Abstract
Background
High-Flow Nasal Cannula (HFNC) therapy is useful treatment in patients with acute respiratory failure (ARF). The ROX index (ratio of pulse oximetry/fraction of inspired oxygen to respiratory rate) has been evaluated to predict success of HFNC in patients with pneumonia.
Objective
The aim of this study was to determine whether the ROX Index could predict HFNC therapy success in patients with ARF due to SARS-CoV-2 pneumonia.
Methods
An observational, prospective study was performed including patients admitted with ARF secondary to SARS-CoV-2 pneumonia who met criteria for HFNC therapy initiation. Demographic, radiological, laboratory and clinical course data were collected. The ROX index was calculated at 1 h, 6 h, 12 h and 24 h after starting HFNC.
Results
In total 85 patients were included (age, 64.51 + 11.78 years; male, 69.4%). HFNC failed in 47 (55.3%) patients, of whom 45 (97.8%) were initially managed with noninvasive ventilation (NIV). ROX index at 24 h was the best predictor of HFNC success (AUC 0.826, 95%CI 0.593–1.00, p = 0.015) with a cut-off point of 5.35 (S 0.91, Sp 0.79, PPV 0.92, NPP 0.79). In multivariate logistic regression analysis ROX index at 24 h proved the best predictor of HFNC success.
Conclusions
ROX index at 24 h with a cut-off point of 5.35 predicts HFNC success in patients with SARS-Cov-2-induced ARF.
Keywords: COVID-19, Respiratory failure, High flow nasal cannula, Mechanical ventilation
1. Introduction
Coronavirus disease 2019 (COVID-19) can present as asymptomatic infection, mild upper respiratory tract illness or severe bilateral pneumonia, which may progress to acute respiratory distress syndrome (ARDS) [1]. Published data show that around 20% of hospitalized patients may need respiratory support and admittance to an intensive care unit (ICU) [2]. Oxygen therapy is the main treatment for acute respiratory failure (ARF) [3], but the optimal management strategy for respiratory failure due to SARS-CoV-2 is still evolving, and besides this, no clear recommendations have been established concerning indications for noninvasive respiratory support or which patients will require tracheal intubation and invasive management.
In viral pneumonias, ventilatory support with noninvasive ventilation (NIV) is associated with high failure rates [4,5]. As a result, high flow nasal cannula (HFNC) therapy is considered an alternative in noninvasive respiratory support in patients with ARF due to SARS-CoV-2 infection, in order to avoid endotracheal ventilation.
HFNC is able of supplying flows of up to 60 L per minute and a fraction of inspired oxygen of 1; moreover, HFNC presents better oropharyngeal dead space washout, provides discrete positive end-expiratory pressure (PEEP), and improves secretion clearance. These actions improve pulmonary mechanics, resulting in improved respiratory patterns, decreased respiratory rate, increased pressure of arterial oxygen to fraction of inspired oxygen ratio (PaO2/FiO2) and decreased pulmonary insufflation pressure. Moreover, HFNC is more comfortable and better tolerated than other noninvasive respiratory support therapies. The risk and benefits of HFNC are currently controversial, as it may delay the need for orotracheal intubation and mechanical ventilation without a clear effect on mortality [[6], [7], [8]]. In this regard, there is a pivotal need for tools to allow us to detect early HFNC failure and transfer patients to intensive care units. Roca et al. described the ROX index ([SpO2/FIO2]/respiratory rate) in a patient population diagnosed with both bacterial and viral non-COVID-19 pneumonia and respiratory failure, and established cut-off points after HFNC initiation that predicted the need for intubation in their cohort [9].
There are few studies, mainly retrospective in nature, that evaluate the usefulness of the ROX index in SARS-CoV-2-caused pneumonias [[10], [11], [12], [13]]. We hypothesized that ROX index could be an effective tool to predict success of HFNC therapy in the respiratory failure produced by SARS-CoV-2.
2. Material and methods
2.1. Study type
This is a prospective observational cohort study of patients admitted to the Respiratory Medicine Department of a tertiary university hospital with diagnosis of bilateral pneumonia with ARF due to SARS-CoV-2.
