Abstract
In light of the shift toward patient-centric clinical trials, a measure of simplifying blood collection process and minimizing the volume of blood samples is on the rise. Volumetric absorptive microsampling (VAMS) is a microsampling device developed for blood sampling in non-hospital settings, which enables accurate hematocrit-independent collection of 10 or 20 µL of whole blood with a simple finger prick. In this study, liquid chromatography (LC)-tandem mass spectrometry workflow for quantification of rosuvastatin after VAMS sampling was developed and validated. The VAMS sample was stabilized by matrix drying and the optimum LC conditions and extraction methods were used to reach adequate sensitivity with lower limit of quantification verified at 1 ng/mL in 10 µL of blood. The bioanalytical method to quantify rosuvastatin from 1 to 100 ng/mL in VAMS sample was qualified by specificity, carryover, linearity, within-run and between-run reproducibility and stability. Inaccuracy was less than ± 6% and imprecision was less than 10% after analyzing the samples on 5 different days at all concentration levels. In addition, the feasibility of delivery to the analytical laboratory after home sampling during the guaranteed stability period of 10 days at room temperature was confirmed by evaluating concentration changes after VAMS sampling without adding pH buffer. Our results suggest that VAMS sampling did not have an effect on the stability of rosuvastatin, and it is a viable option for simple and accurate blood collection at home.
Keywords: Blood Specimen Collection; Rosuvastatin; Chemistry Techniques, Analytical; Tandem Mass Spectrometry; Patient-Centered Care
INTRODUCTION
Hypercholesterolemia increases the risk of ischemic heart disease and stroke, which is estimated to cause 2.6 million deaths worldwide annually [1]. In the United States, more than 12% of all adults and 7% of children and adolescents were found to have hypercholesterolemia in a 2015–2016 survey [2]. Since hypercholesterolemia does not present itself with symptoms, evaluation of blood cholesterol level and cardiovascular risk management are necessary. Since 1980, the American College of Cardiology and American Heart Association have been publishing clinical practice guidelines to improve cardiovascular health; the latest guideline was published in 2018 [3]. According to the guideline, statins are the primary treatment for hypercholesterolemia along with lifestyle modification; there are other non-statin lipid-lowering drugs available such as ezetimibe, but statins remain the cornerstone of the hypercholesterolemia therapy.
Rosuvastatin, a synthetic 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitor, is effective in decreasing the level of low-density lipoprotein-cholesterol in blood among various statin drugs. It can also decrease the levels of total cholesterol, triglycerides, and apolipoprotein B, and is effective in increasing the level of high-density lipoprotein-cholesterol [4,5,6]. It is rapidly absorbed after oral administration, where the maximum plasma concentration is achieved within 3 to 5 hours. About 90% of rosuvastatin is bound to plasma proteins, mostly albumin, and is primarily eliminated by fecal excretion with an elimination half-life of about 19 hours. Although not extensively metabolized, approximately 10% of rosuvastatin undergoes metabolism and the major metabolite, N-desmethyl rosuvastatin, is formed by cytochrome P450 2C9 [7]. Previous bioanalytical methods developed to quantify the concentration of rosuvastatin in clinical trials with conventional blood sampling need more than 200 µL of plasma as analytes, which leads to extensive blood sampling [8,9,10,11]. Conventional blood sampling from veins is done by healthcare professionals, and after the samples are centrifuged, plasma analytes are mixed with sodium acetate buffer and stored in −70°C. This process is tedious and often can only be done in hospital-oriented clinical trials, presenting a major hindrance to realization of patient-centric clinical trials. Because patient-centricity is becoming increasingly important in clinical trials due to difficulties in patient recruitment and compliance maintenance, there is an unmet need to simplify the blood sampling process. To accomplish this, minimizing the blood sample volumes is crucial, along with minimization of time to sample blood and lessening of the pain associated with blood sampling.
