Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19), affecting multiple organ systems, including the respiratory tract and lungs. Several studies have reported that the tryptophan-kynurenine pathway is altered in COVID-19 patients. The tryptophan-kynurenine pathway plays a vital role in regulating inflammation, metabolism, immune responses, and musculoskeletal system biology. In this minireview, we surmise the effects of the kynurenine pathway in COVID-19 patients and how this pathway might impact muscle and bone biology.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the current pandemic, suspected to originate from infected bats [1]. Coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2, has turned out to be a major global catastrophe affecting millions of individuals across the globe [2]. In the United States, as of today, more than 30 million lives have been affected by COVID-19, and over six hundred thousand Americans have lost their lives, according to the Johns Hopkins Coronavirus Resource Center [3]. COVID-19 can present a wide spectrum of symptoms such as cough, fever, shortness of breath, muscle pain, and loss of taste and smell [4]. Mild to severely affected patients may experience elevated proinflammatory cytokines such as IL-1, TNF- α, and IL-6 [5], which negatively affect human health (Figure 1). Excessive activation of these proinflammatory cytokines (cytokine storm) leads to the alteration of several metabolic signaling pathways (e.g., the tryptophan-kynurenine pathway).
Figure 1.
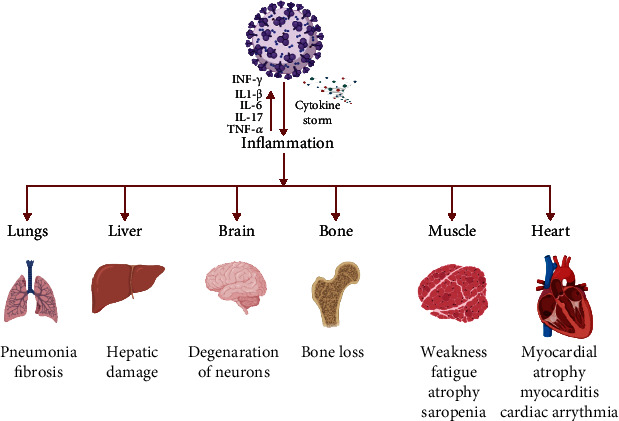
Illustration of impact of COVID-19 caused by infection with SARS-CoV-2 on various human organs-lungs, liver, brain, bone, muscle, and heart. (Figure is created by using http://BioRinder.com.)
Recent studies have shown that the tryptophan-kynurenine pathway (Trp-Kyn) is altered in COVID-19 patients. A study conducted by Thomas et al. analyzed serum metabolites of COVID-19 patients and found that tryptophan (Trp) levels were reduced, and L-kynurenine (Kyn) was elevated [5]. A study performed by Fraser et al. reported similar findings (elevated levels of Kyn in COVID-19 patients) [6]. Another study reported that Kyn levels were elevated, along with kynurenic acid (Kyn-A) and quinolinic acid (QA) in the serum of COVID-19 patients [7]. The study conducted by Lawler et al. demonstrated elevated levels of QA in the blood plasma of COVID-19 patients [8]. Sex-specific differences have also been reported in the levels of Kyn-A and QA metabolites in COVID-19 patients. Serum metabolic analyses performed by Cai et al. reported elevated levels of Kyn-A in male patients compared to female patients [9]. Lionetto et al. assessed serum metabolites in COVID-19 patients and found that Kyn/Trp levels were elevated in male patients [10]. Moreover, Cai et al. (2020) reported an elevated Kyn-A: L-Kyn was associated with increased severity of COVID-19 infection in male patients [9]. The studies mentioned above indicate that activation of the tryptophan-kynurenine pathway might be one of the reasons for the increased susceptibility of males to COVID-19 infection.
Several studies also reported elevated levels of genes involved in tryptophan metabolic pathways [11, 12]. The study conducted by Policard et al. reported that indoleamine-pyrrole 2,3-dioxygenase (IDO-1) is significantly upregulated in COVID-19 patients [11]. Another study also reported similar findings showing elevated levels of IDO-1 in COVID-19 patients [12]. The study conducted by Grunewald et al. in the murine model demonstrated that IDO-1, IDO-2, and TDO-2 were significantly upregulated in murine coronavirus infection [13]. The prevalence and severity of COVID-19 disease are directly associated with age and the underlying condition, such as diabetes, obesity, and cardiovascular disorders [14, 15]. It is well known that the tryptophan-kynurenine pathway elevated with age and above mentioned underlying conditions [16].
