PURPOSE
Medulloblastoma is composed of four clinically and prognostically distinct molecular subgroups (WNT, SHH, group 3, and group 4). However, the clinical implications of these subgroups in the context of the unique challenges of low- to middle-income countries are rarely reported.
METHODS
We assembled an institutional cohort of children (3-18 years) diagnosed with medulloblastoma and treated in Jordan between 2003 and 2016. Tumors were subgrouped by NanoString and correlated with clinical and radiologic characteristics.
RESULTS
Eighty-eight patients were identified (63% male); median age was 6.9 years (interquartile range 4.8-9.2) and median symptom duration was 6 weeks (interquartile range 4-11). Radiotherapy was implemented as standard-risk in 41 patients (47%) and high-risk in 47 patients (53%). Subgrouping revealed 17 WNT (19%), 22 SHH (25%), 21 group 3 (24%), and 28 group 4 tumors (32%). Median time between craniotomy and radiotherapy was 45 days (17-155); 44% of them > 49 days. Median duration of radiotherapy was 44 days (36-74). Seventy-two patients (82%) received chemotherapy afterward. With a median follow-up of 4.6 years (0.2-14.9), 5-year progression-free survival (PFS) and overall survival were 73.5% and 69.4%, respectively, with no statistically significant survival difference between standard-risk and high-risk patients. Metastasis was significant for overall survival (P = .011). Patients with SHH and group 4 tumors had very good PFS (83.4% and 87.0%, respectively) and those with group 3 tumors had dismal outcomes (PFS 44.9%), whereas WNT tumors had less-than expected PFS (70.5%). PFS was statistically significant in patients with nonmetastatic tumors receiving radiotherapy ≤ 49 days (P = .011), particularly group 3 tumors.
CONCLUSION
Patients with SHH and group 4 medulloblastoma had excellent survival comparable with high-income countries. Compliance with treatment protocols and avoiding radiotherapy delays are important in achieving adequate survival in low- to middle-income country settings. Subgroup-driven treatment protocols should be considered in countries with limited resources.
INTRODUCTION
Medulloblastoma is the most common malignant CNS tumor in children. Integrated genomics has identified at least four subgroups (WNT, SHH, group 3, and group 4) with characteristic clinical, radiologic, and prognostic features.1-3 Survival is highly variable across subgroups with WNT tumors having the highest survival and group 3 tumors being more metastatic, with dismal survival.4-7 However, apart from few ongoing clinical trials,8-11 risk stratification and treatment of medulloblastoma are still based on age, degree of tumor resection, and presence or absence of metastasis.12-14
CONTEXT
Key Objective
In pediatric medulloblastoma, would the molecular subgroups be the main prognostic factor even in a low- to middle-income country setting?
Knowledge Generated
Molecular subgroups are the driving prognostic factor in homogenously treated children in Jordan. Excellent progression-free survival was achieved in patients with SHH and group 4 tumors, whereas those with group 3 tumors had dismal outcomes and WNT tumors had less-than expected progression-free survival.
Relevance
Practitioners in low- to middle-income country settings can achieve comparable medulloblastoma survivals with those in high-income countries when they comply with treatment protocols and avoid radiotherapy delays. Nevertheless, molecularly driven treatment protocols are needed to balance survival with quality of survival in these countries.
Treating medulloblastoma in low- to middle-income countries (LMICs) is challenging.15 Specifically, delays in diagnosis, delays to treatment, and a lack of uniform, dedicated pediatric neurosurgical expertise all compromise survival. Furthermore, a lack of community rehabilitation services negatively affects the quality of life of these children who are already challenged in cognition, hearing, and growth. Limited reports of medulloblastoma outcome in the LMIC setting have generally reported lower survival compared with high-income countries (HICs), where this lower survival was mostly attributed to inconsistencies with both chemotherapy and radiation.16-20 There is a paucity of LMIC studies incorporating molecular subgroups.21-25
King Hussein Cancer Center (KHCC) is the only cancer-dedicated hospital in Jordan. The pediatric neuro-oncology service was established in 2003-2004 through a twinning program with the Hospital for Sick Children in Toronto. Monthly video teleconferences are held to review patients' management plans, which are delivered by the local multidisciplinary team.26 We previously reported improved survival in a pilot study of children with medulloblastoma at KHCC following the implementation of this twinning program.27 In this current retrospective study of radiated patients, we demonstrate that analogous to HICs, medulloblastoma subgroups are the most important variable influencing survival. We show that patients with SHH and group 4 tumors have excellent survivals comparable with HICs, despite a delay in treatment, whereas patients with group 3 tumors had poor outcomes consistent with recent cooperative group studies.
