with the Taqman quantitative polymerase chain reaction (qPCR) [20], a most probable number (MPN) culture method, and an enrichment broth culture method, providing quantitative culture-independent results, quantitative culture-dependent results, as well as a qualitative gold-standard culture result, respectively [17, 21, 22]. Environmental samples were preprocessed by homogenizing with a Stomacher® 400 Circulator (Seward, West Sussex, UK), before testing with the Taqman qPCR, enrichment broth and direct dilution plating. The percent recovery (%R) of environmental sampling was determined based on controlled laboratory experiments with pre-inoculated coupons and is further described in the Supplementary Material-methods. Detailed descriptions of sample processing methods are also described in the Supplementary Material.
Statistics and Data Analysis
The relationship between C. auris concentrations in resident skin swabs and associated handrail samples was evaluated using Kendall’s tau-b (τ b) coefficient of rank correlation and the corresponding nonparametric rank test. It is an alternative to the Spearman rank-order correlation coefficient and is recommended in situations with small sample size and many tied ranks [23]. Data analysis and figures were generated using R 4.0.2 and Python 3.7 software.
RESULTS
C. auris Burden on Residents’ Skin
Fifty-seven (82.6%) of 69 residents on the ventilator-capable floor of vSNF A were screened for C. auris skin colonization. Eight refused screening, and 4 were not present at the time of sampling. Twenty (35.1%) of the screened residents were found to be C. auris positive by both culture and qPCR; 11 residents were identified as positive by qPCR but not culture. Thus, a total of 31 (54.4%) residents were positive at the time of sampling. All culture-positive residents were also positive by qPCR. Of the 11 qPCR-positive but culture-negative residents, 9 were known to be C. auris culture-positive from prior PPS. One resident had been sampled 7 times since March 2017 and had been consistently negative by culture. The remaining single culture-negative but qPCR-positive resident had no prior C. auris screening history.
Fourteen of the 30 occupied rooms on the floor (46.6%) housed at least 1 resident that was culture-positive, and an additional 4 rooms had at least 1 qPCR-positive resident (18 rooms, 60.0% total; Figure 1).
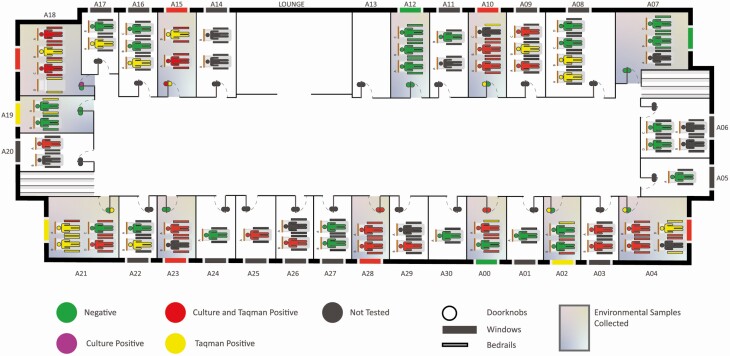
Figure 1.
Facility map with culture-based and qPCR results for residents and associated environmental surfaces. The specific organization of beds within a room may differ from the image.
The mean concentration of C. auris in culture-positive skin swabs was 1.2 × 105 MPN/mL (range 7.1–1.0 × 106), while the mean concentration interpolated from qPCR Cq values was 1.1 × 106 cells/mL (range 410–9.7 × 106).
Environmental Contamination of C. auris
A total of 100 environmental samples were collected from the windowsills, doorknobs, and handrails of the resident beds in 12 rooms. Fifty environmental samples were culture-positive, and 70 were qPCR-positive. All culture-positive samples were qPCR-positive except for the outward facing doorknob in room A18, which was culture-positive but qPCR-negative. The mean concentration of C. auris in culture-positive environmental samples was 92 colony-forming units (CFU)/100 cm2 (range 2.4–970), and the mean concentration interpolated from qPCR was 4.0 × 104 cells/100 cm2 (range 460–4.50 × 105; Table 1).
Table 1.