The findings from these studies strongly indicate that the Trp-Kyn pathway is altered in COVID-19 patients, leading to a decrease in Trp levels and an increase in Kyn and its metabolites. Recent studies also demonstrated reduced muscle mass and bone loss in COVID-19 patients [17–20]. Based on the findings from our group and published literature, we came up with a novel perspective suggesting that the activation of the Trp-Kyn pathway in COVID-19 patients might be involved in bone and muscle loss.
2. The Tryptophan-Kynurenine (Trp-Kyn) Pathway
Tryptophan (Trp) is an essential amino acid that plays a vital role in protein synthesis, growth, mental health, and immune responses [21]. As age advances, proinflammatory cytokines, such as IL-6, IL-1β, and IFN-γ, lead to the activation of indoleamine 2,3-dioxygenase (IDO-1) [22]. An increase in levels/activity of IDO-1 along with inflammaging further leads to immunosuppression, neurodegenerative disorders, cardiovascular diseases, and fragility [21–24]. Augmentation of the levels/activity of IDO-1 decreases Trp levels and leads to the generation of several Trp intermediate metabolites [25]. Trp is catabolized by rate-limiting enzymes such as indoleamine 2,3-dioxygenase-1 (IDO-1), indoleamine 2,3-dioxygenase-2 (IDO-2), and tryptophan 2,3-dioxygenase-2 (TDO-2) into N-formylkynurenine and Kyn [26]. Further, Kyn is broken down into Kyn-A and 3-hydroxykynurenine by kynurenine aminotransferases (KAT) and kynurenine 3-monooxygenase (KMO) [27]. Trp also acts as a substrate for the generation of nicotinamide adenine dinucleotide (NAD+) through the conversion of quinolinic acid. NAD+ plays a crucial role in regulating several cellular processes, including energy production, chromosome stability, immune cell signaling, longevity mechanisms, and DNA repair [28, 29]. The Kyn and its metabolites induce downstream signaling by directly activating Ahr signaling [30] and/or indirect activation of the MEK- (mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) kinase-) ERK1/2 MAPK signaling pathway [31, 32].
IDO-1 is a master regulator of the Kyn pathway and downstream regulator of interferon signaling [33], which is activated during viral infection [34]. On the other hand, it has been reported that interferon-γ stimulates the expression of ACE2 (the receptor for SARS-CoV-2) in COVID-19 infection [35]. Hence, the interferon-γ signaling cascade potentiates inflammation in SARS-CoV-2 pathology [5]. Enhanced inflammation further leads to an increase in IDO-1 activity followed by enhanced degradation of Trp into Kyn and its metabolites. Our group identified the Trp-Kyn catabolic pathway as a novel causal mechanism in age-associated musculoskeletal complications (stem cell dysfunction and muscle and bone loss). We hypothesized that elevated levels of Kyn and its metabolites might be involved in COVID-19 musculoskeletal pathophysiology (Figure 2).
Figure 2.
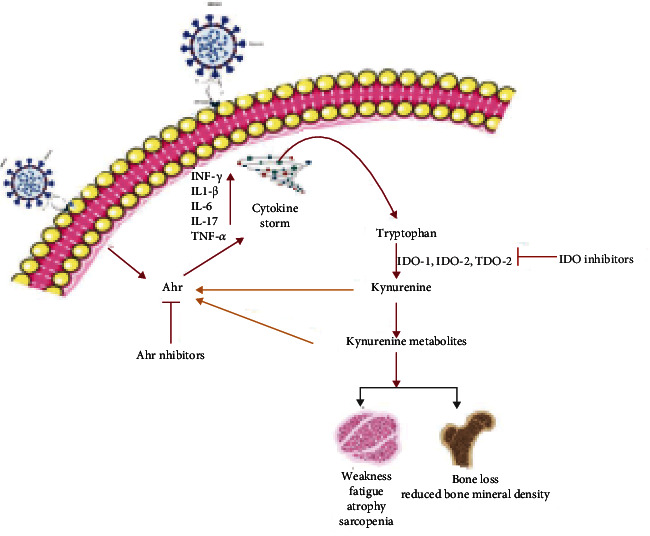
Overview of effects of SARS-CoV-2 infection on the muscle and bone. The SARS-CoV-2 infection elicits systemic inflammation (Cytokine storm), which activates the tryptophan-kynurenine pathway. Kynurenine is broken down into several downstream metabolites, which further activates AhR signaling, affecting the integrity and structure of the musculoskeletal system. (Figure is created by using http://BioRinder.com.)