METHODS
Between January 2003 and December 2016, 88 patients age between 3 and 18 years at presentation were identified at KHCC and fulfilled inclusion criteria. Only patients treated with craniospinal radiotherapy for medulloblastoma with curative intent were included. Patients with sufficient clinical data and tumor tissue referred solely for radiotherapy to KHCC were included.
Pathology was reviewed by a KHCC neuropathologist (M.H.) to confirm the diagnosis of medulloblastoma and to exclude atypical teratoid rhabdoid tumor by BAF-47 and BRG-1 immunohistochemistry (IHC). All tumors were stained for β-catenin and p53. p53 was considered immunopositive if > 50% of tumor cell nuclei were strongly positive; otherwise, it was considered negative. β-catenin IHC was considered immunopositive when at least 10% of tumor nuclei showed positivity. Molecular subgrouping was performed using NanoString, from RNA extracted from formalin-fixed paraffin-embedded tissue, at the Princess Margaret Cancer Centre Genomics Facility as previously described.28 Only tumors with an assigned subgroup were included in this study.
Neuroimaging where available was re-reviewed by a KHCC neuroradiologist (M.S.). Tumors were assessed for metastasis according to Chang's classification. Near-total resection was considered when the residual tumor was ≤ 1.5 cm2 (R0); otherwise, subtotal resection was considered. Tumor progression or relapse was considered when the tumor size increased > 20% or new lesions appeared. If magnetic resonance imaging scans were not available for review, radiology reports or written clinical reports issued at KHCC were used for staging.
Patients were treated uniformly: up-front 3D conformal radiotherapy followed by chemotherapy: 23.4-Gy craniospinal radiation (CSI) for standard-risk (SR; R0M0) and 36- to 39-Gy CSI for high-risk (HR; R+, M+, and since 2012 for large-cell anaplastic histology) with tumor bed boost to 54 Gy. Patients with SR medulloblastoma received concurrent weekly vincristine with radiotherapy for a total of eight doses, then eight cycles (each 6 weeks apart) of CCNU 75 mg/m2 (day 1), cisplatin 75 mg/m2 (day 1), and vincristine 1.5 mg/m2 (days 1, 7, and 14) as per A9961 protocol,13 whereas patients with HR medulloblastoma received 3 monthly cycles of cisplatin 90 mg/m2 (day 1) and etoposide 150 mg/m2 IV (days 3 and 4), then 8 monthly cycles of vincristine 1.5 mg/m2 (day 1) and cyclophosphamide 1 g/m2 (days 1 and 2) as modified from POG-9031 protocol.14
This study was approved by the Institutional Review Boards at KHCC and the Hospital for Sick Children.
Statistical Analysis
Kaplan-Meier survival curves were performed to present patients' progression-free survival (PFS) and overall survival (OS). Log-rank test was used to compare median survival times. Five-year survival rates with their corresponding 95% CIs were reported. Cox regression model was used to adjust the P values and hazards for significant factors. A significance criterion of P ≤ .05 was used in the analysis. All analysis was performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
Patients' Demographics and Subgrouping
Eighty-eight patients were identified; 55 were males with a median age of 6.9 years (interquartile range [IQR] 4.8-9.2; Table 1). Median duration of symptoms before presentation was 6 weeks (IQR 4-11). Near-total resection was achieved in 51 tumors and subtotal resection in 31 (six unknown; Table 1). Ventriculoperitoneal shunts were inserted in 51% of patients. Fifty-nine tumors were nonmetastatic, nine were M1, and 19 were M2-M3 (32% were M+). Forty-seven patients (53%) were treated as HR with 36-Gy CSI (metastatic disease [60%], residual tumor [32%], anaplastic histology [2%], or unknown [6%]).
TABLE 1.
