Abstract
β-Lapachone has been reported to have anticancer and various other therapeutic effects, but is limited in clinical applications by its low bioavailability. pH-Dependent isomerization can be suggested as one plausible factor influencing its low bioavailability. Since it is known that β-lapachone is converted to its isomer, α-lapachone in hydrochloric acid (HCl) solution, isomerization in the human body may be driven by HCl in the gastric fluid. The purpose of this study was to evaluate the possibility of isomerization of β-lapachone in the human body. Chemical reactions were conducted using simulated gastric fluid (SGF, pH 1.2) and simulated intestinal fluid (SIF, pH 7.5) at 37°C. β-Lapachone was observed in SGF at 37°C for 1 hour and SIF for 3 hours. In addition, biofluid analysis was performed on plasma samples 1 hour and 4 hours, and on urine sample 12 hours after oral administration of 100 mg MB12066, a synthetic β-lapachone, in healthy adult male. All samples were analyzed using liquid chromatography-tandem mass spectrometry. Only β-lapachone peaks existed in the spectra obtained from SGF and SIF. No isomerization of β-lapachone was observed in the analysis of any of the human samples. In the current study, the possibility of pH-dependent isomerization of β-lapachone in the human body was not confirmed.
Keywords: Beta-Lapachone, Bioavailability, Liquid Chromatography, Tandem Mass Spectrometry, MB12066
INTRODUCTION
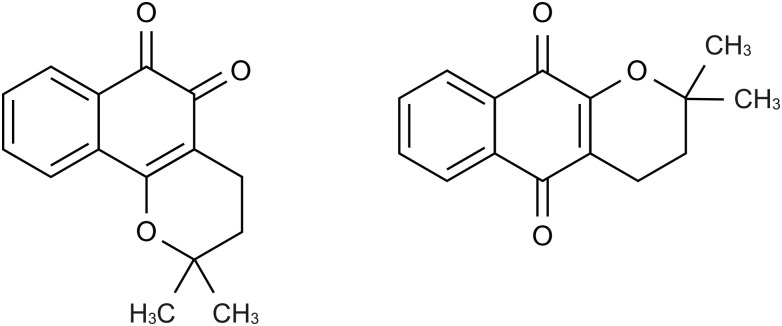
β-Lapachone (3,4-dihydro-2,2-dimethyl-2H-naphthol[1,2-b]pyran-5,6-dione, Fig. 1), an ortho-naphthoquinone naturally originating from the bark of the lapacho tree (Tabebuia avellanedae) [1], has been shown to have many beneficial effects, including anticancer [2,3], anti-inflammatory [4,5], anti-bacterial [6], and anti-aging properties [7]. β-Lapachone has also demonstrated therapeutic effects in various diseases, such as metabolic syndrome [8,9] and neurodegenerative disorders [10]. β-Lapachone is especially well known as an anticancer agent, affecting the activity of nicotinamide adenine dinucleotide (NAD) (P)H:quinone oxidoreductase 1 (NQO1). NQO1 is an antioxidant flavoenzyme involved in quinone reduction [11]. NQO1 is expressed in many tumors, including lung [12,13], breast [14], and pancreatic cancers [15]. The reduction of β-lapachone to hydroquinone occurs via NQO1 in the presence of NAD(P)H [16]. Since hydroquinone is unstable, it spontaneously reverts to stable β-lapachone through autoxidation, leading to a futile redox cycle [2,11]. This futile redox cycle causes the generation of intracellular reactive oxygen species, leading to the death of cancer cells [2,11,16].
Figure 1. Structure of β-lapachone (left) and α-lapachone (right).
Several clinical trials have been conducted to develop β-lapachone as a drug. ARQ 501 (ArQule, Burlington, MA, USA), a synthetic β-lapachone compound, was developed as an anticancer drug, and phase I and phase II clinical trials were conducted in adult patients [17,18].
MB12066 (KT&G Life Sciences, Suwon, Korea), another synthetic β-lapachone compound, was developed, and clinical trials were conducted in healthy Korean male adults to verify its therapeutic effects in metabolic syndrome, including obesity and alcoholic fatty liver disease [19,20]. β-Lapachone can play an important role in the treatment of metabolic syndrome through NQO1-mediated changes of the NAD+/NADH ratio, which is important for energy metabolism in cells [8,21]. MB12066 was shown to induce NAD(P)H oxidation to stimulate adenosine monophosphate (AMP)-activated protein kinase (AMPK) signaling and mitochondrial function, thereby promoting glucose and lipid metabolism [8,21]. This can reduce free fatty acids, cholesterol, and glucose accumulation, which can increase energy consumption by promoting browning in white adipose tissue, and can also be effective in treating obesity and metabolic diseases such as fatty liver and dyslipidemia [8,9,21]. Although β-lapachone has various beneficial effects, it is limited in drug development by its low bioavailability [22].
Drug bioavailability refers to the rate and extent of a drug reaching systemic circulation after administration [23]. Poor bioavailability in the human body is known to be caused by low aqueous solubility and extensive first-pass metabolism. Drugs with poor aqueous solubility have low dissolution amounts and slow dissolution velocities [23]. In a preclinical pharmacokinetic study, the bioavailability of β-lapachone was measured as 15.54% when orally administered to rats [22]. Apart from β-lapachone [22,24,25], paclitaxel [26] and griseofulvin [27] are also examples of low water-soluble drugs. Orally administered drugs enter the liver through the portal circulation before reaching systemic circulation [23]; if a significant amount of a drug is metabolized by various metabolic enzymes due to extensive first-pass metabolism, bioavailability is lowered [23]. In addition to β-lapachone [22,28,29,30,31,32], lovastatin [33] and pentazocine [34] are examples of drugs that have low bioavailability due to extensive first-pass metabolism.
