Abstract
Background and aims
This meta-analysis aims to highlight the impact of cardio-metabolic comorbidities on COVID-19 severity and mortality.
Methods
A thorough search on major online databases was done for studies describing the clinical outcomes of COVID-19 patients. We used random-effects model to compute pooled estimates for critical or fatal disease.
Results
A total of 20,475 patients from 33 eligible studies were included. Maximum risk of development of critical or fatal COVID-19 disease was seen in patients with underlying cardiovascular disease [OR: 3.44, 95% CI: 2.65–4.48] followed by chronic lung disease, hypertension and diabetes mellitus. Of the total cases, 64% had one of the four comorbidities with the most prevalent being hypertension with a pooled prevalence of 27%.
Conclusions
Presence of comorbidities like cardiovascular disease, chronic lung disease, hypertension and diabetes mellitus led to a higher risk of development of critical or fatal COVID-19 disease, with maximum risk seen with underlying cardiovascular disease.
Keywords: COVID-19, SARS-CoV-2, Hypertension, Diabetes mellitus, Cardiovascular disease, Meta-analysis, Systematic review
1. Introduction
Viruses of the Coronaviridae family frequently cause zoonotic and human infections. While most of these coronavirus infections have a mild clinical profile in immunocompetent humans, exceptions to this are the Severe acute respiratory syndrome coronavirus (SARS-CoV) which caused global outbreak during 2002–2003 [1] and the Middle East respiratory syndrome (MERS) coronavirus (MERS-CoV) which was first identified from Saudi Arabia in 2012 [2]. Both of these caused global pandemics with the potential to cause respiratory failure and resulting mortality. An addition to this list is the coronavirus disease 2019 (COVID-19) caused by novel coronavirus strain (2019-nCoV), termed as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which has been spreading worldwide since its first case was reported in December 2019 from Wuhan, China [3]. The disease manifestation is heterogenous, ranging from mild disease to extensive pulmonary disease manifesting as acute respiratory distress syndrome (ARDS) which may culminate in mortality [4]. Increasing age and/or presence of one or more comorbidity have been found to be at associated with increased risk for severe disease and fatality [5]. The Centre for Disease Control (CDC) of United States has enlisted the medical comorbidities that are associated with severe COVID-19 disease. These include cancer, chronic kidney disease, cardiovascular conditions (coronary artery disease, heart failure and/or cardiomyopathy), pulmonary illnesses like chronic obstructive pulmonary disease (COPD), solid organ transplant recipients, obesity, diabetes mellitus, pregnancy, sickle cell disease and smoking [6]. This systematic review was undertaken to establish the risk of critical disease or mortality amongst patients with commonly encountered comorbidities viz. hypertension, diabetes, chronic lung disease and cardiovascular disease. This will help in identifying ‘at-risk’ individuals to predict disease progression in this subset and also direct health resources effectively towards this patient population. The findings of this study can be used to guide decisions in subsequent waves of the pandemic and also in future pandemics.
2. Data and methods
2.1. Search databases and search strategies
This systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Electronic search was carried out in Medline (PubMed) and EMBASE database. We selected relevant studies published online between December 2019 and October 2020. Search strategy relevant for our article was prepared using well-defined keywords and was refined for maximal inclusivity and appropriateness. No language restrictions were applied. The search strategy for PubMed/MEDLINE database was as follows: (“COVID-19″ OR “Coronavirus” OR “COVID” OR coronavirus, sars [MeSH Terms]) AND (“Death” OR “Mortality” OR “Fatality” OR “Outcome”) AND (“Comorbidity” OR “Comorbidities” OR “Risk Factors” OR “Clinical Characteristics”). EMBASE database was also included for this review with the search strategy as follows: (‘coronavirus disease 2019'/exp OR ‘coronavirus disease 2019′) AND (‘death'/exp OR ‘death’ OR ‘fatality'/exp OR ‘fatality’ OR ‘mortality'/exp OR ‘mortality’) AND (‘comorbidity'/exp OR ‘comorbidity’ OR ‘risk factor'/exp OR ‘risk factor’) AND [humans]/lim AND [embase]/lim AND [2019–2020]/py AND [embase]/lim NOT ([embase]/lim AND [medline]/lim). Filters were applied to exclude studies existing in both PubMed and Embase database(s) so as to avoid inclusion of duplicate articles. The title, abstract and relevant full text of the all documents identified by the search criteria were screened independently by two investigators and articles which were meeting our inclusion criteria were finally included. A third author resolved conflicts. We also performed a manual search of the reference list of included studies to prevent exclusion of eligible studies. Relevant pre-print articles were also included for enhancing inclusiveness.