Results From Environmental Sampling Organized by Sample Type
| Taqman Results | Culture Resultsa | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive/Total | Interpolated cells/100 cm2 | Positive /Total | CFU/100 cm2 | ||||||
| Sample Type | Mean | Min | Max | Broth | Plates | Mean | Min | Max | |
| Window | 9/12 | 8.2 × 103 | 660 | 3.2 × 104 | 6/12 | 6/12 | 80 | 4.7 | 410 |
| Indoor knob | 7/12 | 4.6 × 103 | 460 | 1.5 × 105 | 3/12 | 2/12 | 2.9 | 2.4 | 3.4 |
| Outdoor knob | 3/12 | 1.2 × 103 | 840 | 1.8 × 103 | 3/12 | 1/12 | 350 | - | - |
| Left handrail | 26/32 | 5.1 × 104 | 570 | 3.7 × 105 | 18/32 | 17/32 | 58 | 2.7 | 270 |
| Right handrail | 25/32 | 5.4 × 104 | 590 | 4.5 × 105 | 20/32 | 18/32 | 120 | 3.3 | 970 |
| Total | 70/100 | 4.0 × 104 | 460 | 4.5 × 105 | 50/100 | 44/100 | 92 | 2.4 | 970 |
Abbreviation: CFU, colony-forming unit.
aEach sample was cultured using both the qualitative enrichment broth method as well as quantitative dilution plating. The summary statistics provided by culture reflect results from the quantitative dilution plating.
Sampling Efficiency and Recovery of C. auris Recovery From Plastic Surfaces
The percent of C. auris AR 0385 cells recovered from spiked textured plastic surfaces with the sponge sampling method ranged from 1.4 % to 3.7%, with the mean recovery found to be 2.3% (SD 0.008). Overgrowth of other organisms was not observed on any plates.
Skin Colonization Status of Residents in Rooms With Associated Environmental Sampling
Environmental sampling was conducted in 12 rooms that housed 28 residents: 12 residents were positive by both culture and qPCR, 5 were culture-negative but positive by qPCR, and the remaining 11 were negative. Overall, 17 C. auris-positive residents were housed in 8 of the 12 sampled rooms. The remaining 4 rooms were occupied by C. auris negative residents, and 1 resident in room A07 who was not tested (Figure 1). Environmental contamination with C. auris was detected in all 8 rooms with C. auris positive residents, as well as in 2 of the 4 rooms occupied by C. auris negative residents (Figure 1).
All 12 C. auris culture-positive residents with associated environmental samples had at least one culture-positive handrail; 10 of these (83.3%) were culture-positive for both handrails. All handrails of culture-positive residents were also qPCR-positive. Similarly, for the 5 beds occupied by residents that were qPCR-positive but culture-negative, all 10 associated handrails were qPCR-positive, 6 of which were also culture-positive. Therefore, when culture and qPCR results were considered collectively, C. auris contamination was detected on both handrails of all beds associated with all 17 C. auris positive residents (Figure 1).
Relationship Between Skin Colonization Burden and Environment Contamination
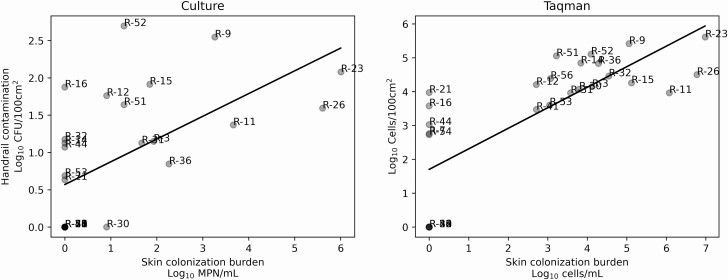
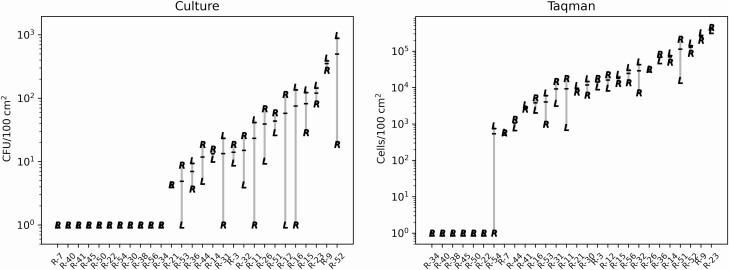
The concentrations of C. auris in residents’ skin swabs were positively related to the averaged concentration recovered from the left and right handrails of their beds (Figure 2). This relationship was observed when using data from both culture-dependent (τ b = 0.536, P = .0004) as well as qPCR (τ b = 0.657, P = 3.832e−6) approaches. Similarly, a positive association was found when the left and right handrails were evaluated individually as well as collectively as independent samples (P < .0001, Supplementary Figure 1). Even though the range of C. auris concentrations observed on all handrails ranged over several orders of magnitude, left-right handrail pairs from the same bed were generally similar in concentration (Figure 3).
