Abstract
Background
Integrase strand transfer inhibitor (InSTI)–based regimens are now recommended as first-line antiretroviral therapy (ART) for adults with human immunodeficiency virus, but evidence on long-term clinical effectiveness of InSTI-based regimens remains limited. We examined whether InSTI-based regimens improved longer-term clinical outcomes.
Methods
We included participants from clinical cohorts in the North American AIDS Cohort Collaboration on Research and Design who initiated their first ART regimen, containing either InSTI (ie, raltegravir, dolutegravir, and elvitegravir-cobicistat) or efavirenz (EFV) as an active comparator, between 2009 and 2016. We estimated observational analogs of 6-year intention-to-treat and per-protocol risks, risk differences (RDs), and hazard ratios (HRs) for the composite outcome of AIDS, acute myocardial infarction, stroke, end-stage renal disease, end-stage liver disease, or death.
Results
Of 15 993 participants, 5824 (36%) initiated an InSTI-based and 10 169 (64%) initiated an EFV-based regimen. During the 6-year follow-up, 440 in the InSTI group and 1097 in the EFV group incurred the composite outcome. The estimated 6-year intention-to-treat risks were 14.6% and 14.3% for the InSTI and EFV groups, respectively, corresponding to a RD of 0.3% (95% confidence interval, −2.7% to 3.3%) and a HR of 1.08 (.97–1.19); the estimated 6-year per-protocol risks were 12.2% for the InSTI group and 11.9% for the EFV group, corresponding to a RD of 0.3% (−3.0% to 3.7%) and a HR of 1.09 (.96–1.25).
Conclusions
InSTI- and EFV-based initial ART regimens had similar 6-year composite clinical outcomes. The risk of adverse clinical outcomes remains substantial even when initiating modern ART.
Keywords: integrase strand transfer inhibitors, treatment-naive adults with HIV, trial emulation, efavirenz, antiretroviral therapy
Our study shows a similar 6-year risk of composite clinical outcomes for initial integrase strand transfer inhibitor–based regimens and the efavirenz-based regimen in a large multisite observational cohort collaboration in the United States and Canada.
Contemporary antiretroviral therapy (ART) is highly effective in suppressing plasma viremia and prolonging survival [1]. Because there is no cure for human immunodeficiency virus (HIV), people living with HIV may be exposed to ART for decades [2], so maximizing the safety and tolerability while maintaining strong potency remains a clinical priority.
Integrase strand transfer inhibitor (InSTI)–based regimens are now recommended widely as first-line ART for adults [3–5]. Randomized trials of InSTI-based regimens have demonstrated clear short-term evidence of strong potency as well as tolerability, compared with other regimens [6–13]. However, most randomized trials have been focused on short-term (48-week) surrogate biomarkers. Limited data are available regarding longer-term clinical effectiveness of InSTI-based regimens. The few existing observational studies that have examined the clinical effects and safety end points of InSTI-based regimens have limitations, such as insufficient sample size and limited follow-up [14, 15].
In a collaboration of cohort studies in the United States and Canada, emulating a randomized trial, we aimed to examine whether those initiating a regimen of InSTI with tenofovir disoproxil fumarate [TDF] or tenofovir alafenamide, and emtricitabine [FTC]) had improved longer-term (6-year) clinical outcomes of AIDS-defining illnesses, all-cause mortality rate, and serious non-AIDS events compared with those initiating a regimen of efavirenz (EFV) with the same backbone.
METHODS
Study Design
The North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) is the largest consortium of clinical and interval HIV cohorts in North America supported by the National Institutes of Health. Details on this collaboration have been published elsewhere [16]. Briefly, the NA-ACCORD consists of >20 single-site and multisite cohorts that prospectively collect data on >180 000 adults living with HIV who had ≥2 care visits within 12 months at >200 clinical sites in the United States and Canada. Cohorts securely transfer demographic, medication, laboratory, diagnostic, and vital status data annually to the central Data Management Core (University of Washington, Seattle), where the data undergo quality control and are harmonized across cohorts for analyses by the Epidemiology/Biostatistics Core (Johns Hopkins University, Baltimore, Maryland). The human subjects research activities of the NA-ACCORD and each participating cohort have been approved by their respective local institutional review boards, the Johns Hopkins School of Medicine, and the University of North Carolina School of Medicine.