Another plausible cause for low bioavailability of β-lapachone is pH-dependent isomerization of β-lapachone in the human body. Isomerization is a reaction changing the chemical structure of a compound without changing its molecular composition. Isomerization is one type of possible drug degradation reaction, rendering drugs inactive or unstable in gastrointestinal fluid [23,35]. Roxithromycin, an antibacterial agent, is one example of an agent with low bioavailability caused by pH-dependent isomerization [36]. Isomerization of roxithromycin in the gastric fluid to Z-roxithromycin affects the bioavailability of roxithromycin [36].
However, pH-dependent isomerization does not necessarily decrease bioavailability. Lycopene, a carotenoid pigment in tomatoes, is known to reduce the risk of prostate cancer [37]. Low pH in the stomach isomerizes lycopene to cis-lycopene, that is absorbed even more compared to the trans isomer, leading to increased bioavailability [38,39,40]. Accordingly, bioavailability may be increased or decreased depending on pH-dependent isomerization. It has long been known that β-lapachone is isomerized to α-lapachone in hydrochloric acid (HCl) solution [41,42,43]. α-Lapachone (Fig. 1), a para-naphthoquinone, has an anticancer effect by blocking deoxyribonucleic acid (DNA) topoisomerase II [44], although it has weaker pharmacological effect than β-lapachone [45,46]. In the stomach, HCl is secreted at a concentration of about 0.2–0.5% to aid in digestion. Therefore, some orally administered β-lapachone may be isomerized in the stomach by HCl. If this were to occur, the level of β-lapachone from the stomach to the small intestine would be reduced, and the amount of β-lapachone reaching the systemic circulation would be decreased, resulting in low bioavailability.
β-Lapachone may not be isomerized into α-lapachone because of low HCl concentration in the stomach. However, since there is no experiment to confirm isomerization of β-lapachone using biofluid samples, the purpose of this study was to evaluate the possibility of the isomerization of β-lapachone in the human body and to examine whether it could contribute to its low bioavailability. Two experiments were conducted: one in which reactions were performed in simulated gastric fluid (SGF, pH 1.2) and simulated intestinal fluid (SIF, pH 7.5) at 37°C; and another in which plasma and urine samples of a healthy adult male were analyzed following oral administration of MB12066.
METHODS
Materials
β-Lapachone (MB12066) was obtained from KT&G Life Sciences. Co., Ltd.. α-Lapachone was purchased from BOC Sciences (Shirley, NY, USA). High-performance liquid chromatography (HPLC)-grade acetonitrile, formic acid (98–100% for analysis), ammonium acetate, and hydrochloric acid (HCl, 37%) were purchased from Merck (Darmstadt, Germany). Sodium chloride (NaCl), sodium hydroxide (NaOH) and potassium phosphate monobasic (KH2PO4) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Deionized water was purified using a Milli-Q system (Merck Millipore, Burlington, MA, USA). Heparinized blank human plasma was purchased from Innovative Research, Inc. (Novi, MI, USA).
Methods
Preparation of β-lapachone standard solution, simulated gastric fluid (SGF), and simulated intestinal fluid (SIF)
β-Lapachone is sensitive to light, so the exposure of light was minimized during sample preparation and analysis [47]. A working standard solution of β-lapachone was prepared in acetonitrile at a concentration of 1 µg/mL. Then, 100 µL of β-lapachone solution was added to 900 µL of SGF (pH 1.2) or SIF (pH 7.5) lacking enzyme, respectively. The process of making SGF and SIF was as follows [48]: SGF was made by adding 100 mg of NaCl and 0.35 mL of HCl to 49.65 mL of deionized water. SIF was made by dissolving 340 mg of KH2PO4 into 12.5 mL of deionized water. Then, 9.5 mL of 0.2 M NaOH and 20 mL of deionized water were added to the solution. To adjust the pH to 7.5, 0.2 M NaOH was used. Deionized water was added to bring the volume of the resulting solution to 50 mL. β-Lapachone in SGF or SIF were incubated in the water bath at 37°C for 1 hour or 3 hours, respectively. Each 100 µL sample was transferred to a separate tube.
Human plasma and urine samples preparation
Human plasma and urine samples from one of the subjects who participated in a pharmacokinetic study conducted in healthy Korean male adults following oral administration of a single 100 mg dose of MB12066 (ClinicalTrials.gov Identifier: NCT02338856) were used for analysis. The study protocol was approved by Institutional Review Board of Kyungpook National University Hospital, Daegu, Republic of Korea, and written informed consents were obtained from all subjects [19]. Plasma samples obtained at 1 hour and 4 hours after oral administration were used. Urine sample obtained from 0 to 12 hours after oral administration was used. Samples were stored at −70°C until analysis.