2.2. Population
Patients admitted to the Respiratory Medicine Department of a tertiary university hospital with microbiological diagnosis of SARS-CoV-2, radiological alterations compatible with pneumonia and respiratory failure with HFNC initiation criteria were included in the study. COVID-19 diagnosis was made according to WHO interim guidance [14] by real-time reverse-transcription polymerase chain reaction assay for nasal and pharyngeal swab specimens. Indication to start HFNC was determined by presence of at least one of the three following criteria in patients on conventional oxygen therapy: severe dyspnea with signs of more labored breathing and use of accessory respiratory muscles, respiratory rate over 30 rpm, and PaO2/FiO2 under 200 despite FiO2 over 0.4 [15]. All patients were managed and received treatment for any SARS-CoV-2-induced disease and related complications following Spanish health authority protocols [16]. These protocols16 recommend mainly prophylaxis of venous thromboembolic disease and treatment of thromboembolic events, corticosteroids treatment if the patient presents severe pneumonia, rapid progression, SpO2 <90% or respiratory rate >30 bpm, anti-IL6 treatment if IL > 40 mg/mL or D-dimer > 1500 ng/mL treatment with Remdesivir in those patients who need oxygentherapy with a maximum of 7 days of symptoms and antibiotic treatment in case of bacterial superinfection. Exclusion criteria were refusal to participate in the study, immediate need of orotracheal intubation and mechanical ventilation due to hemodynamic instability, inability to protect the upper airway or respiratory acidosis, and subjects with non-intubation orders or under 18 years old.
2.3. HFNC therapy and treatment failure
Patients underwent continuous cardio-respiratory monitoring during the entire procedure at the Respiratory Medicine Department and were placed in a negative pressure room. To ensure minimal exposure and risk, the healthcare team managing tracheostomized patients used full personal protective equipment for aerosol-generating procedures, including FFP3 mask, eye protection, fluid-repellent gown and gloves. Patients were instructed to wear surgical masks during HFNC use to reduce aerosol spread. Clinical equipment used for HFNC treatment (AIRVO2, Fisher & Paykel Healthcare, Auckland, New Zealand or OH–70C, Micomme Medical, Hunan, China) was set initially at a temperature of 31–37 °C according to tolerance, a flow of 50–60 L/m and a FiO2 adjusted to maintain SpO2 over 93%. Vital signs and respiratory patterns were continuously monitored (Monitor Vista 120, Dräger, Lübeck, Germany).
HFNC failure was defined as respiratory support upgraded to mechanical ventilation (non-invasive or invasive) or death despite HFNC (severe dyspnea with signs of more labored breathing and use of accessory respiratory muscles, respiratory rate over 30 rpm, PaO2/FiO2 under 200 despite FiO2 1 and flow 60 l/m, pH under 7.34). NIV was initially implemented and if failed, endotracheal intubation (ETI) and mechanical ventilation were performed.
2.4. Ethical issues
This study was approved by the Ethics Research Committee of our institution (HCUV-INCLIVA, project 2021/004). Written informed consent and use of data were waived owing to the severity of the situation. Nonetheless, verbal authorization from patient or caregiver was required.
2.5. ROX index
ROX index, defined as the ratio of SpO2/FiO2to respiratory rate, was calculated at 1 h, 6 h, 12 h and 24 h after start of HFNC therapy.
2.6. Data collection
Demographic, clinical, analytic and radiological data were collected at start of HFNC therapy. Demographic variables assessed included sex, age, body mass index and comorbidities using the Charlson comorbidity index. Analytic data included total lymphocyte count, creatinine, d-dimer, C-reactive protein, IL-6 and procalcitonin. A Covid-19 chest X-ray severity score as adapted by Wong et al. (termed herein Covid19-CXRScore) [17] was used to radiologically determine severity. Clinical course variables included time from symptoms onset to HFNC, time under HFNC, ICU admission and mortality at 7 and 30 days from starting HFNC therapy.
2.7. Endpoints
The primary endpoint of the study was to determine whether ROX index could predict success or failure of HFNC therapy in patients with respiratory failure in the context of pneumonia due to SARS-CoV-2. Secondary endpoints were to analyze HFNC failure rate and the clinical course of patients needing NIV due to failure of HFNC therapy.
2.8. Statistical analysis
We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational studies [18].
Assuming a 5% risk and 80% statistical potency to detect differences, and based on previous studies showing that the ROX index detects approximately 85% of HFNC therapy failures in patients with SARS-COV-2 pneumonia,10-13 the minimum sample size calculated to detect differences was 84 patients.
Binary and categorical variables were summarized using frequency counts and percentages. Continuous distributed variables were expressed as mean + SD. Data comparisons were performed using Student's-t test. Dichotomic variables were compared using the chi-square test. Time using HFNC and probability HFNC success was assessed with Kaplan-Meier charts and comparisons were made using the Log-Rank test. Forward stepwise logistic regression analysis, unadjusted and adjusted for variables related to COVID-19 severity, was used to determine the variables associated with HFNC treatment failure. The multivariate analysis model included variables exhibiting significant association in the univariate model. Receiver operating characteristics (ROC) curves were used to identify a cut-off point in variables that best predict HFNC therapy failure in logistic regression. Statistical significance was set at p < 0.05.