3. The Try-Kyn Pathway in COVID-19-Induced Musculoskeletal Pathophysiology
Kyn is known to increase with age and is involved in deleterious effects on the musculoskeletal system [24, 36–38]. Recently published data have demonstrated a loss of bone and muscle in COVID-19 patients [17–20]. We hypothesize that an increase in cytokine levels leads to activation of the IDO-Kyn pathway, which raises the levels of Kyn and its metabolites, leading to activation of the aryl hydrocarbon receptor (AhR) and downstream signaling. Induction of AhR signaling directly by viral particles [39] or by Kyn metabolites leads to bone and muscle loss. Viral infection activates AhR through an IDO1-AhR-IDO1-positive feedback loop, which eventually causes upregulation of downstream effectors, such as TCDD-inducible PARP (TiPARP), and enhances the expression of cytokines (e.g., interleukin IL-1β, IL-10, and TNF-α) [39]. Therefore, we hypothesize that elevations in the cytokine expression elicit IDO-Kyn-AhR activation that results in bone and muscle loss.
There is conclusive evidence demonstrating that Kyn increases bone resorption by activating the AhR signaling pathway [38, 40, 41]. An increase in Kyn levels accelerates skeletal aging, leading to decreased osteoblast numbers and increased osteoclast numbers and activity, resulting in bone loss via decreased formation and enhanced resorption [42]. The study performed by our group analyzed the direct effects of feeding Kyn on bone mass and also evaluated the short-term effects of intraperitoneal injection of Kyn on bone turnover in CD-1 mice [24]. Micro-CT analysis revealed a significant bone loss upon Kyn feeding in adult mice, and serum analysis revealed an increase in the levels of osteoclastogenic markers such as RANKL and pyridinoline crosslinks (PYD) [24]. Our study also reported an increase in bone marrow adiposity with Kyn treatment. Moreover, bone marrow stromal cells isolated from Kyn-injected mice showed a decrease in the expression of Hdac-3 and its cofactor NcoR1 and augmentation of the expression of lipid storage genes such as Cidec and Plin1 [24], suggesting a phenotype similar to accelerated aging since such changes are also observed in aged bone marrow cells [43]. A study conducted by Kalaska et al. revealed that elevated Kyn levels decrease bone strength in rats [44]. Kyn metabolites may also exert effects on bone: a study performed by Darlington et al. measured the ratio of 3-hydroxyanthranilic acid to anthranilic acid and found that anthranilic acid levels were increased, and 3-hydroxyanthranilic acid levels were decreased in osteoporotic patients [45].
Studies performed by our group have shown that in vitro treatment of RAW264.7 cells, a macrophage-like cells line, with Kyn induces osteoclastogenesis by upregulating osteoclast transcription factors (such as c-fos and NFATc1) which leads to an increase in TRAP+ osteoclasts [40]. Another metabolite, Kyn-A, inhibits the differentiation of osteoblasts and increases osteoclastogenesis through the extracellular signal-regulated kinase (ERK) pathway [36, 46]. Another study conducted by our group demonstrated that Kyn treatment of human and mouse myoblasts increases reactive oxygen species formation [47]. Consistent with this in vitro studies, in vivo treatment of mice with Kyn leads to increased lipid peroxidation accompanied by reduced muscle size and muscle strength [47]. Several Trp downstream metabolites such as Kyn, Kyn-A, and 3-hydroxykynurenine are endogenous AhR ligands likely to induce musculoskeletal damage [38, 40, 41, 48].
The decline in tryptophan levels and elevated levels of Kyn and its metabolites postcovid will affect not only musculoskeletal health but also accelerate other age-related diseases (such as Alzheimer and Parkinson). The decline in tryptophan levels will impair the serotonin and melatonin pathway, which leads to the development of neurological disorders such as depression, cognitive impairment, sleep disorder, Alzheimer, and Parkinson's [49]. Moreover, a decrease in tryptophan levels will also affect protein synthesis leading to weight loss and muscular atrophy [50]. Some of the comorbidities that have been associated with severe COVID-19 are aging, diabetes, hypertension, chronic lung disease, cancer, and HIV. It is well known that the tryptophan-Kyn pathway is activated in the abovementioned conditions [51–55].