Patient and Tumor Characteristics (N = 88 patients)

Classic histology (46 tumors, 53%) and group 4 (28 tumors, 32%) were the most common (Table 1). Nineteen tumors (22%) exhibited nuclear positivity for β-catenin; 14 were subgrouped as WNT and five were non-WNT by NanoString (two SHH, two group 4, and one group 3). Three WNT tumors by NanoString exhibited cytoplasmic β-catenin staining. Four tumors harbored p53 immunopositivity (three WNT and one SHH), not confirmed by sequencing. Sex distribution (P = .033), median age at presentation (P = .003), tumor histology (P = .000), and presence or absence of metastasis (P = .008) were significantly different across subgroups but variables such as time to radiotherapy (P = .392), percentage of SR or HR stratification (P = .093), and proportion of patients who received chemotherapy (P = .233) did not show statistically significant differences (Table 2).
TABLE 2.
Demographics Among Subgroups
CSI was initiated at a median of 45 days from tumor resection (range, 17-155 days); 44% of them > 49 days (Table 2). Median duration of radiotherapy was 44 days (range, 36-74). Seventy-two patients (82%) received maintenance chemotherapy: 66 at KHCC and six at other institutions. Four patients did not receive chemotherapy (three were parental choice and one of unknown etiology). The administration of maintenance chemotherapy was unknown in 12 patients referred to KHCC solely for radiotherapy.
Twenty-five patients (28%) experienced tumor recurrence; 18 were metastatic and only five were documented by tumor biopsy or CSF malignant cells to be medulloblastoma. Ten patients (40%) received treatment at relapse but eventually died.
Medulloblastoma Subgroup Was the Most Significant Prognostic Factor
At a median follow-up of 4.6 years (range, 0.2-14.9), the overall 5-year PFS and OS were 73.5% (95% CI, 62.6 to 83.2) and 69.4% (95% CI, 58.2 to 79.6), respectively (Fig 1A). In univariate analysis, sex, histology, time to start radiotherapy, and duration of radiotherapy were not statistically significant for PFS or OS (Table 3). Five-year PFS of SR and HR groups was 80.6% (95% CI, 64.6 to 92.6) and 66.6% (95% CI, 50.9 to 80.6), respectively (P = .250; Fig 1B). Presence of metastasis was significant for 5-year OS (P = .011) but not PFS (P = .057; Figure 1C). Across subgroups, the proportion of nonmetastatic tumors treated with 36-Gy CSI was not significantly different (P = .387; Table 2).
FIG 1.
Graphs demonstrating 5-year PFS and OS: (A) PFS and OS of all patients (product-limit survival estimate), (B) PFS and OS according to risk stratification (Kaplan-Meier plot), (C) PFS and OS according to presence or absence of metastasis (Kaplan-Meier plot), and (D) PFS and OS according to medulloblastoma subgroup. G, group; mets, metastasis; OS, overall survival; PFS, progression-free survival.
TABLE 3.
Univariate Analysis for PFS/OS for Patients
Five-year PFS and OS (±95% CI) were significantly different across subgroups: WNT—70.5% (38.3 to 94.1) and 64.6% (34.6 to 89.3), respectively; SHH—83.4% (63.0 to 96.6) and 77.8% (56.1 to 93.5), respectively; group 3—44.9% (23.2 to 67.6) and 41.4% (20.6 to 64.0), respectively; group 4—87.0% (70.5 to 97.3) and 85.9% (68.2 to 97.1), respectively (P = 02; Figure 1D). In addition, 5-year PFS was not influenced by time to start radiotherapy (≤ or > 49 days from craniotomy; hazard ratio [HR], 0.861; 95% CI, 0.383 to 1.931; P = .716), but when adjusting for subgroups it was significant between group 3 and group 4 tumors (HR, 6.188; 95% CI, 1.975 to 19.385; P = .002). Similarly, duration of radiotherapy (≤ or > 50 days) was not significant for 5-year PFS (P = .230) but when adjusting for subgroups it was significant between patients with group 3 and group 4 tumors (HR, 6.952; 95% CI, 2.192 to 22.051; P = .001).
Among the 59 nonmetastatic tumors, 5-year PFS by subgroup was as follows: WNT—72.7% (34.9 to 97.4), SHH—87.4% (67.2 to 98.7), group 3—57.1% (21.9 to 88.7), and group 4—81.3% (59.2 to 95.9), with no statistically significant difference (P = .069). However, the PFS was significantly different among patients with nonmetastatic tumors who received radiotherapy ≤ 49 days compared with those who received it > 49 days from craniotomy date, 89.7% (95% CI, 76.2 to 97.9) versus 65.2% (95% CI, 43.1 to 84.4), respectively (P = .011). When adjusting for subgroups, this was only significant between groups 3 and 4 (HR, 5.36; 95% CI, 1.31 to 22.02; adjusted P = .019). Time to starting radiotherapy (≤ or > 49 days from craniotomy) was not significant in the 29 metastatic tumors (PFS, 44.9%; 95% CI, 22.0 to 68.9 v 100%; P = .071) or according to their subgroups.