Study Population and Eligibility Criteria
This prospective cohort study included HIV-seropositive and ART-naive adults aged ≥18 years who initiated an ART regimen consisting of TDF (or tenofovir alafenamide), FTC, and either an InSTI (ie, raltegravir [RAL], dolutegravir [DTG], or elvitegravir-cobicistat [EVG/COBI]) or EFV while under follow-up between July 2009 and December 2016 from 16 clinic cohorts in the NA-ACCORD. We started follow-up in 2009, rather than 2007 when RAL was first approved by the Food and Drug Administration, because RAL was recommended only for those with drug resistance from 2007 through 2009. Patients excluded were (1) those having evidence of prior ART; (2) those with an undetectable HIV viral load measured between 90 days before to 7 days after ART initiation, as undetectable viral load may indicate unreported treatment; and (3) those having a history of acute myocardial infarction (MI) or stroke, end-stage renal disease (ESRD), or end-stage liver disease (ESLD).
Outcome Assessment
The primary outcome was a composite of the first occurrence of an AIDS-defining illness, a serious non-AIDS event, or death from any cause after ART initiation [17]. AIDS-defining illnesses were based on 1993 criteria of the Centers for Disease Control and Prevention (see Supplementary Material, section 1, for specific AIDS-defining illnesses) [18]. Serious non-AIDS events were defined as follows: acute MI or stroke, ESRD (estimated glomerular filtration rate consistently <30 mL/min/1.73 m2 for ≥3 months), and ESLD (2 fibrosis 4 scores >3.25, greater than 6 months apart). ESRD and ESLD outcomes were based on laboratory tests, because the date through which ESRD and ESLD events are validated in the NA-ACCORD did not extend throughout the study follow-up. The diagnoses and laboratory tests were obtained from electronic medical records. The date of death was identified from queries to the US Social Security death index, the national death index, state (or provincial, for Canadian sites) death certificates, and electronic medical records.
Covariates
We selected baseline covariates that we considered to be potential confounders for effect of ART initiation on the composite outcome. Age, sex, race, ethnicity, and HIV acquisition risk group (men who have sex with men, injection drug use, heterosexual behavior, and other) were self-reported at enrollment. Body mass index and history of any clinical AIDS diagnosis, hepatitis C infection (a positive antibody test, detectable RNA, or the presence of hepatitis C genotype test), hepatitis B infection (defined as a positive surface antigen test, a positive e antigen test, or a positive DNA test result), diagnosis of depression or anxiety, diabetes mellitus (glycosylated hemoglobin ≥6.5%, diabetes-specific medication, or a diagnosis with a diabetes-related medication), hypertension (clinical diagnosis and prescription of antihypertensive medication), elevated total cholesterol (≥240 mg/dL), and statin prescription were recorded at ART initiation. Baseline CD4 T-cell count (cells per microliter) and HIV viral load (copies per milliliter) were captured at the closest date to ART initiation within the window from 90 days before to 7 days after ART initiation. Calendar year at initiation was included and coded as dummy variables. Time-varying covariates, which were used to account for differential loss to follow-up and ART treatment changes, included time-updated CD4 T-cell count, HIV viral load, new occurrences of clinical diseases or conditions (ie, diabetes mellitus, depression, anxiety, and hypertension), elevated total cholesterol, and new statin prescription after ART initiation.
Statistical Analyses
Our inclusion and exclusion criteria restricted our study population to those we believe initiated ART while under observation from 2009 to 2016, to avoid selection bias due to the inclusion of prevalent ART users [19]. The study mimicked the setting of a randomized controlled trial, where patients are randomly assigned to either the InSTI-based regimen or the active comparator EFV-based regimen through adjustment for baseline confounders measured in the NA-ACCORD. Each participant was followed up from the date of ART initiation (study entry for individuals and time origin for our study design) until the earliest date of first occurrence of the composite outcome, date of loss to follow-up (defined as 18 months after the date of last CD4 T-cell count or HIV viral load measurement), or administrative end of follow-up (at 6 years, cohort-specific end date, or 31 December 2016).
Missing baseline covariates were imputed 10 times using multiple imputation by chained equations [20, 21] (see Supplementary Material, section 2, for the proportion of missing values for each baseline covariate). The imputation model included all baseline covariates, treatment variable, the binary outcome indicator, and the cumulative reference hazard [21]. Baseline CD4 T-cell count and HIV viral load were log-transformed to avoid negative imputed values.
For primary analyses, we estimated the observational analog of the intention-to-treat effect of initiating an InSTI-based regimen compared with initiating the EFV-based regimen, regardless of ART treatment changes. For the intention-to-treat analysis, in each imputed data set, we accounted for baseline confounding and differential loss to follow-up by constructing inverse probabilities of treatment weights and of censoring weights, respectively. These weights were combined and applied to the Cox proportional hazard model for the composite outcome for initiating an InSTI-based regimen versus initiating the EFV-based regimen. A robust standard error for hazard ratio (HR) was calculated for each imputed data set. We also estimated the 6-year intention-to-treat risk of the composite outcome for each treatment group and the corresponding 6-year intention-to-treat risk difference (RD) using inverse probability-weighted Kaplan-Meier estimators [22, 23].