Plasma and urine samples were thawed at room temperature before preparation. Then, 100 µL of each sample was transferred to a microcentrifuge tube, and 200 µL of acetonitrile was added to each tube. The mixture was vortexed for 5 minutes, then centrifuged at 13,200 rpm for 10 minutes at 4°C. Each supernatant (100 µL) was transferred to a separate vial for analysis.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis
The reaction of β-lapachone in SGF and SIF was analyzed using an Alliance 2695 HPLC (Waters Corp., Milford, MA, USA) coupled with a Finnigan LXQ ion trap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). HPLC was performed using a Zorbax Eclipse XDB-C18 column (2.1 × 150 mm, 3.5 µm, Agilent Technologies, Inc., Santa Clara, CA, USA). The column oven and autosampler temperature were set to 40°C and 20°C, respectively. An isocratic elution mode was used with 5 mM ammonium acetate and 0.1% formic acid in water (mobile phase A) and acetonitrile (mobile phase B) in a 50:50 (v/v) ratio. The flow rate was 0.2 mL/min, and the sample injection volume was 5 µL; each sample was analyzed over a 20 minutes run. Mass detection was performed in MS/MS positive electrospray ionization mode, with a scan range of 100–250 mass-to-charge ratio (m/z). The collision gas was helium, and the collision energy value was 20 eV. Other optimized MS parameters for MS/MS positive mode were as follows: capillary temperature, 320°C; capillary voltage, 32 V; flow rate of aux gas (N2), 5 arbitrary units; flow rate of sheath gas (N2), 20 arbitrary units; and tube lens voltage, 60 V.
For more accurate and precise analysis of human samples, an Agilent 1200 Series HPLC (Agilent) coupled with API 5000 mass spectrometer (SCIEX, Framingham, MA, USA) were used. The column and the column oven temperature were as above, as were the isocratic elution mode and the mobile phases. The autosampler temperature was set to 10°C. The sample injection volume was 2 µL, and each sample was analyzed over a 20 minutes run with a flow rate of 0.2 mL/min. Mass detection was performed in multiple reaction monitoring (MRM) positive electrospray ionization mode. The MRM transition (m/z 243 → 187) was monitored for more selective and sensitive analysis. The following optimized MS parameters for MRM positive mode were used: source temperature, 600°C; ion spray voltage, 5,500 V; declustering potential (DP), 46 V; entrance potential (EP), 8 V; collision energy (CE), 27 V; collision cell exit potential (CXP), 16 V.
RESULTS
The reaction to isomerization of β-lapachone in simulated gastric fluid (SGF) and simulated intestinal fluid (SIF)
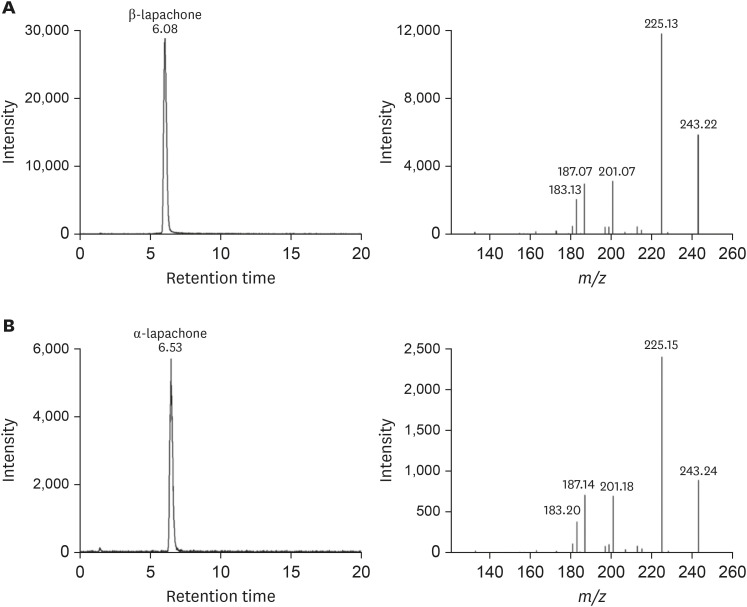
Before analysis, peak chromatograms and MS/MS spectra of the β-lapachone standard solution (100 ng/mL) and α-lapachone standard solution (1,000 ng/mL) were identified (Fig. 2).
Figure 2. Representative liquid chromatography-tandem mass spectrometry (LC-MS/MS) ion chromatograms and MS/MS spectra (scan range: mass to charge ratio (m/z) 100–250) of (A) β-lapachone standard solution (100 ng/mL) and (B) α-lapachone standard solution (1,000 ng/mL).
At a retention time of 6.08 minutes, β-lapachone was detected with a molecular ion ([M+H]+) at m/z 243 (Fig. 2); at a retention time of 6.53 minutes, α-lapachone was detected with the same m/z value and MS/MS spectrum (Fig. 2). The ion at m/z 243 yielded fragments at m/z 225, 201, and 187.
