Abstract
During pregnancy, a series of physiological changes are determined at the molecular, cellular and macroscopic level that make the mother and fetus more susceptible to certain viral and bacterial infections, especially the infections in this and the companion review. Particular situations increase susceptibility to infection in neonates. The enhanced susceptibility to certain infections increases the risk of developing particular diseases that can progress to become morbidly severe. For example, during the current pandemic caused by the SARS-CoV-2 virus, epidemiological studies have established that pregnant women with COVID-19 disease are more likely to be hospitalized. However, the risk for intensive care unit admission and mechanical ventilation is not increased compared with nonpregnant women. Although much remains unknown with this particular infection, the elevated risk of progression during pregnancy towards more severe manifestations of COVID-19 disease is not associated with an increased risk of death. In addition, the epidemiological data available in neonates suggest that their risk of acquiring COVID-19 is low compared with infants (<12 months of age). However, they might be at higher risk for progression to severe COVID-19 disease compared with older children. The data on clinical presentation and disease severity among neonates are limited and based on case reports and small case series. It is well documented the importance of the Zika virus infection as the main cause of several congenital anomalies and birth defects such as microcephaly, and also adverse pregnancy outcomes. Mycoplasma infections also increase adverse pregnancy outcomes. This review will focus on the molecular, pathophysiological and biophysical characteristics of the mother/placental-fetal/neonatal interactions and the possible mechanisms of these pathogens (SARS-CoV-2, ZIKV, and Mycoplasmas) for promoting disease at this level.
Abbreviations: ACE2, angiotensin-converting enzyme 2 receptors; AF, amniotic fluid; Ang 1–7, angiotensin 1–7; APTT, activated partial thromboplastin time; ARDS, acute respiratory distress syndrome; AT1R, angiotensine type 1 receptor; B/L7, cathepsin B/L7; BV, bacterial vaginitis; CDC, Centers for Disease Control and Prevention, USA; CMV, cytomegalovirus; COVID-19, coronavirus disease 2019; CoVs, coronaviruses; CS, cytokine storm; CSF, cerebrospinal fluid; DIC, disseminated intravascular coagulation; FRC, functional residual capacity; GFR, glomerular filtration rate; HIV, human immunodeficiency virus; HSV, Herpes simplex virus; ILs, interleukins; MAS, Macrophage Activation Syndrome; MasR, Mas Receptor (for angiotensin 1–7); MIPL, medically induced premature labor; NK, Natural Killer; NGS, next-generation sequencing; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; PRMBL, premature rupture of membranes before labor; RAS, renin–angiotensin–aldosterone system; S, spike protein; SPL, spontaneous premature labor; SVR, systemic vascular resistance; TMPRSS2, Type II transmembrane serine protease; TORCH, toxoplasmosis, rubella, cytomegalovirus and Herpes virus-like infections; TNF-α, tumor necrosis factor-α; WHO, World Health Organization; ZIKV, Zika virus
Keywords: Viruses, Bacteria, Mycoplasma, Pregnancy, Neonates, Maternal-fetal interphase
1. Physiological changes during pregnancy
To better understand the pathophysiological and molecular considerations of viral and bacterial infections during pregnancy, a general background of the physiological changes during pregnancy is needed. In general, the increased physiological requirements during pregnancy due to the stress of carrying growing offspring in their bodies until delivery may explain the higher incidence of preterm delivery and stillbirths when additional stress is created by certain infectious diseases. This is also compounded by additional pre-existing clinical conditions, such as obesity, metabolic and cardiovascular diseases, and other conditions that can affect pregnant women during and after labor [1,2]. Pregnancy is associated with early vasodilation of the systemic vasculature, and especially the maternal kidneys, and systemic changes in particular, such as changes in hormone levels [3]. These necessities changes allow a pregnant body to adapt to meet the increased circulatory and metabolic demands of the mother and fetus and to ensure adequate uteroplacental circulation for fetal growth and development. These and other factors are relevant to understanding the effects of emerging infectious diseases in pregnancy.
The systemic vasodilation associated with pregnancy occurs as early as 5 weeks after conception and then proceeds until full placentation and the complete development of the uteroplacental circulation [4]. This vasodilation is mediated by endothelium-dependent factors, including nitric oxide synthesis that is upregulated by estradiol, progesterone, and possibly vasodilatory prostaglandins (PGI2) and relaxin [5,6]. Both hormone levels increase substantially and consistently during pregnancy [7,8]. Relaxin is a peptide produced by the corpus luteum during pregnancy, detectable in the luteal phase of the ovulatory cycle [9]. All these changes result in a 25–30% fall in systemic vascular resistance (SVR). To compensate for this the cardiac output increases by approximately 40% during pregnancy [8,10]. The fall in SVR likely triggers the renin-angiotensin–aldosterone system (RAS) to retain sodium and increase plasma volume. Because of the changes in SVR, adaptive responses by the heart also occur during pregnancy to maintain adequate pressure and flow rates. This is achieved mostly by an increase in the heart stroke volume and secondarily by an increase in heart rate. The maximum cardiac output is found at about 20–28 weeks gestation [3]. This higher demand in cardiac function necessitates healthy hearts in pregnant women. Also, during labor, there is a further increase in cardiac output (15% in the first stage and 50% in the second stage). The sympathetic response to pain and anxiety further elevates the heart rate and blood pressure. This is relevant in some adverse outcomes of blood pressure during pregnancy (i.e. preeclampsia, gestational and chronic hypertension, and a mixture of these). Importantly, infectious diseases can affect the cardiovascular system and its functional reserve, which changes during pregnancy, and can have an important impact on pregnant women.
During pregnancy, there are functional changes in the maternal respiratory system. Oxygen demand increases significantly resulting in a 40–50% increase in minute ventilation, mostly due to an increase in tidal volume, rather than in the respiratory rate. This maternal hyperventilation causes arterial pO2 to increase and arterial pCO2 to fall. As a consequence, a mild alkalosis occurs during pregnancy [11]. The diaphragmatic elevation by the gravid uterus and the gestational weight gain are responsible for altered pulmonary volumes leading to a reduced total lung capacity and to an inability to clear effectively pulmonary secretions [[11], [12], [13]]. The expiratory reserve volume and functional residual capacity (FRC) decrease, while vital capacity remains unchanged. This is compensated for by hyperventilation and a tidal volume increment by progesterone-dependent stimulation of the respiratory center [13,14].
Since hyperventilation leads to more air uptake over time, pregnant women are more likely to acquire emerging infectious diseases carried by airborne droplets, aerosols, and other sources of infections [15]. In addition, there are progesterone-mediated changes in the nasal mucosa. As a result, microorganisms are difficult to eliminate because of the inability to clear pulmonary secretions efficiently and the adhesion properties of microorganisms, especially in the upper respiratory tract [[11], [12], [13],15].
Maternal immunological changes during pregnancy are characterized by a strong first-line-of-defense response against viral and bacterial pathogens mediated by effective activations of Natural Killer (NK) cells and monocytes [[15], [16], [17]]. However, once the first host response barrier is overcome, the second line of defense is also partially defective due to the attenuation of cell-mediated immunity responses by helper lymphocytes type 1 (known as the Th1 response) [[15], [16], [17], [18]]. In addition, the Th2 response mediated by helper lymphocytes type 2, which potentiate humoral responses by Th2 cells, is enhanced. The imbalance between these two Th responses contributes to an overall increased infectious disease burden and eventually increased morbidity from intracellular pathogens, like the emerging infectious diseases considered in this special edition [15,[17], [18], [19], [20]]. As the Th1 host immune response declines, the chances of developing “cytokine storms” (CS) are also diminished or dysregulated in comparison with non-pregnant women [16,17,20,21]. In parallel with this, during pregnancy the microbicidal, pro-inflammatory Th1 cytokines, such as interferon-ɣ and interleukins (IL), IL 1α, 1β, 12 and 6), are reduced or diminished in their activities, and the anti-inflammatory Th2 cytokines, such as IL-4, IL-10, IL-13 and transforming growth factor-beta (TGF-β), are increased in their activities [[15], [16], [17]]. There are relative variations in these responses during the different trimesters of gestation in pregnancy [16].
During normal pregnancy, the levels of blood fibrinogen and D-dimers increase, whereas the activated partial thromboplastin time (APTT) and prothrombin time decrease due to increases in the majority of the coagulation factors in plasma. Because of this, pregnancy is a condition with a higher risk of thrombotic incidents [22,23].
Renal diseases are frequently found in pregnant women partially because of the hormonal and hemodynamic changes that occur during pregnancy that can impact renal function, leading to less functional reserve [24,25]. The kidneys increase in length and volume, and physiologic hydronephrosis occurs in up to 80% of women during pregnancy [24,25]. Hormonal changes during pregnancy allow for increased blood flow to the kidneys and altered renal autoregulation, such that glomerular filtration rate (GFR) increases significantly through reductions in net glomerular oncotic pressure and increased renal size. As a result, the GFR increases by approximately 50% with subsequent decreases in serum creatinine, urea, and uric acid values. The antidiuretic hormone secretion is generally depressed, resulting in lower osmolality and serum sodium levels, whereas the rise in progesterone levels protects the pregnant woman from hypokalemia [24,25].
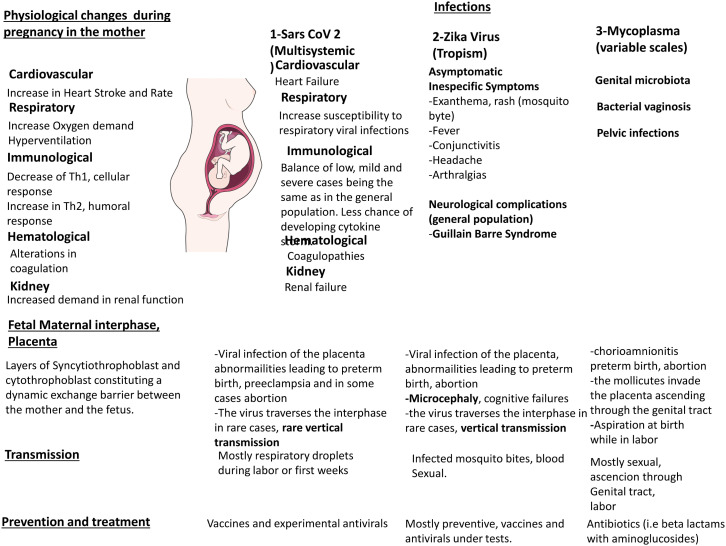
The various physiological changes seen during pregnancy are summarized in Fig. 1 , left panel. In Fig. 1, right panel, we have summarized some of the important properties of the diseases relevant for this review.
Fig. 1.
Overview of the physiological changes during pregnancy in the mother, the placenta and the fetus and implications in SARS-CoV-2, ZIKV and Mycoplasma infections. (A) Scheme showing the physiological changes seen during pregnancy in the mother, the placenta and the fetus and the main mechanisms of transmission, prevention and treatment of the diseases promoted by the infectious pathogens discussed in this contribution. The left panel shows the general issues, whereas the right panel, presents some of the different diseases discussed in this review.
2. The placenta and the possibilities of vertical transmission in emerging diseases like Sars-CoV-2 virus, Zika virus, and Mycoplasma
2.1. The human fetal-maternal interface
The placenta forms the primary barrier between the maternal and fetal compartments throughout pregnancy, mediating the exchange of gases, nutrients, and waste products between these compartments. This transitory organ is formed by the interaction between embryo trophoblastic cells and maternal uterine endometrium. In humans, the primitive trophoblast layer differentiates into various tissues: (a) syncytiotrophoblast, which is an invasive cell layer that allows the blastocyst to erode the endometrium to reach direct contact with the maternal blood supply; (b) cytotrophoblast, which contributes new cells that fuse with the syncytiotrophoblast, allowing their continuous expansion; and (c) invasive extravillous cytotrophoblast, formed by cytotrophoblast cells extending beyond the syncytiotrophoblast and making contact with the endometrium, instigating anchoring villi, and invading the endometrial stroma. The syncytiotrophoblast delineates intercommunicating lacunae filled with maternal blood. The trophoblastic masses between the lacunae are the basis for the further development of the villous trees of the placenta. The core of the placental villi will be gradually filled with extraembryonic mesoderm that will become vascularized with embryonic blood vessels [26].
Syncytiotrophoblast is responsible for the exchange of nutrient molecules as well as placental hormone production, and they provide a defense barrier against pathogenic microorganisms. Its functionality can be diminished by diseases and adverse conditions, such as hypertension and pre-eclampsia [27,28]. Early in pregnancy, the cytotrophoblast forms into a continuous layer beneath the syncytiotrophoblast, and as pregnancy advances, the cytotrophoblastic layer becomes incomplete. The stromal core of the chorionic villi contains fetal blood vessels immediately surrounded by endothelial cells and then by an extracellular matrix containing Hofbauer and mesenchymal cells. Hofbauer cells are a specialized type of macrophage that has been described as anti-inflammatory type-M2 cells that promote maternal tolerance towards the fetus. They have also been implicated in tissue remodeling and control placental angiogenesis and villous tree growth and branching. As immune cells, they have been implied in vertical transmission of several pathogens, since they do not appear to be efficient at controlling viral infections [29]. In the development of the early placenta, fetal vessels are located away from the trophoblast, but as pregnancy progresses they become located in close contact with the trophoblast.
In the third trimester, fetal capillaries within the villi are separated from maternal blood by a very thin layer of syncytiotrophoblast, seen as a vascular-syncytial membrane [30]. Between the syncytiotrophoblast and endothelial cells, the extracellular matrix is reduced to allow the fusion of both basal laminae, which act as filters [31]. All of these changes (relocation of blood vessels, thinning of syncytiotrophoblastic layer, and cytotrophoblast discontinuity) are indicators of placental maturation [26].
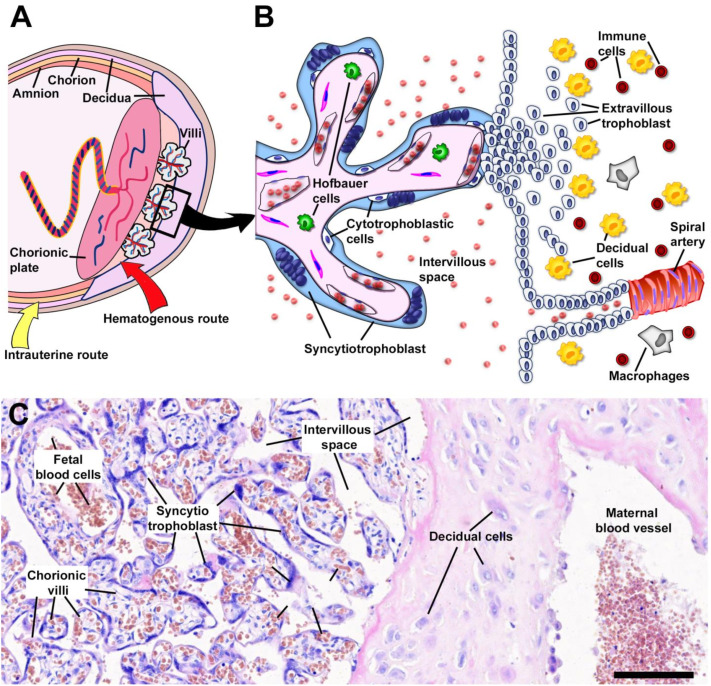
Pre-decidual endometrial changes begin during the menstrual cycle, even without embryo implantation [32]. The endometrial stromal cells further react to trophoblast invasion by increasing decidualization. The decidual stroma is characterized by its edematous structure and strong infiltration of immune cells, such as uterine NK cells, macrophages, and T cells, which play an important role in the placentation process and the remodeling of the spiral arteries [32]. The structural human maternal-placental interphase is shown in Fig. 2 .
Fig. 2.
The intrauterine environment during human pregnancy. (A) Schematic representation of the structure of the human placenta and chorioamniotic membranes. Location of placental structures as chorionic villi and decidua components are schematized. The potential routes of infection are represented. Ascendant microbial colonization is shown (yellow arrow) while the hematogenous via is indicated with a red arrow. (B) Schematic drawing of the region indicated in the square showing the placental villi. The two types of villi are represented in the scheme: two placental floating villi that freely end in the intervillous space and an anchoring villi that attaches to the endometrial wall. The main cell types involved in fetal-mother interaction are indicated. (C) Histological structure of mature normal placenta showing the placental villi and the modifications of the endometrium with the presence of decidual cells. Scale bar: 200 μm.
2.2. Placental transmission of viral and mycoplasmal infections
Several mechanisms may be used by pathogens for maternal-fetal transmission, including hematogenous spread, trophoblastic transcellular or paracellular pathways, or transport within immune cells. Although the syncytiotrophoblast constitutes the main barrier and fetal protection against microorganisms. Some viruses and mycoplasmas may cross this barrier and other sectors of the placenta, such as decidua, extravillous trophoblast. In addition, fetal membranes might be also involved in pathogen transference. Also, infectivity can vary throughout gestation, and it is probably associated with changes related to placental maturation [33,34]. Two main routes are used to infect the placenta: the ascending intrauterine route, which is the predominant one in bacterial infections, and the hematogenous route. Microorganisms invading the maternal-fetal interface via the hematogenous route are less common than those implicated in bacterial ascending infections [[33], [34], [35]]. Microorganisms that invade the placenta by the hematogenous route include Listeria monocytogenes [36], Zika virus [37,38], Treponema pallidum [35], Cytomegalovirus [39], Plasmodium species [40], and microorganisms causing toxoplasmosis, syphilis, varicella-zoster, including parvovirus B19, Rubella, and Herpes infections (TORCH infections) [41]. These microorganisms gain access through the maternal circulation to the intervillous space, from there they invade the villi and eventually the fetal circulation. In contrast to the ascending infections that cause inflammation primarily in the choriodecidua and amnion, microorganisms invading via the hematogenous route cause inflammation primarily in the placental villi and intervillous space [42].