Volumetric absorptive microsampling (VAMS) is a hematocrit-independent microsampling technique developed for patients that enables them to sample blood at their home without necessitating hospital visit. The process of pricking one's fingertip with a lancet, allowing the tip of the VAMS sampler to directly touch the drop of blood on the fingertip, and collecting capillary blood within 3 seconds, is simple and less invasive compared to conventional blood sampling from veins. Reproducible sampling of a small amount of blood (10 µL) is also expected to be useful in collecting blood from children and patients with severe anemia [12,13,14]. The samples collected by VAMS can be dried and stored at room temperature (RT) and can be shipped to bioanalytical companies without extra cost for storage and shipping at low temperatures. VAMS is expected to have advantages of accurate and non-invasive blood volume collection in a hematocrit-independent manner, thereby enabling patient-centric and cost-effective clinical trials to be conducted [15,16]. To quantify the drug concentration from small amount of blood samples collected by VAMS, there is a need for liquid chromatography-tandem mass spectrometry (LC-MS/MS) bioanalytical method with adequate sensitivity. However, only a small number of studies have been published on development of optimized extraction and pre-treatment methods for various drugs collected by VAMS [17,18,19]. The objective of this study was to develop and validate a bioanalytical method using LC-MS/MS to measure rosuvastatin in 10 µL of blood collected by VAMS. To achieve this objective, the LC-MS/MS method with adequate sensitivity and reproducibility was developed and validated, and subsequent evaluation of the proposed method's recovery rate and stability was carried out.
MATERIALS AND METHODS
Chemicals and instruments
Rosuvastatin calcium (purity ≥ 98%) was obtained from Futoro Laboratories Inc. (Pimple Saudagar, India). Carbamazepine, used as the internal standard (IS), was obtained from Toronto Research Chemicals Inc. (Toronto, Canada). Formic acid, acetic acid and sodium acetate were purchased from Sigma-Aldrich (St. Louis, MO, USA). HPLC-grade methanol and acetonitrile were purchased from Thermo Fisher Scientific (Waltham, MA, USA). VAMS devices (Mitra™, 100601-B 10 µL tips in 96-autorack) were purchased from Neoteryx (Torrance, CA, USA). Analytical balance (CP224S) and the pH meter (PP-15) were obtained from Sartorius (Göttingen, Germany).
Chromatographic and mass spectrometric conditions
The samples were measured using an Agilent HPLC 1100 series (Santa Clara, CA, USA) coupled with AB Sciex 4000 QTRAP mass spectrometer (Foster City, CA, USA). Chromatographic analysis was performed on a Phenomenex Kinetex C18 column (50 × 2.1 mm, 2.6 µm) (Torrance, California, USA) maintained at 20°C. Aqueous formic acid (0.1%) (buffer A) and methanol (buffer B) were used as mobile phase at a flow rate of 0.2 mL/min. Accordingly, the composition of the mobile phase with formic acid-water (0.1%) was chosen in order to lower the pH to protonate the acidic rosuvastatin. Gradient elution started at 80% of B, followed by a linear increase of B to 50% within 0.5 minutes and maintained up to 2.0 minutes. Thereafter, the %B was reversed to the initial composition (80%) up to 5.0 minutes. Multiple reaction monitoring (MRM) was carried out at the positive electrospray mode, the MRM transition pairs (precursor ion/product ion) were m/z 482.3/258.2 for rosuvastatin and m/z 237.3/194.1 for carbamazepine (Fig. 1). MRM parameters were optimized and following ionization conditions were obtained. The ion source gas 1, 2 were set at 40 L/min, with source temperature at 400°C. The ion spray voltage, declustering potential, collision energy and collision cell exit potential were set at 5,500 V, 107.9 V, 48.35 eV, and 15.69 V, respectively. Peak area ratios were calculated using AB Sciex Analyst® 1.7 (Foster City, CA, USA).
Figure 1. Precursor and product ion spectra of rosuvastatin (A) and the internal standard (B).
Preparation of standards and quality control (QC) samples on VAMS
Stock solutions of rosuvastatin and IS were prepared by dissolving in 50% methanol at 1 mg/mL and stored at −20°C. Working standard solutions were prepared by diluting with 50% methanol to yield a concentration of 10, 20, 50, 100, 200, 400, 800, and 1,000 ng/mL. Working solution of IS was prepared by diluting with 50% methanol to yield a concentration of 100 ng/mL. Sodium acetate buffer (pH 4.0; 0.2 M) as pH stabilizer was added in pooled drug-free whole blood in the ratio of 3:1 (v/v). Calibration curves with final concentrations of 1, 2, 5, 10, 20, 40, 80, and 100 ng/mL were made by spiking of the diluted blood. QC samples were prepared at 3 concentration levels representing the low QC (LQC, 1 ng/mL), medium QC (40 ng/mL), and high QC (HQC, 80 ng/mL), respectively. VAMS samples were prepared from spiked blood to which pH stabilizer had been added. The hydrophilic polymeric tip of VAMS absorbed blood by capillary action. The VAMS tips were dried for 1 hour at RT.