3. Results
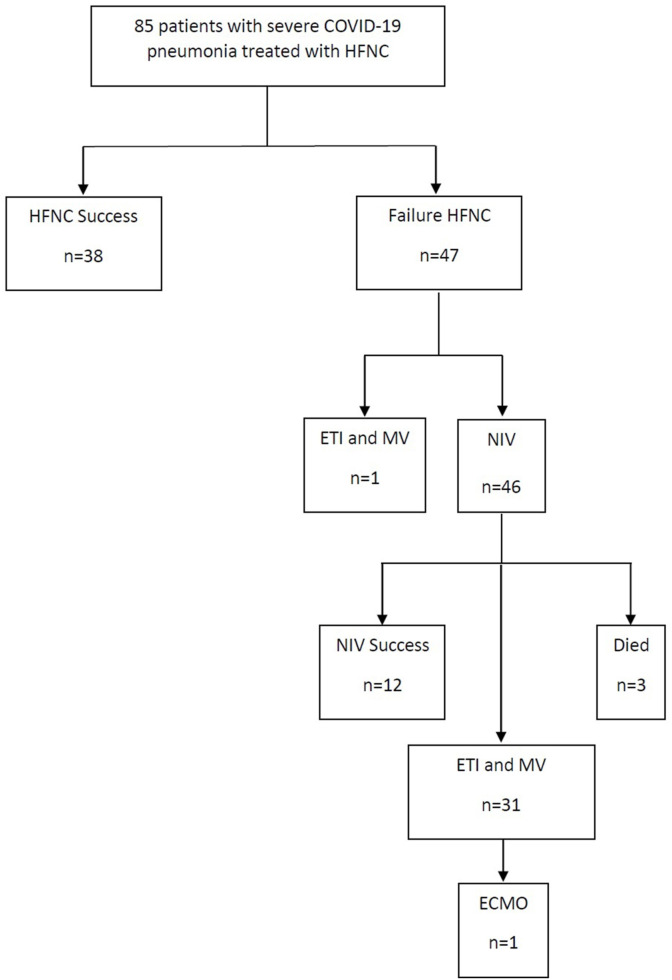
Over a two-month period (January–February 2021) 123 patients with COVID-19 admitted to the Respiratory Medicine ward needed HFNC, of whom 85 met the inclusion criteria and were included in the study. All had severe ARF secondary to bilateral pneumonia due to SARS-CoV-2 and were treated with HFNC (Fig. 1 ). Table 1, Table 2 show demographic and clinical data on HFNC therapy initiation. In total, 38 patients (44.70%) were managed successfully with HFNC (Fig. 1). Time from symptoms onset to HFNC treatment was 9.03 + 4.41 days (9.44 + 0.86 days in HFNC success group and 8.70 + 0.51 days in HFNC failure group, p = 0.442). Statistically significant between-group differences were found in IL-6 and severity of radiologic involvement as measured with the COVID19-CXRScore (Table 1). In patients managed successfully with HFNC, treatment duration was 3.29 + 0.53 days, while in patients with failed HFNC, treatment duration until failure was 1.47 + 021 days. One hour after starting HFNC the failure rate was 12.9%, after 6 h 22.2%, after 12 h 19.1%, and after 24 h 21.6%. At seven days, mortality in the success group was 0% vs. 10.63% in the failure group (p = 0.038) and at 30 days it had risen to 5.26% vs. 36.17% (p = 0.001) respectively.
Fig. 1.
Study flowchart.
Table 1.
Demographic and clinical data according to successful or failed HFNC.