Figure 2.
Environmental contamination of the bed handrails (Y-axis) shown in relationship to the occupying resident’s skin colonization burden (X-axis) with culture-based (panel A) and qPCR-based (panel B) methods. Gray points indicate the average of both the left and right handrails associated with a given resident. Linear regression shown for visual aid. Relationship between environmental contamination on handrails and resident colonization burden assessed with non-parametric Kendall’s tau-B.
Figure 3.
Concentrations of C. auris on the left and right handrails of each resident’s bed. Culture results are shown in the left panel and qPCR results are shown the right panel. Left and Right sides of the bed indicated with an “L” and “R”, respectively. Samples are organized along the Y-axis based on ascending mean of left and right sides shown with black horizontal bar.
Detection of C. auris in the Environment of Residents Who Screened Negative for C. auris
In addition to the 17 C. auris positive beds that were occupied by C. auris colonized residents, we found 3 beds, where both handrails were culture-positive despite being occupied by residents who screened negative for C. auris (Figure 1, Room A00 Bed B, Room A02 Bed B, and Room A21 Bed C). Review of facility records indicated the occupant of Bed C in Room A21 had previously been reported colonized. In contrast, the occupants of Bed B in Room A00 and Bed B in Room A02 had both been sampled numerous times and have no prior record of C. auris colonization. The facility records indicate that both residents were recently relocated into these rooms, which were previously occupied by C. auris colonized residents as recently as early 1 month prior for Bed B in room A00 and 2 months prior for Bed B in Room A02.
DISCUSSION
Controlling C. auris in the healthcare environment is challenging because the mechanisms facilitating transmission are not well understood. Previous investigations have established that extensive contamination of the healthcare setting is common during C. auris outbreaks, but it has not been demonstrated how this contamination occurs [4, 7, 10, 24]. Here we find that colonized residents can harbor high concentrations of C. auris cells on their skin, often hundreds of thousands and even millions of cells per sample. Importantly, we found residents with more C. auris on their skin also had more C. auris on their bed, thus establishing a positive correlation between skin colonization and environmental contamination for this pathogen (Figure 2). Formally relating these 2 variables improves our understanding of how C. auris spreads and helps support evidence-based IPC guidance. Similar observations relating colonization burden and environmental contamination have also been made with bacterial pathogens problematic in the healthcare environment [25–28].
Our findings have important implications for C. auris control strategies. Because colonized residents likely continually contaminate the environment through shedding, diligent and frequent disinfection is necessary for the duration of care [2, 29]. We found C. auris colonization burdens ranged by 4–5 orders of magnitude (Table 1). The reasons why some people have a higher colonization burden compared to others is not currently known but may include factors such as the frequency of CHG and standard bathing or duration of stay at the facility. Colonization burden may also be related to underlying-conditions and host factors, such as genetics or host microbial community. Previous epidemiological studies have shown that exposure to broad-spectrum antibiotics and recent hospitalization are risk factors for C. auris colonization [30].
Given the relationship observed between C. auris colonization burden and environmental contamination, suppressing colonization may help reduce transmission. Daily CHG bathing has been used with some success to control the spread of bacterial pathogens such as Vancomycin-resistant Enterococcus [31, 32]. Additional data are needed to better understand the impact of CHG bathing on C. auris. More broadly, it is important to note C. auris colonization is not fully understood; recent data have highlighted C. auris colonization in the anterior nares and other body sites. Colonization at these sites should be considered when developing strategies to reduce or suppress colonization [33, 34].