Extraction methods for VAMS samples
VAMS tips, which collected blood spiked to QC levels, were dried at RT for 1 hour. VAMS tip and 10 µL of IS were placed in a tube, and then extracted with 250 µL of methanol or acetonitrile as the extraction solvent. The tubes were vortexed at 1,000 rpm for 10 minutes at RT. In addition, the sonication was added at RT for 10 minutes to increase the solubility, and the extraction recovery of the analyte was compared. The supernatant (200 µL) was dispensed into a new tube, with vacuum evaporated at 45°C and reconstituted with 50 µL of 50% methanol. The optimized reproducible extraction methods were performed under the following conditions to identify suitable solvents for desorbing rosuvastatin from dried blood collected on VAMS tips: 1) methanol-50% methanol solvents including vortexing; 2) methanol-50% methanol solvents including sonication after vortexing; 3) acetonitrile-50% methanol solvents including vortexing; and 4) acetonitrile-50% methanol solvents including sonication after vortexing. Solvent extraction recovery of VAMS samples was calculated by comparing the analyte and IS peak areas with those of the standard solution injected at the same concentration. The extraction rate did not have to be 100%, but it must be consistent and reproducible.
Bioanalytical method validation
Specificity, carryover, linearity, within-run and between-run reproducibility and stability were evaluated to validate the assay developed for rosuvastatin measurement in VAMS with pH stabilizer added. The optimized pre-treatment method including sonication was applied. Weighted linear regression with 1/x as the weighting factor was selected. Sensitivity refers to the lower limit of quantification (LLOQ), which is the lowest concentration on the calibration curve, where the response of the analyte must be at least 5 times the blank sample. Specificity was confirmed when the response of interfering substances in the biological samples taken from at least 6 people was less than 20% of the analyte LLOQ and less than 5% of the IS. Carryover was performed by injecting a blank sample after the maximum concentration of calibration curve (upper limit of quantification [ULOQ]). In the blank sample, the response of analyte should be less than 20% and less than 5% for the IS. Accuracy was evaluated by analyzing the QC samples with 5 replicates on the same day (within-run) or within 5 consecutive days (between-run), and confirming the coefficient of variation (CV) values to be within 15%. For the LLOQ concentrations, bias (%) of accuracy and precision were considered acceptable if within 20%. Stability was confirmed according to IS operating procedures and the Ministry of Food and Drug Safety Guidelines for Bioanalytical Method Validation [20]. The stability of rosuvastatin stored as dried blood on VAMS was evaluated by LQC and HQC in triplicate. The stability at the autosampler was evaluated by extracting sample in autosampler for 24 hours at 4°C. Bench-top stability of VAMS samples was evaluated after maintaining the laboratory handling conditions at RT for 5 hours. Freeze-thaw stability was evaluated after 3 freeze and thaw cycles. Short-term and long-term stability were evaluated in VAMS samples that were stored for 1 hour and 30 days after 1 hour of drying at RT, respectively.
Stability evaluation
Long term stability of rosuvastatin in dried blood collected by VAMS at RT, without pH stabilizer added, was assessed up to 10 days after sampling because VAMS samples were expected to be stored at home and shipped to an analytical laboratory within 7 days [21]. VAMS sampling was performed and, without adding pH stabilizer to imitate the sampling process done at home, the samples were dried and stored at RT for 1, 3, 7, and 10 days. The concentration of rosuvastatin in VAMS samples stored for each period was compared with the initial concentration (concentration value after drying for 1 hour); the measured rosuvastatin concentration was required to be higher than 85% of the initial concentration in order to conclude sample stability.
RESULTS
Extraction recovery
Extraction recovery tests were performed under 4 conditions (as described in section ‘Extraction methods for VAMS samples’) to identify suitable extraction solvents for desorbing rosuvastatin from dried blood collected on VAMS tips and to optimize reproducible extraction methods. Similar extraction efficiencies were observed at the 3 concentrations of QC levels for each condition, as shown in Fig. 2. The %recovery of pre- and post-sonicated extraction were 70.62–91.83% and 102.75–117.33%, respectively, showing improved recovery when sonication was added; and the accuracy was also adequate since the value of CV was within 10% when methanol was used as the extraction solvent (Fig. 2A). On the other hand, using acetonitrile as the extraction solvent, the %recovery of pre- and post-sonicated extraction was 1.91–4.03% and 3.35–4.12%, respectively, and it was deemed as not reproducible, with CV exceeding 15% (Fig. 2B).