2.2. Inclusion and exclusion criteria
The inclusion criteria were as follows:
(1) cohort studies, case-control studies and case series studies; (2) the study population included patients with laboratory confirmed SARS-CoV-2 infection; and (3) the primary outcome being reported as fatal or critical case (cases requiring ICU admission or developing ARDS or requiring invasive mechanical ventilatory support or cases labelled as “severe COVID” as per local or national classification criteria) versus non-critical case(s) amongst patients with or without various comorbidities.
The exclusion criteria were as follows: (1) studies with a sample size less than 20; (2) duplicate studies; (3) studies without required information; (4) literature or systematic reviews.
2.3. Data extraction
Two separate authors screened the selected studies based on the inclusion and exclusion criteria. Studies satisfying the same were included and data extraction was done onto predefined forms: study characteristics (sample size, first author, publication year and study design) and prevalence of comorbidities including diabetes mellitus, hypertension, chronic respiratory diseases (bronchial asthma, COPD and bronchiectasis) and cardiovascular diseases. The clinical outcome of patients with and without underlying comorbidities was extracted from the full-texts. Data extraction was done independently by two authors. Disagreements were resolved by discussion with a third author to establish a consensus.
2.4. Data synthesis and statistical analysis
Definition of ‘critical case’ as one or more of the following (1) cases requiring ICU admission; (2) cases developing ARDS; (3) cases requiring invasive mechanical ventilation; or (4) cases labelled as “severe COVID” as per the criteria used nationally or locally in the institution. The primary outcome measure was taken as ‘critical case’ or death. All data analysis was performed using STATA software version 16.0 (Stata Corp, College Station, Texas, USA). Sum of the fatal and critical cases was calculated for patients with and without the above-mentioned comorbidities in each cohort and odds ratio (OR) was computed. I2 statistic was used to determine the degree of heterogeneity between studies and I2 > 50% was considered as statistically significant heterogeneity [7]. A random-effects model was used to estimate the combined effect and precision owing to statistically significant heterogeneity. Pooled analysis was done and pooled OR with 95% confidence interval was computed as a measure of risk of fatal or critical disease among COVID-19 infected patients with a particular comorbidity (diabetes/hypertension/chronic lung disease or cardiovascular disease) versus patients with no comorbidity. Statistical significance was considered for a p-value <0.05 for any test or model.
3. Results
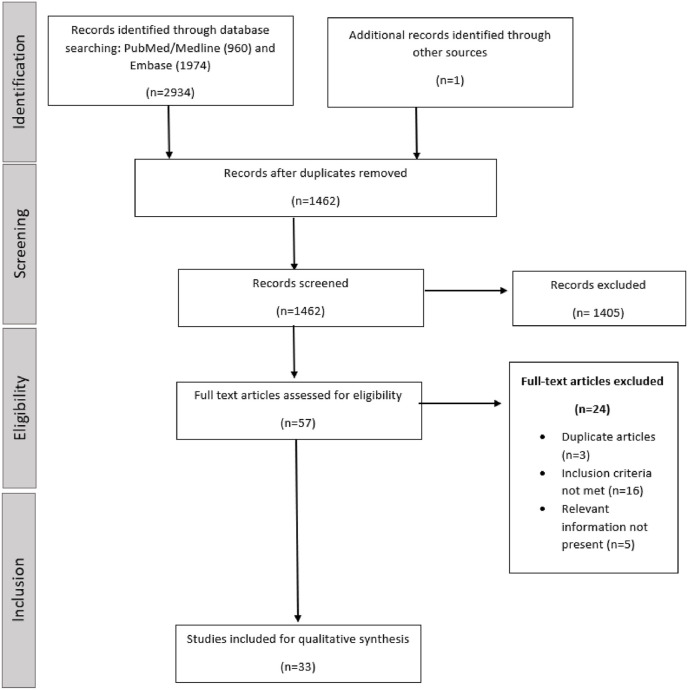
The combined search of the PubMed and Embase Library databases identified 2934 citations. One additional article was identified by citation search of the relevant articles. After the removal of duplicates, the title and abstract were screened for relevance. Articles not meeting the inclusion criteria or not providing the relevant desired information were excluded. The full texts of remaining articles were assessed independently in duplicate by two authors: SS and GG. A total of 33 articles met the inclusion criteria and were included in the synthesis. Majority of the included articles were retrospective cohort studies, with the exception of few studies. PRISMA guidelines were followed for the search and selection process (Fig. 1 ).
Fig. 1.
Flowchart of the search and selection process for the meta-analysis (PRISMA).