We estimated the 6-year risks because few patients in the InSTI group were followed up for >6 years after ART initiation. The standard error for RD was estimated from a nonparametric bootstrap of 200 random samples with replacement for each imputed data set. Rubin’s rule was applied to pool the results across imputed data sets to obtain the pooled HR and the 6-year RD with 95% confidence intervals (CIs).
We also estimated the per-protocol effect of initiating and remaining on an InSTI-based regimen, compared with initiating and remaining on the EFV-based regimen [24, 25]. For per-protocol analyses, participants were additionally censored when they deviated from their initial treatment regimen (ie, treatment changes). Treatment changes included treatment discontinuations and switches. Treatment changes that were considered allowable exceptions and thus were not censored included (1) a change from one InSTI-based regimen to another (for instance, from a RAL-based regimen to a DTG-based regimen); (2) a change from the EFV-based regimen to a nonnucleoside reverse-transcriptase inhibitor–based regimen of rilpivirine, TDF and FTC; and (3) a switch between TDF and tenofovir alafenamide. The inverse probability of censoring weights was revised to censor at the minimum of loss to follow-up or treatment changes [26] and was combined with the inverse probability of treatment weights to estimate the 6-year per-protocol risks, RD, and HR.
We assessed the robustness of our estimates using 3 secondary analyses: (1) we restricted the composite outcome only to the most HIV-relevant events—AIDS and death, as well as restricted to serious non-AIDS events; (2) we also adjusted for individual cohorts as a potential confounder; and (3) we repeated the analyses stratified by baseline CD4 T-cell count (≤200/μL vs >200/μL). SAS software (version 9.4; SAS Institute) was used for all analyses.
RESULTS
Of 15 993 eligible participants, 5824 (36.4%) initiated an InSTI-based regimen, and 10 169 (63.6%) initiated the EFV-based regimen between 2009 and 2016. Among 5824 patients initiating InSTI, 1840 (31.6%) initiated RAL, 3361 (57.7%) initiated EVG/COB, and 639 (10.7%) initiated DTG. Characteristics at ART initiation of the study population are shown in Table 1. Compared with those in the EFV group, the participants in the InSTI group were more likely to be female and nonblack, report male-to-male sexual contact, and have a previous diagnosis of depression or anxiety. Secular trends showed an increased proportion initiating an InSTI-based regimen from 2009 to 2016 (see Supplementary Material, section 3).
Table 1.
Characteristics of 15 993 Human Immunodeficiency Virus–Infected Adults Initiating an Integrase Strand Transfer Inhibitor–Based or Efavirenz-based Antiretroviral Therapy Regimen in the North American AIDS Cohort Collaboration on Research and Design (July 2009 to December 2016)
| Participants by Regimen, No. (%) | |||
|---|---|---|---|
| Characteristic | InSTI Baseda (n = 5824) | EFV Baseda (n = 10 169) | Overall (n = 15 993) |
| Age, median (IQR), y | 37.0 (28.0–48.0) | 41.0 (31.0–50.0) | 40.0 (30.0–50.0) |
| Female sex | 894 (15.3) | 1101 (10.8) | 1995 (12.5) |
| Black race | 2351 (40.4) | 4611 (45.3) | 6962 (43.5) |
| Hispanic ethnicity | 722 (12.4) | 1350 (13.3) | 2072 (13.0) |
| BMI, median (IQR)b | 25.1 (22.3–28.7) | 25.1 (22.3–28.6) | 25.1 (22.3–28.6) |
| Injection drug use | 566 (9.7) | 1033 (10.2) | 1599 (10.0) |
| Male-to-male sexual contact | 3209 (55.1) | 4523 (44.5) | 7732 (48.4) |
| Heterosexual behavior | 1349 (23.2) | 2030 (20.0) | 3379 (21.1) |
| Previous AIDS diagnosis | 480 (8.2) | 735 (7.2) | 1215 (7.6) |
| Hepatitis B | 214 (3.7) | 426 (4.2) | 640 (4.0) |
| Hepatitis C | 564 (9.7) | 1123 (11.0) | 1687 (10.6) |
| Previous depression diagnosis | 859 (14.8) | 1038 (10.2) | 1897 (11.9) |
| Previous anxiety diagnosis | 692 (11.9) | 727 (7.2) | 1419 (8.9) |
| Diabetes mellitus | 284 (4.9) | 545 (5.4) | 829 (5.2) |
| Hypertension | 817 (14.0) | 1792 (17.6) | 2609 (16.3) |
| Statin prescription | 340 (5.8) | 806 (7.9) | 1146 (7.2) |
| Elevated total cholesterol | 224 (3.9) | 508 (5.0) | 732 (4.6) |
| Baseline CD4 T-cell count, median (IQR), cells/μL | 349.0 (173.0–524.0) | 323.0 (178.0–461.0) | 332.0 (177.0–485.0) |
| Baseline viral load, median (IQR), copies/mL | 40 432.0 (8829.0–139 000.0) | 36 754.5 (7482.5–123 182.0) | 38 440.0 (8017.0–128 000.0) |
| Calendar year at initiation, median (IQR) | 2014 (2013–2015) | 2011 (2010–2012) | 2012 (2010–2014) |
Abbreviations: BMI, body mass index; EFV, efavirenz; InSTI, integrase strand transfer inhibitor; IQR, interquartile range.