Prognosis of WNT-Activated Medulloblastoma Was Poor
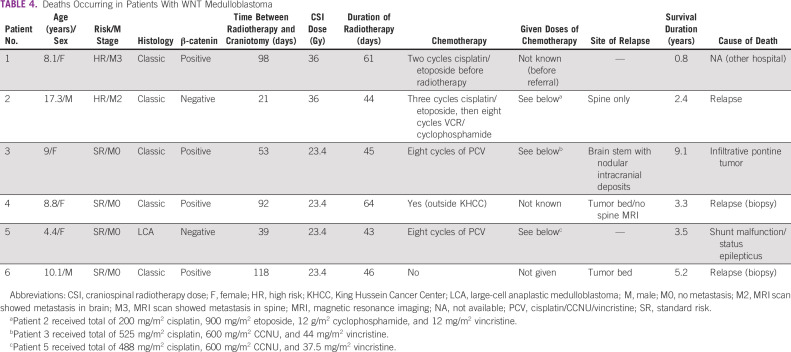
Seventeen patients with WNT tumors had a median age of 9 years (IQR 7.5-11.4) at presentation; 11 of them were females (65%). Two tumors had large-cell anaplastic histology, four were M2/M3 at diagnosis (24%), three were R1, and 14 had nuclear β-catenin immunopositivity. Radiotherapy was given at a median of 53 days from craniotomy date (IQR 35-92). Three patients (two SR and one HR) did not receive any chemotherapy. At a median follow-up of 3.1 years (range, 0.2-13.2 years), six patients died: two patients had biopsy-proven histologic diagnosis of local medulloblastoma relapse but WNT activation could not be confirmed; two patients had metastatic tumors, one in spine and the other resembling a radiation-induced diffuse intrinsic pontine glioma with metastasis (Fig 2); one patient died because of shunt malfunction while in complete remission; and the sixth patient died at an outside hospital of unknown etiology (Table 4). Five-year PFS and OS were 70.5% (95% CI, 38.3 to 94.1) and 64.6% (95% CI, 34.6 to 89.3), respectively.
FIG 2.
Selected MRI images (at the level of the brain stem) from the patient with WNT tumor who developed radiation-induced diffuse intrinsic pontine glioma. (A) Axial T1 WI showing an infiltrative hypointense lesion involving and expanding the pons (more on the left side) and the Lt middle cerebellar peduncle. (B and C) Axial T2 and FLAIR WI, respectively, showing hyperintense signal of the lesion with minimal involvement of the Lt cerebellar white matter. There is also an area of gliosis at the medial aspect of the right cerebellum that appeared stable compared with previous MRI studies (not shown). This is related to the previous surgery. (D and E) Enhanced coronal and sagittal T1 WI, respectively, showing enhancement of the infiltrative brain stem lesion with infiltration of the medulla oblongata. FLAIR, fluid-attenuated inversion recovery; Lt, left; MRI, magnetic resonance imaging; WI, weighted image.
TABLE 4.
Deaths Occurring in Patients With WNT Medulloblastoma
DISCUSSION
Our data demonstrate the driving effect of medulloblastoma subgroups on the prognosis of uniformly treated children from an LMIC. Our results show that, similar to HICs, patients with group 4 and SHH tumors have excellent outcome, those with group 3 tumors have poor survival, and patients with WNT tumors had lower-than expected outcome.
Similar to the published data on medulloblastoma,4,29 our review demonstrated male preponderance (62.5%), younger median age, and the predominance of classic histology and group 4 tumors. Despite having a relatively similar percentage of M+ disease (32%),4,29 our cohort had a disproportionately higher proportion of patients treated as HR (53.4%) compared with the Hospital for Sick Children experience (24%)4 or the SJMB03 study (31%).29 Fifteen patients (17%) in our cohort received 36-Gy CSI solely because of a residual tumor > 1.5 cm2. This is not uncommon in LMICs,17,25 which are challenged with limited number and expertise of neurosurgeons. This is an important issue in LMICs as higher doses of radiation are associated with more neurocognitive deficits while supportive and educational resources are limited in the community. Another important challenge in an LMIC is the delay in diagnosis and referral of children with brain tumors. The median duration of symptoms before diagnosis in our series was 6 weeks (IQR 4-11), which is close to that reported from Morocco30 and Brazil19 and slightly longer than Hospital for Sick Children experience of 4 weeks (IQR 4-12), which was reported to be subgroup-specific (longer in WNT and group 4).31 Our data did not show statistical difference in the prediagnostic interval among the subgroups (P = .208).