C. auris was detected on both handrails of all beds occupied by C. auris colonized residents (Figure 1). Moreover, both left and right handrails of a bed were contaminated with similar concentrations of C. auris, suggesting shedding from the occupant as a common source of contamination (Figure 3). Contaminated beds may facilitate transmission if not disinfected effectively between occupants. We identified 2 instances where residents without C. auris colonization history were found in beds contaminated with viable C. auris. There might be several explanations for this observation. First, facility records indicate both residents were recently moved into the new rooms and therefore, might have been placed into beds that were not properly decontaminated after previous residents. Unfortunately, no data were available whether these residents were relocated with their old bed, which we were told was a common practice in the facility, or placed into a bed already located in the new room. Second, it is possible these residents were colonized by C. auris at other body sites and missed by our colonization screening [29, 30]. Third, in at least 1 case, both a negative and a positive resident were housed in the same room, raising the possibility that cross-contamination occurred. Overall, our data indicate that facilities should ensure beds are regularly and effectively decontaminated.
Our environmental data likely underestimate the full extent of environmental contamination. When investigating the efficiency of the sampling method for C. auris, we found the mean recovery was only 2.3% (SD 0. 8) of the total cells present, when working with an artificially spiked textured plastic surface similar to handrails. This indicates the actual extent of environmental contamination could be up to 100x higher than detected by culture. More colonized residents and environmental samples were detected with qPCR than with culture, which was not surprising because qPCR can detect both viable and nonviable cells. In addition, it was previously shown viable but nonculturable C. auris cells can persist in the environment [17]. The recovery rate of C. auris was lower than that of other organisms using this same sampling and processing method. Bacterial sampling and recovery was found to range from 7.7% (SD 5.2%) for carbapenemase-producing KPC+ Klebsiella pneumoniae to 58.9% (SD 12.7%) for Clostridioides difficile spores [35].
C. auris was also detected on doorknobs and windowsills, demonstrating the ability of C. auris to be spread more broadly within the room [4, 7, 10]. This emphasizes the importance of adherence to current IPC guidelines for C. auris. Although we were unable to verify IPC compliance at the time of this work, these practices were assessed at this facility several months prior to our work [13]. An environmental cleaning assessment found 61% fluorescent marker removal in 7 rooms tested. External auditors observed 75% staff compliance with hand hygiene upon room exit and 48% staff compliance upon on room entrance. Glove and gown use compliance was 73% for patients on contact precautions. Our work highlights the value of environmental cleaning and the adherence to these guidelines [36]. Because many products are infective against C. auris, daily and terminal disinfection should be performed using products with EPA-registered C. auris label claims [19, 37, 38]. Additional work is needed to understand disinfection efficacy of UV light, hydrogen peroxide fogging, and other “no-touch” methods for reducing transmission.
Our work has several limitations. First, our analysis of the relationship between colonization burden on the skin and environmental contamination establishes correlation but not causation. Second, this work was performed at a single facility with a high colonization prevalence. Given the clonal nature of C. auris outbreaks, it is likely that the isolates recovered in this study are highly related and do not represent the genetic diversity known within the species. Although the environmental isolates from this study were not sequenced, whole genome sequencing of clinical isolates from patients from this and other healthcare facilities in Chicago demonstrated that the isolates belonged to clade IV and were highly clonal [16]. Third, these data represent a single point in time and do not address how colonization burden or environmental contamination change over time. Furthermore, 17% of residents were not sampled, and their contribution to environmental contamination at the facility was not known. We also lacked information to verify routines for cleaning, CHG bathing, and other facility practices.