aBoth regimens included the same backbone of tenofovir disoproxil fumarate (or tenofovir alafenamide) and emtricitabine.
bBMI calculated as weight in kilograms divided by height in meters squared.
During a median follow-up of 2.0 years (interquartile range, 1.2–3.2), 440 (7.6%) of the 5824 participants who initiated an InSTI-based regimen incurred the composite outcome. Among these, 288 (5.0%) had an incident diagnosis of AIDS, 16 (0.3%) had a diagnosis of acute MI or stroke, 7 (0.1%) incurred ESRD, 40 (0.7%) incurred ESLD, and 89 (1.5%) died. Of the 10 169 participants initiating the EFV-based regimen with a median follow-up of 3.8 years (interquartile range, 2.3–5.3), 1097 (10.8%) experienced the composite outcome. Among these, 671 (6.6%) had AIDS, 61 (0.6%) had a diagnosis of acute MI or stroke, 26 (0.3%) had ESRD, 116 (1.1%) incurred ESLD, and 223 (2.2%) died. A total of 3104 (19.4%) among 15 993 participants were lost to follow-up, including 13.4% in the InSTI group (782 of 5824) and 22.8% in the EFV group (2322 of 10 169). The crude risk of loss to follow-up is shown in the Supplementary Material (section 4).
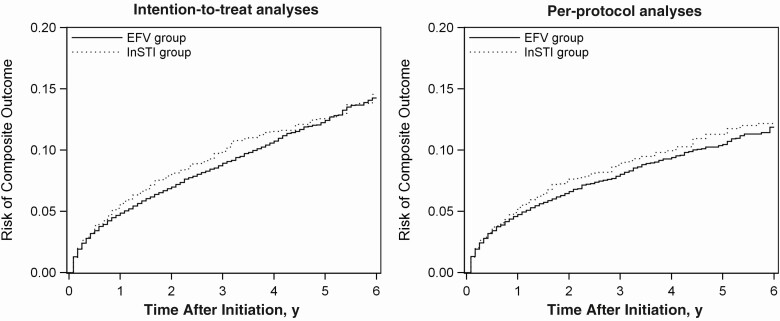
After accounting for baseline confounding and differential loss to follow-up, the intention-to-treat HR of the composite outcome for initiating an InSTI-based regimen versus initiating the EFV-based regimen was 1.08 (95% CI, .97–1.19). The intention-to-treat 6-year risk of the composite outcome was 14.6% for initiating an InSTI-based regimen and 14.3% for initiating the EFV-based regimen, corresponding with a 6-year RD of 0.3% (95% CI, −2.7% to 3.3%) (Table 2). The Kaplan-Meier risk curve for intention-to-treat analyses across 6-year follow-up is depicted in Figure 1.
Table 2.
Estimated 6-Year Risk Differences and Hazard Ratios for the Composite Outcome in Adults Initiating an Integrase Strand Transfer Inhibitor–Based Versus an Efavirenz-based Regimen in the North American AIDS Cohort Collaboration on Research and Design (July 2009 to December 2016)
| Analysis | Participants, no. | Duration, person-years | Outcomes, no.a | 6-y Risk, % | 6-y RD (95% CI), % | HR (95% CI) |
|---|---|---|---|---|---|---|
| Crudeb | ||||||
| EFV | 10 169 | 38 029.3 | 1097 | 14.2 | 0 | 1 |
| InSTI | 5824 | 13 240.8 | 440 | 13.1 | −1.1 (−4.1 to 1.9) | 0.99 (.88–1.10) |
| Intention to treatc | ||||||
| EFV | 10 169 | 38 029.3 | 1097 | 14.3 | 0 | 1 |
| InSTI | 5824 | 13 240.8 | 440 | 14.6 | 0.3 (−2.7 to 3.3) | 1.08 (.97– 1.19) |
| Per protocold | ||||||
| EFV | 10 169 | 25 100.8 | 672 | 11.9 | 0 | 1 |
| InSTI | 5824 | 9313.0 | 325 | 12.2 | 0.3 (−3.0 to 3.7) | 1.09 (.96 –1.25) |
Abbreviations: CI, confidence interval; EFV, efavirenz; HR, hazard ratio; InSTI, integrase strand transfer inhibitor; RD, risk difference.