Figure 2. Solvent-dependent apparent extraction recovery of rosuvastatin at QC levels in dried blood on volumetric absorptive microsampling. Rosuvastatin was extracted by methanol (A) and acetonitrile (B) by means of vortexing only (pre-sonication, purple bar) and sonication combined with vortexing (post-sonication, pink bar). Data represent the arithmetic mean ± standard deviation of 3 replicates.
LQC, low quality control; MQC, medium quality control; HQC, high quality control.
Validation of VAMS assays for rosuvastatin in human blood
Ion chromatograms of rosuvastatin and IS at LLOQ (1 ng/mL) are shown in Fig. 3. The analyte response at LLOQ was at least 5 times greater than the blank sample. Retention time of rosuvastatin was 0.9 minutes. The calibration curves of standards in the range of 1–100 ng/mL were fitted by linear-order calibration curves weighted 1/x, with r > 0.999 (Table 1). Specificity assessment showed no significant interferences, and no continuous carryover reaction was observed when residual analyte or IS was injected after ULOQ. At the QC level, accuracy of within- and between-run was 94.4–100.64% and 98.64–102.4%, respectively, and was within the acceptance criteria. Also, the precision was less than 10% and within the acceptance criteria (Table 2). To confirm that all steps and storage conditions of the assay did not affect the stability of the analyte, the results of the method stability validation were compared, and the comparison showed that rosuvastatin in the VAMS sample was within 15% of the theoretical value for each stability test condition (Table 3).
Figure 3. Ion chromatogram at lower limit of quantification. (A) Double blank, (B) rosuvastatin (1 ng/mL), and (C) internal standard (10 ng/mL).
Table 1. Calibration data (range, 1–100 ng/mL) for the determination of rosuvastatin in volumetric absorptive microsampling samples using liquid chromatography-tandem mass spectrometry.
| Assay | Slope | Intercept | r |
|---|---|---|---|
| Within-run | 0.0141 ± 0.0010 | 0.0021 ± 0.0011 | 0.9991 |
| Between-run | 0.0233 ± 0.0011 | 0.0026 ± 0.0020 | 0.9992 |
Data are reported as the arithmetic mean ± standard deviation of 5 replicates.
Table 2. Within- and between-run accuracy and precision of rosuvastatin in dried blood on volumetric absorptive microsampling.
| Accuracy (%) | %CV | |||||
|---|---|---|---|---|---|---|
| LQC | MQC | HQC | LQC | MQC | HQC | |
| Within-run | 94.4 | 100.64 | 99.76 | 3.84 | 5.29 | 3.34 |
| Between-run | 98.64 | 99.77 | 102.4 | 9.26 | 5.50 | 4.54 |
CV, coefficient of variation; LQC, low quality control; MQC, medium quality control; HQC, high quality control.
Table 3. Stability of rosuvastatin on volumetric absorptive microsampling under various stability conditions of 3 replicates.
| Validation assessments | Stability Condition | Accuracy (%) | %CV | ||
|---|---|---|---|---|---|
| LQC | HQC | LQC | HQC | ||
| Bench top | RT (5 hr) | 110.33 | 104.33 | 0.43 | 3.16 |
| Autosampler | 4°C (24 hr) | 100.53 | 97.10 | 7.12 | 3.09 |
| Freeze and thaw | −70°C (3 cycles) | 112.00 | 101.90 | 0.73 | 1.72 |
| Short-term | RT (1 hr) | 102.17 | 102.77 | 4.77 | 5.77 |
| Long-term | RT (30 days) | 90.67 | 99.37 | 6.99 | 0.21 |
CV, coefficient of variation; RT, room temperature; LQC, low quality control; HQC, high quality control.