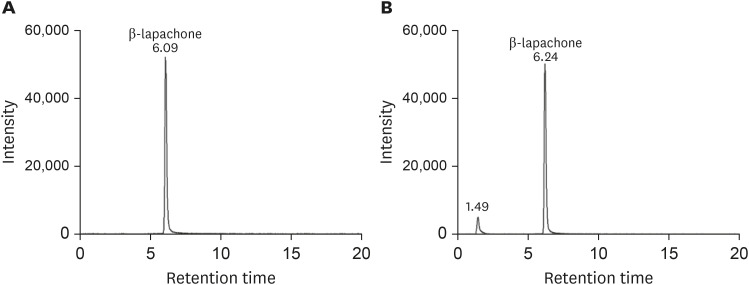
Fig. 3 shows MS/MS chromatograms of β-lapachone in SGF and SIF, respectively. Based on the chromatogram peak of α-lapachone standard solution, there was no α-lapachone peak in SGF after 1 hour. Only β-lapachone peak was observed at a retention time of 6.09 minutes. Similarly, only β-lapachone existed in SIF after 3 hours. One peak at the retention time of 1.49 minutes was observed, but as shown in Fig. 2, this peak did not correspond to a α-lapachone peak.
Figure 3. Representative liquid chromatography-tandem mass spectrometry ion chromatograms of (A) β-lapachone (100 ng/mL) in simulated gastric fluid (pH 1.2) and (B) in simulated intestinal fluid (pH 7.5).
Human plasma and urine samples analysis
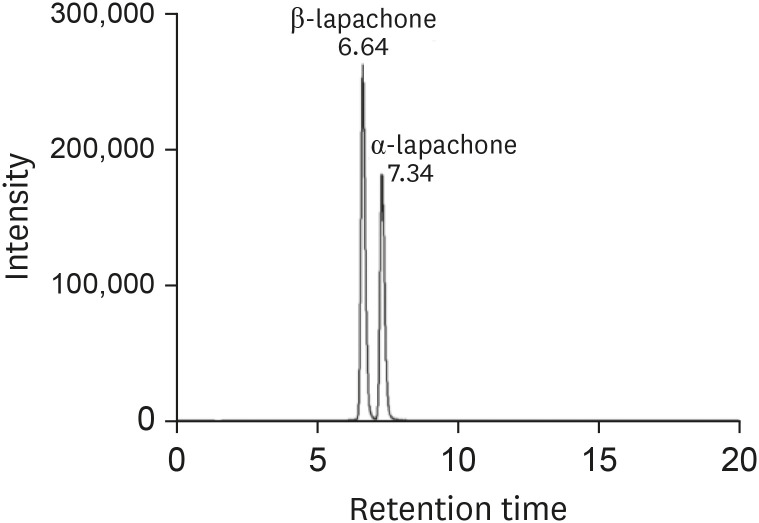
Fig. 4 shows MRM chromatograms of β-lapachone standard solution (100 ng/mL) and α-lapachone standard solution (200 ng/mL) in blank plasma. At a retention time of 6.64 minutes, β-lapachone was detected, and at a retention time of 7.34 minutes, α-lapachone was detected. α-Lapachone concentration was twice as high as β-lapachone, but the intensity was lower than β-lapachone.
Figure 4. Representative multiple reaction monitoring (mass to charge ratio [m/z] 243 → 187) chromatogram of β-lapachone standard solution (100 ng/mL) and α-lapachone standard solution (200 ng/mL) in blank plasma.
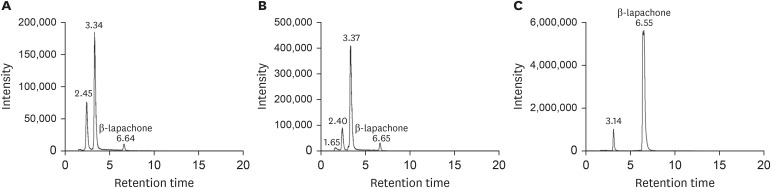
MRM chromatograms of β-lapachone in human plasma and urine samples are shown in Fig. 5, respectively. In plasma samples obtained 1 hour and 4 hours after oral administration of MB12066, (100 mg), based on the chromatogram peak of the α-lapachone standard solution in blank plasma, there were no α-lapachone peaks present in the two plasma samples (Fig. 5A and B). Similarly, in the urine sample obtained 0 to 12 hours after oral administration of MB12066, there was no α-lapachone peak observed (Fig. 5C). Several peaks before the retention time of 6.00 minutes were observed in human plasma and urine samples, but these peaks did not correspond to the peaks of the α-lapachone standard solution.
Figure 5. Representative multiple reaction monitoring (mass to charge ratio [m/z] 243 → 187) chromatograms of β-lapachone in human plasma samples obtained from (A) 1 hour and (B) 4 hours and human urine sample (C) obtained from 0 to 12 hours after oral administration of 100 mg MB12066.
DISCUSSION
Hooker [41] discovered that when β-lapachone was dissolved in HCl, α-lapachone was formed. Due to the continuous addition and removal of HCl to β-lapachone, the side ring of β-lapachone is opened and the β-lapachone structure is converted to α-lapachone [41]. Even though this process has been known for more than a century [41], this isomerization has not been well studied under acidic condition in the human body. In this study, we sought to observe isomerization of β-lapachone in the presence of HCl at concentrations between 0.2% and 0.5% through controlled reaction experiments, and also analyzed plasma and urine samples from a healthy Korean male who orally received MB12066. When making SGF, concentrated 37% HCl was used, accounting for 0.7% of the total SGF, leading to an actual HCl concentration in SGF of 0.259%, similar to the concentration of HCl in real gastric fluid. Liu et al. [25] conducted in vitro dissolution tests of β-lapachone at pH 1.4 for 1 hour and pH 6.5 for 3 hours. According to Wen et al. [49], the stomach transit time is 0.25 hours under fasting condition and 1 hour under fed condition, and the jejunum transit time is 1.7 hours regardless of food intake [49]. Accordingly, we conducted our experiments related to the pH-dependent isomerization of β-lapachone for 1 hour in SGF and 3 hours in SIF. Also, when analyzing human samples, we used plasma samples obtained 1 hour and 4 hours after oral administration of β-lapachone.