aThe composite outcome included AIDS-defining illnesses, acute myocardial infarction or stroke, end-stage renal disease, end-stage liver disease, or death.
bCrude analysis did not account for baseline confounding, differential loss to follow-up, or treatment changes (uncensored).
cIntention-to-treat analyses accounted for baseline confounding and differential loss to follow-up.
dPer-protocol analyses accounted for baseline confounding, differential loss to follow-up, and treatment changes (ie, treatment discontinuations or switches).
Figure 1.
Risk of the composite outcome (AIDS-defining illnesses, acute myocardial infarction or stroke, end-stage renal disease, end-stage liver disease, or death) among 15 993 human immunodeficiency virus–infected adults initiating an integrase strand transfer inhibitor (InSTI)–based or an efavirenz (EFV)–based regimen between July 2009 and December 2016 in the North American AIDS Cohort Collaboration on Research and Design. Left, Intention-to-treat analyses that accounted for baseline confounding and differential loss-to-follow-up. Right, Per-protocol analyses that accounted for baseline confounding, differential loss to follow-up, and treatment changes.
Fifty-four percent of the participants initiating an InSTI-based regimen (3140 of 5824) and 68% of the those initiating the EFV-based regimen (6964 of 10 169) had a treatment change before incurring the composite outcome, being lost to follow-up or completing the study. The crude risk of treatment change is shown in the Supplementary Material (section 4). The 6-year risk of treatment change was 85.9% for the InSTI group and 83.2% for the EFV group. After accounting for baseline confounding, differential loss-to-follow-up, and treatment changes, the adjusted per-protocol HR of the composite outcome for initiating and remaining on an InSTI-based regimen versus initiating and remaining on the EFV-based regimen was 1.09 (95% CI, .96–1.25). The adjusted per-protocol 6-year risk of the composite outcome was 12.2% for initiating and remaining on an InSTI-based regimen, and 11.9% for initiating and remaining on the EFV-based regimen, corresponding with a 6-year RD of 0.3% (95% CI, −3.0% to 3.7%) (Table 2). The Kaplan-Meier risk curve for per-protocol analyses across 6-year follow-up is depicted in Figure 1.
Effect estimates were similar under the secondary analyses for restriction to most relevant clinical events, restriction to serious non-AIDS events, including confounding by cohort as a confounder, and stratification by baseline CD4 T-cell count (see Supplementary Material, section 5).
DISCUSSION
Using this large collaboration of North American HIV cohorts, we found similar effects on the composite clinical outcome between InSTI-based and EFV-based initial ART regimens in both intention-to-treat and per-protocol analyses. This finding is consistent with the previous literature [14], but with a broader spectrum of clinical outcomes, longer follow-up, and larger sample size, these results provide more assurance of the clinical effectiveness of the initiation of InSTI-based ART. However, they do not suggest that InSTI-based regimens have better clinical outcomes than the EFV-based regimen. Furthermore, our study showed that, in this sample with a median age of 40 years, the 6-year risk of clinical outcomes was substantial for both InSTI and EFV groups (14% in intention-to-treat analyses and 12% in per-protocol analyses).
Findings of existing randomized trials focused on short-term biomarkers suggest that patients initiating InSTI-based regimens experienced more rapid antiretroviral activity and fewer adverse events and drug interactions than those initiating other regimens [6–13, 27]. Direct comparisons of the efficacy and safety of InSTI-based regimens with those of EFV-based regimens have been performed in several randomized controlled trials: STARTMRK (RAL vs EFV) [6, 7, 28–30], SINGLE (DTG vs EFV) [9, 31], and Study 102 (EVG vs EFV) [11, 27, 32, 33].