Lack of communication between professionals in LMICs and presence of postoperative complications usually delay starting radiotherapy early. Our median time to starting radiotherapy was 45 days (IQR 35-59), although most international protocols recommend initiation of radiotherapy within 4-5 weeks from craniotomy.29,32 A better PFS was reported in PNET-4 trial32 for SR medulloblastoma when radiotherapy was started < 49 days from surgery (0.81 ± 0.02 v 0.67 ± 0.09; P = .04). Similarly, we could demonstrate a better 5-year PFS in our patients with nonmetastatic tumors who received radiotherapy ≤ 49 days compared with > 49 days from craniotomy (89.7% v 65.2%; P = .011), which was particularly significant between groups 3 and 4. This association was not observed in M+. The ability to complete radiotherapy within 50 days was associated with a better EFS in the PNET-3 trial33 compared with > 50 days (3-year EFS of 78.5% v 53.7%; P = .0092); however, there was no effect in the whole cohort in the PNET-4 trial (P = .931).32 In our experience, duration was significant only when adjusting for subgroups between group 3 and group 4 (HR, 6.952; 95% CI, 2.192 to 22.051; P = .001).
Medulloblastoma subgroups were the most important prognostic factor in our cohort with an excellent 5-year PFS in SHH (83.4%) and group 4 (87.0%) comparable with data reported at the Hospital for Sick Children (61.3% and 88.7%, respectively)4 and in the SJMB03 trial (SHH: average-risk 77.5% and high-risk 25%; group 4: average-risk 87.3% and high-risk 68.1%).29 This was superior to the few reported studies from Brazil25 (52.9% in SHH; 41.7% in non-WNT and non-SHH) and a study from India21 (75.2% in SHH and 39.5% in non-WNT and non-SHH). Compliance in delivering treatment protocols has likely contributed to our superior outcome as all our patients received radiotherapy and 82% had chemotherapy. This is compared with lower compliance in other LMICs reported by Narayan et al21 (55.1% completed radiotherapy and 32.2% were given chemotherapy) and by Jiang et al23 (40% received chemotherapy). Group 3 medulloblastomas are known universally for their poor outcome2; however, some other factors may have contributed to our dismal outcome such as delays in starting radiotherapy or interruptions.
On the other hand, WNT tumors in our cohort did poorly. Three patients died of medulloblastoma progression, which were likely multifactorial. Two patients had significant delays to radiotherapy (92 and 118 days from surgery) without any adjuvant chemotherapy administered, and a third had M2 disease. This requires further investigation across our center and across other LMIC settings.