Inhibiting Trp-Kyn and/or AhR signaling may represent a novel therapeutic approach for preventing COVID-19-dependent musculoskeletal health and other age-related diseases. There are several Trp-Kyn/Ahr inhibitors that are undergoing clinical trials for various diseased conditions [56]. Currently, indoximod (IDO inhibitor), epacadostat (IDO inhibitor), and IK175 (Ahr inhibitor) are being used for inhibiting Trp-Kyn-Ahr signaling [26].
4. Conclusion
Current studies regarding the activation of the IDO-Kyn-AhR pathway in COVID-19 patients have opened up a new frontier for the scientific research community. Based on the available literature, it seems inevitable that activation of the IDO-Kyn-AhR pathway in COVID-19 patients should lead to bone and muscle loss, inducing significant musculoskeletal damage. However, there is currently advancement in COVID-19 therapies (Figure 3), but no strategies are available to address musculoskeletal-related issues. Given that the IDO-Kyn-AhR pathway is activated in COVID-19 patients, the use of inhibitors of IDO and/or AhR might be beneficial to reduce or prevent bone and muscle loss in this disease. IDO1 inhibitors (such as indoximod) and AhR inhibitors (e.g., IK 175) may help prevent bone and muscle loss. Some of these inhibitors are currently in clinical trials to treat several cancers and related complications. However, we suggest the necessity of conducting detailed clinical studies to design therapeutic strategies using these inhibitors to prevent bone and muscle loss in COVID-19 patients. The above-discussed literature is based on old variants of COVID-19. It will be interesting to know how delta and other recent variants circulating in the population will affect the IDO-Kyn-AhR pathway.
Figure 3.
Illustration of various strategies used for COVID-19 treatment. (Figure is created by using http://BioRinder.com.)
Acknowledgments
This publication is based upon work supported in part by the National Institutes of Health, AG036675 (National Institute on Aging-AG036675 S. F, MML, W.D.H, M. H, C.I.).
Data Availability
The data supporting this review are from previously reported studies and datasets, which have been cited.
Conflicts of Interest
The authors also declare that there is no other conflict of interest regarding the publication of this manuscript. The abovementioned funding did not lead to any conflict of interest regarding the publication of this manuscript.
Authors' Contributions
Conceptualization was contributed by SF. Methodology was contributed by SF, SV, CN, SK, and RK. Formal analysis was contributed by CN, SV, SK, RK, MML, and WDH. Resources were contributed by SF, CI, MML, and WDH MWH. Original draft preparation was contributed by SF and SV. Writing—review and editing was contributed by SF, SV, CN, CI, MWH, and WBB. Funding acquisition was contributed by SF, CI, MML, WDH, MWH, and WBB.