| Total population (n = 85) | HFNC Success (n = 38) | HFNC Failure (n = 47) | p | |
|---|---|---|---|---|
| Sex (M/F) | 59/26 | 26/12 | 33/14 | 0.859 |
| Age (y) | 64.51 + 11.78 | 62.47 + 11.32 | 66.17 + 12.01 | 0.152 |
| Charlson index | 1.22 + 1.77 | 0.94 + 1.87 | 1.44 + 1.67 | 0.199 |
| Lymphocytes (x109/L) | 1.01 + 1.16 | 0.91 + 0.44 | 1.09 + 1.53 | 0.482 |
| D-dimer (mg/mL) | 2404.89 + 3881.73 | 2097.54 + 3670.84 | 2657.60 + 4070.46 | 0.519 |
| CRP (mg/L) | 119.93 + 92.77 | 109.39 + 89.25 | 128.63 + 95.68 | 0.347 |
| Procalcitonin (mg/mL) | 2.17 + 11.22 | 3.43 + 15.62 | 1.12 + 5.29 | 0.364 |
| IL-6 (pg/mL) | 189.11 + 304.10 | 11.72 + 133.91 | 258.96 + 389.02 | 0.027 |
| Creatinine (mg/dL) | 0.90 + 0.42 | 0.85 + 0.27 | 0.94 + 0.51 | 0.347 |
| COVID19-CXRScore | 4.57 + 1.52 | 4.07 + 1.47 | 4.97 + 1.45 | 0.006 |
| ROX1h | 5.99 + 1.58 | 6.84 + 1.26 | 5.30 + 1.47 | 0.000 |
| ROX6h | 6.10 + 1.73 | 6.98 + 1.36 | 5.12 + 1.58 | 0.000 |
| ROX12h | 6.41 + 1.91 | 7.32 + 1.54 | 4.85 + 1.43 | 0.000 |
| ROX24h | 6.61 + 2.05 | 7.29 + 1.77 | 4.81 + 1.63 | 0.000 |
Table 2.
Comorbidities of patients comparing successful and failed HFNC.
| HFNC Success | HFNC Failure | p | |
|---|---|---|---|
| BMI >30 kg/m2 | 17 (44.73%) | 21 (44.68%) | 0.996 |
| Smoker | 3 (7.89%) | 1 (22.12%) | 0.444 |
| Hypertension | 16 (42.10%) | 23 (48.93%) | 0.530 |
| Diabetes | 5 (13.15%) | 11 (23.40%) | 0.230 |
| Heart disease | 5 (13.15%) | 6 (12.76%) | 0.957 |
| COPD | 4 (10.52%) | 0 (0%) | 0.023 |
| Asthma | 5 (13.15%) | 3 (6.38%) | 0.288 |
| Cancer | 1 (2.63%) | 6 (12.76%) | 0.091 |
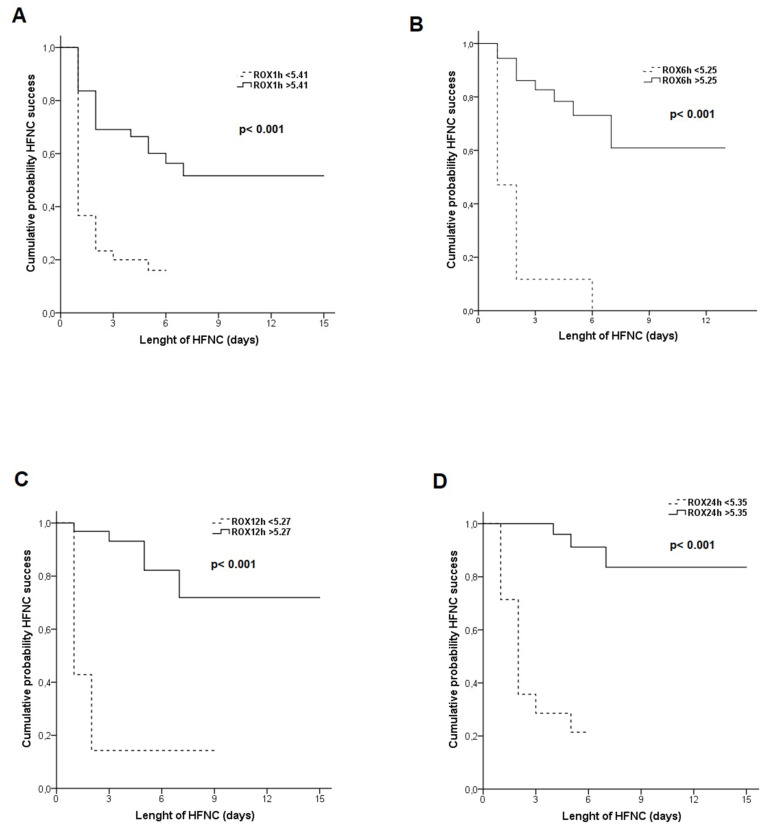
Table 3 shows the results of logistic regression univariate analysis of predictive factors for HFNC success. In multivariate analysis the only variable found to predict HFNC success was ROX24h (OR 0.47, 95%CI 0.23–0.95, p = 0.036). In adjusted multivariate analysis including variables related to COVID-19 severity, the only variable found to predict HFNC success was ROX24h (OR 0.11, 95%CI 0.01–0.89, p = 0.039). The results of ROC curve analysis for ROX index measured at different hours show a significant area under the curve for ROX1h (AUC 0.80, 95%CI 0.70–0.80, p = 0.000), ROX6h (AUC 0.83, 95%CI 0.71–0.95, p = 0.000), ROX12h (AUC 0.88, 95%CI 0.77–0.99, p = 0.000) and ROX24h (AUC 0.85, 95%CI 0.71–0.98, p = 0.000). Table 4 shows the cut-off point at different hours best predicting HFNC treatment success. Fig. 2 shows the Kaplan-Meier curve for the probability of patients with HFNC success.