Pathogenic microorganisms have developed distinct strategies to invade and colonize maternal and fetal tissues. This can vary throughout gestation or with the level of maternal infection or corresponding host immune responses. For example, cytomegalovirus replication takes place in cytotrophoblast cells where the virus arrive using a transcytosis mechanism involving the neonatal Fc receptor (FcRn), which also transports IgG immunoglobulins throughout the syncytiotrophoblast during the second half of pregnancy [39]. The extravillous trophoblast is the main target for initial infections by Toxoplasma gondii, Listeria monocytogenes, and Zika virus. Most of these pathogens also infect maternal leucocytes in the decidua, which might facilitate transfer to extravillous trophoblasts [35]. Since Hofbauer cells are localized in villi, they have been implicated in the spread of some infections, such as Zika or HIV-1 [43]. Less information is available about mycoplasma vertical transmission, although congenital infections in newborns are associated with the presence of mycoplasma in placental tissues [44,45]. As of this date, precise data on the vertical transmission of the SARS-CoV-2 virus in pregnant women has not yet become available. In mothers with mild or moderate COVID-19 disease, the newborn's delivery is usually at term; however, in those patients with severe COVID-19 disease, it is common to find preterm births, either spontaneous births or because of medical indications [46,47,56,[48], [49], [50], [51], [52], [53], [54], [55]]. Though they are not commonly observed, spontaneous abortions and fetal demise have been reported [49,54,57]. Despite this, there is no direct evidence of SARS-CoV-2 placental infection in pregnant women with COVID-19 disease, although there are several recent reports on abnormalities in the morphologies of the placenta in these women [58,59]. The abnormalities include perivillous fibrin, fetal and maternal vascular malperfusion, and infarctions in several places [60,61]. These pathologies in the placenta of pregnant women with COVID-19 disease have been found despite the rare detection of viral RNA or proteins of the SARS-CoV-2 virus [[60], [61], [62]]. It is also important that this has been observed in women with mild to moderate COVID-19, usually with comorbidities like hypertension, eclampsia, or gestational diabetes [59].
Viremia caused by the SARS-CoV-2 virus is generally low and found only in 1% of symptomatic COVID-19 cases [63], which may play a role in the low incidence of vertical transmission detected in pregnant women. This virus has been detected in the amniotic fluid, the umbilical cord blood, and in the placentas of infected women [51,64,65]. However, recent literature provides some evidence that vertical transmission of SARS-CoV-2 is possible, although this appears to occur only rarely [66,67]. In general, the passage of this coronavirus through the placenta increases with increasing gestational age. However, the severity of fetal injuries decreases, once embryo/fetal development proceeds towards the end of pregnancy. Some of the damage may be driven by abnormal immune responses [59]. Studies suggest that maternal COVID-19 disease in the late second or third trimester is associated with more severe disease morbidity, and this requires increased surveillance than is necessary for early pregnancy [68,69]. Recently, a mother infected in the last trimester with SARS-CoV-2 delivered an infected neonate. In this particular case, the placenta showed signs of acute and chronic intervillous inflammation and was positive for the SARS-CoV-2 virus. Both maternal and neonatal blood samples were also positive for this virus, indicating for the first time that transmission may have occurred through the placenta [70]. Also, direct evidence of SARS-VoV-2 viral invasion in the placental tissue exists, as virions were observed invading into the syncytiotrophoblast in the placental villi [71]. However, there is a pressing need for more research in this area, as the data remain inconclusive.
2.3. Understanding the molecular basis of virus placental infections
A key factor in preventing the deleterious effects of COVID-19 disease during pregnancy is to understand the mechanisms of vertical transmission of the emergent SARS-CoV-2 virus. Specifically, if the machinery that permits the entrance and replication of the virus is present in the placenta and chorion amnion, we can speculate that the virus uses the same mechanisms of host invasion as in other tissues, despite viremia being low [63]. However, the syncytiotrophoblast can modulate the immune response to a viral infection, since it is a part of the maternal-interface barriers [39,72]. Placental cells can initiate local and paracrine immune responses through membrane vesicles containing microRNAs that confer resistance to viral infections [72,73].
Research on SARS COV-2 host epithelial cell entry has been focused on ACE2, known to be the receptor of SARS-CoV-2. The expression pattern of ACE2 across more than 150 different cell types corresponding to all major human tissues and organs has shown expression of ACE2 in the placenta [74]. However, cell entry and the spread of SARS-CoV-2 viruses are widely thought to depend on the ACE2 receptor [75] and also the serine protease TMPRSS2 [76], with likely participation of B/L7 [77] and furin protease [78]. Using single-cell sequencing, Pique-Regi et al. (2020) found that very few cells co-express ACE2 and TMPRSS2 in the placenta, concluding that this is not a likely pathway for vertical transmission of the SARS-CoV-2 virus [79]. ACE2 has been localized by immunocytochemistry in cytotrophoblasts, syncytiotrophoblast, endothelium, and vascular smooth muscle [80]. It is worth mentioning that ACE2 plays a central role in placentation, the migration of trophoblasts, vasodilation in the mother, and vascular remodeling [81]. Because of this, the presence of ACE2 receptors seems to be related to adverse outcomes during viral involvement in pregnancy, such as miscarriages, preeclampsia, and ectopic pregnancy occurring during viral infections [80]. In contrast with ACE2 receptors, the expression of TMPRSS2 has not been consistently observed in pregnancy-associated complications [77,82]. The receptor for ZIKV in host cells has not yet been identified, although laboratory studies suggest that the receptors could be AXL or TIM1 receptors [83,84]. AXL is a cell surface receptor tyrosine kinase, part of the TAM family of kinases (abbreviation for Tyro-3, AXL, and Mer Tyrosine-kinase receptors) [84,85]. It is involved in the stimulation of cell proliferation, aggregation, and survival through the activation of several downstream pathways (PI3K-AKT-mTOR, MEKERK, NF-κB, and JAK/STAT) [85]. ALX is important in processes related to cell growth, differentiation, and proliferation, and it is also involved in oncogenesis and cell development. It is additionally an inhibitor of host immune responses [86], and it is expressed in many cells in which ZIKV shows positive tropism. The results on ZIKV receptors with human cells, however, have been controversial [83]. The TIM1 receptors (T cell immunoglobulin mucin domain-1 receptors) are glycoproteins that can interact with phosphatidylserine, either derived from apoptotic cells (contributing to the dead cells' clearance) or viral membranes [87]. These receptors have been found on most of the cells for which ZIKV has shown positive tropism [37]. Other proteases that could contribute to viral entry have not been thoroughly investigated. Epidemiological and experimental studies have made it clear that the ZIKV is attracted to different tissue and cell types, especially the special stem neural cell types (neurotropism). In these cells, ZIKV exhibits high levels of viral replication, apoptosis, and alterations of the cell cycle. This might explain the sequelae observed in newborns exposed to the virus while being in an intrauterine environment [88,89]. As mentioned above, ZIKV expresses neurotropism for neural stem cells, promoting direct damage and producing sequelae and deformations as a result of this damage [89]. In mimicking this type of neurotropism in vitro it has been found that the infection of neural cells by ZIKV replicating at high efficiency in tissue cultures of human cell lines induces apoptosis and damage of neural tissue through the activation of caspase-3 [90,91].
The relationship of ZIKV with microcephaly is statistically significant for the entire affected population infected with ZIKV. In addition, because of the detection of the ZIKV RNA in amniotic fluid, placenta, and fetal brains, this virus has also been closely associated with microcephaly [[92], [93], [94]]. ZIKV invades the villi without infecting the syncytiotrophoblast and thus the virus does not cross the placental barrier [37]. This is enough for immune evasion of ZIKV [95], resulting in a key role of the infection of the maternal residual tissues to explain the pathogenesis of ZIKV infection in the maternal-fetal interface, with its consequences in the embryo and fetus [96]. ZIKV replicates in proliferating explants from first-trimester human placentas, being these cells possible locations for the propagation of the infection [97]. During this stage, it has been established that ZIKV has a wide cell and tissue tropism in the maternal-fetal interface [98]. Positive tissue tropism and replication of the virus in placental cells during pregnancy has been demonstrated in vivo and in vitro [37]. ZIKV does not only infect placental cells, but it can also infect placental macrophages [99]. This can lead to congenital anomalies alongside placental insufficiency and delayed fetal growth. Fetal death is also associated with ZIKV infection during pregnancy [100,101].
During maternal infection by ZIKV, the ZIKV released into blood can easily reach the placenta and be transmitted to the fetus [102]. However, the degree of fetal infection by the virus is determined by the time when the infection took place during pregnancy. If acquired during the first trimester of pregnancy, the more frequent appearance of the encephalic sequels and the greater degree of microcephaly is usually observed [103]. It is estimated that the probability of vertical ZIKV transmission is about 20 to 30%, but symptoms do not always develop immediately in newborns after infection [104]. It is believed that other neurocognitive problems, not well understood and recognized, are also due to the ZIKV and can take place after intrauterine exposure. Some complications, such as the size of the skull circumference during birth have been linked to neurocognitive development and some neurological and anatomical anomalies in children that do not exhibit microcephaly have been reported after intrauterine exposure to ZIKV [105].
Additional research is needed to evaluate and understand the molecular basis of virus placental infections.
3. SARS-CoV-2, Zika, and Mycoplasma presentation during pregnancy and its pathophysiological and molecular correlations
3.1. Pathogenesis and clinical forms of presentation of SARS-CoV-2 during pregnancy
Based on available information the USA Centers for Disease Control and Prevention (CDC) has declared that pregnant women appear to have a similar risk of presentation of COVID-19 disease as the non-pregnant adult population (85%, 10%, and 5%, respectively) [106,107]. In addition, the most common initial symptoms of COVID-19 in pregnant women are quite similar to those reported for non-pregnant patients, such as fever, cough, shortness of breath, and diarrhea [59]. However, it is worth mentioning that pregnant women with comorbidities are at increased risk of developing severe COVID-19 illness requiring mechanical ventilation [52]. Although various reports indicate that pregnancy does not intensify the severity of maternal disease caused by SARS-CoV-2, increased case numbers of preeclampsia and preterm birth have been reported in women with COVID-19 disease [58].
Pregnant women are substantially more susceptible to viral respiratory illnesses that are associated with higher morbidity and mortality rates [108]. During pregnancy, the physiological ventilatory changes make pregnant women more susceptible to infection by lightweight airborne viral particles. The decreased FRC and consequent hyperventilation increase the chance of inhaling airborne pathogens like the SARS-CoV-2 virus. In addition, the adhesion of the virus particles to cells is benefited by the high levels of progesterone found during pregnancy, which hinders viral clearance [109]. Because of these reasons, pregnancy is usually considered a condition of high susceptibility to viral infections, especially to those directly affecting the respiratory system, such as the SARS-CoV-2 virus. This is especially apparent during the third trimester. Certain symptoms, such as shortness of breath, can be normally reported in some pregnant women without COVID-19 disease, and it should be differentiated from the changes mentioned above in Section 1 of this review. The higher respiratory demands of pregnant women may also promote the need for mechanical ventilation during the first hours of severe presentations of COVID-19 disease with severe acute respiratory distress syndrome [48,[110], [111], [112], [113]], but this rarely results in death [114,115].
Recent investigations have suggested the presence of co-infections in patients with SARS and MERS infections, in particular co-pathogens like Streptococcus pneumoniae, Staphylococcus aureus, Mycoplasma pneumoniae, Chlamydia pneumonia, among other possibilities [116]. These co-pathogens, along with possible activation of latent bacterial infections, could be a strong determinant of a fatal disease course [117]. For example, Mycoplasma pneumonia has been found in COVID-19 disease, and the types and severity of signs and symptoms in these patients could be due, in part, to this co-pathogen and/or other bacterial and viral co-infections [117]. Thus, the co-infections found in patients with COVID-19 disease might be extremely important in determining the prognosis of the disease due to the promotion of a pro-inflammatory state and other conditions important in influencing survival.
3.1.1. The importance of ACE 2 receptors in pregnancy and SARS-CoV-2 pathogenesis
An important unifying concept during pregnancy is that at the molecular level there is an increased expression of the angiotensin-converting enzyme receptor type 2 (ACE2), and this is routinely seen in pregnant mammalian kidneys. ACE2 plays a central role in the regulation of blood pressure [118,119]. As a membrane-bound aminopeptidase enzyme, ACE2's main function is to hydrolyze angiotensin I into angiotensin 1–9, as well as to transform angiotensin II into angiotensin 1–7 (MasR) [118,119]. This type of enzyme reaction has physiological relevance, especially when taking into account the changes in vasoactive substances starting from a vasoconstrictor agent like angiotensin II. The role of angiotensin II is to raise blood pressure upon binding with the Angiotensin type I receptor (AT1R) and also to be converted into vasodilator agents such as angiotensin 1–7. This latter conversion will contribute to lowering blood pressure through MasR agonism and AT1R antagonism, leading to the reduction of vascular resistance, and therefore a decrease in blood pressure [118,119]. We will discuss below the ramifications of this finding for women who are pregnant with certain viral infections like infections caused by SARS-CoV-2 virus.
Regarding SARS-CoV-2 infectivity and its effects before and during pregnancy, it is important to consider the distribution of ACE2 receptors in women and how pregnancy affects this [120]. The ACE2 receptor is an important component of the RAS axis. It is a zinc metalloprotease, expressed in several tissues and organs, and it is also the molecular receptor for the SARS-CoV-2 virus that binds to this protein through the S protein on its surface during the initial steps of cell infection [119]. The ubiquity and variability of expression of this receptor in several tissues of the female reproductive tract is intriguing, in particular, with regards to how it could explain SARS-CoV-2 pathogenesis and its implications in human fertility, the short- and long-term viability of gametes, and its possible adverse effects on pregnancy [119,121]. The ACE2 receptor is expressed in the vagina, uterus, fallopian tube, and ovary (specifically in ovarian gametes) [[119], [120], [121], [122], [123]]. In the ovary, ACE2 seems to have an important role in maintaining the balance between angiotensin II and angiotensin 1–7. This balance regulates the secretions of hormones like estradiol and progesterone in favor of the oocyte maturation cycle as well as the generation and progression of the corpus luteum. The expression of ACE2 seems to increase as the oocyte maturation cycle progresses [124]. Thus, it is important to consider female fertility and the possible effects of SARS-CoV-2 in women infected before their pregnancy.
The abundance of ACE2 receptors that fluctuates during the menstrual cycle might make ovary cells more susceptible to SARS-CoV-2 viral infection and also susceptible to the effects of ACE2 blockade. This could have important implications in the ovulation process, and consequently in female fertility [119]. In guinea pigs, which seem to be similar to humans with regards to ACE2 and angiotensin 1–7 (Ang 1–7) expression, ACE2 expression played a critical role in the decidualization process, trophoblastic invasion, and also in regulating placental blood flow [81]. Based on previous reports researchers have maintained that human fertility might be compromised by SARS-CoV-2 infections, especially in males, though the impact of SARS-CoV-2 in human fertility is still controversial and further studies are needed [[124], [125], [126], [127], [128], [129], [130], [131], [132], [133]]. It has been recommended that non-pregnant, fertile women that have recently acquired SARS-CoV-2 infections have a close follow-up consultation until more evidence is available [119]. Table 1 summarizes the locations where ACE2 expression could affect human fertility and pregnancy in females as well as its possible function. Importantly, there is also an incremental change in the expression of ACE2 receptors during pregnancy [134]. Given its binding to the ACE2 receptors, the SARS-CoV-2 virus can cause a down-regulation of the RAS axis. This is a possible trigger of cardiovascular pathologies that could plausibly affect not only a pregnant women's health but also the health of her fetus [135]. According to experimental evidence, ACE2 knockout mice have low levels of Ang 1–7 that have been associated with preeclampsia and intrauterine growing restriction as well as high levels of Ang II, a vasoconstrictor hormone that at high concentrations contributes to low blood flux and a subsequent restriction in fetal nutrition [119]. The main organs and tissues where ACE2 expression increases during pregnancy are shown in Table 1 (left column). Also shown is the role of ACE2 receptors located in those organs and tissues during pregnancy (right column). Since the ACE2 receptor is the plasma membrane binding site for the SARS-CoV-2 Spike protein and is necessary to allow virus entry into host cells, all the organs and tissues with increased expression of ACE2 receptors are potential sites of infection of SARS-CoV-2 in pregnant women.
Table 1.
ACE2 expression during pregnancy and its normal function.
| ACE2 expression | Function |
|---|---|
| Ovary | Hormonal secretion influence and further regulation of oocyte maturation and luteus corpum. |
| Endometrium | Regulation of embryonic implantation and decidua's formation. |
| Decidua | Decidualization. Trophoblastic invasion. |
| Placenta | Decrease in vascular resistance. |
| Kidney | Blood pressure regulation. |
In the lungs, SARS-CoV-2 virus infects host respiratory epithelial cells through the ACE2 receptor, expressed primarily in type II alveolar cells, although ACE2 is also expressed in many extrapulmonary sites [108]. The mechanism of entry of the SARS-CoV-2 virus into a host cell is initiated by binding of the S proteins to ACE2 receptors on the cell surface, as it is with the SARS-CoV-1 virus. However, the affinity between the SARS-CoV-2 S protein and ACE2 is much higher than that with the SARS-CoV-1 S protein [109]. Despite the relatively low general case fatality rate (CFR) estimated at 2.3% in SARS-CoV-1 and MERS, SARS-CoV-2 is much more contagious, and this could explain, in part, the increased deaths in absolute numbers in COVID-19 disease [136]. Concerning the susceptibility of pregnant women to COVID-19 disease, they may be more vulnerable to SARS-CoV-2 viral infection because of their increased general susceptibility to viral respiratory infections, as it has been mentioned above.
ACE2 receptors are also abundant in the heart, explaining the observation that during generalized SARS-CoV-2 infections hearts might be affected. Because of this, the SARS-CoV-2 virus is a potential cause of cardiac inflammation and heart failure, and this can be more severe during pregnancy because of the higher heart function demand in pregnant women, especially those with severe COVID-19 illness [137]. Consistently, some cases of cardiomyopathies in pregnant women with COVID-19 disease have been reported [138]. In summary, the increased heart rate and stroke volume were seen in the second trimester of gestation, during labor, and immediately after delivery, which could result in heart failure in pregnant women with COVID-19 disease and cardiac comorbidities. This could be especially apparent during more severe forms of presentation of the illness when pregnant women have a lower cardiac function reserve and their hearts are already stressed by these physiological events (Section 1, this review).