References
- 1.Sharun K., Dhama K., Pawde A. M., et al. SARS-CoV-2 in animals: potential for unknown reservoir hosts and public health implications. Veterinary Quarterly . 2021;41(1):181–201. doi: 10.1080/01652176.2021.1921311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saghir S. A. M., AlGabri N. A., Alagawany M. M., et al. Chloroquine and Hydroxychloroquine for the prevention and treatment of COVID-19: a fiction, Hope or hype? An updated review. Therapeutics and Clinical Risk Management . 2021;17:371–387. doi: 10.2147/TCRM.S301817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. The Lancet Infectious Diseases . 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology . 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas T., Stefanoni D., Reisz J. A., et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight . 2020;5(14, article e140327) doi: 10.1172/jci.insight.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser D. D., Slessarev M., Martin C. M., et al. Metabolomics profiling of critically ill coronavirus disease 2019 patients: identification of diagnostic and prognostic biomarkers. Critical Care Explorations . 2020;2(10, article e0272) doi: 10.1097/CCE.0000000000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen B., Yi X., Sun Y., et al. Proteomic and metabolomic characterization of COVID-19 patient sera. Cell . 2020;182(1):59–72.e15. doi: 10.1016/j.cell.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawler N. G., Gray N., Kimhofer T., et al. Systemic perturbations in amine and kynurenine metabolism associated with acute SARS-CoV-2 infection and inflammatory cytokine responses. Journal of Proteome Research . 2021;20(5):2796–2811. doi: 10.1021/acs.jproteome.1c00052. [DOI] [PubMed] [Google Scholar]
- 9.Cai Y., Kim D. J., Takahashi T., et al. Kynurenic acid underlies sex-specific immune responses to COVID-19. medRxiv . 2020 doi: 10.1101/2020.09.06.20189159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lionetto L., Ulivieri M., Capi M., et al. Increased kynurenine-to-tryptophan ratio in the serum of patients infected with SARS-CoV2: An observational cohort study. Biochimica et Biophysica Acta - Molecular Basis of Disease . 2021;1867(3, article 166042) doi: 10.1016/j.bbadis.2020.166042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Policard M., Jain S., Rego S., Dakshanamurthy S. Immune characterization and profiles of SARS-CoV-2 infected patients reveals potential host therapeutic targets and SARS-CoV-2 oncogenesis mechanism. bioRxiv . 2021 doi: 10.1101/2021.02.17.431721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sungnak W., Huang N., Bécavin C., et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nature Medicine . 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grunewald M. E., Shaban M. G., Mackin S. R., Fehr A. R., Perlman S. Murine coronavirus infection activates the aryl hydrocarbon receptor in an indoleamine 2,3-dioxygenase-independent manner, contributing to cytokine modulation and proviral TCDD-inducible-PARP expression. Journal of Virology . 2020;94 doi: 10.1128/JVI.01743-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasmi A., Peana M., Pivina L., et al. Interrelations between COVID-19 and other disorders. Clinical Immunology . 2021;224, article 108651 doi: 10.1016/j.clim.2020.108651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Hearn M., Liu J., Cudhea F., Micha R., Mozaffarian D. Coronavirus disease 2019 hospitalizations attributable to cardiometabolic conditions in the United States: a comparative risk assessment analysis. Journal of the American Heart Association . 2021;10(5, article e019259) doi: 10.1161/JAHA.120.019259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oxenkrug G. Insulin resistance and dysregulation of tryptophan-kynurenine and kynurenine-nicotinamide adenine dinucleotide metabolic pathways. Molecular Neurobiology . 2013;48(2):294–301. doi: 10.1007/s12035-013-8497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narla R. R., Adler R. A. Osteoporosis care amidst the prolonged pandemic. Journal of Endocrinological Investigation . 2021;44(7):1353–1361. doi: 10.1007/s40618-021-01542-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramani S. L., Samet J., Franz C. K., et al. Musculoskeletal involvement of COVID-19: review of imaging. Skeletal Radiology . 2021;50(9):1763–1773. doi: 10.1007/s00256-021-03734-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giraudo C., Librizzi G., Fichera G., et al. Reduced muscle mass as predictor of intensive care unit hospitalization in COVID-19 patients. PLoS One . 2021;16(6, article e0253433) doi: 10.1371/journal.pone.0253433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim M. A., Kurniawan A. A. Dreadful Consequences of Sarcopenia and Osteoporosis Due to COVID-19 Containment. Geriatric Orthopaedic Surgery & Rehabilitation . 