Table 3.
Univariate analysis for HFNC success.
| OR | 95%CI | p | |
|---|---|---|---|
| ROX1h | 0.44 | 0.29–0.66 | 0.000 |
| ROX6h | 0.40 | 0.24–0.68 | 0.001 |
| ROX12h | 0.29 | 0.14–0.59 | 0.001 |
| ROX24h | 0.39 | 0.23–0.68 | 0.001 |
Table 4.
Cut-off points to predict HFNC success at 1, 6, 12 and 24 h of starting therapy.
| Cut-off point | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|
| ROX1h | 5.41 | 0.86 | 0.56 | 0.60 | 0.83 |
| ROX6h | 5.25 | 0.96 | 0.64 | 0.75 | 0.94 |
| ROX12h | 5.27 | 0.93 | 0.71 | 0.84 | 0.86 |
| ROX24h | 5.35 | 0.91 | 0.79 | 0.92 | 0.79 |
Fig. 2.
Accumulated probability of HFNC success according to ROX index at A = 1 h, B = 6 h, C = 12 h, D = 24 hours
Among patients in whom HFNC was not effective, one patient was intubated and received mechanical ventilation and 46 patients received treatment with NIV. This latter treatment was successful in 12 patients (26.1%); of those with failed NIV, 3 patients died, 30 were intubated and received mechanical ventilation and one patient needed ECMO despite invasive mechanical ventilation. Thus, of the 85 patients included in the study, noninvasive respiratory therapies (HFNC±NIV) successfully avoided ETI or death in 55 patients (58.82%).
4. Discussion
The findings of the present study show that the ROX index is a useful tool to assess HFNC success in patients suffering SARS-CoV-2-based pneumonia; a cut-off point of 5.35 after 24 h with treatment with HFNS predicts success of the therapy. Furthermore, noninvasive respiratory therapies (HFNC±NIV) are able to avoid ETI or death in 58.82% of patients with COVID-19.
According to the International Severe Acute Respiratory and emerging Infections Consortium, 19% of patients with COVID-19 need ICU or high dependence unit admission, and 29% of this subgroup require noninvasive respiratory support [19] Scala et al. proposed a management strategy for ARF in SARS-CoV-2-associated severe pneumonia, based on progression from conventional oxygen therapy to noninvasive respiratory support therapies (HFNC, NIV), invasive MV, and as a last step extracorporeal membrane oxygenation (ECMO).3 At the beginning of the pandemic, use of HFNC in COVID-19 patients was not recommend in different guidelines due to the potential risks of aerosol transmission and spread of the virus. However, the results of subsequent studies led to reassessment of these recommendations [20,21]. As an additional measure proposed to reduce the possibility of virus spread during HFNC, Leonard et al. [22] found that concomitant use of a surgical mask with HFNC can reduce droplets in exhaled breath. Moreover, recent studies have reported that compared with conventional oxygen therapy, HFNC reduces the need for ETI in patients with ARF due to SARS-CoV-2 [[23], [24], [25]].−25
In previous viral pandemics (MERS and SARS) the NIV failure rate was around 30%, while in ARF secondary to H1N1, NIV failed in up to 77% of patients. Scientific society guidelines have made no recommendations for or against NIV use in COVID-19 patients due to insufficient evidence, however this technique could be considered in carefully selected patients in a protected environment to avoid ETI and VM-related complications such as pneumonia or ventilator lung injuries.5.
Nonetheless, use of noninvasive respiratory support techniques should not result in delayed ETI given the high mortality rate when ETI and IMV is postponed. This highlights an urgent need for parameters that predict technique failure in COVID-19 patients receiving noninvasive respiratory support. The ROX index has been proposed as a potential tool to detect failed HFNC therapy. Roca et al. [26] described the ROX index for the first time in 2016 and subsequently validated it as a predictor of HNC success in non-COVID pneumonia patients, reporting good performance in identifying patients managed with HFNC with a low failure risk in whom ETI could be avoided. These authors established a cut-off point of 4.88, 12 h after starting therapy, as the best predictor of success [27].