Our study suggests that comparable survival with HICs is feasible across LMIC settings through strict adherence to treatment protocols, proper staging, and prompt initiation of radiotherapy. Indeed, our findings suggest that when correcting for subgroup, comparable outcomes can be achieved, and subgroup-specific strategies can and should be implemented in LMIC settings. Specifically, recent studies from the Children's Oncology Group have suggested that carboplatin radiosensitization significantly improves outcome in metastatic group 334 and reductions in radiotherapy to 18 Gy have poor survivals restricted to group 4,35 suggesting that subgroup-specific therapies should not be restricted to HICs. Although a limitation for many LMIC centers is lack of a constant twinning partner, we would advocate for protocol implementation in LMIC centers to ensure uniform staging, and being cognizant of the risks of delaying therapy, specifically developing protocols unique to the limitations of each center. For example, although KHCC has access to in-house radiotherapy, in some LMIC settings, initiation of radiotherapy within 49 days is not feasible, and other strategies such as sandwich chemotherapy could be considered.36
The recent 2021 WHO Classification of central nervous system tumors includes a robust molecular stratification of medulloblastoma. Although this represents a huge leap forward in moving beyond morphologic classification, there are major barriers in the LMIC settings with incorporating real-time molecular characterization. Although IHC is one of the cheapest methods, it is also the most inaccurate and fraught with technical difficulties. A major limitation of IHC is the inability of distinguishing group 3 from group 4, but even WNT tumors are not reliably identified with nuclear β-catenin; indeed, the subgroup-specific results of ACNS0332 support the urgent need for the development of low-cost, reliable, reproducible, and rapid subgrouping of all four groups in LMICs. Moreover, there is batch-to-batch variability such as with the four polyclonal antibody strategy previously proposed, and accurate strategies using monoclonal antibodies may negate this issue.1 Although capital costs are an issue, several LMICs have incorporated subgrouping, including a real-time polymerase chain reaction method developed in Mumbai,37 a six-probe methylation signature developed in Spain,38 and the 22-gene signature we used using NanoString.28 Although NanoString carries significant capital costs, it has been successfully implemented in some LMICs such as Barretos, Brazil.24 Several efforts have shown that subgrouping can be reliably inferred from magnetic resonance imaging,39 which may represent an attractive orthogonal method of subgrouping in LMICs. As North American and Western European cooperative groups move further toward personalized protocols, efforts need to be focused on ensuring the implementation of subgroup-specific treatments that have the potential to improve functional outcomes and quality of life in LMIC survivors.
Our study is limited by the inherent bias of the retrospective design and unavailability of many tumor samples for children treated during the same study time. However, this study is the largest that demonstrates the relationship between medulloblastoma subgroups and outcome in uniformly treated children in a single center in an LMIC.
In conclusion, medulloblastoma subgroups are an important prognostic factor in LMICs as they are in HICs. Our SHH and group 4 medulloblastomas had comparable excellent survival despite incomplete resections and treatment delays. However, it also highlights the importance of treatment compliance within a unified protocol. More efforts are needed in LMICs to be compliant with time frames in relation to starting radiotherapy and avoiding interruptions, which may specifically be important in nonmetastatic tumors or specific subgroups. Proper identification of subgroups is important as future treatment protocols will likely integrate them in the risk stratification. This may be a challenge in LMICs with the associated cost and needed technology; thus, reliable cheap and accurate methods are desperately needed. Nevertheless, LMICs are ideal settings to validate molecularly stratified medulloblastoma protocols where survival needs to be balanced wisely with quality of survival to best use the limited health care resources.
ACKNOWLEDGMENT
We acknowledge the help by Mrs Dalia Al-Rimawi from the Biostatics Unit at KHCC in finalizing the statistical data.
Eric Bouffet
Consulting or Advisory Role: Novartis
Research Funding: Roche, Bristol Myers Squibb
Vijay Ramaswamy
Honoraria: AstraZeneca
Consulting or Advisory Role: AstraZeneca Canada
No other potential conflicts of interest were reported.
DISCLAIMER
Any opinion, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of ASCO or CCF.
PRIOR PRESENTATION
Presented, in part, as an oral presentation at ISPNO 2018, Denver, CO, July 1, 2018, and SIOP-Asia 2019, Abu Dhabi, United Arab Emirates, April 4, 2019.
SUPPORT
N.A. is a recipient of LIFe award funded by a Conquer Cancer Foundation (CCF) of ASCO Long-Term International Fellowship. This work was also supported by funds from the Intramural Grant Program at King Hussein Cancer Center. V.R. is supported by operating grants from the Canadian Institutes of Health Research, Canadian Cancer Society, Meagan's Hug Foundation, C.R. Younger Foundation, Nelina's Hope, b.r.a.i.n.child, and Garron Family Cancer Center.
M.A.-H. and V.R. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Nisreen Amayiri, Ahmed Ibrahimi, Awni Musharbash, Eric Bouffet, Maysa Al-Hussaini, Vijay Ramaswamy
Financial support: Nisreen Amayiri, Vijay Ramaswamy
Administrative support: Vijay Ramaswamy
Provision of study materials or patients: Maysa Al-Hussaini
Collection and assembly of data: Nisreen Amayiri, Maisa Swaidan, Ahmed Ibrahimi, Nader Hirmas, Eric Bouffet, Maysa Al-Hussaini, Vijay Ramaswamy
Data analysis and interpretation: Nisreen Amayiri, Ahmed Ibrahimi, Eric Bouffet, Vijay Ramaswamy
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Eric Bouffet
Consulting or Advisory Role: Novartis
Research Funding: Roche, Bristol Myers Squibb
Vijay Ramaswamy
Honoraria: AstraZeneca
Consulting or Advisory Role: AstraZeneca Canada
No other potential conflicts of interest were reported.