As mentioned above, the vertical transmission of the SARS-CoV-2 virus from the mother to the offspring seems to be quite rare [59,106,139,140]. In vitro fertilization experiments using infected oocytes do not support evidence for vertical transmission nor alterations in fertilization [124]. Concerning embryonic implantation, although ACE2 endometrial expression has proved to be low, its levels increase during the first half of the secretory phase, which could mean that the blockade of this receptor by the virus could have some impact on the viability of the embryo, though this remains unclear [120].
Cases of preeclampsia and preterm birth have been reported and are more common in severe COVID-19 presentations [140,141]. It is more common to observe a preeclampsia-like syndrome in pregnant women with COVID-19 disease than the classical preeclampsia presentation during pregnancy [142]. At variance with classical preeclampsia, in which some typical biomarkers/signs of this syndrome are correlated with an increase in uterine artery pulsatility index (UtAPI) and angiogenic factors (soluble fms-like tyrosine kinase-1/placental growth factor [sFlt-1/PlGF]), these biomarkers are usually not increased in the preeclampsia-like syndrome of pregnant women with COVID-19 [142].
A nearly two-fold increase in the incidence rate of pre-term deliveries as well as stillbirths (fetal death at ≥24 weeks gestation) has been reported for pregnant women with SARS-CoV-2 infections [143,144]. The genes associated with very early preterm birth and pathophysiological alterations associated with these cases involve growth signaling, inflammation, and immunity-related pathways, as discussed above in the overview of physiological changes (Section 1, this review). SARS-CoV-2 infections promote the alterations that favor preterm birth and stillbirth, especially in pregnant women with more severe COVID-19 disease [145]. The increment in cases of preeclampsia and preterm birth caused by SARS-CoV-2 infections have also been linked, at least partially, to the hemodynamic changes during pregnancy described previously (Section 1, this review). In association with this, the expression and activity of the ACE2 receptor linked to the RAS response increases concerning their linkage to negative feedback functions. ACE2 receptors are expressed in most of the blood vessels of the body, and as such many organs can be affected by SARS-CoV-2 infection. In particular, ACE2 receptor expression has been shown to increase in single-cell transcriptome studies at the maternal-fetal interface as well as in some fetal organs [146]. This could be the pathophysiological basis of the preeclampsia-like syndrome observed in some pregnant women with severe COVID-19 disease [142].
3.1.2. Special considerations regarding immunological, hematological, and kidney changes implicated in SARS-CoV-2 pathogenesis and prognosis
The immunological changes occurring during pregnancy could play a central role in determining the rate of progression towards severe cases in SARS-CoV-2 infected women. These immunological changes lead us to hypothesize that pregnant or recently pregnant women, infected by SARS-CoV-2, are at higher risk of developing more severe presentations of COVID-19 disease according to surveillance by the CDC and the US Public Health Service in the USA [147]. From an immunological perspective, pregnancy is linked to maternal immunological adaptation that is dependent on the gestational stage; therefore, infection with the SARS-CoV-2 virus during pregnancy may result in a variable inflammatory response. The relative anti-inflammatory responses seem to vary during pregnancy. The anti-inflammatory responses are less compromised during the first and third trimesters of gestation. These trimesters compared to the rest of the pregnancy yield more risk for the development of CS, leading to more severe inflammatory states of the disease at the beginning and the end of gestation [148]. The inflammatory cascade or CS promoted in the host by SARS-CoV-2 could play a key role in the development of ARDS, leading to the development of the most severe cases of COVID-19 disease during the first and third trimester, or immediately after delivery [149,150].
In patients that have severe COVID-19 disease with interstitial pneumonia elevated serum levels of proinflammatory cytokines have been found. CS and its clinical manifestations are characterized by increased plasma concentrations of various proinflammatory cytokines, such as IL-1, 2, 6, 7, and 10; granulocyte-colony stimulating factor, tumor necrosis factor-alpha (TNF-α), interferon γ-inducible protein (IFN-γ), and several others [109,151,152]. Thus, elevated proinflammatory cytokine levels are a key factor in the development of CS with subsequent progression to ARDS. CS is also importantly involved in Macrophage Activation Syndrome (MAS) that is associated with severe presentations and death, especially in children with chronic rheumatic diseases [153]. The hyperactivation of the immune response initially can be restricted to the lung parenchyma and lymphoid tissue of alveolar bronchi, and later the pulmonary vasculature where it promotes vascular dysfunction promoted by MAS-like inflammation. The MAS-like inflammation and vascular dysfunction can result in pulmonary intravascular coagulopathy with micro thrombosis and hemorrhage. That is why measuring the levels of the proinflammatory cytokines like IL-6 produced via macrophage activation induced by a Th1 immune response is considered as a biomarker and predictor of poor prognosis in COVID-19 disease [154]. In severe cases of COVID-19 patients, heparin is thought to be beneficial, because of its anti-inflammatory action, decreasing the risk of vascular thrombosis.
Hematological alterations related to coagulation during COVID-19 disease have been reported [[155], [156], [157]]. It should be appreciated that the most severely ill patients present with coagulopathy and disseminated intravascular coagulation (DIC), and the most typical features found are the higher levels of D-Dimer and Fibrinogen Degradation Products (FDP) and prothrombin prolongation. Although the mechanisms of coagulopathy are not fully known, it is estimated to play a key role in the involvement of dysregulated immune responses directed by inflammatory cytokines, lymphocyte cell death, hypoxia, and endothelial damage [158], as mentioned in the paragraph above and in Section 1. As the levels of fibrinogen, D-Dimers, and coagulation factors found in plasma increase with a decrement of APTT and prothrombin times, pregnancy by itself is associated with increased thromboembolic risk, and during infection, with the SARS-CoV-2 virus, the risk might be greatly increased due to additional coagulation changes [155].
Because of the diminished functional reserve of the kidneys during pregnancy (Section 1, this review), there is an additional increase in kidney diseases. Some women have been found to have chronic kidney disease for the first time during pregnancy [159]. Around 20% of women who develop early pre-eclampsia (≤30 weeks gestation), especially those with heavy proteinuria, have previously unrecognized chronic kidney disease [160]. Taking all of these changes into consideration, it is not surprising that COVID-19 disease affects pregnant women in more severe ways, especially in those women that have kidney comorbidities. Pregnant women with COVID-19 and kidney diseases are a high-risk group that should be managed by a multidisciplinary team approach, including a nephrologist and neonatologist [161]. There are some reports of acute tubular necrosis in pregnant women with COVID-19 disease, possibly due to the extreme functional demand of kidney function during pregnancy and medications received to treat COVID-19 symptoms [143,162]. Because of this, the impact of medications on the kidneys during treatment of COVID-19 and other diseases in pregnant women has to be taken into special consideration.
3.2. Pathogenesis and clinical forms of presentation of Zika Virus during pregnancy
Zika virus (ZIKV) disease is usually acute and self-limited. Transmission of ZIKV can occur via a vector or non-vector-borne process. The cycle of the vector-borne transmission of ZIKV (the most frequently observed), starts via virus acquisition by mosquitoes (mostly Aedes Agypti and Ades Albopictus) during a blood meal, similar to the insect-borne vectors that cause Dengue and Chikungunya diseases. When the virus replicates inside mosquitoes and eventually reaches the salivary glands, it can be secreted and subsequently injected into a new host during the next blood meal. In its new human host, ZIKV can replicate in fibroblasts, keratinocytes, and immature dendritic cells in the skin [163]. Then the replicated virus can penetrate lymphatic vessels and ganglia and can become systemic through viremia. The incubation period in humans can last from 3 to 12 days [164]. This period is followed by an illness state with several forms of presentation. In most patients, ZIKV is asymptomatic (80% of cases), and it can be present also as a moderate dengue-like fever disease (20% of cases). During this period the virus can be found in patients´ blood [165]. The symptoms found during a moderate disease course are fever, macular or papular rash, conjunctivitis (red eyes), arthralgia, headache, myalgia, and occasionally retro-orbital pain [165]. These symptoms are usually present between 4 and 7 days after infection [166,167].
It can be difficult to distinguish ZIKV symptoms from those produced by diseases caused by other Arboviruses, especially Dengue and Chikungunya. An exanthema (pruriginous maculopapular rash) that starts in the face and/or trunk suggests ZIKV infection [104,168]. ZIKV can also have complications and sequelae like the Guillain-Barré syndrome and hemorrhagic symptoms [169]. Guillain-Barré syndrome is an autoimmune disease of the nerves, starting with weakness to later present as flaccid paralysis. A temporal coincidence of Guillain-Barré syndrome with ZIKV has been found. Guillain-Barré syndrome patients found in ZIKV outbreaks were found to have high levels of IgM antibodies against ZIKV. They also experience rapid development of neurological symptoms [170].
The hemorrhagic complications during ZIKV infections are usually mild [171]. ZIKV infections are in general systemic, and the virus can be found in body secretions for weeks after the infection [172]. ZIKV also shows positive tissue tropism by targeting testicular tissue. ZIKV can be secreted into the semen for months after infection, and thus ZIKV infection is considered a sexually transmitted disease (STV) [173]. The non-vector-borne transmission of ZIKV occurs by sexual, transplacental, or perinatal transfer as well as accidents in the laboratory or during blood transfusions. This ability of ZIKV to be acquired by human-to-human transmission makes ZIKV unique among other Flaviviruses.
The possibility of acquiring a ZIKV infection during pregnancy and displaying clinical symptoms are not modified by the pregnancy itself [104]. Most of the ZIKV-infected women who are pregnant are asymptomatic or paucisymptomatic. Nor has ZIKV been described as causing a more severe infection or a major risk for complications in the mother during pregnancy. The non-vector-borne transmission of ZIKV is sexual, transplacental, perinatal, or due to laboratory accidents or blood transfusions. The vertical transmission of ZIKV can happen at any time during pregnancy. When the infection by ZIKV takes place in the mother, it is irrelevant if the mother was displaying symptoms or was without symptoms for the vertical transmission to occur.
The outbreaks reported in French Polynesia between 2013 and 2014 and Brazil in 2015 were important in establishing the association between ZIKV infection, Guillain-Barré Syndrome, and microcephaly. Other less frequent complications can occur, usually in less than 1% of ZIKV patients, and can include transverse myelitis, meningoencephalitis, myocarditis, and thrombocytopenic purpura [104,174,175]. In 2015, Brazil reported an increase in the prevalence of newborns with microcephaly in the North-East region of the country, which was temporally associated with an outbreak of ZIKV infections. The WHO declared a public health emergency due to the observed effects of ZIKV in Brazil and the spread of the ZIKV from Brazil to Argentina in 2016 [[176], [177], [178]].
ZIKV complications, such as teratogenesis linked to the ZIKV, are related to an ample spectrum of fetal malformations whose landmark is the congenital Zika syndrome. Other malformations have been described, such as those in the genitourinary tract, skeletal muscle, ophthalmologic, and lung and craniofacial malformations, among others [89,179]. In particular, almost 30% of ZIKV-infected children have significant vision anomalies due to the infection of retinal cells and optical nerves [180]. In addition, congenital alterations due to ZIKV have also been described as an incremental increase in the risk of spontaneous abortion, fetal death, low weight at birth, preterm birth, and restriction to intrauterine growth [89,102,178]. Supporting evidence is the finding of ZIKV antigens and nucleic acids in the amniotic fluid, the placenta, and the fetal tissues in most of the cases described in places where outbreaks of ZIKV have been confirmed [93].
The diagnosis of ZIKV infections in pregnant women and their offspring can be difficult because the detection of the ZIKV genetic material occurs during a limited time in the infected mother, and also because a fetal infection can be present for several months or years later [104]. Though a PCR assay for the ZIKV in the amniotic fluid can be useful on some occasions, it is not routinely recommended [104]. Imaging studies, such as ultrasound and magnetic resonance, play a chief supporting role in the diagnosis of fetal viral infections during pregnancy. These diagnostic tests should be carried out in endemic regions or in people returning from endemic regions, especially in patients with Guillain-Barré-like syndromes and pregnant women suspected of having ZIKV infections [169]. In these regions, organs and blood donations should be also tested for ZIKV infections [181].
There is no specific treatment for ZIKV during the infection process, and there are no specific and effective antiviral drugs available to treat ZIKV in neonates. The recommendations are to treat ZIKV symptoms and provide support treatments [176]. Prevention and control, including avoiding mosquito bites, are the main protective measures available to avoid ZIKV infection, such as the use of mosquito repellent, adequate clothes, mosquito nets, among other approaches. Vector control, blood and tissue bank tests, and prevention of sexual and vertical transmission are also useful as reinforcement and protective policies.
As of the preparation of this contribution, there were no available vaccines against the Zika virus to prevent the disease, though some vaccines are currently under development and are being tested [176]. Some advances in antiviral therapeutics and vaccines are under investigation but remain at an early phase of development. The preconception recommendations and also those during pregnancy to avoid being infected by ZIKV are the only methods available worldwide to currently prevent vertical transmission. In many countries, it is still difficult or impossible to obtain generalized access to reliable anti-conception methods, and safe abortion procedures are out of reach for many women, particularly in Africa and in some Latin-American countries. Though during 2020, the scientific community and the world have paid attention to the SARS-CoV-2 pandemic, ZIKV is also an important public health problem in many countries around the world [182].
3.3. Pathogenesis and clinical forms of presentation of Mycoplasma during pregnancy
The adherence of mycoplasmas to host cells is a prerequisite for its pathogeneses, and the cell adhesion membrane protein-lipoproteins (adhesins) from mycoplasmas play an essential role to achieve this. For example, an adhesion molecule has been found in Mycoplasma genitalium (M. genitalium), a 140 kD protein, and it is probably the most studied adhesin molecule isolated from mycoplasmas [183,184]. The M. genitalium adhesion molecule is expressed on a distinctive, protruding curved terminal organelle that appears to be important for cell adhesion and motility of this mycoplasma. The proteins and array of this organelle have strong similarities to those found in Mycoplasma pneumoniae [185]. The proteins and array of molecules in this organelle have strong similarities to similar molecules found in Mycoplasma pneumoniae [185]. To achieve cell adhesion rapid antigenic variations of the surface proteins of mycoplasmas occur, which also allows them to evade the immune responses of the host [185]. The successful colonization of host cells, in addition, appears to be determined by the synthesis of mycoplasma bacterial endotoxins and other non-adhesion lipoproteins, which in turn can activate fetal and decidual membranes to locally produce host cytokines and chemokines [186]. The secretion of endotoxins and cytokines can also stimulate the synthesis and release of prostaglandins, which in turn can activate proteases and other bioactive substances that can lead to adverse pregnancy outcomes [187]. As mentioned above, several Mycoplasma species, their membranes or their membrane components can directly activate macrophages and monocytes, leading to the expression and secretion of important pro-inflammatory cytokines like TNF- α and several interleukins (IL - 1, IL - 1β, IL-6, IL-8, IL-12, IL-16) and interferon-γ [184]. Mycoplasma infection and resulting inflammation, either local or systemic, are important in producing deleterious symptoms during pregnancy. After the adhesion and entry of mycoplasmas into host cells, several mycoplasma metabolites can cause cellular damage and can interfere with the normal cellular metabolism [183,184].
Mycoplasmal infections during pregnancy have been linked with certain complications and neonatal risks, and this aspect of mycoplasma and disease has been debated for many years [188]. Since these pathogens are generally able to colonize mucosal tissues, the maternal vaginal colonization by some Mycoplasma species, such as M. hominis and M. genitalium, can be an important risk factor in the occurrence of ascending intrauterine infection and/or during fetal passage through the birth canal. Concerning M. hominis, its vaginal presence during bacterial vaginitis (BV) is associated with pre-term delivery and spontaneous miscarriage, as is also found with M. genitalium infections [188]. In addition, it should be noted that during pregnancy the vaginal microbiome undergoes notable changes, especially due to the increased levels of progesterone and estrogens, resulting in an increase in the abundance of Lactobacillus spp., commonly known to be lactic-acid producing bacteria, leading to a decrease in vaginal pH, which is protective against most bacterial and viral pathogen infections [189].
As mentioned above in various cases, certain mycoplasmas can colonize the urogenital tract, whereas other mycoplasmas, such as M. pneumoniae, primarily colonize the respiratory tract. However, various mycoplasmas can disseminate through the bloodstream during or after a respiratory tract infection and spread to the placenta. Interestingly, vertical transmission cases of invasive M. pneumoniae are relatively rare [45]. However, certain associations between M. pneumoniae during pregnancy and hematological complications, such as hemolytic anemia, are among the most common pregnancy complications. Less frequently found are: thrombocytopenia, thrombotic thrombocytopenic purpura (TTP), DIC, aplastic anemia, hemophagocytosis, and transient acquired thrombin deficiency [190].
Premature labor (PL) is often classified following clinical presentation as spontaneous premature labor (SPL) (50% of cases), premature rupture of membranes before labor (PRMBL) (30% of cases), and medically induced premature labor (MIPL) (20% of cases) [191]. Although there are multifunctional causes to these complications of pregnancy, several pathogenic microorganisms have been associated with PL, especially during their movement from the lower genital tract, entering and invading the chorioamnion, followed by chorioamnionitis, and invasion of the amnion to finally promote a fetal infection. The detection of bacteria, including various Mycoplasma species, in the amniotic fluid (AF), is a significant finding, because the AF is usually sterile in women that are not in labor. Extraction of AF with positive cultures for bacteria and/or mycoplasma (it should be noted here that mycoplasmas rarely grow in such cultures) is found in 13% of the women under SPL with intact membranes. In those women that enter into labor prematurely positive cultures for bacteria in the AF increase to 22%. Cultures can be positive for pathogenic bacteria in up to 32% of the women with PRMBL before labor, though it increases to 75% at the moment of labor [192]. When these situations arise during pregnancy, the frequency of positive AF cultures increases.