In summary, we found that colonized individuals can harbor high concentrations of C. auris on their skin. C. auris concentrations on residents’ beds were positively related to the amount on their skin, emphasizing the importance of source control methods as well as diligent environmental cleaning needed to reduce the transmission of C. auris. Further work to improve our understanding of colonization, mechanisms of transmission, and modes of environmental contamination will help improve our ability to control this pathogen.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We would like to acknowledge Steve Hurst and Kizee A. Etienne from the Centers for Disease Control for assistance processing samples. We’d also like to thank Snigdha Vallabhaneni from the Centers for Disease Control and Prevention for helpful review of the manuscript. The use of product names in this manuscript does not imply their endorsement by the US Department of Health and Human Services. The finding and conclusions in this article are those of the authors and do not necessarily represent the views of the CDC.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Vallabhaneni S, Jackson BR, Chiller TM. Candida auris: an emerging antimicrobial resistance threat. Ann Intern Med 2019; 171:432–3. [DOI] [PubMed] [Google Scholar]
- 2.Forsberg K, Woodworth K, Walters M, et al. . Candida auris: the recent emergence of a multidrug-resistant fungal pathogen. Med Mycol 2019; 57:1–12. [DOI] [PubMed] [Google Scholar]
- 3.Clancy CJ, Nguyen MH. Emergence of Candida auris: an international call to arms. Clin Infect Dis 2017; 64:141–3. [DOI] [PubMed] [Google Scholar]
- 4.Adams E, Quinn M, Tsay S, et al. . Candida auris in healthcare facilities, New York, USA, 2013–2017. Emerg Infect Dis 2018; 24:1816–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Southwick K, Adams EH, Greenko J, et al. . 2039. New York State 2016–2018: progression from Candida auris colonization to bloodstream infection. Open Forum Infect Dis 2018; 5:S594–5. [Google Scholar]
- 6.Lockhart SR. Candida auris and multidrug resistance: defining the new normal. Fungal Genet Biol 2019; 131:103243. [DOI] [PubMed] [Google Scholar]
- 7.Schelenz S, Hagen F, Rhodes JL, et al. . First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control 2016; 5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eyre DW, Sheppard AE, Madder H, et al. . A Candida auris outbreak and its control in an intensive care setting. N Engl J Med 2018; 379:1322–31. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz-Gaitán A, Moret AM, Tasias-Pitarch M, et al. . An outbreak due to Candida auris with prolonged colonisation and candidaemia in a tertiary care European hospital. Mycoses 2018; 61:498–505. [DOI] [PubMed] [Google Scholar]
- 10.Escandón P, Chow NA, Caceres DH, et al. . Molecular epidemiology of Candida auris in Colombia reveals a highly related, countrywide colonization with regional patterns in amphotericin B resistance. Clin Infect Dis 2019; 68:15–21. [DOI] [PubMed] [Google Scholar]
- 11.Chow NA, Gade L, Tsay SV, et al. ; US Candida auris Investigation Team . Multiple introductions and subsequent transmission of multidrug-resistant Candida auris in the USA: a molecular epidemiological survey. Lancet Infect Dis 2018; 18:1377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almaghrabi RS, Albalawi R, Mutabagani M, et al. . Molecular characterisation and clinical outcomes of Candida auris infection: single-centre experience in Saudi Arabia. Mycoses 2020; 63:452–60. [DOI] [PubMed] [Google Scholar]
- 13.Pacilli M, Kerins JL, Clegg WJ, et al. . Regional emergence of Candida auris in Chicago and lessons learned from intensive follow-up at 1 ventilator-capable skilled nursing facility. Clin Infect Dis 2020; 71:e718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karmarkar E, Karmarkar E, O’Donnell K, et al. . LB1. Regional assessment and containment of Candida auris transmission in post-acute care settings—Orange County, California, 2019. Open Forum Infect Dis 2019: 6:S993-S. [Google Scholar]
- 15.Chow NA, Muñoz JF, Gade L, et al. . Tracing the evolutionary history and global expansion of Candida auris using population genomic analyses. mBio 2020; 11:e03364–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chow NA, de Groot T, Badali H, Abastabar M, Chiller TM, Meis JF. Potential fifth clade of Candida auris, Iran, 2018. Emerg Infect Dis 2019; 25:1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welsh RM, Bentz ML, Shams A, et al. . Survival, persistence, and isolation of the emerging multidrug-resistant pathogenic yeast Candida auris on a plastic health care surface. J Clin Microbiol 2017; 55:2996–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piedrahita CT, Cadnum JL, Jencson AL, Shaikh AA, Ghannoum MA, Donskey CJ. Environmental surfaces in healthcare facilities are a potential source for transmission of Candida auris and other Candida species. Infect Control Hosp Epidemiol 2017; 38:1107–9. [DOI] [PubMed] [Google Scholar]