Our study did not identify isomerization of β-lapachone into α-lapachone in the human body. Delarmelina et al. [50] explained isomerization of β-lapachone and α-lapachone using the microsolvation model, and found that the isomerization of β-lapachone and α-lapachone varies depending on the concentration of HCl. The microsolvation model explains the energetics behind intermolecular interactions in the solvation process [51]. In HCl solution, HCl reacts with H2O and donates H+ to H2O. Hydronium ions (H3O+) are formed by combining protons with water molecules, because hydrogen ions cannot exist stably in an aqueous solution. If the concentration of HCl is low, H+ will not be released, resulting in fewer hydronium ions and more water molecules. Upon analysis of the microsolvation of the protonated sites and the solvation of the protonated sites with one more water molecule of β-lapachone and α-lapachone, the relative enthalpy values showed that β-lapachone is more stable than α-lapachone [50]. In addition, the isomerization of β-lapachone and α-lapachone can be related to diprotonated lapachol, but there is no sufficient proton donor in the low acid solution, resulting in the formation of monoprotonated lapachol [50]. When monoprotonated lapachol is formed, β-lapachone which is more stable than α-lapachone, is formed in a protonated state, because nucleophilic attack on the carbonyl group C4=O occurs without an enthalpic barrier [50]. Delarmelina et al. [49] showed that more β-lapachone existed than α-lapachone in 9% HCl/acetic acid; the HCl concentration of SGF in our experiment was lower than 9%, so more β-lapachone would exist in that solution. In addition, we analyzed urine sample to identify the isomerization in the process of urine excretion following the metabolism of β-lapachone, but were unable to identify α-lapachone. Based on previous studies, β-lapachone is metabolized in the liver into various forms, but the metabolism of α-lapachone is not observed [28,29,30]. Lee et al. [29] explained that β-lapachone is metabolized by cytochrome P450 (CYP) to produce mono-oxygenated metabolites, while O-methylated metabolites are produced by membrane-bound catechol-O-methyltransferase (MB-COMT). Also, Cheng et al. [28] and Miao et al. [30] reported that the major metabolic pathway of β-lapachone was via glucuronidation, a phase II metabolic pathway, and several metabolites were produced by uridine diphosphate (UDP)-glucuronosyltransferase (UGT) using human liver S9 fractions and human hepatocytes, respectively.
There are several limitations to this study. First, SGF and SIF were used to make simulated gastrointestinal conditions, but only the pH of the stomach and the small intestine were applied, without any digestive enzymes. Second, since human plasma samples were taken in January 2015 [19], a lot of time passed prior to sample analysis. The stability of the plasma samples was not verified, and the β-lapachone peak after oral administration was low, even though the tmax of MB12066 is reported to be 3 hours [19]. Also, analyzing small number of human subject (n = 1) is a limitation in this study. After verifying the stability of plasma samples, it will be necessary to repeat this analysis with more human samples in the future. Several peaks were observed in both plasma and urine samples before the retention time of the β-lapachone peak. Miao et al. [30] conducted β-lapachone metabolism study using human hepatocytes. Based on this study, we presume that these peaks are related to β-lapachone glucuronide-conjugated metabolites [30]. Further study is needed to identify the substance responsible for each peak before the β-lapachone peak.
In this study, LC-MS/MS analysis was conducted to evaluate the possibility of pH-dependent isomerization of β-lapachone in the human body. Isomerization of β-lapachone into α-lapachone was not observed in SGF after 1 hour, SIF after 3 hours, or in human plasma or urine samples, respectively. Through this study, the possibility of pH-dependent isomerization of β-lapachone in the human body was not confirmed.
Further studies are needed to analyze factors such as size reduction [25], complex formation [52,53,54], and water-soluble prodrug development [55] to improve the low solubility of β-lapachone, which limits its potential for drug development.
Footnotes
Funding: This research was supported by a grant of the Korea Health technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HR15C0001) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1I1A1A01064906).
Reviewer: This article was reviewed by peer experts who are not TCP editors.
Conflict of Interest: - Authors: Nothing to declare
- Reviewers: Nothing to declare
- Editors: Nothing to declare
- Conceptualization: Lee KM.
- Data curation: Gwon MR, Seong SJ.
- Formal analysis: Lee KM, Seong SJ.
- Investigation: Gwon MR, Lee HW.
- Methodology: Lee KM, Lee HW.
- Resources: Gwon MR.
- Supervision: Yoon YR.
- Writing - original draft: Lee KM.
- Writing - review & editing: Yoon YR, Seong SJ.