These studies included a total of 20,475 patients. Most of the studies were reported from China since it was the initial epicentre of the pandemic. Other studies were reported from various countries across the world including Unites States of America, Egypt, Italy, Denmark, Poland, Spain and Iran. Data regarding the prevalence and clinical outcome of patients with and without these four comorbidities (diabetes, hypertension, chronic lung disease and cardiovascular disease) was extracted individually from these studies.
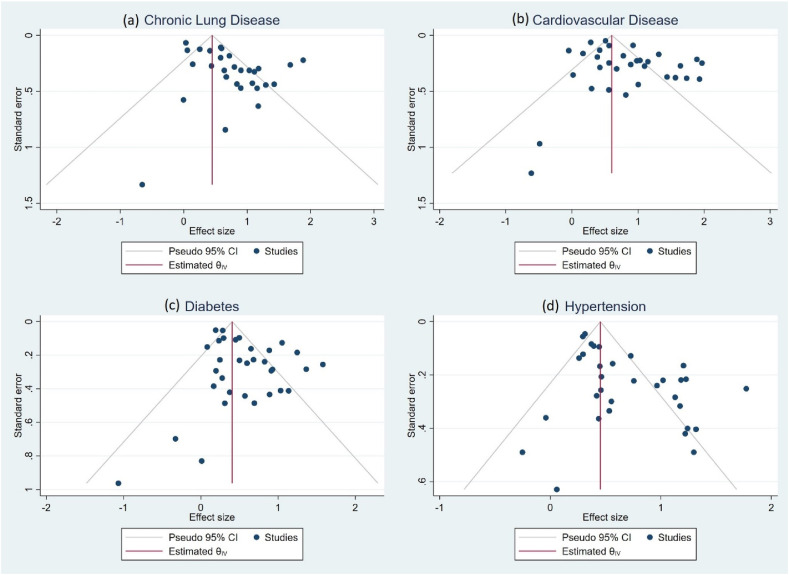
Thirty-three studies provided the data in terms of risk of development of severe/critical/fatal COVID-19 among patients with four comorbidities namely diabetes, hypertension, cardiovascular disease and chronic lung disease. Of the total cases, 64% of the patients had one of the four comorbidities (hypertension, diabetes mellitus, chronic lung disease or cardiovascular disease). The most prevalent comorbidity among the patients was hypertension with a pooled prevalence of 27% [95% CI: 19–35%], followed by diabetes mellitus [14%; 95% CI: 10–18%], cardiovascular diseases [11%; 95% CI: 7–15%] and chronic lung diseases [5%; 95% CI: 3–7%]. The heterogeneity test showed high heterogeneity among these studies [I2 > 50%] owing to inclusion of heterogenous population and varied definitions for severity, and thus a random effects model was used for the meta-analysis to compute pooled estimates. Pooled OR was calculated for each comorbidity as a measure of risk of development of critical or fatal COVID-19 disease. Funnel plots for assessing publication bias are depicted in Fig. 4.
Fig. 4.
Funnel plots depicting publication bias.
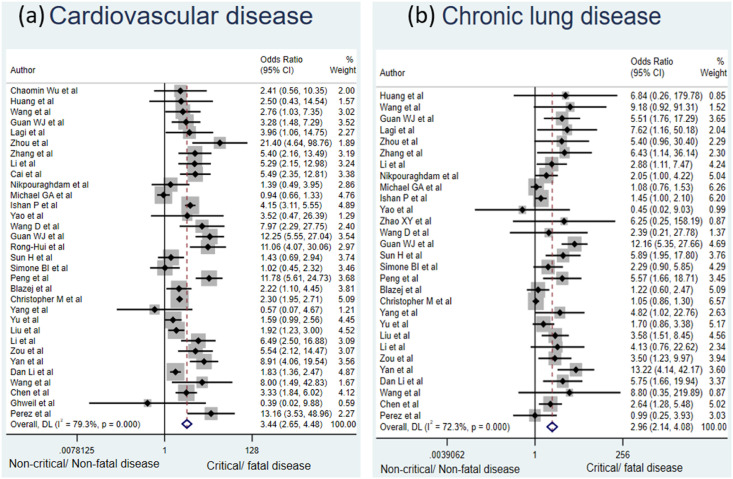
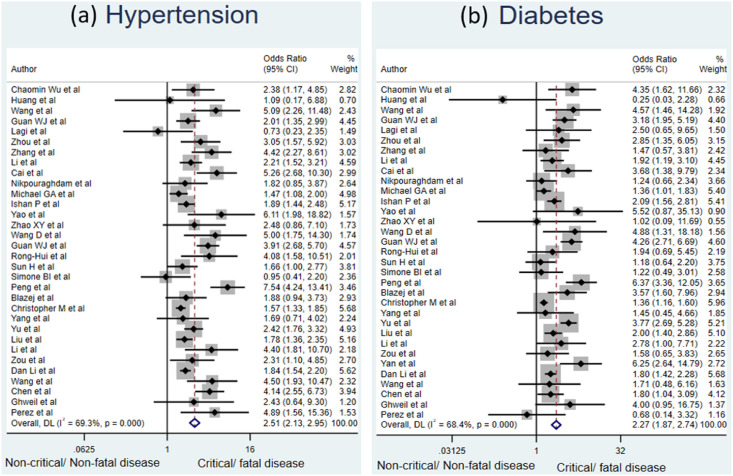
Patients with underlying cardiovascular disease had the maximum risk of development of critical or fatal COVID-19 disease compared to patients without this comorbidity with a pooled OR of 3.44 [95% CI: 2.65–4.48]. This was followed by chronic lung disease [OR: 2.96, 95% CI: 2.14–4.08], hypertension [OR: 2.51, 95% CI: 2.13–2.95] and diabetes mellitus [OR: 2.27, 95% CI: 1.87–2.74]. All results were statistically significant. The forest plots shown (Fig. 2 and Fig. 3 ) depict the same findings. The data pertaining to the various included studies is depicted in Table 1 . This emphasizes the fact that presence of all these comorbidities is associated with significantly higher risk of critical or fatal outcome amongst COVID-19 patients. Such patients are more likely to develop disease complications with progression to ARDS, requirement of ICU admission, mechanical ventilatory support and thus have a higher risk of fatality from COVID-19.
Fig. 2.
Forest plot depicting the relationship between (a) cardiovascular disease and (b) chronic lung disease with the risk of critical or fatal COVID-19 disease.
Fig. 3.
Forest plot depicting the relationship between (a) diabetes mellitus and (b) hypertension with the risk of critical or fatal COVID-19 disease.
Table 1.
Details of the studies included in the meta-analysis.
| Study ID | Author | Location | Year | Type of study | Sample size | Reference |
| 1 | Chaomin Wu et al. | Wuhan | 2020 | Retrospective Cohort | 201 | [18] |
| 2 | Huang et al. | Hubei | 2020 | Prospective Cohort | 41 | [19] |
| 3 | Wang et al. | Wuhan | 2020 | Retrospective Case Series | 138 | [20] |