REFERENCES
- 1.Northcott PA, Korshunov A, Witt H, et al. : Medulloblastoma comprises four distinct molecular variants. J Clin Oncol 29:1408-1414, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramaswamy V, Remke M, Bouffet E, et al. : Risk stratification of childhood medulloblastoma in the molecular era: The current consensus. Acta Neuropathol 131:821-831, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramaswamy V, Taylor MD: Medulloblastoma: From myth to molecular. J Clin Oncol 35:2355-2363, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Ramaswamy V, Remke M, Adamski J, et al. : Medulloblastoma subgroup-specific outcomes in irradiated children: Who are the true high-risk patients? Neuro Oncol 18:291-297, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kool M, Korshunov A, Remke M, et al. : Molecular subgroups of medulloblastoma: An international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, group 3, and group 4 medulloblastomas. Acta Neuropathol 123:473-484, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pietsch T, Schmidt R, Remke M, et al. : Prognostic significance of clinical, histopathological, and molecular characteristics of medulloblastomas in the prospective HIT2000 multicenter clinical trial cohort. Acta Neuropathol 128:137-149, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shih DJ, Northcott PA, Remke M, et al. : Cytogenetic prognostication within medulloblastoma subgroups. J Clin Oncol 32:886-896, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ClinicalTrials.gov : International Society of Paediatric Oncology (SIOP) PNET 5 Medulloblastoma, ClinicalTrials.gov Identifier: NCT02066220, https://clinicaltrials.gov/ct2/show/NCT02066220 [Google Scholar]
- 9.ClinicalTrials.gov : Reduced Craniospinal Radiation Therapy and Chemotherapy in Treating Younger Patients With Newly Diagnosed WNT-Driven Medulloblastoma, ClinicalTrials.gov Identifier: NCT02724579, https://clinicaltrials.gov/ct2/show/NCT02724579 [Google Scholar]
- 10.ClinicalTrials.gov : A Clinical and Molecular Risk-Directed Therapy for Newly Diagnosed Medulloblastoma, ClinicalTrials.gov Identifier: NCT01878617, https://www.clinicaltrials.gov/ct2/show/NCT01878617 [Google Scholar]
- 11.ClinicalTrials.gov : HeadStart4: Newly Diagnosed Children (10 y/o) With Medulloblastoma and Other CNS Embryonal Tumors, ClinicalTrials.gov Identifier: NCT02875314, https://clinicaltrials.gov/ct2/show/NCT02875314 [Google Scholar]
- 12.Gajjar A, Chintagumpala M, Ashley D, et al. : Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): Long-term results from a prospective, multicentre trial. Lancet Oncol 7:813-820, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Packer RJ, Gajjar A, Vezina G, et al. : Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol 24:4202-4208, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Tarbell NJ, Friedman H, Polkinghorn WR, et al. : High-risk medulloblastoma: A pediatric oncology group randomized trial of chemotherapy before or after radiation therapy (POG 9031). J Clin Oncol 31:2936-2941, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parkes J, Hendricks M, Ssenyonga P, et al. : SIOP PODC adapted treatment recommendations for standard-risk medulloblastoma in low and middle income settings. Pediatr Blood Cancer 62:553-564, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Wahba HA, Abu-Hegazy M, Wasel Y, et al. : Adjuvant chemotherapy after reduced craniospinal irradiation dose in children with average-risk medulloblastoma: A 5-year follow-up study. J BUON 18:425-429, 2013 [PubMed] [Google Scholar]
- 17.Akyuz C, Varan A, Kupeli S, et al. : Medulloblastoma in children: A 32-year experience from a single institution. J Neurooncol 90:99-103, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Aguilar E, Sepulveda-Vildosola AC, Rivera-Marquez H, et al. : Clinical and molecular parameters for risk stratification in Mexican children with medulloblastoma. Arch Med Res 38:769-773, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Bleil CB, JWJ Bizzi, Bedin A, et al. : Survival and prognostic factors in childhood medulloblastoma: A Brazilian single center experience from 1995 to 2016. Surg Neurol Int 10:120, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehrvar A, Tashvighi M, Hedayati Asl AA, et al. : Management and outcomes of treating pediatric medulloblastoma: An eight years' experience in an Iranian pediatric center. Childs Nerv Syst 34:639-647, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Narayan V, Sugur H, Jaiswal J, et al. : Medulloblastoma: Distinctive histo-molecular correlation with clinical profile, radiologic characteristics, and surgical outcome. Pediatr Neurosurg 54:329-340, 2019 [DOI] [PubMed] [Google Scholar]