Stability
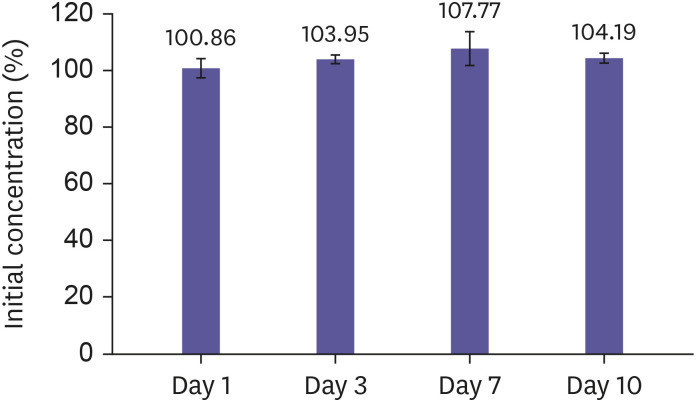
Stability at RT was evaluated without addition of pH stabilizer. The rosuvastatin concentrations as expressed as the percentage of initial rosuvastatin concentration as time passed were 100.83, 103.96, 100.71, and 104.17% after storage of 1, 3, 7, and 10 days, respectively (Fig. 4). The results showed no significant decrease in concentration after storage at RT for 10 days.
Figure 4. Stability of rosuvastatin on volumetric absorptive microsampling at room temperature for 1 day, 3 days, 7 days, and 10 days. Data are represented as arithmetic mean ± standard deviation of 3 replicates.
DISCUSSION
The LC-MS/MS method of this study to analyze rosuvastatin concentration from 10 µL of diluted blood collected by VAMS showed more than 85% of extraction recovery and adequate precision and accuracy within 15% of actual value within the concentration range of 1–100 ng/mL. An ideal IS selected to guarantee high accuracy of LC-MS/MS assay should be extracted with adequate reproducibility. There should not be significant interfering peak observed during analysis, and the IS should be eluted close to the analyte on the column in similar chemical property. Hence carbamazepine, with suitable retention time, acceptable matrix effects, and extraction recoveries, was selected as the IS in this study [8,22]. Previous methods for rosuvastatin concentration analysis required retention time of up to 4.13 minutes, analyte volume of up to 1,700 µL and solvent volume of up to 5 mL [23,24,25]. This new bioanalytic method, compared to the previous method, has the retention times of rosuvastatin and IS within 1 minute, and requires only one tenth of the analyte and solvent volume.
Rosuvastatin belongs to Biopharmaceutics Classification System Class II drugs, which is a classification of drug substances based on aqueous solubility and intestinal permeability, which means that rosuvastatin shows low solubility and high permeability. In order to effectively extract drugs from VAMS porous tips, methanol was used as a suitable solvent for desorbing rosuvastatin from dried blood collected on VAMS tips. In addition, the extraction method including sonication treatment has proven to be an effective way to obtain consistently high extraction rates compared to vortexing. Sonication of liquids produces the sound waves that propagate into the liquid, which results in cycles with different pressures; small vacuum bubbles created during low pressure cycles eventually collapse during high pressure cycles [26]. This phenomenon is a method of improving physical solubility due to impact force when a bubble is crushed and dissipated under sound waves [27].
Conventional bioanalytical methods of rosuvastatin concentration measurement in blood have added buffers such as 0.1 M sodium acetate and 25% ammonia solution to stabilize rosuvastatin, because of the possibility of pH-dependent interconversion of lactone ring present in rosuvastatin metabolites by carboxylesterase through hydrolysis. A study had specifically evaluated the extent of lactone ring-containing metabolite (rosuvastatin 5S-lactone) converted to parent drug according to different pre-treatment pH [23]; further studies of interconversion of lactone to rosuvastatin are needed to evaluate the effect of pre-treatment pH on rosuvastatin metabolite in samples collected by VAMS. Meanwhile, matrix drying has been reported to reduce drug degradation in the blood by hydrolysis and increase drug stability [28,29]. The same approach was applied in the current study, and as a result, VAMS samples without pH stabilizer added that were stored in dried state to prevent reverse pH-dependent interconversion of rosuvastatin, were found to be stable. These results suggest that blood sampling at home using VAMS could be applied in clinical trials, and the VAMS samples could be delivered to the analytical laboratory for extraction within a guaranteed stability period at RT.