| 4 | Guan WJ et al. | China | 2020 | Retrospective Cohort | 1099 | [21] |
| 5 | Lagi et al. | Italy | 2020 | Retrospective Cohort | 84 | [22] |
| 6 | Zhou et al. | Wuhan | 2020 | Retrospective Cohort | 191 | [23] |
| 7 | Zhang et al. | Wuhan | 2020 | Retrospective Case Series | 221 | [24] |
| 8 | Li et al. | Wuhan | 2020 | Ambispective Cohort | 548 | [25] |
| 9 | Cai et al. | Hubei | 2020 | Retrospective Cohort | 298 | [26] |
| 10 | Nikpouraghdam et al. | Iran | 2020 | Retrospective Cohort | 2964 | [27] |
| 11 | Michael GA et al. | New York | 2020 | Retrospective Case Series | 1000 | [28] |
| 12 | Ishan P et al. | New York | 2020 | Retrospective Cohort | 1078 | [29] |
| 13 | Yao et al. | China | 2020 | Retrospective Cohort | 108 | [30] |
| 14 | Zhao XY et al. | Jingzhou | 2020 | Retrospective Cohort | 91 | [31] |
| 15 | Wang D et al. | Wuhan | 2020 | Retrospective Cohort | 107 | [32] |
| 16 | Guan WJ et al. | China | 2019 | Retrospective Cohort | 1590 | [33] |
| 17 | Rong-Hui et al. | Wuhan | 2019 | Prospective Cohort | 179 | [34] |
| 18 | Sun H et al. | Wuhan | 2020 | Retrospective Case Control | 244 | [35] |
| 19 | Simone BI et al. | Denmark | 2020 | Retrospective Cohort | 175 | [36] |
| 20 | Peng et al. | China | 2020 | Retrospective Cohort | 703 | [37] |
| 21 | Blazej et al. | Poland | 2020 | Retrospective Cohort | 169 | [38] |
| 22 | Christopher M et al. | New York | 2020 | Prospective Cohort | 2729 | [39] |
| 23 | Yang et al. | China | 2020 | Retrospective Cohort | 200 | [40] |
| 24 | Yu et al. | Wuhan | 2020 | Retrospective Cohort | 1464 | [41] |
| 25 | Liu et al. | Wuhan | 2020 | Cohort | 1190 | [42] |
| 26 | Li et al. | Wuhan | 2019 | Retrospective Cohort | 245 | [43] |
| 27 | Zou et al. | Wuhan | 2020 | Retrospective Cohort | 121 | [44] |
| 28 | Yan et al. | China | 2020 | Retrospective Cohort | 256 | [45] |
| 29 | Dan Liu et al. | Wuhan | 2020 | Retrospective Cohort | 2044 | [46] |
| 30 | Wang et al. | China | 2020 | Retrospective | 175 | [47] |
| 31 | Chen et al. | Wuhan | 2020 | Cohort | 660 | [48] |
| 32 | Ghweil et al. | Egypt | 2020 | Retrospective Cohort | 66 | [49] |
| 33 | Perez et al. | Spain | 2020 | Retrospective Cohort | 96 | [50] |
4. Discussion
This study evaluated the risk of critical or fatal coronavirus disease amongst patients with underlying comorbidities. Various published studies describing the clinical outcome of patients infected with SARS-CoV-2 virus were screened on major online journal databases (PubMed and EMBASE). This was followed by data extraction and statistical analysis to evaluate the association between poor clinical outcome due to COVID-19 and presence of various comorbidities. Studies were included from across the globe and a fairly large sample size was analyzed. The four comorbidities chosen were hypertension, diabetes mellitus, cardiovascular disease and chronic lung disease since these were the most frequently encountered comorbidities in clinical practice.
The most prevalent comorbidity among the studied cohorts was hypertension with a pooled prevalence of 27% [95% CI: 19–35%]. Hypertension was associated with a significantly greater risk of critical or fatal outcome from COVID-19 with an OR of 2.51 [95% CI: 2.13–2.95]. Global estimates from 2010 suggest that as high as 31.1% of adults which translates into approximately 1.39 billion adults worldwide [8]. The findings from the present study becomes of paramount importance owing to the ongoing global pandemic and the staggering prevalence of hypertension. Hypertensive patients constitute a high-risk population for severe COVID-19 illness and should be prioritized for healthcare services in future waves of this pandemic for greater public health impact in reduction of mortality due to COVID-19.