References
- 1.Pardee AB, Li YZ, Li CJ. Cancer therapy with beta-lapachone. Curr Cancer Drug Targets. 2002;2:227–242. doi: 10.2174/1568009023333854. [DOI] [PubMed] [Google Scholar]
- 2.Kung HN, Lu KS, Chau YP. The chemotherapeutic effects of lapacho tree extract: β-lapachone. Chemo Open Access. 2014;3:131–135. [Google Scholar]
- 3.Park EJ, Choi KS, Kwon TK. β-Lapachone-induced reactive oxygen species (ROS) generation mediates autophagic cell death in glioma U87 MG cells. Chem Biol Interact. 2011;189:37–44. doi: 10.1016/j.cbi.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Sitônio MM, Carvalho Júnior CH, Campos IA, Silva JB, Lima MC, Góes AJ, et al. Anti-inflammatory and anti-arthritic activities of 3,4-dihydro-2,2-dimethyl-2H-naphthol[1,2-b]pyran-5,6-dione (β-lapachone) Inflamm Res. 2013;62:107–113. doi: 10.1007/s00011-012-0557-0. [DOI] [PubMed] [Google Scholar]
- 5.Manna SK, Gad YP, Mukhopadhyay A, Aggarwal BB. Suppression of tumor necrosis factor-activated nuclear transcription factor-kappaB, activator protein-1, c-Jun N-terminal kinase, and apoptosis by beta-lapachone. Biochem Pharmacol. 1999;57:763–774. doi: 10.1016/s0006-2952(98)00354-2. [DOI] [PubMed] [Google Scholar]
- 6.Cruz FS, Docampo R, de Souza W. Effect of beta-lapachone on hydrogen peroxide production in Trypanosoma cruzi. Acta Trop. 1978;35:35–40. [PubMed] [Google Scholar]
- 7.Lee JS, Park AH, Lee SH, Lee SH, Kim JH, Yang SJ, et al. Beta-lapachone, a modulator of NAD metabolism, prevents health declines in aged mice. PLoS One. 2012;7:e47122. doi: 10.1371/journal.pone.0047122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang JH, Kim DW, Jo EJ, Kim YK, Jo YS, Park JH, et al. Pharmacological stimulation of NADH oxidation ameliorates obesity and related phenotypes in mice. Diabetes. 2009;58:965–974. doi: 10.2337/db08-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi WH, Ahn J, Jung CH, Jang YJ, Ha TY. β-Lapachone prevents diet-induced obesity by increasing energy expenditure and stimulating the browning of white adipose tissue via downregulation of miR-382 expression. Diabetes. 2016;65:2490–2501. doi: 10.2337/db15-1423. [DOI] [PubMed] [Google Scholar]
- 10.Lee M, Ban JJ, Chung JY, Im W, Kim M. Amelioration of Huntington's disease phenotypes by beta-lapachone is associated with increases in Sirt1 expression, CREB phosphorylation and PGC-1α deacetylation. PLoS One. 2018;13:e0195968. doi: 10.1371/journal.pone.0195968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang K, Chen D, Ma K, Wu X, Hao H, Jiang S. NAD (P) H: quinone oxidoreductase 1 (NQO1) as a therapeutic and diagnostic target in cancer. J Med Chem. 2018;61:6983–7003. doi: 10.1021/acs.jmedchem.8b00124. [DOI] [PubMed] [Google Scholar]
- 12.Bey EA, Bentle MS, Reinicke KE, Dong Y, Yang CR, Girard L, et al. An NQO1- and PARP-1-mediated cell death pathway induced in non-small-cell lung cancer cells by beta-lapachone. Proc Natl Acad Sci U S A. 2007;104:11832–11837. doi: 10.1073/pnas.0702176104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z, Zhang Y, Jin T, Men J, Lin Z, Qi P, et al. NQO1 protein expression predicts poor prognosis of non-small cell lung cancers. BMC Cancer. 2015;15:207. doi: 10.1186/s12885-015-1227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y, Zhang Y, Wu Q, Cui X, Lin Z, Liu S, et al. Clinical implications of high NQO1 expression in breast cancers. J Exp Clin Cancer Res. 2014;33:14. doi: 10.1186/1756-9966-33-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ough M, Lewis A, Bey EA, Gao J, Ritchie JM, Bornmann W, et al. Efficacy of beta-lapachone in pancreatic cancer treatment: exploiting the novel, therapeutic target NQO1. Cancer Biol Ther. 2005;4:95–102. doi: 10.4161/cbt.4.1.1382. [DOI] [PubMed] [Google Scholar]
- 16.Siegel D, Yan C, Ross D. NAD(P)H:quinone oxidoreductase 1 (NQO1) in the sensitivity and resistance to antitumor quinones. Biochem Pharmacol. 2012;83:1033–1040. doi: 10.1016/j.bcp.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shapiro GI, Supko JG, Ryan DP, Appelman L, Berkenblit A, Craig AR, et al. Phase I trial of ARQ 501, an activated checkpoint therapy (ACT) agent, in patients with advanced solid tumors. J Clin Oncol. 2005;23(Suppl 16):3042. [Google Scholar]
- 18.Khong HT, Dreisbach L, Kindler HL, Trent DF, Jeziorski KG, Bonderenko I, et al. A phase 2 study of ARQ 501 in combination with gemcitabine in adult patients with treatment naive, unresectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25(Suppl 18):15017. [Google Scholar]