Recent studies have analyzed the performance of the ROX index to detect HFNC failure in SARS-CoV-2-induced ARF, which showed high discriminative value to predict HFNC failure within 24 h of HFNC initiation, although these studies are limited mainly by their retrospective design. Previous studies reported HFNC failure rates in SARS-CoV-2 patients of between 38 and 55%, while NIV failure rates ranged from 44 to 72%. Our results are in agreement with those previously reported. Moreover, our noninvasive strategy (HFNC–NIV) circumvented the need for ETI in 58.82% of patients; using a similar treatment strategy (HFNC–NIV) to ours, Wang et al.12 avoided ETI in 55% of patients with severe ARF. Regarding ROX index cut-off points to predict success of HFNC therapy in COVID-19, different studies reported similar points to those proposed by Roca et al. [27] and Hu et al.11, with a population similar to ours in terms of demographics and comorbidities, concluded that 6 h after HFNC onset with a cut-off point at 5.55 was the most suitable predictor of HFNC success, albeit with a relatively low sensitivity (61.1%) and relatively high specificity (84.6%); in contrast, in the present study the best predictor of HFNC success is the ROX index 24 h after therapy onset, with a cut-off point of 5.35, which yielded better results in sensitivity, specificity, PPV, NPV and AUC than those proposed by other studies.10, 11, 13 [28], Recently have been published prospective studies evaluating utility of ROX index in COVID [[29], [30], [31]]. Mellado-Artigas et al. [29], in a multicenter prospective study that included 259 patients with severe pneumonia due to SARS-CoV-2 who reveived HFNC at ICU admission, found that baseline non-respiratory Sequential Organ Failure Assessment (SOFA) score and ROX index were associated with endotracheal intubation and mechanical ventilation. Suliman et al., 30 in a prospective study that included 69 patients with moderate and severe COVID found that ROX index measured on the first day of admission was independent predictor factor of intubation with a cut-off point <25.26; a very high cut-off point is striking in this study, 30 fact for which it was not included in a recent meta-analysis [32]. Finally, Gianstefani et al., 31 enrolled 554 patients admitted to emergency department with SARS-Cov-2 infection and found that ROX index at admission was associated with hospitalization and 30 days mortality; again, the cut-off points of this study were very high (25.7 and 22.3 respectively), included patients with mild upper respiratory tract illness and pneumonia and the authors do not mention the use of HFNC.
The study has several limitations: first, despite being a prospective study, it was conducted at a single-center hospital. Second, at the time this study was conducted, our hospital and center was under very high pressure, which could affect patient management in terms of available intensive care resources. Finally, another limitation regards use of SpO2 instead of PaO2 in the ROX index. However, SpO2/FiO2 has a good correlation with PaO2/FiO2 showing its usefulness in patients with ARDS [33]. 9.
In conclusion, our results suggest that the ROX index predicts HFNC success in patients suffering severe SARS-CoV-2-related pneumonia at a score of 5.35 or below 24 h after HFNC initiation. Moreover, sequential respiratory support with HFNC and NIV as rescue therapy in failed HFNC served to avoid ETI or death in 58.82% of patients with COVID-19.
Ethical issues
This study was approved by the Ethics Research Committee of our institution (HCUV-INCLIVA, project 2021/004). Written informed consent and use of data were waived owing to the severity of the situation. Nonetheless, verbal authorization from patient or caregiver was required.
Credit author statement
Santos Ferrer conceived and designed the study, collected, analyzed and interpreted the data, drafted the article and approved the submitted version.
Jesus Sancho conceived and designed the study, collected, analyzed and interpreted the data, drafted the article and approved the submitted version. JS is responsible for the overall content of the manuscript as guarantor.
Enric Bures conceived and designed the study, collected, analyzed and interpreted the data, drafted the article and approved the submitted version.
Irene Bocigas acquired the data, drafted the article and approved the submitted version.
Alba Mulet acquired the data, drafted the article and approved the submitted version.
Heidi Mora acquired the data, drafted the article and approved the submitted version.
Erik Monclou acquired the data, drafted the article and approved the submitted version.
Antonio Quezada acquired the data, drafted the article and approved the submitted version.
Pablo Royo acquired the data, drafted the article and approved the submitted version.
Declaration of competing interest
All the authors declare that they have no conflict of interest.