With regards to BV, several studies have shown that asymptomatic or symptomatic BV elevates the risk of having a spontaneous abortion or preterm labor [193]. Usually, late-term abortions and preterm deliveries during pregnancy are considered together when analyzing their causes, as they share many etiological factors. After the 2000s, congenital fetal infections were documented at higher frequencies compared with the frequencies found before the 2000s. Indeed, one of every four premature newborns during the 23rd and 32nd weeks of pregnancy are born with bacteriemia, usually with M. genitalium and Ureaplasma species. M. hominis has been isolated in 24% of the patients during preterm labor and in 8% of those without preterm labor. This difference was not considered statistically significant. However, higher numbers of M. hominis (≥105 units, measured as color change units) have been detected in patients with preterm labor, compared to none that were found in controls (p < 0.05). When high numbers of these microorganisms are found, it appears to be a signature of BV. Though M. hominis is likely to play a role in BV per se, and as a consequence of the adverse results of pregnancy, it is not known if this is a peculiarity of BV or if this can also be applied to other bacteria that can play an important role in BV. As expected, it is relatively common to find low numbers of M. hominis in the absence of BV, where it is likely that adverse pregnancy outcomes will not occur [194].
Bacterial transmission from the mother to offspring can happen after intrauterine infection where the bacteria can multiply in the AF and also be transmitted to the fetal lungs. This type of infection can also happen at the beginning of pregnancy where epithelial membranes are intact. Alternatively, hematogenous infections can occur that involve the umbilical blood vessels in an infected placenta. Finally, infections may also happen through the respiratory mucosa or the skin, while the newborn is being delivered during labor.
The pathogenic effects of mycoplasmas during pregnancy and their adverse effects in various tissues are currently under study, including special attention being directed at premature contractions that can induce premature labor, preterm labor, spontaneous abortion, and other adverse events. Their role in neonate infections is also under study. For example, M. genitalium, Ureaplasma parvum, and Ureaplasma urealyticum can be sexually transmitted, and thus they are sexually transmitted diseases. Other afflictions occur due to mycoplasmal infections, such as infertility and illnesses such as urethritis in men, and pelvic inflammatory disease in women. Although the female diseases are frequently associated with bacteria in the AF and placenta, these bacteria are not routinely sought in pregnant women, probably because it is quite difficult to grow mycoplasmas in cultures outside the body and the need to perform only routine diagnostic PCRs. The association between various pathogens and complications of pregnancy has been investigated more intensely during recent years [195]. Next-generation sequencing (NGS) can detect the microbial cell-free DNA in the maternal plasma of women with clinically and/or histologically evident chorioamnionitis, but this usually does not include intracellular species of bacteria. Differences among the microorganisms living in the genital microbiota have been observed when comparing pregnant women under preterm labor or delivery to term and healthy labor. The most frequently observed bacterial species are Lactobacillus spp., and these species are abundant in the specimens obtained during delivery at term and healthy labor. U. parvum, M. hominis, and Haemophilus influenza were found almost exclusively when there was chorioamnionitis. Streptococcus mitis, U, parvum, and M. hominis were significantly correlated between paired samples of maternal plasma and umbilical cord, suggesting that several associated defects in pregnant women and fetuses like those reported above are caused by these colonizing pathogenic microorganisms [196,197].
Until recently, most diagnostic studies on fetal infections were performed with cultures of AF or performing molecular tests directly on the AF [198]. However, there could be complications with this approach. For example, Oh et al. [199] have shown that 24% of the patients with clinical chorioamnionitis and preterm delivery do not show any evidence of amniotic inflammation when cultures or molecular assays were performed using AF. Nearly 66% of the patients showed negative AF cultures when there were signs of infection by other methods. This demonstrates that the in vitro cultures of AF represents a low sensitivity method for the diagnosis of AF infections. Also, amniocentesis is an invasive procedure with additional medical risks.
The results obtained with NGS have identified many common microorganisms as part of the infections found during pregnancy complications, including those microorganisms that we know are specifically pathogenic and thought to be causing chorioamnionitis [196]. The examination of NGS cell-free microbiological DNA in plasma is a fast non-invasive test that can be achieved after obtaining small amounts of maternal plasma. However, this procedure is unlikely to be useful in the diagnosis of mycoplasmal infections, because these microorganisms are rarely found in plasma. This method aims to diagnose infections during pregnancy using the maternal plasma without the need of performing risky amniocentesis procedures. This allows an appropriate therapeutic approach using the correct antimicrobials before and after delivery. This should be an improvement over the current methods used to diagnose intrauterine microbial infections during pregnancy, because antimicrobials like antibiotics can alter the neonate microbiota and have toxic effects, and this would be avoided. This diagnostic technique seems to be promising to identify possible causes of chorioamnionitis [196], but it may not allow the diagnosis of mycoplasmal infections.
4. Special considerations about the neonates and breastfeeding
4.1. COVID-19 disease
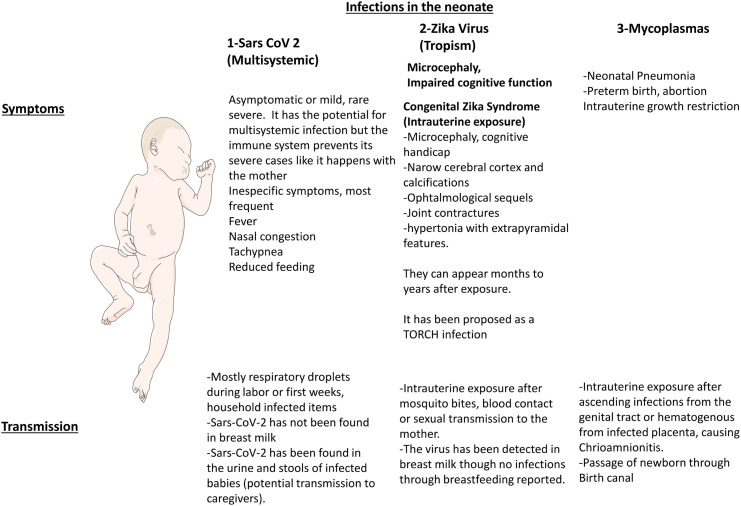
As of this date, the vertical intrauterine transmission of viral and bacterial pathogens remains under investigation, especially in COVID-19 disease, and recent determinations of the rates of SARS-Cov-2 infection seem to be independent of the mode of delivery in newborns. The most probable way of acquiring the virus for a newborn is through respiratory droplets from the mother or other infected household contacts, or eventually by direct contact with contaminated surfaces [49]. There has been no demonstration of breastfeeding transmission in neonates from mothers with COVID-19 [200]. The symptoms and the most common methods of transmission in neonates for the diseases such as COVID-19 are summarized in Fig. 3 .
Fig. 3.
SARS-CoV-2 virus, Zika virus and Mycoplasma species in the neonate. A scheme summarizing the main symptoms and transmission pathways for various diseases in the neonate caused by SARS-CoV-2 virus, Zika virus and Mycoplasma species.
The pandemic behavior of the SARS-Cov-2 virus excludes partially the newborn period since the incidence of transmission of this infection in this group is uncommon. The clinical presentation of COVID-19 disease is frequently asymptomatic or a mild disease process with low to moderate morbidity, without requiring hospitalization. Some factors thought to be important in diminishing the severity of SARS-CoV-2 infections in neonates and children compared to adults are: (i) the differences in the intensities of innate and adaptive immune responses to infections and injuries; (ii) exposure to more diverse infectious pathogens that are increasing in frequency and concurrency; (iii) pre-existing immunity to some coronaviruses; (iv) the presence of different microbiota; (v) higher levels of melatonin than in adults; (vi) protective side-effects of required vaccines and (vii) reduced exposure to SARS-CoV-2 virus compared to adults [201]. Severe illness presentations of COVID-19 disease have been reported, even if generally considered uncommon. It is certainly possible that SARS-CoV-2 infections can occur in preterm or neonates with underlying medical conditions. This makes these newborns potentially at risk of developing severe COVID-19 symptoms [55]. There have also been reports describing newborns with symptoms similar to COVID-19 that required admission to a neonatal intensive care unit (NICU) because of fever, augmented labored breathing, gastrointestinal symptoms, tachycardia, and signs of pulmonary infection. Interestingly, several babies with symptoms of COVID-19 disease tested negative for the SARS-CoV-2 virus. Only 40% of these patients had positive tests for SARS-CoV-2 [48,49,55,[202], [203], [204]].
It is difficult to determine at this time whether full-term neonates during the first month of life diagnosed with COVID-19 follow a similar clinical course compared to older children. This could be due, in part, because the immune system in the early stages of life has unique characteristics [205]. The possible way that neonates fend off viral (and bacterial) infections in the absence of maternally transmitted IgG antibodies could be directly related to their innate immune systems and the presence of larger numbers of immature T cells. This is a general characteristic of newborns that makes them generally less vulnerable to nearly all viral infections, including those caused by coronaviruses. The SARS-CoV-2 virus has the potential to be systemic in neonates, affecting multiple organs, including the kidney and the gastrointestinal tract [205,206]. The more frequent presentations of COVID-19 found in neonates are fever and a respiratory tract infection, and they also display other mild symptoms, such as nasal congestion, tachypnea, reduced feeding, or more severe signs, such as cyanosis, fatigue, and oxygen requirement, and even ARDS. Gastrointestinal tract symptoms are occasionally present, and in exceptional cases, central nervous system symptoms (e.g. seizures) have been found. The most common course of COVID-19 disease in children is the complete recovery from the illness, but in exceptional cases, a fatal outcome has occurred [207]. With regards to Regarding a mother's infection with SARS-CoV-2 during pregnancy and the subsequent transmission of maternal IgG antibodies to the fetus via placental route or to the newborn via breastfeeding, there is no evidence of protection mediated by mother's breast milk [208].
Although respiratory transmission is the primary route for SARS-CoV-2 infectivity, the oropharyngeal presence of SARS-CoV-2 RNA in both urine and stool in the baby is an important finding because these sources could serve as additional vehicles for virus transmission. Whether the virus detected in urine and stool is viable and infective requires further investigation. As preventive measures, caregivers should be counseled to wear face masks and practice proper hand-washing, especially when changing the diapers of neonates, to prevent the spread of the SARS-CoV-2 virus among household contacts [209].
4.2. Zika virus and its similarity to TORCH infections
In recent years evidence has accumulated of an association between ZIKV infection during pregnancy and a syndrome characterized by fetal microcephaly. Microcephaly is a severe brain developmental disorder, which is accompanied by impaired cognitive functions and neurodevelopment. In the case of ZIKV, this can involve the arrest of brain growth and partial collapse of the skull [210,211].
Neonates that were exposed to ZIKV through vertical intra-uterine transmission had several symptoms and affections linked to ZIKV infections of the mother during pregnancy [212,213]. A pattern in newborns that had intrauterine exposure to ZIKV has been described as five characteristics that have been termed congenital Zika syndrome. These are: (a) severe microcephaly with partial skull collapse, (b) narrowing of the cerebral cortex with subcortical calcifications, (c) ophthalmologic sequels with macular scars and focal pigmentation in the retina, (d) congenital contractures of one or several joints, and (e) early hypertonia with extrapyramidal failure [103,212,213]. It is unclear if mild neurocognitive issues and other disorders are linked to ZIKV infections [211,214]. Most of the cases of transmission by ZIKV occur via a vector, such as the Aedes mosquito. However, as pointed out above, transmissions via blood, sexual or vertical routes have been confirmed with ZIKV [215,216].
Manifestations due to mother-to-child transmissions of ZIKV have been detected before birth, at the time of delivery, or later. This suggests that when ZIKV disease appears months or even several years after birth, these diseases or manifestations could be due to events that occurred during pregnancy or at birth. The reported abnormalities or symptoms are seen during or after obvious ZIKV infections, such as microcephaly, are calcifications at the cortical-subcortical level, the union of the white matter, atrophy in the parenchyma, agenesis/hypoplasia of the corpus callosum, cerebellar and brainstem hypoplasia, and lissencephaly-pachygyria [217]. Currently, the transmission of the ZIKV has not been detected via breast milk, although the virus has been detected in breast milk samples [218]. Therefore, current evidence suggests that breastfeeding of infected mothers should be maintained as usual with neonates [218].
Some authors have proposed that the Zika virus be included with the traditional TORCH pathogens, because of the similarities in pathogenesis, fetal infection, and developmental defects that are caused by the infection. The similarities between the congenital disorders that are induced by the TORCH and ZIKV pathogens include neurotropism with specific congenital malformations, depending on the gestational age at the time of fetal infection [219]. Additional knowledge about the disease(s) caused by ZIKV infections remains to be revealed; however, other maternal viral infections that result in the transmission to the fetus provide instructive lessons that can be applied to the prospective evaluation of ZIKV infections and the diseases that this virus causes.
One of the most obvious properties of ZIKV is that it can have cytopathic effects on various tissues and cell types. Viral antigens have been found in the cytoplasm of neurons, especially those with characteristics of degeneration and necrosis, and within glial cells. Although there is an absence of substantial inflammatory responses in the brain during ZIKV infections, a specific cytopathic viral effect has been found that is similar to that reported in fatal congenital rubella syndrome [219]. Congenital rubella infections can only be prevented by vaccination. The same is expected with ZIKV in endemic areas if an effective vaccine can be developed. Diseases caused by congenital cytomegalovirus (CMV) infections are probably the diseases that most closely resemble the diseases caused by ZIKV infections, since both can produce profound effects on the fetus, such as microcephaly, sensorineural hearing loss, chorioretinitis, and other neurological defects. The information on CMV infections during pregnancy has provided a robust framework for current and future studies of ZIKV infections and the diseases that this virus causes. There are similarities in the proposed mechanisms of CNS damage in early-occurring CMV and ZIKV infections that occur during the development of the CNS. Some of the proposed pathogenic mechanisms are virus-induced destruction of neural progenitor cells, alterations in the proliferative capacity of neural progenitor cells, damage to the vasculature, and significant structural damage to the central nervous system and spinal cord [220].
In addition to CMV, human immunodeficiency virus (HIV-1) infections in infants are similar to congenital ZIKV infections in their timing and mechanisms of mother-to-child transmission. Like HIV-1, ZIKV-infected infants may be asymptomatic at birth but then develop symptoms of the disease within the first few months of life. Few effective treatments currently exist to prevent vertical transmission in viral diseases like ZIKV, CMV, and HIV-1. In HIV-1 infections, treatment with antiretrovirals has produced reductions in vertical transmission from mother to fetus. A better understanding of the mechanisms of placental and fetal infections will allow the development of new therapeutic strategies that reduce fetal ZIKV infections [221].
Similar to ZIKV primary maternal infections, primary maternal infections by Herpes simplex virus (HSV) are usually asymptomatic [222,223]. However, there are also some contrasts between HSV and ZIKV infections, and the data on these infections can be analyzed to learn more about them. An antiviral drug exists for the treatment of neonatal HSV infections (acyclovir), whereas none is currently available for the prevention or treatment of ZIKV infections during pregnancy or childhood. Interestingly, when suppressive acyclovir therapy was administered for 6 months following a newborn HSV infection, an improved neurologic outcome was forthcoming, implying that HSV can be suppressed in the CNS without causing CNS symptoms. This outcome remains unknown for ZIKV infections involving the CNS.
There remain some uncertainties about the postnatal development and evolution of the disease in ZIKV affected infants. Prospective cohort studies for symptomatic and for asymptomatic ZIKV-infected newborns whose mothers have Zika infections should strengthen our knowledge on the subject. Recent evidence indicates that infants with a normal head circumference at birth may still have underlying brain and/or eye defects in the first 18 months to 4 years after infection with ZIKV [224].
4.3. Mycoplasmas
Mycoplasmas, at least initially during their human colonization, are primarily mucosal inhabitants, along with genital mycoplasmas and ureaplasmas that colonize the urogenital tract. These mycoplasmas are among the most frequently found superficial infections in humans, and at these sites, they rarely cause threatening symptoms. However, when mycoplasmas invade further into various organs, they can produce more severe signs and symptoms. For example, M. hominis, U. urealyticum, and U. parvum are examples of pathogens that can invade pregnant mothers and are closely associated with neonatal pneumonias [225]. Among the more than 200 known species of Mycoplasma, at least 13 species are known to be human pathogens, such as M. pneumoniae, M. hominis, and U. urealyticum, which are among the most well-known Mycoplasma species involved in various human diseases and conditions, some of which are discussed in another review in this special issue [1]. In this section, M. hominis is a relatively uncommon infection, but it can cause life-threatening disease in both full-term and preterm newborns. In contrast, M. pneumoniae is a commonly found infection, especially in atypical childhood pneumonia and other diseases.
As also discussed elsewhere in this contribution, during pregnancy bacterial transmission from a mother to her child can occur via intrauterine infection from the blood, via ascending pathways through the genitourinary tract, where the bacterium multiplies in the AF and is then transmitted to the fetal lungs, or by the passage of the newborn through an infected maternal birth canal. The ascending pathway type of infection can occur early in pregnancy when the fetal membranes remain intact [226]. Hematogenous intrauterine infections can occur through the umbilical blood vessels of an infected placenta, causing subsequently an intrauterine infection. In this context, infections caused by Ureaplasma spp. can result in chorioamnionitis, the spread of infection throughout fetal organs, and the inception of congenital pneumonia [227]. Finally, during fetal passage through an infected maternal birth canal subsequent colonization of the newborn's skin, mucous membranes, and respiratory tract can occur [228].
When intrauterine mycoplasmal infections arise, they are often associated with birth complications, such as premature rupture of birth membranes, premature delivery, and spontaneous abortion. For example, the identification of Ureaplasma spp. inside the chorioamnios has been consistently associated with histologic chorioamnionitis and inversely related to birth weight. Such microorganisms can invade the amniotic cavity and persist for several weeks when the fetal membranes are intact, and subsequently, they can initiate a severe inflammatory reaction in the absence of labor. Maternal and fetal colonization by Ureaplasma spp. has also been associated with the development of bronchopulmonary dysplasia in preterm infants of less than 32 weeks of GA [229].