- 19.Lee HW, Seong SJ, Ohk B, Kang WY, Gwon MR, Kim BK, et al. Pharmacokinetic and safety evaluation of MB12066, an NQO1 substrate. Drug Des Devel Ther. 2017;11:2719–2725. doi: 10.2147/DDDT.S142339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S, Lee S, Cho JY, Yoon SH, Jang IJ, Yu KS. Pharmacokinetics and tolerability of MB12066, a beta-lapachone derivative targeting NAD(P)H: quinone oxidoreductase 1: two independent, double-blind, placebo-controlled, combined single and multiple ascending dose first-in-human clinical trials. Drug Des Devel Ther. 2017;11:3187–3195. doi: 10.2147/DDDT.S151269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin S, Park J, Li Y, Min KN, Kong G, Hur GM, et al. β-Lapachone alleviates alcoholic fatty liver disease in rats. Cell Signal. 2014;26:295–305. doi: 10.1016/j.cellsig.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Kim I, Kim H, Ro J, Jo K, Karki S, Khadka P, et al. Preclinical pharmacokinetic evaluation of β-lapachone: characteristics of oral bioavailability and first-pass metabolism in rats. Biomol Ther (Seoul) 2015;23:296–300. doi: 10.4062/biomolther.2015.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hetal T, Bindesh P, Sneha T. A review on techniques for oral bioavailability enhancement of drugs. Int J Pharm Sci Rev Res. 2010;4:203–223. [Google Scholar]
- 24.Bermejo M, Mangas-Sanjuan V, Gonzalez-Alvarez I, Gonzalez-Alvarez M. Enhancing oral absorption of β-lapachone: progress till date. Eur J Drug Metab Pharmacokinet. 2017;42:1–10. doi: 10.1007/s13318-016-0369-7. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, Liu Z, Chen Y, Chen Z, Chen H, Pui Y, et al. Oral bioavailability enhancement of β-lapachone, a poorly soluble fast crystallizer, by cocrystal, amorphous solid dispersion, and crystalline solid dispersion. Eur J Pharm Biopharm. 2018;124:73–81. doi: 10.1016/j.ejpb.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Peltier S, Oger JM, Lagarce F, Couet W, Benoît JP. Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded lipid nanocapsules. Pharm Res. 2006;23:1243–1250. doi: 10.1007/s11095-006-0022-2. [DOI] [PubMed] [Google Scholar]
- 27.Jadhav P, Deshmukh V, Patil P, Dhawale S. Enhancement of solubility and dissolution rate of griseofulvin by microparticulate systems. J Curr Pharm Res. 2011;2:432–438. [Google Scholar]
- 28.Cheng X, Liu F, Yan T, Zhou X, Wu L, Liao K, et al. Metabolic profile, enzyme kinetics, and reaction phenotyping of β-lapachone metabolism in human liver and intestine in vitro . Mol Pharm. 2012;9:3476–3485. doi: 10.1021/mp300296m. [DOI] [PubMed] [Google Scholar]
- 29.Lee S, Kim IS, Kwak TH, Yoo HH. Comparative metabolism study of β-lapachone in mouse, rat, dog, monkey, and human liver microsomes using liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2013;83:286–292. doi: 10.1016/j.jpba.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 30.Miao XS, Zhong C, Wang Y, Savage RE, Yang RY, Kizer D, et al. In vitro metabolism of beta-lapachone (ARQ 501) in mammalian hepatocytes and cultured human cells. Rapid Commun Mass Spectrom. 2009;23:12–22. doi: 10.1002/rcm.3835. [DOI] [PubMed] [Google Scholar]
- 31.Kim IS, Kim Y, Kwak TH, Yoo HH. Effects of β-lapachone, a new anticancer candidate, on cytochrome P450-mediated drug metabolism. Cancer Chemother Pharmacol. 2013;72:699–702. doi: 10.1007/s00280-013-2230-x. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Li Q, Cheng X, Wang H, Wang G, Hao H. UDP-glucuronosyltransferase 1A determinates intracellular accumulation and anti-cancer effect of β-lapachone in human colon cancer cells. PLoS One. 2015;10:e0117051. doi: 10.1371/journal.pone.0117051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar S, Nagpal K, Singh S, Mishra D. Improved bioavailability through floating microspheres of lovastatin. Daru. 2011;19:57–64. [PMC free article] [PubMed] [Google Scholar]
- 34.Ehrnebo M, Boréus LO, Lönroth U. Bioavailability and first-pass metabolism of oral pentazocine in man. Clin Pharmacol Ther. 1977;22:888–892. doi: 10.1002/cpt1977226888. [DOI] [PubMed] [Google Scholar]
- 35.Stuurman FE, Nuijen B, Beijnen JH, Schellens JH. Oral anticancer drugs: mechanisms of low bioavailability and strategies for improvement. Clin Pharmacokinet. 2013;52:399–414. doi: 10.1007/s40262-013-0040-2. [DOI] [PubMed] [Google Scholar]
- 36.Zhang S, Xing J, Zhong D. pH-dependent geometric isomerization of roxithromycin in simulated gastrointestinal fluids and in rats. J Pharm Sci. 2004;93:1300–1309. doi: 10.1002/jps.20023. [DOI] [PubMed] [Google Scholar]