The STARTMRK trial found noninferiority of the RAL-based regimen to the EFV-based regimen on viral suppression at week 48 and 96, but findings also suggested significantly fewer drug-related clinical adverse events in the RAL group [6, 7, 28–30]. At week 240, the study showed that RAL induced significantly better viral suppression [28]. In the SINGLE study directly comparing DTG plus abacavir-lamivudine with the coformulated EFV/FTC/TDF, the DTG-based regimen was found to have a more favorable tolerability profile and a lower rate of treatment discontinuations [9, 31]. The Study 102 trial, which compared coformulated EVG/COBI/FTC/TDF versus coformulated EFV/FTC/TDF, showed significantly better immunological recovery in the EVG group but comparable rates of viral suppression and similar numbers of treatment discontinuations [11, 27, 32, 33].
Overall, these trials suggested that InSTIs had a better virological response and tolerability with fewer treatment discontinuations, in contrast with our finding that InSTIs lead to a higher 6-year risk of treatment changes and had comparable long-term clinical effects with EFV. The discrepancies between clinical trials and observational data may be due in part to the shorter duration of follow-up in clinical trials, because some adverse effects may occur only after prolonged treatment exposure. Therefore, further research is warranted to incorporate and investigate the reasons for treatment changes and provide additional insights on the tolerability of InSTI-based initial antiretroviral therapy in a real-world observational setting.
Our study is subject to limitations. First, one key challenge in analyzing observational data is that the treatment was not randomly assigned. That means that all baseline confounders should be measured and adjusted for to achieve comparability of treatment groups and to emulate a randomized trial. It is possible that residual confounding was present. Second, ESRD and ESLD outcomes were based on laboratory tests. In addition, acute MI or stroke were based on diagnosis data, because adjudicated events in NA-ACCORD were not available for all cohorts throughout the follow-up period. Thus, our outcome data may suffer from measurement error. Future studies on validated outcomes, including validated MI and cancer outcomes, are a worthwhile addition.
A third limitation was that we did not assess additional chronic disease outcomes or their proxies, such as weight gain or metabolic disorders, for which there is some evidence of enhanced risk when comparing InSTI-based with other regimens. Although there are currently both trial and observational data supporting the association of some InSTI-based regimens with more rapid weight gain [34–37], and increases in waist circumference [38], these may be intermediates of hard outcomes that we did assess in this analysis. We further felt that quantifying these relationships would be beyond the scope of this analysis and may merit separate study. Finally, we only estimated the overall clinical effects of InSTI class and did not distinguish the clinical outcomes for each distinct InSTI agent, because such detailed analyses would require a larger study.
Several strengths of our study are worth noting. We used a large observational pooling project and emulated a randomized trial [39]. First, the size, breadth, prolonged follow-up, and representativeness of the NA-ACCORD data allowed us to more accurately quantify the clinical effectiveness of InSTI-based initial ART regimens with adjustment for many potential confounders. Second, we restricted analyses to participants starting ART so that prior ART could not bias the results [19]. We also set time zero of our analyses to align with time zero of a randomized trial when eligibility criteria are met and treatment strategies are assigned. Furthermore, we adopted modern causal and statistical approaches for intention-to-treat and per-protocol analyses to ameliorate the concerns of baseline confounding, differential loss to follow-up, and treatment discontinuations and switches, which are difficult to address using traditional regression methods, and provide comprehensive evidence about the effectiveness of InSTIs. Our analytical approaches also allowed us to estimate the more patient-relevant measures of interest (ie, absolute risks and RDs), in addition to HRs.
In conclusion, using a rigorous methodological approach, our study found similar 6-year risks of composite clinical outcomes for initial InSTI-based regimens and the EFV-based regimen by leveraging a large multisite observational cohort collaboration in the United States and Canada. Given the lack of existing or forthcoming longer-term randomized evidence, prospective cohort studies in real-world settings contribute to the evidence base and complement findings from randomized clinical trials. With principled causal and statistical methods, one can leverage observational data to obtain more accurate effect estimates, provide the best available evidence, and address timely and important questions that inform policy decision making and treatment guidelines.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Centers for Disease Control and Prevention.