In conclusion, a bioanalytical method of rosuvastatin concentration analysis, using LC-MS/MS, from 10 µL of whole blood collected on VAMS was developed and validated with adequate reproducibility, reliability and sensitivity. Sample collection was convenient, and the dried samples remained stable in RT for 10 days after sampling without adding pH buffer. The fully validated LC-MS/MS method may be applied to clinical trials including, but not limited to, bioequivalence and pharmacokinetic studies of rosuvastatin to bolster patient-centricity.
Footnotes
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2017R1C1B2011095).
Reviewer: This article was reviewed by peer experts who are not TCP editors.
Conflict of Interest: - Authors: Nothing to declare
- Reviewers: Nothing to declare
- Editors: Nothing to declare
- Conceptualization: Kim MG.
- Formal analysis: Lee SE.
- Methodology: Lee SE, Kim MG.
- Writing - original draft: Moon SJ, Lee SE.
- Writing - review & editing: Moon SJ, Kwak YG, Kim MG.
References
- 1.World Health Organization. Global Health Observatory (GHO) data, raised cholesterol: situation and trends [Internet] [Accessed March 24, 2020]. https://www.who.int/gho/ncd/risk_factors/cholesterol_text/en/
- 2.Carroll MD, Kit BK, Lacher DA. Total and high-density lipoprotein cholesterol in adults: National Health and Nutrition Examination Survey, 2009–2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 3.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 4.McTaggart F. Comparative pharmacology of rosuvastatin. Atheroscler Suppl. 2003;4:9–14. doi: 10.1016/s1567-5688(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 5.Walley T, Folino-Gallo P, Stephens P, Van Ganse E. Trends in prescribing and utilization of statins and other lipid lowering drugs across Europe 1997–2003. Br J Clin Pharmacol. 2005;60:543–551. doi: 10.1111/j.1365-2125.2005.02478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortese F, Gesualdo M, Cortese A, Carbonara S, Devito F, Zito A, et al. Rosuvastatin: beyond the cholesterol-lowering effect. Pharmacol Res. 2016;107:1–18. doi: 10.1016/j.phrs.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration. Crestor (rosuvastatin calcium) tablets [Internet] [Accessed March 24, 2020]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021366s016lbl.pdf.
- 8.Trivedi RK, Kallem RR, Mullangi R, Srinivas NR. Simultaneous determination of rosuvastatin and fenofibric acid in human plasma by LC-MS/MS with electrospray ionization: assay development, validation and application to a clinical study. J Pharm Biomed Anal. 2005;39:661–669. doi: 10.1016/j.jpba.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Gao J, Zhong D, Duan X, Chen X. Liquid chromatography/negative ion electrospray tandem mass spectrometry method for the quantification of rosuvastatin in human plasma: application to a pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;856:35–40. doi: 10.1016/j.jchromb.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Kumar PP, Murthy TE, Basaveswara Rao MV. Development, validation of liquid chromatography-tandem mass spectrometry method for simultaneous determination of rosuvastatin and metformin in human plasma and its application to a pharmacokinetic study. J Adv Pharm Technol Res. 2015;6:118–124. doi: 10.4103/2231-4040.157982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin PD, Warwick MJ, Dane AL, Brindley C, Short T. Absolute oral bioavailability of rosuvastatin in healthy white adult male volunteers. Clin Ther. 2003;25:2553–2563. doi: 10.1016/s0149-2918(03)80316-8. [DOI] [PubMed] [Google Scholar]
- 12.Velghe S, Stove CP. Volumetric absorptive microsampling as an alternative tool for therapeutic drug monitoring of first-generation anti-epileptic drugs. Anal Bioanal Chem. 2018;410:2331–2341. doi: 10.1007/s00216-018-0866-4. [DOI] [PubMed] [Google Scholar]