Diabetes mellitus was the second most prevalent comorbidity in this study with a pooled prevalence across various included studies to be 14% [95% CI: 10–18%]. Diabetes mellitus and metabolic syndrome constitute a major upcoming non-communicable disease pandemic with approximately 462 million people diagnosed with type 2 diabetes mellitus as of data from 2017 [9]. The simultaneous COVID-19 pandemic has given rise to “interaction” between the two pandemics of diabetes and COVID-19. The present study reported that diabetic individuals had an increased risk of developing a critical disease or dying from COVID-19 infection compared to non-diabetic individuals. Guo et al. [10] reported that COVID-19 patients with diabetes mellitus as the sole comorbidity had a greater risk of severe lung involvement and uncontrolled systemic inflammation. In addition, levels of inflammation and coagulation-related biomarkers such as IL-6, ferritin, C-reactive protein and D-dimer were significantly greater in diabetic patients compared to non-diabetics. Diabetic patients were more likely to develop inflammatory cytokine storm which contributed to poorer clinical outcomes and resulting mortality. Diabetes mellitus is associated with immune dysregulation which leads to impairment of innate and adaptive immune responses culminating in hyper-inflammatory responses as witnessed by cytokine storm [[11], [12], [13]] [[11], [12], [13]] [[11], [12], [13]]. The reported OR for critical or fatal disease among diabetic patients with COVID-19 in this study was 2.27 which was statistically significant [95% CI: 1.87–2.74]. This highlights that individuals with diabetes mellitus also constitute an “at-risk” population, having more than two-fold higher risk of development of poor clinical outcomes from COVID-19.
Cardiovascular disease (excluding hypertension and cerebrovascular disease) as a comorbidity had a pooled prevalence of 11% [95% CI: 7–15%] in this meta-analysis and was identified as the strongest risk factor for COVID-19 severity and death from the same, with a 3.44-times greater risk of development of poor clinical outcomes [OR = 3.44; 95% CI: 1.96–2.77]. Cardiovascular diseases included in this review were coronary artery disease, chronic heart failure and congenital heart disease (cerebrovascular diseases were excluded for the purpose of analysis and hypertension was considered as a separate comorbidity). In addition to the high mortality rates among patients with cardiovascular diseases infected with SARS-CoV-2, COVID-19 can itself cause diverse cardiovascular disorders including arrhythmias, acute coronary syndrome, venous thromboembolism and direct myocardial injury [14]. These acute events can decompensate underlying heart disease and lead to worsening and progressive contractile dysfunction [15]. There is abundant expression of ACE2 (angiotensin converting enzyme) receptors in the heart and viral- ACE2 interaction has been proposed as the underlying mechanism for the ‘bidirectional interaction’ of SARS-CoV-2 with the cardiovascular system.