Several Mycoplasma species are considered normal commensals, but at least six in the genus Mycoplasma are considered human pathogens, among them M. pneumoniae, M. genitalium, and M. hominis [225]. Recently, findings of great interest were published indicating that M. hominis infections can produce bacteremia, pneumonia, meningitis, among other symptoms in newborns, but the significance of M. hominis as a pathogen causing neonatal meningitis remains unclear [230]. M. hominis rarely appears to invade the chorioamnios and amniotic fluid in the absence of other microorganisms, and data that support an independent role for this mycoplasma in histologic or clinical amnionitis is modest at best [231,232]. Recently reported M. hominis infections in newborns to indicate that the clinical findings, which can include pleocytosis with or without inflammatory reactions, are not always in agreement with the results obtained by testing of the CSF. In addition, M. hominis can be isolated from the CSF of newborns without signs of meningitis [230].
Traditional procedures for the diagnosis of bacteria in the blood and tissues have relied on Gram staining, but the lack of bacterial cell wall components makes mycoplasmas undetectable by Gram staining. Another diagnostic limitation is that the growth of mycoplasmas in bacterial cultures is very slow, limited to only certain species, and requires specific growth media, a situation that can lead to patients with undiagnosed mycoplasmal infections, such as in cases of meningitis and especially chronic illnesses. Unexplained increases in morbidity and mortality are often found in patients with undiagnosed mycoplasmal infections [233,234].
The problems associated with the specific identification of mycoplasmal involvement in pregnancy and neonatal infections can also result in poor treatment decisions. For example, M. hominis is intrinsically resistant to many antibiotics, such as β-lactams, glycopeptides, sulfonamides, and macrolides. For this reason, the most frequently used antibiotics (β-lactams combined with aminoglycosides) in neonates suspected of sepsis are not effective against M. hominis. Since the majority of antibiotics active against M. hominis are the tetracyclines, lincosamides, chloramphenicol, and fluoroquinolones, these options are usually only selected if the clinical course of the newborn patient worsens. This can lead to delays in employing more optimal treatments, resulting in more fatal outcomes [230,235]. As discussed here and elsewhere [236,237], there are several difficulties related to the clinical testing and identification of mycoplasma pathogens in patients with acute or chronic diseases, as well as the association of these infections with specific diagnoses. Unless these problems can be overcome, the recognition of clinical presentations and specific diseases and their infectious causes may result in ineffective empirical therapies.
5. Final comment
Continuing societal exposures to emerging and reemerging infectious diseases are among the most challenging threats to our species. Among the pathogens involved, we have chosen to focus on three infections that are important in pregnancy and neonatal health: SARS-CoV-2 virus, Zika virus, and Mycoplasma species.
During and immediately after pregnancy there are a significant number of physiological changes in the mother and offspring. A temporary organ controlling the relationship between the mother's intracorporal environment and offspring, the placenta, must be created. After delivery, neonates have not fully developed all of the abilities of their homeostatic systems, and under normal conditions are dependent on breastfeeding from their mothers. Pregnant women have an increased risk of developing cardiorespiratory, hematological, immunological, and kidney-related diseases, because of the physiological adaptations that they must endure for the delivery and development of their offspring. These physiological changes explain the increased susceptibility and risk of developing diseases promoted by certain intracellular pathogens, such as the examples discussed in this contribution. Often these microorganisms are difficult to diagnose and treat, and any delay in either their diagnosis and/or treatment can lead to difficult and potentially life-threatening situations for the mother, the fetus, or the neonate.
Sources of funding
Gonzalo Ferreira thanks the support from CSIC – Universidad de la República, Montevideo, Uruguay [project numbers 91 and 137], International Cooperation Programs from CSIC, ANII and PEDECIBA, Uruguay. Garth Nicolson thanks the support from the Institute for Molecular Medicine, CA, USA. Luis Sobrevia thanks the support from the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) [grant number 1190316] and International Sabbatical (University Medical Centre Groningen, University of Groningen, The Netherlands) from the Vicerectorate of Academic Affairs, Academic Development Office of the Pontificia Universidad Católica de Chile, Chile.
Declaration of competing interest
The authors confirm that there is no conflict of interest.
References
- 1.Carlin A., Alfirevic Z. Physiological changes of pregnancy and monitoring, Best Pract. Res. Clin. Obstet. Gynaecol. 2008;22:801–823. doi: 10.1016/j.bpobgyn.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Racicot K., Mor G. Risks associated with viral infections during pregnancy. J. Clin. Invest. 2017;127:1591–1599. doi: 10.1172/JCI87490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanghavi M., Rutherford J.D. Cardiovascular physiology of pregnancy. Circulation. 2014;130:1003–1008. doi: 10.1161/CIRCULATIONAHA.114.009029. [DOI] [PubMed] [Google Scholar]
- 4.Osol G., Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology. 2009;24:58–71. doi: 10.1152/physiol.00033.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leo C.H., Jelinic M., Ng H.H., Marshall S.A., Novak J., Tare M., Conrad K.P., Parry L.J. Vascular actions of relaxin: nitric oxide and beyond, Br. Aust. J. Pharm. 2017;174:1002–1014. doi: 10.1111/bph.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boeldt D.S., Bird I.M. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J. Endocrinol. 2017;232:R27–R44. doi: 10.1530/JOE-16-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristiansson P., Wang J.X. Reproductive hormones and blood pressure during pregnancy, Hum. Reprod. 2001;16:13–17. doi: 10.1093/humrep/16.1.13. [DOI] [PubMed] [Google Scholar]
- 8.Shagana J.A., Dhanraj M., Jain A.R., Nirosa T. Physiological changes in pregnancy, Drug Invent. Today. 2018;10:1594–1597. doi: 10.5005/jp/books/12974_8. [DOI] [Google Scholar]
- 9.Conrad K.P. Emerging role of relaxin in the maternal adaptations to normal pregnancy: implications for preeclampsia, Semin. Nephrol. 2011;31:15–32. doi: 10.1016/j.semnephrol.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahendru A.A., Everett T.R., Wilkinson I.B., Lees C.C., McEniery C.M. A longitudinal study of maternal cardiovascular function from preconception to the postpartum period. J. Hypertens. 2014;32:849–856. doi: 10.1097/HJH.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 11.Hegewald M.J., Crapo R.O. Respiratory physiology in pregnancy, Clin. Chest Med. 2011;32:1–13. doi: 10.1016/j.ccm.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Wise R.A., Polito A.J., Krishnan V. Respiratory physiologic changes in pregnancy, Immunol. Allergy Clin. North Am. 2006;26:1–12. doi: 10.1016/j.iac.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 13.LoMauro A., Aliverti A. Respiratory physiology of pregnancy. Breathe. 2015;11:297–301. doi: 10.1183/20734735.008615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan E.K., Tan E.L. Alterations in physiology and anatomy during pregnancy, Best Pract. Res. Clin. Obstet. Gynaecol. 2013;27:791–802. doi: 10.1016/j.bpobgyn.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Sappenfield E., Jamieson D.J., Kourtis A.P. Pregnancy and susceptibility to infectious diseases, Infect. Dis. Obstet. Gynecol. 2013;2013 doi: 10.1155/2013/752852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Racicot K., Kwon J.Y., Aldo P., Silasi M., Mor G. Understanding the complexity of the immune system during pregnancy. Am. J. Reprod. Immunol. 2014;72:107–116. doi: 10.1111/aji.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berger A. Th1 and Th2 responses: what are they? Bmj. 2000;321:424. doi: 10.1136/bmj.321.7258.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aghaeepour N., Ganio E.A., Mcilwain D., Tsai A.S., Tingle M., Van Gassen S., Gaudilliere D.K., Baca Q., McNeil L., Okada R., Ghaemi M.S., Furman D., Wong R.J., Winn V.D., Druzin M.L., El-Sayed Y.Y., Quaintance C., Gibbs R., Darmstadt G.L., Shaw G.M., Stevenson D.K., Tibshirani R., Nolan G.P., Lewis D.B., Angst M.S., Gaudilliere B. An immune clock of human pregnancy, Sci. Immunol. 2017;2 doi: 10.1126/sciimmunol.aan2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D. Shevyrev, V. %J F. in I. Tereshchenko, Treg Heterogeneity, Function, and Homeostasis, 10 (2019). [DOI] [PMC free article] [PubMed]
- 20.Saito S., Nakashima A., Shima T., Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am. J. Reprod. Immunol. 2010;63:601–610. doi: 10.1111/j.1600-0897.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- 21.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012;76:16–32. doi: 10.1128/mmbr.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James A.H., Tapson V.F., Goldhaber S.Z. Thrombosis during pregnancy and the postpartum period. Am. J. Obstet. Gynecol. 2005;193:216–219. doi: 10.1016/j.ajog.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 23.Greer I.A. Thrombosis in pregnancy: maternal and fetal issues. Lancet. 1999;353:1258–1265. doi: 10.1016/S0140-6736(98)10265-9. [DOI] [PubMed] [Google Scholar]
- 24.Lipkin G.W., Day C.J., Jurawan N., Johnston T.A., Knox E.M. Pregnancy and the kidney, Pract. Nephrol. 2014;20:359–379. doi: 10.1007/978-1-4471-5547-8_33. [DOI] [Google Scholar]
- 25.Cheung K.L., Lafayette R.A. Renal physiology of pregnancy, Adv. Chronic Kidney Dis. 2013;20:209–214. doi: 10.1053/j.ackd.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burton G.J., Fowden A.L. The placenta: a multifaceted, transient organ, Philos. B Biol. Sci. 2015;370 doi: 10.1098/rstb.2014.0066. Trans. R. Soc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nteeba J., Nteeba J., Varberg K.M., Varberg K.M., Scott R.L., Scott R.L., Simon M.E., Simon M.E., Iqbal K., Iqbal K., Soares M.J., Soares M.J., Soares M.J., Soares M.J., Soares M.J. Poorly controlled diabetes mellitus alters placental structure, efficiency, and plasticity, BMJ Open Diabetes Res. Care. 2020;8 doi: 10.1136/bmjdrc-2020-001243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts J.M., Escudero C. The placenta in preeclampsia, Pregnancy Hypertens. 2012;2:72–83. doi: 10.1016/j.preghy.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reyes L., Wolfe B., Golos T. Results Probl. Cell Differ. Springer; 2017. Hofbauer cells: placental macrophages of fetal origin; pp. 45–60. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., Granger D.N., Granger J.P. Vascular Biology of the Placenta: Second Edition. Biota Publishing. 2017:1–113. https://books.google.com.uy/books?id=GhQqDwAAQBAJ [Google Scholar]
- 31.Kay H.H., Nelson D.M., Wang Y. Wiley; 2011. The Placenta: From Development to Disease. [DOI] [Google Scholar]
- 32.Gellersen B., Brosens J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 2014;35:851–905. doi: 10.1210/er.2014-1045. [DOI] [PubMed] [Google Scholar]
- 33.Heerema-McKenney A. Defense and infection of the human placenta. Apmis. 2018;126:570–588. doi: 10.1111/apm.12847. [DOI] [PubMed] [Google Scholar]
- 34.León-Juárez M., Martínez-Castillo M., González-García L.D., Helguera-Repetto A.C., Zaga-Clavellina V., García-Cordero J., Flores-Pliego A., Herrera-Salazar A., Vázquez-Martínez E.R., Reyes-Muñoz E. Cellular and molecular mechanisms of viral infection in the human placenta, Pathog. Dis. 2017;75 doi: 10.1093/femspd/ftx093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arora N., Sadovsky Y., Dermody T.S., Coyne C.B. Microbial vertical transmission during human pregnancy. Cell Host Microbe. 2017;21:561–567. doi: 10.1016/j.chom.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gellin B.G., Broome C.V., Bibb W.F., Weaver R.E., Gaventa S., Mascola L. The epidemiology of listeriosis in the United States-1986. Am. J. Epidemiol. 1991;133:392–401. doi: 10.1093/oxfordjournals.aje.a115893. [DOI] [PubMed] [Google Scholar]
- 37.Tabata T., Petitt M., Puerta-Guardo H., Michlmayr D., Wang C., Fang-Hoover J., Harris E., Pereira L. Zika Virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe. 2016;20:155–166. doi: 10.1016/j.chom.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez-Sobrido L., Almazán F. New advances on zika virus research. Viruses. 2019;11 doi: 10.3390/v11030258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pereira L. Congenital viral infection: traversing the uterine-placental interface, Annu. Rev. Virol. 2018;5:273–299. doi: 10.1146/annurev-virology-092917-043236. [DOI] [PubMed] [Google Scholar]
- 40.Sharma L., Shukla G. Placental malaria: a new insight into the pathophysiology, Front. Med. 2017;4:117. doi: 10.3389/fmed.2017.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stegmann B.J., Carey J.C. TORCH infections. Toxoplasmosis, other (syphilis, varicella-zoster, parvovirus B19) Rubella, Cytomegalovirus (CMV), and Herpes infections., Curr. Womens. Health Rep. 2002;2:253–258. [PubMed] [Google Scholar]
- 42.Cappelletti M., Presicce P., Kallapur S.G. Immunobiology of acute chorioamnionitis, Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simoni M.K., Jurado K.A., Abrahams V.M., Fikrig E., Guller S. Zika virus infection of Hofbauer cells. Am. J. Reprod. Immunol. 2017;77 doi: 10.1111/aji.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srinivasjois R.M., Kohan R., Keil A.D., Smith N.M. Congenital mycoplasma pneumoniae pneumonia in a neonate, Pediatr. Infect. Dis. J. 2008;27:474–475. doi: 10.1097/INF.0b013e318165ebaf. [DOI] [PubMed] [Google Scholar]
- 45.Huber B.M., Sauteur P.M. Meyer, Unger W.W.J., Hasters P., Eugster M.R., Brandt S., Bloemberg G.V., Natalucci G., Berger C. Vertical transmission of Mycoplasma pneumoniae infection. Neonatology. 2018;114:332–336. doi: 10.1159/000490610. [DOI] [PubMed] [Google Scholar]
- 46.Chen L., Li Q., Zheng D., Jiang H., Wei Y., Zou L., Feng L., Xiong G., Sun G., Wang H., Zhao Y., Qiao J. Clinical characteristics of pregnant women with Covid-19 in Wuhan. China, N. Engl. J. Med. 2020;382 doi: 10.1056/nejmc2009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., Li J., Zhao D., Xu D., Gong Q., Liao J., Yang H., Hou W., Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.R. Nie, S.S. Wang, Q. Yang, C.F. Fan, Y.L. Liu, W.C. He, M. Jiang, C.C. Liu, W.J. Zeng, J.L. Wu, K. Oktay, L. Feng, L. Jin, Clinical features and the maternal and neonatal outcomes of pregnant women with coronavirus disease 2019, MedRxiv. (2020). doi: 10.1101/2020.03.22.20041061. [DOI]
- 49.Fan C., Lei D., Fang C., Li C., Wang M., Liu Y., Bao Y., Sun Y., Huang J., Guo Y., Yu Y., Wang S. Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry?, Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y., Zhao R., Zheng S., Chen X., Wang J., Sheng X., Zhou J., Cai H., Fang Q., Yu F., Fan J., Xu K., Chen Y., Sheng J. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China, Emerg. Infect. Dis. 2020;26:1335–1336. doi: 10.3201/eid2606.200287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patanè L., Morotti D., Giunta M.R., Sigismondi C., Piccoli M.G., Frigerio L., Mangili G., Arosio M., Cornolti G. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019–positive mothers and neonates at birth. Am. J. Obstet. Gynecol. MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pierce-Williams R.A.M., Burd J., Felder L., Khoury R., Bernstein P.S., Avila K., Penfield C.A., Roman A.S., DeBolt C.A., Stone J.L., Bianco A., Kern-Goldberger A.R., Hirshberg A., Srinivas S.K., Jayakumaran J.S., Brandt J.S., Anastasio H., Birsner M., O’Brien D.S., Sedev H.M., Dolin C.D., Schnettler W.T., Suhag A., Ahluwalia S., Navathe R.S., Khalifeh A., Anderson K., Berghella V. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: a United States cohort study. Am. J. Obstet. Gynecol. MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong S.F., Chow K.M., Leung T.N., Ng W.F., Ng T.K., Shek C.C., Ng P.C., Lam P.W.Y., Ho L.C., To W.W.K., Lai S.T., Yan W.W., Tan P.Y.H. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am. J. Obstet. Gynecol. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.J. Yan, J. Guo, C. Fan, J. Juan, X. Yu, J. Li, L. Feng, C. Li, H. Chen, Y. Qiao, D. Lei, C. Wang, G. Xiong, F. Xiao, W. He, Q. Pang, X. Hu, S. Wang, D. Chen, Y. Zhang, L.C. Poon, H. Yang, Coronavirus disease 2019 in pregnant women: a report based on 116 cases, Am. J. Obstet. Gynecol. 223 (2020) 111.e1–111.e14. doi: 10.1016/j.ajog.2020.04.014. [DOI] [PMC free article] [PubMed]