2021;12 doi: 10.1177/2151459321992746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palego L., Betti L., Rossi A., Giannaccini G. Tryptophan biochemistry: structural, nutritional, metabolic, and medical aspects in humans. Amino Acids . 2016;2016, article 8952520:1–13. doi: 10.1155/2016/8952520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhan Y., Zhou Y., Zheng W., et al. Alterations of multiple peripheral inflammatory cytokine levels after repeated ketamine infusions in major depressive disorder. Translational Psychiatry . 2020;10(1):p. 246. doi: 10.1038/s41398-020-00933-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lovelace M. D., Varney B., Sundaram G., et al. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology . 2017;112(Part B):373–388. doi: 10.1016/j.neuropharm.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Refaey M. E., McGee-Lawrence M. E., Fulzele S., et al. Kynurenine, a tryptophan metabolite that accumulates with age, induces bone loss. Journal of Bone and Mineral Research . 2017;32(11):2182–2193. doi: 10.1002/jbmr.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Platten M., Nollen E. A. A., Röhrig U. F., Fallarino F., Opitz C. A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nature Reviews. Drug Discovery . 2019;18(5):379–401. doi: 10.1038/s41573-019-0016-5. [DOI] [PubMed] [Google Scholar]
- 26.Tang K., Wu Y. H., Song Y., Yu B. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. Journal of Hematology & Oncology . 2021;14(1):p. 68. doi: 10.1186/s13045-021-01080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kindler J., Lim C. K., Weickert C. S., et al. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Molecular Psychiatry . 2020;25(11):2860–2872. doi: 10.1038/s41380-019-0401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxidants & Redox Signaling . 2008;10(2):179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 29.Fania L., Mazzanti C., Campione E., Candi E., Abeni D., Dellambra E. Role of nicotinamide in genomic stability and skin cancer chemoprevention. International Journal of Molecular Sciences . 2019;20(23):p. 5946. doi: 10.3390/ijms20235946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H., Do D. C., Liu J., et al. Functional role of kynurenine and aryl hydrocarbon receptor axis in chronic rhinosinusitis with nasal polyps. Journal of Allergy and Clinical Immunology . 2018;141(2):586–600.e6. doi: 10.1016/j.jaci.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y., Kilani R. T., Rahmani-Neishaboor E., Jalili R. B., Ghahary A. Kynurenine increases matrix metalloproteinase-1 and -3 expression in cultured dermal fibroblasts and improves scarring _in vivo_. The Journal of Investigative Dermatology . 2014;134(3):643–650. doi: 10.1038/jid.2013.303. [DOI] [PubMed] [Google Scholar]
- 32.Lee H. J., Bach J. H., Chae H. S., et al. Mitogen-activated protein kinase/extracellular signal-regulated kinase attenuates 3-hydroxykynurenine-induced neuronal cell death. Journal of Neurochemistry . 2004;88(3):647–656. doi: 10.1111/j.1471-4159.2004.02191.x. [DOI] [PubMed] [Google Scholar]
- 33.Powers R. K., Culp-Hill R., Ludwig M. P., et al. Trisomy 21 activates the kynurenine pathway via increased dosage of interferon receptors. Nature Communications . 2019;10(1):p. 4766. doi: 10.1038/s41467-019-12739-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen K. B., Watford W. T., Salomon R., et al. Critical role for STAT4 activation by type 1 interferons in the interferon gamma response to viral infection. Science . 2002;297(5589):2063–2066. doi: 10.1126/science.1074900. [DOI] [PubMed] [Google Scholar]
- 35.Onabajo O. O., Banday A. R., Stanifer M. L., et al. Interferons and viruses induce a novel truncated ACE2 isoform and not the full-length SARS-CoV-2 receptor. Nature Genetics . 2020;52(12):1283–1293. doi: 10.1038/s41588-020-00731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin T. H., Yang R. S., Tang C. H., Wu M. Y., Fu W. M. Regulation of the maturation of osteoblasts and osteoclastogenesis by glutamate. European Journal of Pharmacology . 2008;589(1–3):37–44. doi: 10.1016/j.ejphar.2008.04.060. [DOI] [PubMed] [Google Scholar]
- 37.Martin K. S., Azzolini M., Lira Ruas J. The kynurenine connection: how exercise shifts muscle tryptophan metabolism and affects energy homeostasis, the immune system, and the brain. American Journal of Physiology-Cell Physiology . 2020;318(5):C818–C830. doi: 10.1152/ajpcell.00580.2019. [DOI] [PubMed] [Google Scholar]