Chronic lung diseases included in this review were COPD, bronchial asthma and bronchiectasis. Presence of underlying chronic lung disease (COPD, bronchial asthma or bronchiectasis) in patients with COVID-19 was associated with a nearly three-fold greater likelihood of development of critical or fatal illness [OR: 2.96, 95% CI: 2.14–4.08]. The pooled prevalence of chronic lung diseases was 5% [95% CI: 3–7%]. Type-1 interferon mediated immune response constitute important innate response in viral respiratory illnesses by their direct antiviral effects, by inducing early apoptosis and also by producing phagocytosis of virus-infected respiratory epithelial cells resulting in enhanced viral clearance [16]. Many asthmatic patients have been found to have inadequate and delayed innate anti-viral immune responses to common viruses by the bronchial epithelium and alveolar macrophages, especially interferon-mediated immune responses [17]. These findings can be extrapolated to explain the increased risk of critical or fatal COVID-19 disease in this population.
Previously reported reviews pertaining to risk of critical or fatal disease were mainly confined to China which was the initial epicentre of the pandemic. The strength of this study was inclusion of larger number of studies from across the globe including Unites States of America, Egypt, Italy, Denmark, Poland, Spain, China and Iran. The results produced are more reflective of the global picture and accounts for regional differences, if any. However, we would like to acknowledge certain limitations of this study. We had to use a random effects model for analysis owing to presence of significant heterogeneity among the included studies. Heterogeneity in the studies resulted due to the sizeable variation in the sample size of various studies and also in the baseline characteristics of the participants. Second, we established a definition of ‘critical disease’ for purpose of deciding severity of the illness. However, the disease stratification would vary from study to study, largely dependent on local or regional classification criteria. The threshold for ICU admission would also differ among centres and nations and would produce ambiguity in categorisation as “critical” vs “non-critical illness. Third, presence of various confounding factors such as presence of more than one comorbidity in a single participant and their interaction with each other might affect the results. This study did not account for presence of multiple comorbidities in the same participant.
5. Conclusion
Various comorbidities like chronic cardiovascular disease, chronic lung disease, hypertension and diabetes mellitus are risk factors for poor clinical outcomes (development of severe disease or death) among patients infected with COVID-19. Hypertension was the most prevalent comorbidity and underlying cardiovascular disease was associated with the maximum risk. This information is useful to identify individuals at high risk of developing severe or fatal disease so that health resources can be directed effectively towards this subset. The findings of this study can be used to guide decision making in subsequent waves of this pandemic and also in future pandemics of this magnitude.
Author contributions
Conceptualization: Shubham Sahni, Gaurav Gupta, Sanjeev Sinha. Search strategy and data extraction: Shubham Sahni, Gaurav Gupta, Radhika Sarda. Formal Analysis: Shubham Sahni, Shivam Pandey, RM Pandey. Supervision: Sanjeev Sinha, RM Pandey. Writing: Shubham Sahni, Radhika Sarda. Review and editing: Sanjeev Sinha, Gaurav Gupta.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors report no conflict of interest relevant to the published work.
References
- 1.Anderson R.M., Fraser C., Ghani A.C., Donnelly C.A., Riley S., Ferguson N.M., et al. Epidemiology, transmission dynamics and control of SARS: the 2002-2003 epidemic. Philos Trans R Soc Lond B Biol Sci. 2004 Jul 29;359(1447):1091–1105. doi: 10.1098/rstb.2004.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Omari A., Rabaan A.A., Salih S., Al-Tawfiq J.A., Memish Z.A. MERS coronavirus outbreak: implications for emerging viral infections. Diagn Microbiol Infect Dis. 2019 Mar;93(3):265–285. doi: 10.1016/j.diagmicrobio.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhama K., Khan S., Tiwari R., Sircar S., Bhat S., Malik Y.S., et al. Coronavirus disease 2019-COVID-19. Clin Microbiol Rev. 2020 Sep 16;33(4) doi: 10.1128/CMR.00028-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.García L.F. Immune response, inflammation, and the clinical spectrum of COVID-19. Front Immunol. 2020;11:1441. doi: 10.3389/fimmu.2020.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 May;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.website cdc Centers for Disease Control and Prevention People who are at higher risk for severe illness. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html Accessed on.