- 19.Cadnum JL, Shaikh AA, Piedrahita CT, et al. . Effectiveness of disinfectants against Candida auris and other Candida species. Infect Control Hosp Epidemiol 2017; 38:1240–3. [DOI] [PubMed] [Google Scholar]
- 20.Ahmad A, Spencer JE, Lockhart SR, et al. . A high-throughput and rapid method for accurate identification of emerging multidrug-resistant Candida auris. Mycoses 2019; 62:513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bentz ML, Sexton DJ, Welsh RM, Litvintseva AP. Phenotypic switching in newly emerged multidrug-resistant pathogen Candida auris. Med Mycol 2018; 57:636–8. [DOI] [PubMed] [Google Scholar]
- 22.Alexander M. Most probable number method for microbial populations. In: Page A. Methods of Soil Analysis: American Society of Agronomy, Inc., Soil Science Society of America, Inc., 2015:815–20. [Google Scholar]
- 23.Kendall MG. The treatment of ties in ranking problems. Biometrika 1945; 33:239–51. [DOI] [PubMed] [Google Scholar]
- 24.Kumar J, Eilertson B, Cadnum JL, et al. . Environmental contamination with Candida species in multiple hospitals including a tertiary care hospital with a Candida auris outbreak. Pathog Immun 2019; 4:260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donskey CJ, Chowdhry TK, Hecker MT, et al. . Effect of antibiotic therapy on the density of vancomycin-resistant Enterococci in the stool of colonized patients. N Engl J Med 2000; 343:1925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sethi AK, Al-Nassir WN, Nerandzic MM, Bobulsky GS, Donskey CJ. Persistence of skin contamination and environmental shedding of Clostridium difficile during and after treatment of C. difficile infection. Infect Control Hosp Epidemiol 2010; 31:21–7. [DOI] [PubMed] [Google Scholar]
- 27.Livorsi DJ, Livorsi DJ, Arif S, et al. . Methicillin-resistant Staphylococcus aureus (MRSA) nasal real-time PCR: a predictive tool for contamination of the hospital environment. Infect Control Hosp Epidemiol 2015; 36:34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lerner A, Adler A, Abu-Hanna J, Meitus I, Navon-Venezia S, Carmeli Y. Environmental contamination by carbapenem-resistant Enterobacteriaceae. J Clin Microbiol 2013; 51:177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobs Slifka KM, Kabbani S, Stone ND. Prioritizing prevention to combat multidrug resistance in n ursing homes: a call to action. J Am Med Dir Assoc 2020; 21:5–7. [DOI] [PubMed] [Google Scholar]
- 30.Rossow J, Ostrowsky B, Adams E, et al. . Factors associated with Candida auris colonization and transmission in skilled nursing facilities with ventilator units, New York, 2016–2018. Clin Infect Dis 2020; 72:e753–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiner LM, Webb AK, Walters MS, Dudeck MA, Kallen AJ. Policies for controlling multidrug-resistant organisms in US healthcare facilities reporting to the National Healthcare Safety Network, 2014. Infect Control Hosp Epidemiol 2016; 37:1105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vernon MO, Hayden MK, Trick WE, Hayes RA, Blom DW, Weinstein RA; Chicago Antimicrobial Resistance Project (CARP) . Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med 2006; 166:306–12. [DOI] [PubMed] [Google Scholar]
- 33.Hayden MK, Dangana TE, Yelin RD, et al. . 897. Prevalence of Candida auris at body sites, characterization of skin microbiota, and relation of chlorhexidine gluconate (CHG) skin concentration to C. auris detection among patients at a high-prevalence ventilator-capable skilled nursing facility (vSNF) with established CHG bathing. Open Forum Infect Dis 2019; 6:S25–S6. [Google Scholar]
- 34.Zhu Y, O’Brien B, Leach L, et al. . Laboratory analysis of an outbreak of Candida auris in New York from 2016 to 2018: impact and lessons learned. J Clin Microbiol 2020; 58:e01503–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houston H, Rose LJ, West-Deadwyler R, Noble-Wang JA. Recovery of four healthcare associated organisms from hospital surfaces using sponge sticks. American Society for Microbiology (ASM) Microbe. Boston, MA: ASM, 2016. [Google Scholar]
- 36.Caceres DH, Forsberg K, Welsh RM, et al. . Candida auris: a review of recommendations for detection and control in healthcare settings. J Fungi 2019; 5:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutala WA, Kanamori H, Gergen MF, Sickbert-Bennett EE, Weber DJ. Susceptibility of Candida auris and Candida albicans to 21 germicides used in healthcare facilities. Infect Control Hosp Epidemiol 2019; 40:380–2. [DOI] [PubMed] [Google Scholar]
- 38.Sexton DJ, Welsh RM, Bentz ML, et al. . Evaluation of nine surface disinfectants against Candida auris using a quantitative disk carrier method: EPA SOP-MB-35. Infect Control Hosp Epidemiol 2020; 41:1219–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.