- 38.Kondrikov D., Elmansi A., Bragg R. T., et al. Kynurenine inhibits autophagy and promotes senescence in aged bone marrow mesenchymal stem cells through the aryl hydrocarbon receptor pathway. Exp Gerontol. . 2020;130, article 110805 doi: 10.1016/j.exger.2019.110805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turski W. A., Wnorowski A., Turski G. N., Turski C. A., Turski L. AhR and IDO1 in pathogenesis of Covid-19 and the "systemic AhR activation syndrome:" a translational review and therapeutic perspectives. Restorative Neurology and Neuroscience . 2020;38(4):343–354. doi: 10.3233/RNN-201042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eisa N. H., Reddy S. V., Elmansi A. M., et al. Kynurenine promotes RANKL-induced osteoclastogenesis in vitro by activating the aryl hydrocarbon receptor pathway. International Journal of Molecular Sciences . 2020;21(21):p. 7931. doi: 10.3390/ijms21217931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duan Z., Lu J. Involvement of aryl hydrocarbon receptor in L-kynurenine-mediated parathyroid hormone–related peptide expression. Hormones and Cancer . 2019;10(2-3):89–96. doi: 10.1007/s12672-019-0357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim B.-J., Hamrick M. W., Yoo H. J., et al. The detrimental effects of kynurenine, a tryptophan metabolite, on human bone metabolism. The Journal of Clinical Endocrinology & Metabolism . 2019;104(6):2334–2342. doi: 10.1210/jc.2018-02481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGee-Lawrence M. E., Carpio L. R., Schulze R. J., et al. Hdac3 deficiency increases marrow adiposity and induces lipid storage and glucocorticoid metabolism in osteochondroprogenitor cells. Journal of Bone and Mineral Research . 2016;31(1):116–128. doi: 10.1002/jbmr.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalaska B., Pawlak K., Domaniewski T., et al. Elevated levels of peripheral kynurenine decrease bone strength in rats with chronic kidney disease. Frontiers in Physiology . 2017;8:p. 836. doi: 10.3389/fphys.2017.00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Darlington L. G., Forrest C. M., Mackay G. M., et al. On the biological importance of the 3-hydroxyanthranilic acid: anthranilic acid ratio. International Journal of Tryptophan Research . 2010;3:51–59. doi: 10.4137/ijtr.s4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stone T. W., Stoy N., Darlington L. G. An expanding range of targets for kynurenine metabolites of tryptophan. Trends in Pharmacological Sciences . 2013;34(2):136–143. doi: 10.1016/j.tips.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Kaiser H., Yu K., Pandya C., et al. Kynurenine, a Tryptophan Metabolite That Increases with Age, Induces Muscle Atrophy and Lipid Peroxidation. Oxidative Medicine and Cellular Longevity . 2019;2019:9894239. doi: 10.1155/2019/9894238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al Saedi A., Sharma S., Summers M. A., Nurgali K., Duque G. The multiple faces of tryptophan in bone biology. Experimental Gerontology . 2020;129:110778–115565. doi: 10.1016/j.exger.2019.110778. [DOI] [PubMed] [Google Scholar]
- 49.Richard D. M., Dawes M. A., Mathias C. W., Acheson A., Hill-Kapturczak N., Dougherty D. M. L-tryptophan: basic metabolic functions, behavioral research and therapeutic indications. International Journal of Tryptophan Research . 2009;2:45–60. doi: 10.4137/ijtr.s2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ninomiya S., Nakamura N., Nakamura H., et al. Low levels of serum tryptophan underlie skeletal muscle atrophy. Nutrients . 2020;12(4):p. 978. doi: 10.3390/nu12040978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffman W. H., Whelan S. A., Lee N. Tryptophan, kynurenine pathway, and diabetic ketoacidosis in type 1 diabetes. PLoS One . 2021;16(7, article e0254116) doi: 10.1371/journal.pone.0254116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cussotto S., Delgado I., Anesi A., et al. Tryptophan metabolic pathways are altered in obesity and are associated with systemic inflammation. Frontiers in Immunology . 2020;11:p. 557. doi: 10.3389/fimmu.2020.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naz S., Bhat M., Ståhl S., et al. Dysregulation of the tryptophan pathway evidences gender differences in COPD. Metabolites . 2019;9(10):p. 212. doi: 10.3390/metabo9100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mor A., Tankiewicz-Kwedlo A., Pawlak D. Kynurenines as a novel target for the treatment of malignancies. Pharmaceuticals . 2021;14(7):p. 606. doi: 10.3390/ph14070606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou Y. H., Sun L., Chen J., et al. Tryptophan metabolism activates aryl hydrocarbon receptor-mediated pathway to promote HIV-1 infection and reactivation. mBio . 2019;10(6) doi: 10.1128/mBio.02591-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Opitz C. A., Somarribas Patterson L. F., Mohapatra S. R., et al. The therapeutic potential of targeting tryptophan catabolism in cancer. British Journal of Cancer . 2020;122(1):30–44. doi: 10.1038/s41416-019-0664-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this review are from previously reported studies and datasets, which have been cited.