- 22.Zhang ZY, Xu J, Ren Y, et al. : Medulloblastoma in China: Clinicopathologic analyses of SHH, WNT, and non-SHH/WNT molecular subgroups reveal different therapeutic responses to adjuvant chemotherapy. PLoS One 9:e99490, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang T, Zhang Y, Wang J, et al. : A retrospective study of progression-free and overall survival in pediatric medulloblastoma based on molecular subgroup classification: A single-institution experience. Front Neurol 8:198, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leal LF, Evangelista AF, de Paula FE, et al. : Reproducibility of the NanoString 22-gene molecular subgroup assay for improved prognostic prediction of medulloblastoma. Neuropathology 38:475-483, 2018 [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann IL, Cardinalli IA, Yunes JA, et al. : Clinical, demographic, anatomopathological, and molecular findings in patients with medulloblastoma treated in a single health facility. Rev Paul Pediatr 39:e2019298, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amayiri N, Swaidan M, Abuirmeileh N, et al. : Video-teleconferencing in pediatric neuro-oncology: Ten years of experience. J Glob Oncol 4:1-7, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qaddoumi I, Musharbash A, Elayyan M, et al. : Closing the survival gap: Implementation of medulloblastoma protocols in a low-income country through a twinning program. Int J Cancer 122:1203-1206, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Northcott PA, Shih DJ, Remke M, et al. : Rapid, reliable, and reproducible molecular sub-grouping of clinical medulloblastoma samples. Acta Neuropathol 123:615-626, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gajjar A, Robinson GW, Smith KS, et al. : Outcomes by clinical and molecular features in children with medulloblastoma treated with risk-adapted therapy: Results of an international phase III trial (SJMB03). J Clin Oncol 39:822-835, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boutahar FZ, Benmiloud S, El Kababri M, et al. : Time to diagnosis of pediatric brain tumors: A report from the Pediatric Hematology and Oncology Center in Rabat, Morocco. Childs Nerv Syst 34:2431-2440, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramaswamy V, Remke M, Shih D, et al. : Duration of the pre-diagnostic interval in medulloblastoma is subgroup dependent. Pediatr Blood Cancer 61:1190-1194, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Lannering B, Rutkowski S, Doz F, et al. : Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: Results from the randomized multicenter HIT-SIOP PNET 4 trial. J Clin Oncol 30:3187-3193, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Taylor RE, Bailey CC, Robinson K, et al. : Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: The International Society of Paediatric Oncology/United Kingdom Children's Cancer Study Group PNET-3 study. J Clin Oncol 21:1581-1591, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Leary SES, Packer RJ, Li Y, et al. : Efficacy of carboplatin and isotretinoin in children with high-risk medulloblastoma: A randomized clinical trial from the Children's oncology Group. JAMA Oncol 10.1001/jamaoncol.2021.2224 [epub ahead of print on July 22, 2021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michalski JM, Janss AJ, Vezina LG, et al. : Children's Oncology Group phase III trial of reduced-dose and reduced-volume radiotherapy with chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol 39:2685-2697, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verlooy J, Mosseri V, Bracard S, et al. : Treatment of high risk medulloblastomas in children above the age of 3 years: A SFOP study. Eur J Cancer 42:3004-3014, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Kunder R, Jalali R, Sridhar E, et al. : Real-time PCR assay based on the differential expression of microRNAs and protein-coding genes for molecular classification of formalin-fixed paraffin embedded medulloblastomas. Neuro Oncol 15:1644-1651, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomez S, Garrido-Garcia A, Garcia-Gerique L, et al. : A novel method for rapid molecular subgrouping of medulloblastoma. Clin Cancer Res 24:1355-1363, 2018 [DOI] [PubMed] [Google Scholar]
- 39.Perreault S, Ramaswamy V, Achrol AS, et al. : MRI surrogates for molecular subgroups of medulloblastoma. AJNR Am J Neuroradiol 35:1263-1269, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]