- 13.Protti M, Mandrioli R, Mercolini L. Tutorial: Volumetric absorptive microsampling (VAMS) Anal Chim Acta. 2019;1046:32–47. doi: 10.1016/j.aca.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y, Jeon JY, Han SH, Ha N, Jang K, Kim MG. Quantitative analysis of acetylsalicylic acid in human blood using volumetric absorptive microsampling. Transl Clin Pharmacol. 2018;26:32–38. doi: 10.12793/tcp.2018.26.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nys G, Cobraiville G, Kok MG, Wéra O, Servais AC, Fillet M. Comparison of nanofluidic and ultra-high performance liquid chromatography-tandem mass spectrometry for high sensitive pharmacokinetic studies of estrogens starting from whole blood microsampling. J Chromatogr A. 2017;1524:160–168. doi: 10.1016/j.chroma.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Qu Y, Brady K, Apilado R, O'Malley T, Reddy S, Chitkara P, et al. Capillary blood collected on volumetric absorptive microsampling (VAMS) device for monitoring hydroxychloroquine in rheumatoid arthritis patients. J Pharm Biomed Anal. 2017;140:334–341. doi: 10.1016/j.jpba.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 17.Marahatta A, Megaraj V, McGann PT, Ware RE, Setchell KD. Stable-isotope dilution HPLC-electrospray ionization tandem mass spectrometry method for quantifying hydroxyurea in dried blood samples. Clin Chem. 2016;62:1593–1601. doi: 10.1373/clinchem.2016.263715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen IKL, Rosting C, Gjelstad A, Halvorsen TG. Volumetric absorptive MicroSampling vs. other blood sampling materials in LC-MS-based protein analysis - preliminary investigations. J Pharm Biomed Anal. 2018;156:239–246. doi: 10.1016/j.jpba.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 19.Xie I, Xu Y, Anderson M, Wang M, Xue L, Breidinger S, et al. Extractability-mediated stability bias and hematocrit impact: high extraction recovery is critical to feasibility of volumetric adsorptive microsampling (VAMS) in regulated bioanalysis. J Pharm Biomed Anal. 2018;156:58–66. doi: 10.1016/j.jpba.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Ministry of Food and Drug Safety. Guideline on bioanalytical method validation. Cheongju: Ministry of Food and Drug Safety; 2013. [Google Scholar]
- 21.John H, Willoh S, Hörmann P, Siegert M, Vondran A, Thiermann H. Procedures for analysis of dried plasma using microsampling devices to detect sulfur mustard-albumin adducts for verification of poisoning. Anal Chem. 2016;88:8787–8794. doi: 10.1021/acs.analchem.6b02199. [DOI] [PubMed] [Google Scholar]
- 22.Bai X, Wang XP, He GD, Zhang B, Huang M, Li JL, et al. Simultaneous determination of rosuvastatin, rosuvastatin-5 S-lactone, and N-desmethyl rosuvastatin in human plasma by UPLC-MS/MS and its application to clinical study. Drug Res (Stuttg) 2018;68:328–334. doi: 10.1055/s-0043-123576. [DOI] [PubMed] [Google Scholar]
- 23.Hull CK, Penman AD, Smith CK, Martin PD. Quantification of rosuvastatin in human plasma by automated solid-phase extraction using tandem mass spectrometric detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;772:219–228. doi: 10.1016/s1570-0232(02)00088-0. [DOI] [PubMed] [Google Scholar]
- 24.Hull CK, Martin PD, Warwick MJ, Thomas E. Quantification of the N-desmethyl metabolite of rosuvastatin in human plasma by automated SPE followed by HPLC with tandem MS detection. J Pharm Biomed Anal. 2004;35:609–614. doi: 10.1016/j.jpba.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 25.Xu DH, Ruan ZR, Zhou Q, Yuan H, Jiang B. Quantitative determination of rosuvastatin in human plasma by liquid chromatography with electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:2369–2375. doi: 10.1002/rcm.2542. [DOI] [PubMed] [Google Scholar]
- 26.Suslick KS. Kirk-Othmer encyclopedia of chemical technology. 4th ed. New York (NY): John Wiley & Sons; 1998. pp. 517–541. [Google Scholar]
- 27.da Fonseca Antunes AB, De Geest BG, Vervaet C, Remon JP. Solvent-free drug crystal engineering for drug nano- and micro suspensions. Eur J Pharm Sci. 2013;48:121–129. doi: 10.1016/j.ejps.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Strnadová KA, Holub M, Mühl A, Heinze G, Ratschmann R, Mascher H, et al. Long-term stability of amino acids and acylcarnitines in dried blood spots. Clin Chem. 2007;53:717–722. doi: 10.1373/clinchem.2006.076679. [DOI] [PubMed] [Google Scholar]
- 29.Alfazil AA, Anderson RA. Stability of benzodiazepines and cocaine in blood spots stored on filter paper. J Anal Toxicol. 2008;32:511–515. doi: 10.1093/jat/32.7.511. [DOI] [PubMed] [Google Scholar]