- 37.Wertz K, Siler U, Goralczyk R. Lycopene: modes of action to promote prostate health. Arch Biochem Biophys. 2004;430:127–134. doi: 10.1016/j.abb.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 38.Boileau TW, Boileau AC, Erdman JW., Jr Bioavailability of all-trans and cis-isomers of lycopene. Exp Biol Med (Maywood) 2002;227:914–919. doi: 10.1177/153537020222701012. [DOI] [PubMed] [Google Scholar]
- 39.Re R, Fraser PD, Long M, Bramley PM, Rice-Evans C. Isomerization of lycopene in the gastric milieu. Biochem Biophys Res Commun. 2001;281:576–581. doi: 10.1006/bbrc.2001.4366. [DOI] [PubMed] [Google Scholar]
- 40.Honest KN, Zhang HW, Zhang L. Lycopene: isomerization effects on bioavailability and bioactivity properties. Food Rev Int. 2011;27:248–258. [Google Scholar]
- 41.Hooker SC. LVII.—The constitution of “lapachic acid”(lapachol) and its derivatives. J Chem Soc Trans. 1892;61:611–650. [Google Scholar]
- 42.Ettlinger MG, Hydroxynaphthoquinones II. Cyclization and the basicity and interconversion of ortho and para quinones. J Am Chem Soc. 1950;72:3090–3095. [Google Scholar]
- 43.Estévez-braun A, Pérez-sacau E. The chemistry and biology of lapachol and related natural products α- and β-lapachones. Stud Nat Prod Chem. 2003;29:719–760. [Google Scholar]
- 44.Krishnan P, Bastow KF. Novel mechanisms of DNA topoisomerase II inhibition by pyranonaphthoquinone derivatives-eleutherin, alpha lapachone, and beta lapachone. Biochem Pharmacol. 2000;60:1367–1379. doi: 10.1016/s0006-2952(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 45.Moreira CS, Nicoletti CD, Pinheiro DP, de Moraes LG, Futuro DO, Ferreira VF, et al. Synthesis of dehydro-α-lapachones, α-and β-lapachones, and screening against cancer cell lines. Med Chem Res. 2019;28:2109–2117. [Google Scholar]
- 46.Ferreira DC, Tapsoba I, Arbault S, Bouret Y, Alexandre Moreira MS, Ventura Pinto A, et al. Ex vivo activities of beta-lapachone and alpha-lapachone on macrophages: a quantitative pharmacological analysis based on amperometric monitoring of oxidative bursts by single cells. ChemBioChem. 2009;10:528–538. doi: 10.1002/cbic.200800517. [DOI] [PubMed] [Google Scholar]
- 47.Cunha-Filho MS, Estévez-Braun A, Pérez-Sacau E, Echezarreta-López MM, Martínez-Pacheco R, Landín M. Light effect on the stability of β-lapachone in solution: pathways and kinetics of degradation. J Pharm Pharmacol. 2011;63:1156–1160. doi: 10.1111/j.2042-7158.2011.01323.x. [DOI] [PubMed] [Google Scholar]
- 48.Sotiropoulus JB, Deutsch T, Plakogiannis FM. Comparative bioavailability of three commercial acetaminophen tablets. J Pharm Sci. 1981;70:422–425. doi: 10.1002/jps.2600700420. [DOI] [PubMed] [Google Scholar]
- 49.Wen H, Park K. In: Oral controlled release formulation design and drug delivery: theory to practice. Wen H, Park K, editors. Hoboken (NJ): John Wiley & Sons, Inc.; 2010. Introduction and overview of oral controlled release formulation design; pp. 1–19. [Google Scholar]
- 50.Delarmelina M, Nicoletti CD, de Moraes MC, Futuro DO, Bühl M, de C da Silva F, et al. α- and β-Lapachone Isomerization in Acidic Media: Insights from Experimental and Implicit/Explicit Solvation Approaches. ChemPlusChem. 2019;84:52–61. doi: 10.1002/cplu.201800485. [DOI] [PubMed] [Google Scholar]
- 51.Brändas EJ, Kryachko ES. Fundamental world of quantum chemistry: a tribute to the memory of Per-Olov Löwdin, Volume III. Dordrecht: Springer Netherlands; 2013. [Google Scholar]
- 52.Nasongkla N, Wiedmann AF, Bruening A, Beman M, Ray D, Bornmann WG, et al. Enhancement of solubility and bioavailability of beta-lapachone using cyclodextrin inclusion complexes. Pharm Res. 2003;20:1626–1633. doi: 10.1023/a:1026143519395. [DOI] [PubMed] [Google Scholar]
- 53.Ferreira VF, Nicoletti CD, Ferreira PG, Futuro DO, da Silva FC. Strategies for increasing the solubility and bioavailability of anticancer compounds: β-lapachone and other naphthoquinones. Curr Pharm Des. 2016;22:5899–5914. doi: 10.2174/1381612822666160611012532. [DOI] [PubMed] [Google Scholar]
- 54.Cunha-Filho MS, Alvarez-Lorenzo C, Martínez-Pacheco R, Landin M. Temperature-sensitive gels for intratumoral delivery of β-lapachone: effect of cyclodextrins and ethanol. Sci World J. 2012;2012:126723. doi: 10.1100/2012/126723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerber DE, Beg MS, Fattah F, Frankel AE, Fatunde O, Arriaga Y, et al. Phase 1 study of ARQ 761, a β-lapachone analogue that promotes NQO1-mediated programmed cancer cell necrosis. Br J Cancer. 2018;119:928–936. doi: 10.1038/s41416-018-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]