- 7.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002 Jun 15;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 8.Mills K.T., Stefanescu A., He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020 Apr;16(4):223–237. doi: 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan M.A.B., Hashim M.J., King J.K., Govender R.D., Mustafa H., Al Kaabi J. Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020 Mar;10(1):107–111. doi: 10.2991/jegh.k.191028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C., et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 Mar 31:e3319. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodgson K., Morris J., Bridson T., Govan B., Rush C., Ketheesan N. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology. 2015 Feb;144(2):171–185. doi: 10.1111/imm.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guzmán-Flores J.M., López-Briones S. [Cells of innate and adaptive immunity in type 2 diabetes and obesity] Gac Med Mex. 2012 Aug;148(4):381–389. [PubMed] [Google Scholar]
- 13.Shu C.J., Benoist C., Mathis D. The immune system's involvement in obesity-driven type 2 diabetes. Semin Immunol. 2012 Dec;24(6):436–442. doi: 10.1016/j.smim.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020 Sep;17(9):543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fried J.A., Ramasubbu K., Bhatt R., Topkara V.K., Clerkin K.J., Horn E., et al. The variety of cardiovascular presentations of COVID-19. Circulation. 2020 Jun 9;141(23):1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wark P.A.B., Johnston S.L., Bucchieri F., Powell R., Puddicombe S., Laza-Stanca V., et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005 Mar 21;201(6):937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Contoli M., Message S.D., Laza-Stanca V., Edwards M.R., Wark P.A.B., Bartlett N.W., et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006 Sep;12(9):1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 18.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, China. JAMA Intern Med. 2020 Jul 1;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. J Am Med Assoc. 2020 Mar 17;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagi F., Piccica M., Graziani L., Vellere I., Botta A., Tilli M., et al. Early experience of an infectious and tropical diseases unit during the coronavirus disease (COVID-19) pandemic, Florence, Italy, February to March 2020. Euro Surveill. 2020 Apr;25(17) doi: 10.2807/1560-7917.ES.2020.25.17.2000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang G., Hu C., Luo L., Fang F., Chen Y., Li J., et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020 Jun;127:104364. doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020 Jul;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai Q., Huang D., Ou P., Yu H., Zhu Z., Xia Z., et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020 Jul;75(7):1742–1752. doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 27.Nikpouraghdam M., Jalali Farahani A., Alishiri G., Heydari S., Ebrahimnia M., Samadinia H., et al. Epidemiological characteristics of coronavirus disease 2019 (COVID-19) patients in Iran: a single center study. J Clin Virol. 2020 Jun;127:104378. doi: 10.1016/j.jcv.2020.104378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Argenziano M.G., Bruce S.L., Slater C.L., Tiao J.R., Baldwin M.R., Barr R.G., et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020 May 29;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paranjpe I., Russak A.J., De Freitas J.K., Lala A., Miotto R., Vaid A., et al. Infectious Diseases (except HIV/AIDS); 2020 Apr. Clinical characteristics of hospitalized covid-19 patients in New York city.http://medrxiv.org/lookup/doi/10.1101/2020.04.19.20062117 [cited 2021 Mar 15]. Available from. [Google Scholar]
- 30.Yao Q., Wang P., Wang X., Qie G., Meng M., Tong X., et al. A retrospective study of risk factors for severe acute respiratory syndrome coronavirus 2 infections in hospitalized adult patients. Pol Arch Intern Med. 2020 May 29;130(5):390–399. doi: 10.20452/pamw.15312. [DOI] [PubMed] [Google Scholar]
- 31.Zhao X.-Y., Xu X.-X., Yin H.-S., Hu Q.-M., Xiong T., Tang Y.-Y., et al. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infect Dis. 2020 Apr 29;20(1):311. doi: 10.1186/s12879-020-05010-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D., Yin Y., Hu C., Liu X., Zhang X., Zhou S., et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care. 2020 Apr 30;24(1):188. doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan W.-J., Liang W.-H., Zhao Y., Liang H.-R., Chen Z.-S., Li Y.-M., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020 May;55(5) doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du R.-H., Liang L.-R., Yang C.-Q., Wang W., Cao T.-Z., Li M., et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020 May;55(5) doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun H., Ning R., Tao Y., Yu C., Deng X., Zhao C., et al. Risk factors for mortality in 244 older adults with COVID-19 in wuhan, China: a retrospective study. J Am Geriatr Soc. 2020 Jun;68(6):E19–E23. doi: 10.1111/jgs.16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Israelsen S.B., Kristiansen K.T., Hindsberger B., Ulrik C.S., Andersen O., Jensen M., et al. Characteristics of patients with COVID-19 pneumonia at hvidovre hospital, march-april 2020. Dan Med J. 2020 May 15;67(6) [PubMed] [Google Scholar]