References
- 1.World Health Organization Coronavirus disease (COVID-19) outbreak. Disposable at. https://www.who.int
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 Apr 30;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. Epub 2020 Feb 28. PMID: 32109013; PMCID: PMC7092819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scala R., Heunks L. Highlights in acute respiratory failure. Eur. Respir. Rev. 2018;27:180008. doi: 10.1183/16000617.0008-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uyeki T.M., Bernstein H.H., Bradley J.S., Englund J.A., File T.M., Fry A.M., et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 Update on diagnosis, treatment chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin. Infect. Dis. 2019;68:e1–47. doi: 10.1093/cid/ciy866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rochwerg B., Brochard L., Elliott M.W., et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur. Respir. J. 2017;50:1602426. doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 6.Roca O., Hernández G., Diaz-Lobato S., Carratalá J.M., Gutiérrez R., Masclans J.R. Spanish Multidisciplinary Group of High Flow Supportive Therapy (HiSpaFlow). Current evidence for the effectiveness of heated and humidified high flow nasal cannula supportive therapy in adult patients with respiratory failure. Crit. Care. 2016;20:109. doi: 10.1186/s13054-016-1263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mauri T., Turrini C., Eronia N., Grasselli G., Volta C.A., Bellani G., et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am. J. Respir. Crit. Care Med. 2017;195:1207–1215. doi: 10.1164/rccm.201605-0916OC. [DOI] [PubMed] [Google Scholar]
- 8.Möller W., Celik G., Freng S., Bartenstein P., Meyer G., Eickelberg O., et al. Nasal high flow clears anatomical dead. space in upper airway models. J. Appl. Physiol. 2015;118:1207–1215. doi: 10.1152/japplphysiol.00934.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roca O., Messika J., Caralt B., García-de-Acilu M., Sztrymf B., Ricard J.D., Masclans J.R. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: the utility of the ROX index. J. Crit. Care. 2016 Oct;35:200–205. doi: 10.1016/j.jcrc.2016.05.022. Epub 2016 May 31. PMID: 27481760. [DOI] [PubMed] [Google Scholar]
- 10.Zucman N., Mullaert J., Roux D., Roca O., Ricard J.D., Contributors Prediction of outcome of nasal high flow use during COVID-19-related acute hypoxemic respiratory failure. Intensive Care Med. 2020 Oct;46(10):1924–1926. doi: 10.1007/s00134-020-06177-1. Epub 2020 Jul 15. PMID: 32671470; PMCID: PMC7362315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu M., Zhou Q., Zheng R., Li X., Ling J., Chen Y., Jia J., Xie C. Application of high-flow nasal cannula in hypoxemic patients with COVID-19: a retrospective cohort study. BMC Pulm. Med. 2020 Dec 24;20(1):324. doi: 10.1186/s12890-020-01354-w. PMID: 33357219; PMCID: PMC7758183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang K., Zhao W., Li J., Shu W., Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann. Intensive Care. 2020 Mar 30;10(1):37. doi: 10.1186/s13613-020-00653-z. PMID: 32232685; PMCID: PMC7104710, 10.1186/s13613-020-00653-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panadero C., Abad-Fernández A., Rio-Ramirez M.T., et al. High-flow nasal cannula for acute respiratory distress syndrome (ARDS) due to COVID-19. Multidiscip Respir Med. 2020;15(1):693. doi: 10.4081/mrm.2020.693. Published 2020 Sep. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organisation Clinical management of severe acute respiratory infection when novel coronavirus (nCov) is suspected. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected
- 15.Cinesi Gómez C., Peñuelas Rodríguez Ó. Luján Torné M., Egea Santaolalla C., Masa Jiménez J.F., García Fernández J., et al. Clinical consensus recommendations regarding non-invasive respiratory support in the adult patient with acute respiratory failure secondary to SARS-CoV-2 infection. Arch. Bronconeumol. 2020 Jul;56(Suppl 2):11–18. doi: 10.1016/j.arbres.2020.03.005. English, Spanish, Epub 2020 Mar 30. PMID: 32336563; PMCID: PMC7270645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de España Gobierno. Ministerio de Sanidad. Manejo Clínico del COVID-19: Atención hospitalaria. 2020. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/Protocolo_manejo_clinico_ah_COVID-19.pdf