- 55.Yu N., Li W., Kang Q., Xiong Z., Wang S., Lin X., Liu Y., Xiao J., Liu H., Deng D., Chen S., Zeng W., Feng L., Wu J. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study, Lancet Infect. Dis. 2020;20:559–564. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeng H., Xu C., Fan J., Tang Y., Deng Q., Zhang W., Long X. Antibodies in infants born to mothers with COVID-19 pneumonia, JAMA. J. Am. Med. Assoc. 2020;323:1848–1849. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baud D., Greub G., Favre G., Gengler C., Jaton K., Dubruc E., Pomar L. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection, JAMA. J. Am. Med. Assoc. 2020;323:2198–2200. doi: 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghi T., di Pasquo E., Mekinian A., Calza L., Frusca T. Sars-CoV-2 in pregnancy: why is it better than expected? Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;252:476–478. doi: 10.1016/j.ejogrb.2020.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Golden T.N., Simmons R.A. Maternal and neonatal response to COVID-19. Am. J. Physiol. - Endocrinol. Metab. 2020;319:E315–E319. doi: 10.1152/AJPENDO.00287.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.H. Hosier, S. Farhadian, R.A. Morotti, U. Deshmukh, A. Lu-Culligan, K.H. Campbell, Y. Yasumoto, C.B.F. Vogels, A. Casanovas-Massana, P. Vijayakumar, B. Geng, C.D. Odio, J. Fournier, A.F. Brito, J.R. Fauver, F. Liu, T. Alpert, R. Tal, K. Szigeti-Buck, S. Perincheri, C. Larsen, A.M. Gariepy, G. Aguilar, K.L. Fardelmann, M. Harigopal, H.S. Taylor, C.M. Pettker, A.L. Wyllie, C. Dela Cruz, A.M. Ring, N.D. Grubaugh, A.I. Ko, T.L. Horvath, A. Iwasaki, U.M. Reddy, H.S. Lipkind, SARS-CoV-2 infection of the placenta, MedRxiv. (2020). doi: 10.1101/2020.04.30.20083907. [DOI]
- 61.Mulvey J.J., Magro C.M., Ma L.X., Nuovo G.J., Baergen R.N. Analysis of complement deposition and viral RNA in placentas of COVID-19 patients, Ann. Diagn. Pathol. 2020;46 doi: 10.1016/j.anndiagpath.2020.151530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID-19. MedRxiv. 2020;154:23–32. doi: 10.1101/2020.05.08.20093229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens, JAMA. J. Am. Med. Assoc. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsu A.L., Guan M., Johannesen E., Stephens A.J., Khaleel N., Kagan N., Tuhlei B.C., Wan X.F. Placental SARS-CoV-2 in a pregnant woman with mild COVID-19 disease. J. Med. Virol. 2021;93:1038–1044. doi: 10.1002/jmv.26386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hecht J.L., Quade B., Deshpande V., Mino-Kenudson M., Ting D.T., Desai N., Dygulska B., Heyman T., Salafia C., Shen D., Bates S.V., Roberts D.J. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19-positive mothers, Mod. Pathol. 2020;33:2092–2103. doi: 10.1038/s41379-020-0639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.A.M. Kotlyar, O. Grechukhina, A. Chen, S. Popkhadze, A. Grimshaw, O. Tal, H.S. Taylor, R. Tal, Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis, Am. J. Obstet. Gynecol. 224 (2021) 35–53.e3. doi: 10.1016/j.ajog.2020.07.049. [DOI] [PMC free article] [PubMed]
- 67.Auriti C., De Rose D.U., Tzialla C., Caforio L., Ciccia M., Manzoni P., Stronati M. Vertical transmission of SARS-CoV-2 (COVID-19): are hypotheses more than evidences? Am. J. Perinatol. 2020;37:S31–S38. doi: 10.1055/s-0040-1714346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crovetto F., Crispi F., Llurba E., Figueras F., Gómez-Roig M.D., Gratacós E. Seroprevalence and presentation of SARS-CoV-2 in pregnancy. Lancet. 2020;396:530–531. doi: 10.1016/S0140-6736(20)31714-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.M. Knight, K. Bunch, N. Vousden, E. Morris, N. Simpson, C. Gale, P. Obrien, M. Quigley, P. Brocklehurst, J.J. Kurinczuk, Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study, BMJ. 369 (2020) m2107. doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed]
- 70.Vivanti A.J., Vauloup-Fellous C., Prevot S., Zupan V., Suffee C., Cao J. Do, Benachi A., De Luca D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020;11:3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Algarroba G.N., Hanna N.N., Rekawek P., Vahanian S.A., Khullar P., Palaia T., Peltier M.R., Chavez M.R., Vintzileos A.M. Confirmatory evidence of the visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am. J. Obstet. Gynecol. 2020;223:953–954. doi: 10.1016/j.ajog.2020.08.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.L.J. Yockey, C. Lucas, A. Iwasaki, Contributions of maternal and fetal antiviral immunity in congenital disease, Science (80-. ). 368 (2020) 608–612. doi: 10.1126/science.aaz1960. [DOI] [PubMed]
- 73.Delorme-Axford E., Donker R.B., Mouillet J.F., Chu T., Bayer A., Ouyang Y., Wang T., Stolz D.B., Sarkar S.N., Morelli A.E., Sadovsky Y., Coyne C.B. Human placental trophoblasts confer viral resistance to recipient cells, Proc. Natl. Acad. Sci. U. S. A. 2013;110:12048–12053. doi: 10.1073/pnas.1304718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hikmet F., Méar L., Edvinsson Å., Micke P., Uhlén M., Lindskog C. The protein expression profile of ACE2 in human tissues. BioRxiv. 2020;16 doi: 10.1101/2020.03.31.016048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.M. Hoffmann, H. Kleine-Weber, S. Schroeder, N. Krüger, T. Herrler, S. Erichsen, T.S. Schiergens, G. Herrler, N.H. Wu, A. Nitsche, M.A. Müller, C. Drosten, S. Pöhlmann, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell. 181 (2020) 271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed]
- 77.W. Sungnak, N. Huang, C. Bécavin, M. Berg, R. Queen, M. Litvinukova, C. Talavera-López, H. Maatz, D. Reichart, F. Sampaziotis, K.B. Worlock, M. Yoshida, J.L. Barnes, SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes /ACE2+TMPRSS2 Expression different organs/mainly in nasal goblet and ciliated cells, Nat. Med. 26 (2020) 1–7. http://www.nature.com/articles/s41591-020-0868-6. [DOI] [PMC free article] [PubMed]
- 78.D. Bestle, M.R. Heindl, H. Limburg, T.V.L. Van, O. Pilgram, H. Moulton, D.A. Stein, K. Hardes, M. Eickmann, O. Dolnik, C. Rohde, S. Becker, H.D. Klenk, W. Garten, T. Steinmetzer, E. Böttcher-Friebertshäuser, TMPRSS2 and furin are both essential for proteolytic activation and spread of SARS-CoV-2 in human airway epithelial cells and provide promising drug targets, BioRxiv. (2020). doi: 10.1101/2020.04.15.042085. [DOI]
- 79.Pique-Regi R., Romero R., Tarca A.L., Luca F., Xu Y., Alazizi A., Leng Y., Hsu C.D., Gomez-Lopez N. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? BioRxiv. 2020;9 doi: 10.1101/2020.05.18.101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valdés G., Neves L.A.A., Anton L., Corthorn J., Chacón C., Germain A.M., Merrill D.C., Ferrario C.M., Sarao R., Penninger J., Brosnihan K.B. Distribution of angiotensin-(1-7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta. 2006;27:200–207. doi: 10.1016/j.placenta.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 81.Valdés G., Corthorn J., Bharadwaj M.S., Joyner J.N., Schneider D., Brosnihan K.B. Utero-placental expression of angiotensin-(1-7) and ACE2 in the pregnant guinea-pig, Reprod. Biol. Endocrinol. 2013;11:5. doi: 10.1186/1477-7827-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miner J.J., Diamond M.S. Zika Virus pathogenesis and tissue tropism. Cell Host Microbe. 2017;21:134–142. doi: 10.1016/j.chom.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Linger R.M.A., Keating A.K., Earp H.S., Graham D.K. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer, Adv. Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gay C.M., Balaji K., Byers L.A. Giving AXL the axe: targeting AXL in human malignancy, Br. J. Cancer. 2017;116:415–423. doi: 10.1038/bjc.2016.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rothlin C.V., Ghosh S., Zuniga E.I., Oldstone M.B.A., Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 87.Moller-Tank S., Kondratowicz A.S., Davey R.A., Rennert P.D., Maury W. Role of the phosphatidylserine receptor TIM-1 in enveloped-virus entry. J. Virol. 2013;87:8327–8341. doi: 10.1128/jvi.01025-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yi L., Pimentel H., Pachter L. Zika infection of neural progenitor cells perturbs transcription in neurodevelopmental pathways. PLoS One. 2017;12 doi: 10.1371/journal.pone.0175744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alvarado M.G., Schwartz D.A. Zika virus infection in pregnancy, microcephaly, and maternal and fetal health: what we think, what we know, and what we think we know, Arch. Pathol. Lab. Med. 2017;141:26–32. doi: 10.5858/arpa.2016-0382-RA. [DOI] [PubMed] [Google Scholar]
- 90.P.P. Garcez, E.C. Loiola, R.M. Da Costa, L.M. Higa, P. Trindade, R. Delvecchio, J.M. Nascimento, R. Brindeiro, A. Tanuri, S.K. Rehen, Zika virus: Zika virus impairs growth in human neurospheres and brain organoids, Science (80-.). 352 (2016) 816–818. doi: 10.1126/science.aaf6116. [DOI] [PubMed]
- 91.Tang B.L. Zika virus as a causative agent for primary microencephaly: the evidence so far, Arch. Microbiol. 2016;198:595–601. doi: 10.1007/s00203-016-1268-7. [DOI] [PubMed] [Google Scholar]
- 92.Calvet G., Aguiar R.S., Melo A.S.O., Sampaio S.A., de Filippis I., Fabri A., Araujo E.S.M., de Sequeira P.C., de Mendonça M.C.L., de Oliveira L., Tschoeke D.A., Schrago C.G., Thompson F.L., Brasil P., dos Santos F.B., Nogueira R.M.R., Tanuri A., de Filippis A.M.B. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study, Lancet Infect. Dis. 2016;16:653–660. doi: 10.1016/S1473-3099(16)00095-5. [DOI] [PubMed] [Google Scholar]
- 93.R.W. Driggers, C.-Y. Ho, E.M. Korhonen, S. Kuivanen, A.J. Jääskeläinen, T. Smura, A. Rosenberg, D.A. Hill, R.L. DeBiasi, G. Vezina, J. Timofeev, F.J. Rodriguez, L. Levanov, J. Razak, P. Iyengar, A. Hennenfent, R. Kennedy, R. Lanciotti, A. du Plessis, O. Vapalahti, Zika Virus infection with prolonged maternal viremia and fetal brain abnormalities, Obstet. Anesth. Dig. 37 (2017) 51–51. doi: 10.1097/01.aoa.0000512046.09676.84. [DOI]
- 94.Mlakar J., Korva M., Tul N., Popović M., Poljšak-Prijatelj M., Mraz J., Kolenc M., Rus K. Resman, Vipotnik T. Vesnaver, Vodušek V. Fabjan, Vizjak A., Pižem J., Petrovec M., Županc T. Avšič. Zika virus associated with microcephaly, N. Engl. J. Med. 2016;374:951–958. doi: 10.1056/nejmoa1600651. [DOI] [PubMed] [Google Scholar]
- 95.Estévez-Herrera J., Pérez-Yanes S., Cabrera-Rodríguez R., Márquez-Arce D., Trujillo-González R., Machado J.D., Madrid R., Valenzuela-Fernández A. Zika virus pathogenesis: a battle for immune evasion. Vaccines. 2021;9:294. doi: 10.3390/vaccines9030294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weisblum Y., Oiknine-Djian E., Vorontsov O.M., Haimov-Kochman R., Zakay-Rones Z., Meir K., Shveiky D., Elgavish S., Nevo Y., Roseman M., Bronstein M., Stockheim D., From I., Eisenberg I., Lewkowicz A.A., Yagel S., Panet A., Wolf D.G. Zika Virus infects early- and midgestation human maternal decidual tissues, inducing distinct innate tissue responses in the maternal-fetal interface. J. Virol. 2017;91:e01905`–16. doi: 10.1128/jvi.01905-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tabata T., Petitt M., Puerta-Guardo H., Michlmayr D., Harris E., Pereira L. Zika Virus replicates in proliferating cells in explants from first-trimester human placentas. potential sites for dissemination of infection, J. Infect. Dis. 2018;217:1202–1213. doi: 10.1093/infdis/jix552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.El Costa H., Gouilly J., Mansuy J.M., Chen Q., Levy C., Cartron G., Veas F., Al-Daccak R., Izopet J., Jabrane-Ferrat N. ZIKA virus reveals broad tissue and cell tropism during the first trimester of pregnancy, Sci. Rep. 2016;6:1–9. doi: 10.1038/srep35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.K.M. Quicke, J.R. Bowen, E.L. Johnson, C.E. McDonald, H. Ma, J.T. O'Neal, A. Rajakumar, J. Wrammert, B.H. Rimawi, B. Pulendran, R.F. Schinazi, R. Chakraborty, M.S. Suthar, Zika virus infects human placental macrophages, Cell Host Microbe 20 (2016) 83–90. doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed]
- 100.Triunfol M. A new mosquito-borne threat to pregnant women in Brazil an apparent connection between the emergence of Zika virus and an unusual rise in. Lancet Infect. Dis. 2016;16:156–157. doi: 10.1016/S1473-3099(15)00548-4. [DOI] [PubMed] [Google Scholar]
- 101.Brasil P., Pereira J.P., Moreira M.E., Nogueira R.M. Ribeiro, Damasceno L., Wakimoto M., Rabello R.S., Valderramos S.G., Halai U.-A., Salles T.S., Zin A.A., Horovitz D., Daltro P., Boechat M., Gabaglia C. Raja, de Sequeira P. Carvalho, Pilotto J.H., Medialdea-Carrera R., da Cunha D. Cotrim, de Carvalho L.M. Abreu, Pone M., Siqueira A. Machado, Calvet G.A., Baião A.E. Rodrigues, Neves E.S., de Carvalho P.R. Nassar, Hasue R.H., Marschik P.B., Einspieler C., Janzen C., Cherry J.D., de Filippis A.M. Bispo, Nielsen-Saines K. Zika virus infection in pregnant women in Rio de Janeiro, N. Engl. J. Med. 2016;375:2321–2334. doi: 10.1056/nejmoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rosenberg A.Z., Weiying Y., Hill D.A., Reyes C.A., Schwartz D.A. Placental pathology of zika virus: viral infection of the placenta induces villous stromal macrophage (Hofbauer Cell) proliferation and hyperplasia, Arch. Pathol. Lab. Med. 2017;141:43–48. doi: 10.5858/arpa.2016-0401-OA. [DOI] [PubMed] [Google Scholar]
- 103.A.K.T. Mendes, M.R.C. Ribeiro, F. Lamy-Filho, G.A. Amaral, M.C.R. Borges, L.C. Costa, T.B. Cavalcante, R.F.L. Batista, P. da S. Sousa, A.A.M. da Silva, Congenital zika syndrome: association between the gestational trimester of maternal infection, severity of brain computed tomography findings and microcephaly at birth, Rev. Inst. Med. Trop. Sao Paulo 62 (2020) 1–8. doi: 10.1590/S1678-9946202062056. [DOI] [PMC free article] [PubMed]
- 104.Musso D., Ko A.I., Baud D. Zika virus infection — after the pandemic, N. Engl. J. Med. 2019;381:1444–1457. doi: 10.1056/nejmra1808246. [DOI] [PubMed] [Google Scholar]
- 105.Cranston J.S., Tiene S.F., Nielsen-Saines K., Vasconcelos Z., Pone M.V., Pone S., Zin A., Salles T.S., Pereira J.P., Orofino D., Brasil P., Kerin T., Adachi K., Soares F.M., de Abranches A. Dunshee, da Costa A.C.C., Moreira M.E. Lopes. Association between antenatal exposure to Zika virus and anatomical and neurodevelopmental abnormalities in children, JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ryan G.A., Purandare N.C., McAuliffe F.M., Hod M., Purandare C.N. Clinical update on COVID-19 in pregnancy: a review article. J. Obstet. Gynaecol. Res. 2020;46:1235–1245. doi: 10.1111/jog.14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Berghella V. Coronavirus disease 2019 (COVID-19): pregnancy issues, UpToDate. 2020;1:1–22. https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-pregnancy-issues/print?search=coronavirus&source=search_result&selectedTitle=3~150&usage_type=default&display_rank=3 [Google Scholar]
- 108.Dashraath P., Wong J.L.J., Lim M.X.K., Lim L.M., Li S., Biswas A., Choolani M., Mattar C., Su L.L. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am. J. Obstet. Gynecol. 2020;222:521–531. doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhao X., Jiang Y., Zhao Y., Xi H., Liu C., Qu F., Feng X. Analysis of the susceptibility to COVID-19 in pregnancy and recommendations on potential drug screening. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:1209–1220. doi: 10.1007/s10096-020-03897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mahase E. Covid-19: most patients require mechanical ventilation in first 24 hours of critical care. BMJ. 2020;368:m1201. doi: 10.1136/bmj.m1201. [DOI] [PubMed] [Google Scholar]