- 37.Xu P.P., Tian R.H., Luo S., Zu Z.Y., Fan B., Wang X.M., et al. Risk factors for adverse clinical outcomes with COVID-19 in China: a multicenter, retrospective, observational study. Theranostics. 2020;10(14):6372–6383. doi: 10.7150/thno.46833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nowak B., Szymański P., Pańkowski I., Szarowska A., Życińska K., Rogowski W., et al. Clinical characteristics and short-term outcomes of patients with coronavirus disease 2019: a retrospective single-center experience of a designated hospital in Poland. Pol Arch Intern Med. 2020 May 29;130(5):407–411. doi: 10.20452/pamw.15361. [DOI] [PubMed] [Google Scholar]
- 39.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O'Donnell L., Chernyak Y., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020 May 22;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L., Liu J., Zhang R., Li M., Li Z., Zhou X., et al. Epidemiological and clinical features of 200 hospitalized patients with corona virus disease 2019 outside Wuhan, China: a descriptive study. J Clin Virol. 2020 Aug;129:104475. doi: 10.1016/j.jcv.2020.104475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu C., Lei Q., Li W., Wang X., Liu W., Fan X., et al. Clinical characteristics, associated factors, and predicting COVID-19 mortality risk: a retrospective study in wuhan, China. Am J Prev Med. 2020 Aug;59(2):168–175. doi: 10.1016/j.amepre.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J., Zhang S., Wu Z., Shang Y., Dong X., Li G., et al. Clinical outcomes of COVID-19 in Wuhan, China: a large cohort study. Ann Intensive Care. 2020 Dec;10(1):99. doi: 10.1186/s13613-020-00706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li M., Cheng B., Zeng W., Chen S., Tu M., Wu M., et al. Analysis of the risk factors for mortality in adult COVID-19 patients in wuhan: a multicenter study. Front Med. 2020 Aug 25;7:545. doi: 10.3389/fmed.2020.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou L., Dai L., Zhang Y., Fu W., Gao Y., Zhang Z., et al. Front Med; 2020. Clinical characteristics and risk factors for disease severity and death in patients with coronavirus disease 2019 in wuhan, China.https://www.frontiersin.org/articles/10.3389/fmed.2020.00532/full [Internet] [cited 2021 Feb 17];7. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan X., Han X., Peng D., Fan Y., Fang Z., Long D., et al. Clinical characteristics and prognosis of 218 patients with COVID-19: a retrospective study based on clinical classification. Front Med. 2020 Aug 11;7:485. doi: 10.3389/fmed.2020.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu D., Cui P., Zeng S., Wang S., Feng X., Xu S., et al. Risk factors for developing into critical COVID-19 patients in Wuhan, China: a multicenter, retrospective, cohort study. EClinicalMedicine. 2020 Aug;25:100471. doi: 10.1016/j.eclinm.2020.100471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang G., Zhang Q., Wu C., Wu F., Yu B., Lv J., et al. Clinical characteristics of adult fevered COVID-19 patients and predictors for developing severe events. Front Med. 2020 Jul 3;7:324. doi: 10.3389/fmed.2020.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen F., Sun W., Sun S., Li Z., Wang Z., Yu L. Clinical and Translational Medicine; 2020 Jun. Clinical characteristics and risk factors for mortality among inpatients with COVID-19 in Wuhan, China.https://onlinelibrary.wiley.com/doi/abs/10.1002/ctm2.40 [Internet] [cited 2021 Mar 15];10(2). Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghweil A.A., Hassan M.H., Mohamed A.K., Mohamed A.O., Mohammed H.M., Abdelazez A.A., et al. Characteristics, outcomes and indicators of severity for COVID-19 among sample of esna quarantine hospital's patients, Egypt: a retrospective study. ID. 2020 Jul;13:2375–2383. doi: 10.2147/IDR.S263489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martos Pérez F., Luque del Pino J., Jiménez García N., Mora Ruiz E., Asencio Méndez C., García Jiménez J.M., et al. English Edition. Revista Clínica Española; 2020 Aug. Comorbidity and prognostic factors on admission in a COVID-19 cohort of a general hospital. S2254887420300928. [DOI] [PMC free article] [PubMed] [Google Scholar]