Financial support. This work was supported by the National Institutes of Health (grant numbers U01AI069918, F31AI124794, F31DA037788, G12MD007583, K01AI093197, K01AI131895, K23EY013707, K24AI065298, K24AI118591, K24DA000432, KL2TR000421, N01CP01004, N02CP055504, N02CP91027, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050409, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01AI157758, R01DA011602, R01DA012568, R01 AG053100, R24AI067039, U01AA013566, U01AA020790, U01AI038855, U01AI038858, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01DA03629, U01DA036935, U10EY008057, U10EY008052, U10EY008067, U01HL146192, U01HL146193, U01HL146194, U01HL146201, U01HL146202, U01HL146203, U01HL146204, U01HL146205, U01HL146208, U01HL146240, U01HL146241, U01HL146242, U01HL146245, U01HL146333, U24AA020794,U54MD007587, UL1RR024131, UL1TR000004, UL1TR000083, Z01CP010214, and Z01CP010176; and grant number K01AI125087 to J. K. E.), the Centers for Disease Control and Prevention (contracts CDC-200–2006–18797 and CDC-200–2015–63931), the Agency for Healthcare Research and Quality (contract 90047713), the Health Resources and Services Administration (contract 90051652), the Canadian Institutes of Health Research (grant numbers CBR-86906, CBR-94036, HCP-97105, and TGF-96118), the Ontario Ministry of Health and Long Term Care, the Government of Alberta, Canada, the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, the National Heart, Lung, and Blood Institute, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Human Genome Research Institute, the National Institute for Mental Health, the National Institute on Drug Abuse, the National Institute on Aging, the National Institute of Dental and Craniofacial Research, the National Institute of Neurological Disorders and Stroke, the National Institute of Nursing Research, the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Deafness and Other Communication Disorders, the National Institute of Diabetes and Digestive and Kidney Diseases, ViiV Healthcare (fellowship to H. L.), and Emory University Center for AIDS Research (grant number AI050409 to V. C. M.).
Potential conflicts of interest. H. L. received a predoctoral fellowship from ViiV Healthcare. A. A. A. receives personal fees from Merck and ViiV and personal fees and grants from Gilead, outside the submitted work. K. N. A. serves on the scientific advisory board for TrioHealth and is a consultant to the chief medical officer of the “All of Us” study. M. J. S. has a research grant to his institution from Gilead Sciences. P. F. R. has received support from the National Institute of Allergy and Infectious Diseases (grant numbers K01-AI131895 and R21-AI145686) and Gilead (contract VUMC63815). V. D. L. receives grants from the Canadian Institutes of Health Research and other support from the Michael Smith Foundation for Health Research , outside the submitted work. V. C. M. has been a consultant for or received research support from ViiV Healthcare, Gilead Sciences, Lilly, and Bayer. M. J. G. receives personal fees as an ad hoc member of the National HIV advisory boards for Merck, Gilead, and ViiV Healthcare, outside the submitted work. J. J. E. receives grants and personal fees from ViiV Healthcare, Janssen, and Gilead Sciences and personal fees from Merck, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013; 382:1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Althoff KN, Chandran A, Zhang J, et al. North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA . Life-expectancy disparities among adults with HIV in the United States and Canada: the impact of a reduction in drug- and alcohol-related deaths using the lives saved simulation model. Am J Epidemiol 2019; 188:2097–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Panel on Antiretroviral Guidelines for Adults and Adolescents DHHS. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV.2019. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed 4 September 2020.
- 4. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the international antiviral society-USA panel. JAMA 2018; 320:379–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. Update of recommendations on first- and second-line antiretroviral regimens.2019. Available at: https://www.who.int/hiv/pub/arv/arv-update-2019-policy/en/. Accessed 4 September 2020.
- 6. Lennox JL, DeJesus E, Lazzarin A, et al. STARTMRK investigators . Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet 2009; 374:796–806. [DOI] [PubMed] [Google Scholar]
- 7. Lennox JL, Dejesus E, Berger DS, et al. STARTMRK Investigators . Raltegravir versus efavirenz regimens in treatment-naive HIV-1-infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J Acquir Immune Defic Syndr 2010; 55:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clotet B, Feinberg J, van Lunzen J, et al. ING114915 Study Team . Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 2014; 383:2222–31. [DOI] [PubMed] [Google Scholar]
- 9. Walmsley SL, Antela A, Clumeck N, et al. SINGLE Investigators . Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369:1807–18. [DOI] [PubMed] [Google Scholar]
- 10. Eron JJ, Cooper DA, Steigbigel RT, et al. BENCHMRK Study Teams . Efficacy and safety of raltegravir for treatment of HIV for 5 years in the BENCHMRK studies: final results of two randomised, placebo-controlled trials. Lancet Infect Dis 2013; 13:587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sax PE, DeJesus E, Mills A, et al. GS-US-236-0102 study team . Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet 2012; 379:2439–48. [DOI] [PubMed] [Google Scholar]