- 111.Schnettler W.T., Al Ahwel Y., Suhag A. Severe acute respiratory distress syndrome in coronavirus disease 2019–infected pregnancy: obstetric and intensive care considerations. Am. J. Obstet. Gynecol. MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hirshberg A., Kern-Goldberger A.R., Levine L.D., Pierce-Williams R., Short W.R., Parry S., Berghella V., Triebwasser J.E., Srinivas S.K. Care of critically ill pregnant patients with coronavirus disease 2019: a case series. Am. J. Obstet. Gynecol. 2020;223:286–290. doi: 10.1016/j.ajog.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.M.Z. Yin, L.J. Zhang, G.T. Deng, C.F. Han, M.X. Shen, H.Y. Sun, F.R. Zeng, W. Zhang, L. Chen, Q.Q. Luo, D.J. Yao, M. Wu, S.H. Yu, H. Chen, D. Baud, X. Chen, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during pregnancy in China: a retrospective cohort study, MedRxiv. (2020). doi: 10.1101/2020.04.07.20053744. [DOI]
- 114.Blitz M.J., Rochelson B., Minkoff H., Meirowitz N., Prasannan L., London V., Rafael T.J., Chakravarthy S., Bracero L.A., Wasden S.W., Shetty S.L. Pachtman, Santandreu O., Chervenak F.A., Schwartz B.M., Nimaroff M. Maternal mortality among women with coronavirus disease 2019 admitted to the intensive care unit. J. Clean. Prod. 2020 doi: 10.1016/j.ajog.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.S. Hantoushzadeh, A.A. Shamshirsaz, A. Aleyasin, M.D. Seferovic, S.K. Aski, S.E. Arian, P. Pooransari, F. Ghotbizadeh, S. Aalipour, Z. Soleimani, M. Naemi, B. Molaei, R. Ahangari, M. Salehi, A.D. Oskoei, P. Pirozan, R.F. Darkhaneh, M.G. Laki, A.K. Farani, S. Atrak, M.M. Miri, M. Kouchek, S. Shojaei, F. Hadavand, F. Keikha, M.S. Hosseini, S. Borna, S. Ariana, M. Shariat, A. Fatemi, B. Nouri, S.M. Nekooghadam, K. Aagaard, Maternal death due to COVID-19, Am. J. Obstet. Gynecol. 223 (2020) 109.e1–109.e16. doi: 10.1016/j.ajog.2020.04.030. [DOI] [PMC free article] [PubMed]
- 116.Lai C.C., Wang C.Y., Hsueh P.R. Co-infections among patients with COVID-19: the need for combination therapy with non-anti-SARS-CoV-2 agents? J. Microbiol. Immunol. Infect. 2020;53:505–512. doi: 10.1016/j.jmii.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.G.L. Nicolson, G.F. de Mattos, COVID-19 Coronavirus: is infection along with <i>mycoplasma</i> or other bacteria linked to progression to a lethal outcome?, Int. J. Clin. Med. 11 (2020) 282–302. doi: 10.4236/ijcm.2020.115029. [DOI]
- 118.Jia H., Yue X., Lazartigues E. ACE2 mouse models: a toolbox for cardiovascular and pulmonary research. Nat. Commun. 2020;11:5165. doi: 10.1038/s41467-020-18880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jing Y., Run-Qian L., Hao-Ran W., Hao-Ran C., Ya-Bin L., Yang G., Fei C. Potential influence of COVID-19/ACE2 on the female reproductive system, Mol. Hum. Reprod. 2020;26:367–373. doi: 10.1093/molehr/gaaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Henarejos-Castillo I., Sebastian-Leon P., Devesa-Peiro A., Pellicer A., Diaz-Gimeno P. SARS-CoV-2 infection risk assessment in the endometrium: viral infection-related gene expression across the menstrual cycle, Fertil. Steril. 2020;114:223–232. doi: 10.1016/j.fertnstert.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Segars J., Katler Q., McQueen D.B., Kotlyar A., Glenn T., Knight Z., Feinberg E.C., Taylor H.S., Toner J.P., Kawwass J.F. Prior and novel coronaviruses, Coronavirus Disease 2019 (COVID-19), and human reproduction: what is known?, Fertil. Steril. 2020;113:1140–1149. doi: 10.1016/j.fertnstert.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fu J., Zhou B., Zhang L., Balaji K.S., Wei C., Liu X., Chen H., Peng J., Fu J. Expressions and significances of the angiotensin-converting enzyme 2 gene, the receptor of SARS-CoV-2 for COVID-19, Mol. Biol. Reprod. 2020;47:4383–4392. doi: 10.1007/s11033-020-05478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zupin L., Pascolo L., Zito G., Ricci G., Crovella S. SARS-CoV-2 and the next generations: which impact on reproductive tissues? J. Assist. Reprod. Genet. 2020;37:2399–2403. doi: 10.1007/s10815-020-01917-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stanley K.E., Thomas E., Leaver M., Wells D. Coronavirus disease-19 and fertility: viral host entry protein expression in male and female reproductive tissues, Fertil. Steril. 2020;114:33–43. doi: 10.1016/j.fertnstert.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang N., Qin L., Ma L., Yan H. Effect of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) on reproductive system. Stem Cell Res. 2021;52 doi: 10.1016/j.scr.2021.102189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Abobaker A., Raba A.A. Does COVID-19 affect male fertility? World J. Urol. 2021;39:975–976. doi: 10.1007/s00345-020-03208-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barragan M., Guillén J.J., Martin-Palomino N., Rodriguez A., Vassena R. Undetectable viral RNA in oocytes from SARS-CoV-2 positive women. Hum. Reprod. 2021;36:390–394. doi: 10.1093/humrep/deaa284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dutta S., Sengupta P. SARS-CoV-2 and male infertility: possible multifaceted pathology, Reprod. Sci. 2021;28:23–26. doi: 10.1007/s43032-020-00261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.D. Li, M. Jin, P. Bao, W. Zhao, S. Zhang, Clinical characteristics and results of semen tests among men with coronavirus disease 2019, JAMA Netw. Open. 3 (2020) e208292. doi: 10.1001/jamanetworkopen.2020.8292. [DOI] [PMC free article] [PubMed]
- 130.Li R., Yin T., Fang F., Li Q., Chen J., Wang Y., Hao Y., Wu G., Duan P., Wang Y., Cheng D., Zhou Q., Zafar M.I., Xiong C., Li H., Yang J., Qiao J. Potential risks of SARS-CoV-2 infection on reproductive health, Reprod. Biomed. Online. 2020;41:89–95. doi: 10.1016/j.rbmo.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Q. Shen, Xiao X., Aierken A., Yue W., Wu X., Liao M., Hua J. The ACE2 expression in Sertoli cells and germ cells may cause male reproductive disorder after SARS-CoV-2 infection. J. Cell. Mol. Med. 2020;24:9472–9477. doi: 10.1111/jcmm.15541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vishvkarma R., Rajender S. Could SARS-CoV-2 affect male fertility? Andrologia. 2020;52 doi: 10.1111/and.13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jing Y., Run-Qian L., Hao-Ran W., Hao-Ran C., Ya-Bin L., Yang G., Fei C. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol. Hum. Reprod. 2020;26:367–373. doi: 10.1093/molehr/gaaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Levy A., Yagil Y., Bursztyn M., Barkalifa R., Scharf S., Yagil C. ACE2 expression and activity are enhanced during pregnancy. Am. J. Physiol. - Regul. Integr. Comp. Physiol. 2008;295:R1953–R1961. doi: 10.1152/ajpregu.90592.2008. [DOI] [PubMed] [Google Scholar]
- 135.Datta P.K., Liu F., Fischer T., Rappaport J., Qin X. SARS-CoV-2 pandemic and research gaps: understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics. 2020;10:7448–7464. doi: 10.7150/thno.48076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rajewska A., Mikołajek-Bedner W., Lebdowicz-Knul J., Sokołowska M., Kwiatkowski S., Torbé A. COVID-19 and pregnancy-where are we now? A review. J. Perinat. Med. 2020;48:428–434. doi: 10.1515/jpm-2020-0132. [DOI] [PubMed] [Google Scholar]
- 137.Maisch B. SARS-CoV-2 as potential cause of cardiac inflammation and heart failure. Is it the virus, hyperinflammation, or MODS? Herz. 2020;45:321–322. doi: 10.1007/s00059-020-04925-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Juusela A., Nazir M., Gimovsky M. Two cases of coronavirus 2019–related cardiomyopathy in pregnancy. Am. J. Obstet. Gynecol. MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Alzamora M.C., Paredes T., Caceres D., Webb C.M., Webb C.M., Valdez L.M., Valdez L.M., La Rosa M., La Rosa M. Severe COVID-19 during pregnancy and possible vertical transmission. Am. J. Perinatol. 2020;37:861–865. doi: 10.1055/s-0040-1710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pulinx B., Kieffer D., Michiels I., Petermans S., Strybol D., Delvaux S., Baldewijns M., Raymaekers M., Cartuyvels R., Maurissen W. Vertical transmission of SARS-CoV-2 infection and preterm birth. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:2441–2445. doi: 10.1007/s10096-020-03964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.J.N. Hansen, J. Hine, T.D. Strout, COVID-19 and preeclampsia with severe features at 34-weeks gestation, Am. J. Emerg. Med. 39 (2021) 252.e3–252.e5. doi: 10.1016/j.ajem.2020.06.052. [DOI] [PMC free article] [PubMed]
- 142.Mendoza M., Garcia-Ruiz I., Carreras E., Suy A. Authors’ reply re: pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study, BJOG An Int. J. Obstet. Gynaecol. 2020;127:1576–1577. doi: 10.1111/1471-0528.16401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Khalil A., Kalafat E., Benlioglu C., O’Brien P., Morris E., Draycott T., Thangaratinam S., Le Doare K., Heath P., Ladhani S., von Dadelszen P., Magee L.A. SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Khalil A., Von Dadelszen P., Draycott T., Ugwumadu A., O’Brien P., Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA - J. Am. Med. Assoc. 2020;324:705–706. doi: 10.1001/jama.2020.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Knijnenburg T.A., Vockley J.G., Chambwe N., Gibbs D.L., Humphries C., Huddleston K.C., Klein E., Kothiyal P., Tasseff R., Dhankani V., Bodian D.L., Wong W.S.W., Glusman G., Mauldin D.E., Miller M., Slagel J., Elasady S., Roach J.C., Kramer R., Leinonen K., Linthorst J., Baveja R., Baker R., Solomon B.D., Eley G., Iyer R.K., Maxwell G.L., Bernard B., Shmulevich I., Hood L., Niederhuber J.E. Genomic and molecular characterization of preterm birth. Proc. Natl. Acad. Sci. U. S. A. 2019;116:5819–5827. doi: 10.1073/pnas.1716314116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li M., Chen L., Zhang J., Xiong C., Li X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS One. 2020;15 doi: 10.1371/journal.pone.0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ko J.Y., DeSisto C.L., Simeone R.M., Ellington S., Galang R.R., Oduyebo T., Gilboa S.M., Lavery A.M., Gundlapalli A.V., Shapiro-Mendoza C.K. Adverse pregnancy outcomes, maternal complications, and severe illness among US delivery hospitalizations with and without a coronavirus disease 2019 (COVID-19) diagnosis, Clin. Infect. Dis. 2021;73:S24–S31. doi: 10.1093/cid/ciab344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu H., Wang L.L., Zhao S.J., Kwak-Kim J., Mor G., Liao A.H. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J. Reprod. Immunol. 2020;139 doi: 10.1016/j.jri.2020.103122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J. Clin. Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kutleša M. The cytokine storm and COVID-19. Medicus. 2020;29:151–153. [Google Scholar]
- 151.Shimizu M. Cytokine Storm Syndr., Springer; in: 2019. Clinical features of cytokine Storm Syndrome; pp. 31–41. [DOI] [Google Scholar]
- 152.Ragab D., Eldin H. Salah, Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far, Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Otsuka R., Seino K.I. Macrophage activation syndrome and COVID-19. Inflamm. Regen. 2020;40:19. doi: 10.1186/s41232-020-00131-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease, Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Benhamou D., Keita H., Ducloy-Bouthors A.S. Coagulation changes and thromboembolic risk in COVID-19 obstetric patients, Anaesth. Crit. Care Pain Med. 2020;39:351–353. doi: 10.1016/j.accpm.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Totura A.L., Baric R.S. SARS coronavirus pathogenesis: host innate immune responses and viral antagonism of interferon, Curr. Opin. Virol. 2012;2:264–275. doi: 10.1016/j.coviro.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.T. Iba, J.H. Levy, M. Levi, J. Thachil, Coagulopathy in COVID-19, J. Thromb. Haemost. 18 (2020) 2103–2109. doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed]
- 159.Hui D., Hladunewich M.A. Chronic kidney disease and pregnancy, Obstet. Gynecol. 2019;133:1182–1194. doi: 10.1097/AOG.0000000000003256. [DOI] [PubMed] [Google Scholar]
- 160.Williams D., Davison J. Pregnancy plus: chronic kidney disease in pregnancy. Bmj. 2008;336:211–215. doi: 10.1136/bmj.39406.652986.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bajpai D., Shah S. COVID-19 pandemic and pregnancy in kidney disease, Adv. Chronic Kidney Dis. 2020;27:397–403. doi: 10.1053/j.ackd.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Taghizadieh A., Mikaeili H., Ahmadi M., Valizadeh H. Acute kidney injury in pregnant women following SARS-CoV-2 infection: a case report from Iran, Respir. Med. Case Reports. 2020;30 doi: 10.1016/j.rmcr.2020.101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Suthar M.S., Diamond M.S., Gale M. West Nile virus infection and immunity. Nat. Rev. Microbiol. 2013;11:115–128. doi: 10.1038/nrmicro2950. [DOI] [PubMed] [Google Scholar]
- 164.Hamel R., Dejarnac O., Wichit S., Ekchariyawat P., Neyret A., Luplertlop N., Perera-Lecoin M., Surasombatpattana P., Talignani L., Thomas F., Cao-Lormeau V.-M., Choumet V., Briant L., Desprès P., Amara A., Yssel H., Missé D. Biology of Zika virus infection in human skin cells, J. Virol. 2015;89:8880–8896. doi: 10.1128/jvi.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.G.S. Campos, A.C. Bandeira, S.I. %J E. Infectious Diseases Sardi, Zika Virus Outbreak, Bahia, brazil, 21 (2015) 1885. [DOI] [PMC free article] [PubMed]
- 166.Martins M.M., Medronho R.D.A., Da Cunha A.J.L.A. Zika virus in Brazil and worldwide: a narrative review, Paediatr. Int. Child Health. 2020:1–8. doi: 10.1080/20469047.2020.1776044. [DOI] [PubMed] [Google Scholar]
- 167.Ioos S., Mallet H.P., Goffart I. Leparc, Gauthier V., Cardoso T., Herida M. Current Zika virus epidemiology and recent epidemics, Med. Mal. Infect. 2014;44:302–307. doi: 10.1016/j.medmal.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 168.Duffy M.R., Chen T.-H., Hancock W.T., Powers A.M., Kool J.L., Lanciotti R.S., Pretrick M., Marfel M., Holzbauer S., Dubray C., Guillaumot L., Griggs A., Bel M., Lambert A.J., Laven J., Kosoy O., Panella A., Biggerstaff B.J., Fischer M., Hayes E.B. Zika virus outbreak on Yap Island. Federated States of Micronesia, N. Engl. J. Med. 2009;360:2536–2543. doi: 10.1056/nejmoa0805715. [DOI] [PubMed] [Google Scholar]
- 169.L. Barzon, M. Trevisan, A. Sinigaglia, E. Lavezzo, G. Palù, Zika virus: from pathogenesis to disease control, FEMS Microbiol. Lett. 363 (2016) fnw202. doi: 10.1093/femsle/fnw202. [DOI] [PubMed]
- 170.Cao-Lormeau V.M., Blake A., Mons S., Lastère S., Roche C., Vanhomwegen J., Dub T., Baudouin L., Teissier A., Larre P., Vial A.L., Decam C., Choumet V., Halstead S.K., Willison H.J., Musset L., Manuguerra J.C., Despres P., Fournier E., Mallet H.P., Musso D., Fontanet A., Neil J., Ghawché F. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study, Obstet. Gynecol. Surv. 2016;71:451–452. doi: 10.1097/01.ogx.0000489564.35748.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Karimi O., Goorhuis A., Schinkel J., Codrington J., Vreden S.G.S., Vermaat J.S., Stijnis C., Grobusch M.P. Thrombocytopenia and subcutaneous bleedings in a patient with Zika virus infection. Lancet. 2016;387:939–940. doi: 10.1016/S0140-6736(16)00502-X. [DOI] [PubMed] [Google Scholar]
- 172.Gourinat A.C., O’Connor O., Calvez E., Goarant C., Dupont-Rouzeyrol M. Detection of zika virus in urine, Emerg. Infect. Dis. 2015;21:84–86. doi: 10.3201/eid2101.140894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Musso D., Roche C., Robin E., Nhan T., Teissier A., Cao-Lormeau V.M. Potential sexual transmission of zika virus, Emerg. Infect. Dis. 2015;21:359–361. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Baud D., Gubler D.J., Schaub B., Lanteri M.C., Musso D. An update on Zika virus infection. Lancet. 2017;390:2099–2109. doi: 10.1016/S0140-6736(17)31450-2. [DOI] [PubMed] [Google Scholar]
- 175.K.M. Christian, Song H., Ming G.L. Pathophysiology and mechanisms of Zika virus infection in the nervous system, Annu. Rev. Neurosci. 2019;42:249–269. doi: 10.1146/annurev-neuro-080317-062231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Silva N.M., Santos N.C., Martins I.C. Dengue and zika viruses: epidemiological history, potential therapies, and promising vaccines, trop. Med. Infect. Dis. 2020;5:150. doi: 10.3390/tropicalmed5040150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.A. Pastrana, M. Albarracín, M. Hoffmann, G. Delturco, R. López, R. Gil, A. Guzmán, M. Del Barco, A. %J A. argent. pediatr Espeche, Síndrome de Zika congénito en la Argentina: presentación de dos casos clínicos, Arch. Argent. Pediatr. 117 (2019) 635–639. doi: 10.5546/aap.2019.e635. [DOI] [PubMed]