- 17.Wong H.Y.F., Lam H.Y.S., Fong A.H., Leung S.T., Chin T.W., Lo C.S.Y., et al. Frequency and distribution of chest radiographic findings in COVID-19 positive patients. Radiology. 2019;27:201160. doi: 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 19.Escher M, Hall M, Baillie JK, Baruch J, Blumberg L, Carlson G, et al, ISARIC clinical data report 10 february 2021 International Severe Acute Respiratory and emerging Infections Consortium. DOI: 10.1101/2020.07.17.20155218. [DOI]
- 20.Alhazzani W., Møller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E., et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020 May;46(5):854–887. doi: 10.1007/s00134-020-06022-5. Epub 2020 Mar 28. PMID: 32222812; PMCID: PMC7101866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L., Camporota L. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020 Jun;46(6):1099–1102. doi: 10.1007/s00134-020-06033-2. Epub 2020 Apr 14. PMID: 32291463; PMCID: PMC7154064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonard S., Atwood C.W., Jr., Walsh B.K., DeBellis R.J., Dungan G.C., Strasser W., et al. Preliminary findings on control of dispersion of aerosols and droplets during high-velocity nasal insufflation therapy using a simple surgical mask: implications for the high-flow nasal cannula. Chest. 2020 Sep;158(3):1046–1049. doi: 10.1016/j.chest.2020.03.043. Epub 2020 Apr 2. PMID: 32247712; PMCID: PMC7130245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalmers J.D., Crichton M.L., Goeminne P.C., et al. Management of hospitalised adults with coronavirus disease 2019 (COVID-19): a European Respiratory Society living guideline. Eur. Respir. J. 2021;57(4):2100048. doi: 10.1183/13993003.00048-2021. Published 2021 Apr 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demoule A., Vieillard Baron A., Darmon M., Beurton A., Géri G., Voiriot G., et al. High-flow nasal cannula in critically III patients with severe COVID-19. Am. J. Respir. Crit. Care Med. 2020 Oct 1;202(7):1039–1042. doi: 10.1164/rccm.202005-2007LE. PMID: 32758000; PMCID: PMC7528777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonnet N., Martin O., Boubaya M., Levy V., Ebstein N., Karoubi P., et al. High flow nasal oxygen therapy to avoid invasive mechanical ventilation in SARS-CoV-2 pneumonia: a retrospective study. Ann. Intensive Care. 2021 Feb 27;11(1):37. doi: 10.1186/s13613-021-00825-5. PMID: 33638752; PMCID: PMC7910764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roca O., Messika J., Caralt B., García-de-Acilu M., Sztrymf B., Ricard J.-D., et al. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: the utility of the ROX index. J. Crit. Care. 2016;35:200–205. doi: 10.1016/j.jcrc.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 27.Roca O., Caralt B., Messika J., Samper M., Sztrymf B., Hernández G., et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am. J. Respir. Crit. Care Med. 2019 Jun 1;199(11):1368–1376. doi: 10.1164/rccm.201803-0589OC. PMID: 30576221. [DOI] [PubMed] [Google Scholar]
- 28.Chandel A., Patolia S., Brown A.W., Collins A.C., Sahjwani D., Khangoora V., et al. High-flow nasal cannula therapy in COVID-19: using the ROX index to predict success. Respir. Care. 2021 Jun;66(6):909–919. doi: 10.4187/respcare.08631. Epub 2020 Dec 16. PMID: 33328179. [DOI] [PubMed] [Google Scholar]
- 29.Mellado-Artigas R., Mujica L.E., Ruiz M.L., Ferreiro B.L., Angriman F., Arruti E., et al. Predictors of failure with high-flow nasal oxygen therapy in COVID-19 patients with acute respiratory failure: a multicenter observational study. J. Intensive Care. 2021;9(1):23. doi: 10.1186/s40560-021-00538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suliman L.A., Abdelgawad T.T., Farrag N.S., Abdelwahab H.W. Validity of ROX index in prediction of risk of intubation in patients with COVID-19 pneumonia. Adv. Respir. Med. 2021;89(1):1–7. doi: 10.5603/ARM.a2020.0176. [DOI] [PubMed] [Google Scholar]
- 31.Gianstefani A., Farina G., Salvatore V., Alvau F., Artesiani M.L., Bonfatti S., et al. Role of ROX index in the first assessment of COVID-19 patients in the emergency department. Intern. Emerg. Med. 2021 Mar 1:1–7. doi: 10.1007/s11739-021-02675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prakash J., Bhattacharya P.K., Yadav A.K., Kumar A., Tudu L.C., Prasad K. ROX index as a good predictor of high flow nasal cannula failure in COVID-19 patients with acute hypoxemic respiratory failure: a systematic review and meta-analysis. J. Crit. Care. 2021 Sep 7;66:102–108. doi: 10.1016/j.jcrc.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rice T.W., Wheeler A.P., Bernard G.R., Hayden D.L., Schoenfeld D.A., Ware L.B. Comparision of the SpO2/FiO2 ratio and the PaO2/FiO2 ratio in patients with acute lung injury of ARDS. Chest. 2007 Aug;132(2):410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]