- 12. Sax PE, Pozniak A, Montes ML, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet 2017; 390:2073–82. [DOI] [PubMed] [Google Scholar]
- 13. Gallant J, Lazzarin A, Mills A, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet 2017; 390:2063–72. [DOI] [PubMed] [Google Scholar]
- 14. Cole SR, Edwards JK, Hall HI, et al. Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) Investigators . Incident AIDS or death after initiation of human immunodeficiency virus treatment regimens including raltegravir or efavirenz among adults in the United States. Clin Infect Dis 2017; 64:1591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horberg MA, Oakes AH, Hurley LB, et al. Association of raltegravir use with long-term health outcomes in HIV-infected patients: an observational post-licensure safety study in a large integrated healthcare system. HIV Clin Trials 2018; 19:177–87. [DOI] [PubMed] [Google Scholar]
- 16. Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Int J Epidemiol 2007; 36:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. CDC. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MWR Morb Mortal Wkly Rep 1993; 41:1–20. [PubMed] [Google Scholar]
- 19. Brookhart MA. Counterpoint: the treatment decision design. Am J Epidemiol 2015; 182:840–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011; 30:377–99. [DOI] [PubMed] [Google Scholar]
- 21. White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med 2009; 28:1982–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53:457–81. [Google Scholar]
- 23. Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed 2004; 75:45–9. [DOI] [PubMed] [Google Scholar]
- 24. Hernán MA, Robins JM. Per-protocol analyses of pragmatic trials. N Engl J Med 2017; 377:1391–8. [DOI] [PubMed] [Google Scholar]
- 25. Lu H, Cole SR, Hall HI, et al. Generalizing the per-protocol treatment effect: the case of ACTG A5095. Clin Trials 2019; 16:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS clinical trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics 2000; 56:779–88. [DOI] [PubMed] [Google Scholar]
- 27. Zolopa A, Sax PE, DeJesus E, et al. ; GS-US-236-0102 Study Team . A randomized double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr 2013; 63:96–100. [DOI] [PubMed] [Google Scholar]
- 28. Rockstroh JK, DeJesus E, Lennox JL, et al. STARTMRK Investigators . Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naive HIV-1-infected patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr 2013; 63:77–85. [DOI] [PubMed] [Google Scholar]
- 29. Rockstroh JK, Lennox JL, Dejesus E, et al. STARTMRK Investigators . Long-term treatment with raltegravir or efavirenz combined with tenofovir/emtricitabine for treatment-naive human immunodeficiency virus-1-infected patients: 156-week results from STARTMRK. Clin Infect Dis 2011; 53:807–16. [DOI] [PubMed] [Google Scholar]
- 30. DeJesus E, Rockstroh JK, Lennox JL, et al. STARTMRK Investigators . Efficacy of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naïve HIV-1-infected patients: week-192 overall and subgroup analyses from STARTMRK. HIV Clin Trials 2012; 13:228–32. [DOI] [PubMed] [Google Scholar]
- 31. Walmsley S, Baumgarten A, Berenguer J, et al. Dolutegravir plus abacavir/lamivudine for the treatment of HIV-1 infection in antiretroviral therapy-naive patients: week 96 and week 144 results from the SINGLE randomized clinical trial. J Acquir Immune Defic Syndr 2015; 70:515–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zolopa AR, Berger DS, Lampiris H, et al. Activity of elvitegravir, a once-daily integrase inhibitor, against resistant HIV Type 1: results of a phase 2, randomized, controlled, dose-ranging clinical trial. J Infect Dis 2010; 201:814–22. [DOI] [PubMed] [Google Scholar]
- 33. Wohl DA, Cohen C, Gallant JE, et al. GS-US-236-0102 Study Team . A randomized, double-blind comparison of single-tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF versus single-tablet regimen efavirenz/emtricitabine/tenofovir DF for initial treatment of HIV-1 infection: analysis of week 144 results. J Acquir Immune Defic Syndr 2014; 65:e118–20. [DOI] [PubMed] [Google Scholar]
- 34. Bourgi K, Rebeiro PF, Turner M, et al. Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis 2020; 70:1267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Menard A, Meddeb L, Tissot-Dupont H, et al. Dolutegravir and weight gain: an unexpected bothering side effect? AIDS 2017; 31:1499–500. [DOI] [PubMed] [Google Scholar]
- 36. Norwood J, Turner M, Bofill C, et al. Weight gain in persons with HIV switched from efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr 2017; 76:527–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rizzardo S, Lanzafame M, Lattuada E, et al. Dolutegravir monotherapy and body weight gain in antiretroviral naïve patients. AIDS 2019; 33:1673–4. [DOI] [PubMed] [Google Scholar]
- 38. Bhagwat P, Ofotokun I, McComsey GA, et al. Changes in waist circumference in HIV-infected individuals initiating a raltegravir or protease inhibitor regimen: effects of sex and race. Open Forum Infect Dis 2018; 5:ofy201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol 2016; 183:758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.