- 178.T.V.B. de Araújo, L.C. Rodrigues, R.A. de Alencar Ximenes, D. de Barros Miranda-Filho, U.R. Montarroyos, A.P.L. de Melo, S. Valongueiro, M. de F.P.M. de Albuquerque, W.V. Souza, C. Braga, S.P.B. Filho, M.T. Cordeiro, E. Vazquez, D. Di Cavalcanti Souza Cruz, C.M.P. Henriques, L.C.A. Bezerra, P.M. da Silva Castanha, R. Dhalia, E.T.A. Marques-Júnior, C.M.T. Martelli, Association between Zika virus infection and microcephaly in Brazil, January to may, 2016: preliminary report of a case-control study, Lancet Infect. Dis. 16 (2016) 1356–1363. doi: 10.1016/S1473-3099(16)30318-8. [DOI] [PubMed]
- 179.Molnár Z., Kennedy S. Neurodevelopmental disorders: risks of Zika virus during the first trimester of pregnancy. Nat. Rev. Neurol. 2016;12:315–316. doi: 10.1038/nrneurol.2016.71. [DOI] [PubMed] [Google Scholar]
- 180.Ventura C.V., Maia M., Bravo-Filho V., Góis A.L., Belfort R. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet. 2016;387:228. doi: 10.1016/S0140-6736(16)00006-4. [DOI] [PubMed] [Google Scholar]
- 181.M.J. Kuehnert, S. V. Basavaraju, R.R. Moseley, L.L. Pate, S.A. Galel, P.C. Williamson, M.P. Busch, J.O. Alsina, C. Climent-Peris, P.W. Marks, J.S. Epstein, H.L. Nakhasi, J.P. Hobson, D.A. Leiby, P.N. Akolkar, L.R. Petersen, B. Rivera-Garcia, Screening of blood donations for Zika virus infection — Puerto Rico, April 3–June 11, 2016, MMWR. Morb. Mortal. Wkly. Rep. 65 (2016) 627–628. doi:10.15585/mmwr.mm6524e2. [DOI] [PubMed]
- 182.Pergolizzi J., LeQuang J.A., Umeda-Raffa S., Fleischer C., Pergolizzi J., Pergolizzi C., Raffa R.B. The Zika virus: lurking behind the COVID-19 pandemic? J. Clin. Pharm. Ther. 2020 doi: 10.1111/jcpt.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rosengarten R., Citti C., Glew M., Lischewski A., Droeße M., Much P., Winner F., Brank M., Spergser J. Host-pathogen interactions in mycoplasma pathogenesis: virulence and survival strategies of minimalist prokaryotes. Int. J. Med. Microbiol. 2000;290:15–25. doi: 10.1016/S1438-4221(00)80099-5. [DOI] [PubMed] [Google Scholar]
- 184.Benedetti F., Curreli S., Zella D. Mycoplasmas–host interaction: mechanisms of inflammation and association with cellular transformation. Microorganisms. 2020;8:1–21. doi: 10.3390/microorganisms8091351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.McGowin C.L., Totten P.A. The unique microbiology and molecular pathogenesis of Mycoplasma genitalium. J. Infect. Dis. 2017;216:S382–S388. doi: 10.1093/infdis/jix172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Murtha A.P., Edwards J.M. The role of Mycoplasma and Ureaplasma in adverse pregnancy outcomes, Obstet. Gynecol. Clin. North Am. 2014;41:615–627. doi: 10.1016/j.ogc.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 187.B. Larsen, J. Hwang, Mycoplasma, Ureaplasma, and adverse pregnancy outcomes: a fresh look, Infect. Dis. Obstet. Gynecol. 2010 (2010). doi: 10.1155/2010/521921. [DOI] [PMC free article] [PubMed]
- 188.Donders G.G.G., Ruban K., Bellen G., Petricevic L. Mycoplasma/Ureaplasma infection in pregnancy: To screen or not to screen. J. Perinat. Med. 2017;45:505–515. doi: 10.1515/jpm-2016-0111. [DOI] [PubMed] [Google Scholar]
- 189.Neuman H., Koren O. Nestle Nutr. Inst. Workshop Ser. Karger Publishers; 2017. The pregnancy microbiome; pp. 1–9. [DOI] [PubMed] [Google Scholar]
- 190.Nishikawa A., Mimura K., Kanagawa T., Maeda T., Tomimatsu T., Kimura T. Thrombocytopenia associated with Mycoplasma pneumonia during pregnancy: case presentation and approach for differential diagnosis. J. Obstet. Gynaecol. Res. 2015;41:1273–1277. doi: 10.1111/jog.12713. [DOI] [PubMed] [Google Scholar]
- 191.Goldenberg R.L., Culhane J.F., Iams J.D., Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.R. Romero, S.K. Dey, S.J. Fisher, Preterm labor: one syndrome, many causes, Science (80-.). 345 (2014) 760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed]
- 193.Martius J., Eschenbach D.A. The role of bacterial vaginosis as a cause of amniotic fluid infection, chorioamnionitis and prematurity - a review, Arch. Gynecol. Obstet. 1990;247:1–13. doi: 10.1007/BF02390649. [DOI] [PubMed] [Google Scholar]
- 194.Taylor-Robinson D., Lamont R.F. Mycoplasmas in pregnancy, BJOG An Int. J. Obstet. Gynaecol. 2011;118:164–174. doi: 10.1111/j.1471-0528.2010.02766.x. [DOI] [PubMed] [Google Scholar]
- 195.Peretz A., Tameri O., Azrad M., Barak S., Perlitz Y., Dahoud W.A., Ben-Ami M., Kushnir A. Mycoplasma and Ureaplasma carriage in pregnant women: the prevalence of transmission from mother to newborn. BMC Pregnancy Childbirth. 2020;20:456. doi: 10.1186/s12884-020-03147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Witt R.G., Blair L., Frascoli M., Rosen M.J., Nguyen Q.H., Bercovici S., Zompi S., Romero R., Mackenzie T.C. Detection of microbial cell-free DNA in maternal and umbilical cord plasma in patients with chorioamnionitis using next generation sequencing. PLoS One. 2020;15 doi: 10.1371/journal.pone.0231239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yin A. Hua, Peng C. Fang, Zhao X., Caughey B.A., Yang J. Xia, Liu J., Huang W. Wei, Liu C., Luo D. Hong, Liu H. Liang, Chen Y. Yi, Wu J., Hou R., Zhang M., Ai M., Zheng L., Xue R.Q., Mai M. Qin, Guo F. Fang, Qi Y. Ming, Wang D. Mei, Krawczyk M., Zhang D., Wang Y. Nan, Huang Q. Fei, Karin M., Zhang K. Noninvasive detection of fetal subchromosomal abnormalities by semiconductor sequencing of maternal plasma DNA, Proc. Natl. Acad. Sci. U. S. A. 2015;112:14670–14675. doi: 10.1073/pnas.1518151112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.DiGiulio D.B., Romero R., Amogan H.P., Kusanovic J.P., Bik E.M., Gotsch F., Kim C.J., Erez O., Edwin S., Relman D.A. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3 doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.K.J. Oh, S.M. Kim, J.S. Hong, E. Maymon, O. Erez, B. Panaitescu, N. Gomez-Lopez, R. Romero, B.H. Yoon, Twenty-four percent of patients with clinical chorioamnionitis in preterm gestations have no evidence of either culture-proven intraamniotic infection or intraamniotic inflammation, Am. J. Obstet. Gynecol. 216 (2017) 604.e1–604.e11. doi: 10.1016/j.ajog.2017.02.035. [DOI] [PMC free article] [PubMed]
- 200.da Costa A. Vasques, Goes C. Purcell, Gama P. Breastfeeding importance and its therapeutic potential against SARS-CoV-2. Physiol Rep. 2021;9 doi: 10.14814/phy2.14744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.P. Zimmermann, N. Curtis, Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections, Arch. Dis. Child. (2020) archdischild-2020-320338. doi: 10.1136/archdischild-2020-320338. [DOI] [PubMed]
- 202.Wang S., Guo L., Chen L., Liu W., Cao Y., Zhang J., Feng L. A case report of neonatal 2019 coronavirus disease in China, Clin. Infect. Dis. 2020;71:853–857. doi: 10.1093/cid/ciaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zeng L., Xia S., Yuan W., Yan K., Xiao F., Shao J., Zhou W. Vol. 174. JAMA Pediatr; in Wuhan, China: 2020. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19; pp. 722–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhu H., Wang L., Fang C., Peng S., Zhang L., Chang G., Xia S., Zhou W. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl. Pediatr. 2020;9:51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Goenka A., Kollmann T.R. Development of immunity in early life. J. Inf. Secur. 2015;71:S112–S120. doi: 10.1016/j.jinf.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 206.Chi H., Chiu N.C., Tai Y.L., Chang H.Y., Lin C.H., Sung Y.H., Tseng C.Y., Liu L.Y.M., Lin C.Y. Clinical features of neonates born to mothers with coronavirus disease-2019: a systematic review of 105 neonates. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Liu W., Cheng H., Wang J., Ding L., Zhou Z., Liu S., Chang L., Rong Z. Clinical analysis of neonates born to mothers with or without COVID-19: a retrospective analysis of 48 cases from two neonatal intensive care units in Hubei Province. Am. J. Perinatol. 2020;37:1317–1323. doi: 10.1055/s-0040-1716505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dang D., Wang L., Zhang C., Li Z., Wu H. Potential effects of SARS-CoV-2 infection during pregnancy on fetuses and newborns are worthy of attention. J. Obstet. Gynaecol. Res. 2020;46:1951–1957. doi: 10.1111/jog.14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Han M.S., Seong M.W., Heo E.Y., Park J.H., Kim N., Shin S., Cho S.I., Park S.S., Choi E.H. Sequential analysis of viral load in a neonate and her mother infected with severe acute respiratory syndrome coronavirus 2, Clin. Infect. Dis. 2020;71:2236–2239. doi: 10.1093/cid/ciaa447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.L. Schuler-Faccini, E.M. Ribeiro, I.M.L. Feitosa, D.D.G. Horovitz, D.P. Cavalcanti, A. Pessoa, M.J.R. Doriqui, J.I. Neri, J.M. de P. Neto, H.Y.C. Wanderley, M. Cernach, A.S. El-Husny, M.V.S. Pone, C.L.C. Serao, M.T. V. Sanseverino, Possible association between Zika virus infection and microcephaly — Brazil, 2015, MMWR Morb. Mortal. Wkly Rep. 65 (2016) 59–62. doi:10.15585/mmwr.mm6503e2. [DOI] [PubMed]
- 211.Rasmussen S.A., Jamieson D.J., Honein M.A., Petersen L.R. Zika virus and birth defects — reviewing the evidence for causality, N. Engl. J. Med. 2016;374:1981–1987. doi: 10.1056/nejmsr1604338. [DOI] [PubMed] [Google Scholar]
- 212.Moore C.A., Staples J.E., Dobyns W.B., Pessoa A., Ventura C.V., Da Fonseca E.B., Ribeiro E.M., Ventura L.O., Neto N.N., Arena J.F., Rasmussen S.A. Characterizing the pattern of anomalies in congenital zika syndrome for pediatric clinicians. JAMA Pediatr. 2017;171:288–295. doi: 10.1001/jamapediatrics.2016.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Moreira M.E. Lopes, Nielsen-Saines K., Brasil P., Kerin T., Damasceno L., Pone M., Carvalho L.M.A., Pone S.M., Vasconcelos Z., Ribeiro I.P., Zin A.A., Tsui I., Adachi K., Gaw S.L., Halai U.-A., Salles T.S., da Cunha D.C., Bonaldo M.C., Gabaglia C. Raja, Guida L., Malacarne J., Costa R.P., Gomes S.C., Reis A.B., Soares F.V.M., Hasue R.H., Aizawa C.Y.P., Genovesi F.F., Aibe M., Einspieler C., Marschik P.B., Pereira J.P., Portari E.A., Janzen C. J.D. Cherry, Neurodevelopment in infants exposed to Zika virus in utero. 2018;N. Engl. J. Med. 379:2377–2379. doi: 10.1056/nejmc1800098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Rasmussen S.A., Meaney-Delman D.M., Petersen L.R., Jamieson D.J. Studying the effects of emerging infections on the fetus: experience with West Nile and Zika viruses. Birth Defects Res. 2017;109:363–371. doi: 10.1002/bdr2.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Grischott F., Puhan M., Hatz C., Schlagenhauf P. Non-vector-borne transmission of Zika virus: a systematic review, Travel Med. Infect. Dis. 2016;14:313–330. doi: 10.1016/j.tmaid.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 216.Blohm G.M., Lednicky J.A., Márquez M., White S.K., Loeb J.C., Pacheco C.A., Nolan D.J., Paisie T., Salemi M., Rodríguez-Morales A.J., Morris J. Glenn, Pulliam J.R.C., Paniz-Mondolfi A.E. Evidence for mother-to-child transmission of Zika virus through breast Milk, Clin. Infect. Dis. 2018;66:1120–1121. doi: 10.1093/cid/cix968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.V. de M. Marques, C.S. Santos, I.G. Santiago, S.M. Marques, M. das G. Nunes Brasil, T.T. Lima, P.S. Costa, Neurological complications of congenital Zika virus infection, Pediatr. Neurol. 91 (2019) 3–10. doi: 10.1016/j.pediatrneurol.2018.11.003. [DOI] [PubMed]
- 218.C.L. Sampieri, H. Montero, Breastfeeding in the time of Zika: a systematic literature review, PeerJ. 2019 (2019) e6452. doi: 10.7717/peerj.6452. [DOI] [PMC free article] [PubMed]
- 219.Coyne C.B., Lazear H.M. Zika virus-reigniting the TORCH. Nat. Rev. Microbiol. 2016;14:707–715. doi: 10.1038/nrmicro.2016.125. [DOI] [PubMed] [Google Scholar]
- 220.Williamson W.D., Percy A.K., Yow M.D., Gerson P., Catlin F.I., Koppelman M.L. Asymptomatic congenital cytomegalovirus infection: audiologic, neuroradiologic, and neurodevelopmental abnormalities during the first year. Am. J. Dis. Child. 1990;144:1365–1368. doi: 10.1001/archpedi.1990.02150360091031. [DOI] [PubMed] [Google Scholar]
- 221.J. Stevens, H. Lyall, Mother to child transmission of HIV: what works and how much is enough?, J. Inf. Secur. 69 Suppl 1 (2014) S56–62. doi: 10.1016/j.jinf.2014.07.018. [DOI] [PubMed]
- 222.S.H. James, J.S. Sheffield, D.W. Kimberlin, Mother-to-child transmission of herpes simplex virus, J Pediatr. Infect Dis Soc. 3 Suppl 1 (2014) S19–23. doi: 10.1093/jpids/piu050. [DOI] [PMC free article] [PubMed]
- 223.Pinninti S.G., Kimberlin D.W. Maternal and neonatal herpes simplex virus infections. Am. J. Perinatol. 2013;30:113–119. doi: 10.1055/s-0032-1332802. [DOI] [PubMed] [Google Scholar]
- 224.Honein M.A., Woodworth K.R., Gregory C.J. Neurodevelopmental abnormalities associated with in utero Zika virus infection in infants and children-the unfolding story. JAMA Pediatr. 2020;174:237–238. doi: 10.1001/jamapediatrics.2019.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Waites K.B., Katz B., Schelonka R.L. Mycoplasmas and ureaplasmas as neonatal pathogens, Clin. Microbiol. Rev. 2005;18:757–789. doi: 10.1128/CMR.18.4.757-789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Cassell G.H., Davis R.O., Waites K.B., Brown M.B., Marriott P.A., Stagno S., Davis J.K. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16-20 weeks of gestation: potential effect on outcome of pregnancy. Sex. Transm. Dis. 1983;10:294–302. https://www.ncbi.nlm.nih.gov/pubmed/6665671 [PubMed] [Google Scholar]
- 227.Kafetzis D.A., Skevaki C.L., Skouteri V., Gavrili S., Peppa K., Kostalos C., Petrochilou V., Michalas S. Maternal genital colonization with Ureaplasma urealyticum promotes preterm delivery: association of the respiratory colonization of premature infants with chronic lung disease and increased mortality. Clin. Infect. Dis. 2004;39:1113–1122. doi: 10.1086/424505. [DOI] [PubMed] [Google Scholar]
- 228.Chua K.B., Ngeow Y.F., Lim C.T., Ng K.B., Chye J.K. Colonization and transmission of Ureaplasma urealyticum and Mycoplasma hominis from mothers to full and preterm babies by normal vaginal delivery. Med J Malaysia. 1999;54:242–246. https://www.ncbi.nlm.nih.gov/pubmed/10972036 [PubMed] [Google Scholar]
- 229.Chun J., Chun S.H., Han Y.S., Sung T.J. Different degrees of maternal Ureaplasma colonization and its correlation with bronchopulmonary dysplasia in <32 weeks’ preterm infants, Pediatr. Neonatol. 2019;60:441–446. doi: 10.1016/j.pedneo.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 230.Kersin S.G., Bozan T., Ozdemir H., Bilgen H.S., Soyletir G., Memisoglu A., Ozek E. Clinical and laboratory awareness for an under recognized pathogen in newborn meningitis: Mycoplasma Hominis: a case report. Turk. J. Pediatr. 2020;62:280–283. doi: 10.24953/turkjped.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 231.Cassell G.H., Waites K.B., Watson H.L., Crouse D.T., Harasawa R. Ureaplasma urealyticum intrauterine infection: role in prematurity and disease in newborns, Clin. Microbiol. Rev. 1993;6:69–87. doi: 10.1128/CMR.6.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Foulon W., Naessens A., Dewaele M., Lauwers S., Amy J.J. Chronic Ureaplasma urealyticum amnionitis associated with abruptio placentae, Obs. Gynecol. 1986;68:280–282. https://www.ncbi.nlm.nih.gov/pubmed/3488530 [PubMed] [Google Scholar]
- 233.Alonso-Vega C., Wauters N., Vermeylen D., Muller M.F., Serruys E. A fatal case of Mycoplasma hominis meningoencephalitis in a full-term newborn. J. Clin. Microbiol. 1997;35:286–287. doi: 10.1128/JCM.35.1.286-287.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Mignard S., Flandrois J.P. 16S rRNA sequencing in routine bacterial identification: a 30-month experiment. J. Microbiol. Methods. 2006;67:574–581. doi: 10.1016/j.mimet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 235.Wildenbeest J.G., Said I., Jaeger B., van Hest R.M., van de Beek D., Pajkrt D. Neonate with Mycoplasma hominis meningoencephalitis given moxifloxacin. Lancet Infect. Dis. 2016;16:e261–e266. doi: 10.1016/S1473-3099(16)30162-1. [DOI] [PubMed] [Google Scholar]
- 236.Nicolson G.L. Chronic bacterial and viral infections in neurodegenerative and neurobehavioral diseases, lab. Med. 2008;39:291–299. doi: 10.1309/96M3BWYP42L11BFU. [DOI] [Google Scholar]
- 237.Nicolson G.L. Pathogenic mycoplasma infections in chronic illnesses: general considerations in selecting conventional and integrative treatments. Int. J. Clin. Med. 2019;10:477–522. doi: 10.4236/ijcm.2019.1010041. [DOI] [Google Scholar]