Abstract
Objective
To determine the levels of carcinoembryonic antigen (CEA), proliferating nuclear antigen Ki67 and p53 in pseudomyxoma peritonei (PMP) of appendiceal origin and to correlate the levels with clinicopathological characteristics and overall survival.
Methods
This retrospective study collected data on clinicopathological features and immunohistochemical staining of CEA, Ki67 and p53 in patients with PMP of appendiceal origin. Overall survival was evaluated using Kaplan–Meier plots. Median survival time was estimated by Log-rank tests. Potential prognostic factors were evaluated by Cox proportional hazards regression models.
Results
A total of 141 patients with PMP of appendiceal origin were enrolled in the study with a median age of 54 years. Of these, 93 (66.0%) were diagnosed with low-grade mucinous carcinoma, 43 (30.5%) with high-grade mucinous carcinoma and five (3.5%) with high-grade with signet ring cells. CEA exhibited ubiquitous immunopositivity in most cases and was not associated with overall survival. Ki67 labelling index (LI) and p53 status were related to histological grade and overall survival. The main pathological indicators affecting survival included histological grade, lymph node involvement, angiolymphatic invasion, Ki67 LI and p53.
Conclusion
Combined analysis of high Ki67 LI and aberrant p53 may provide the basis for evaluating the biological behaviour of PMP and predicting clinical outcome.
Keywords: Pseudomyxoma peritonei, appendiceal origin, immunohistochemistry, peritoneum
Introduction
Pseudomyxoma peritonei (PMP) is an extremely rare syndrome with an estimated incidence of 1–2 per million per year.1,2 PMP is characterized by extensive intraperitoneal mucinous effusions resulting from an accumulation of gelatinous ascites or disseminated lesions of neoplastic mucinous epithelia.1,2 Clinical manifestations include abdominal pain and abdominal distension. Imaging indicates non-specific space-occupying lesions in the abdomen and pelvis.3 Abdominal puncture for the collection of mucinous ascites samples and laparotomy surgery in parallel with histopathology facilitate the diagnosis of PMP.4,5 Carcinoembryonic antigen (CEA) is one of the most widely used tumour serum markers, especially for diagnosing gastrointestinal tumours and for predicting tumour progression or remission.6 Previous studies have indicated that proliferating nuclear antigen Ki67 and p53 status were closely related to development, differentiation, metastasis and prognosis of various malignancies.7–13 However, the significance of these factors in PMP has not been previously reported. This current study retrospectively reviewed PMP cases of appendiceal origin that had undergone cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). The study collected clinicopathological data, evaluated the expression profiles of CEA, Ki67 and p53, and explored their relationship with the clinicopathological characteristics and prognosis.
Patients and methods
Patient recruitment
This retrospective study reviewed demographic and clinical characteristics from a chart review of all consecutive patients with PMP of appendiceal origin that were treated with CRS and HIPEC between February 2013 and March 2019 at the Department of Peritoneal Cancer Surgery, Beijing Shijitan Hospital, Capital Medical University, Beijing, China. Clinicopathological findings were reviewed including histological grade, lymph node involvement and perineural and angiolymphatic invasion. Pathological diagnosis was confirmed by haematoxylin and eosin staining of tissue sections. Expression of CEA, Ki67 and p53 was determined by immunohistochemistry on formalin fixed paraffin-embedded tissue samples as described below. All patients that met the diagnostic criteria were enrolled in the current study. PMP was classified into four categories according to Peritoneal Surface Oncology Group International standards.1 Patients with PMP presenting as ‘acellular mucin’ were excluded from this study.
The study protocol was approved by the Medical Ethics Committee of Beijing Shijitan Hospital, Capital Medical University, Beijing, China (no. sjtkyll-lx-2020-51) and the study was conducted in accordance with the declaration of Helsinki. All patients or their authorized legal guardians provided written informed consent.
Immunohistochemical staining
Paraffin-embedded biopsy tissue blocks were cut into 4 µm sections, deparaffinized and rehydrated. The tissue sections were then incubated overnight at 4°C in a moist chamber. Immunohistochemical staining was performed using primary mouse monoclonal antibodies against Ki67 (clone UMAB107 ready to use; OriGene China, Beijing, China), p53 (clone DO7 ready to use; OriGene China) and CEA (clone COL1 ready to use; OriGene China) that were processed in automated staining equipment (INTELLIPATH FLX®; Biocare Medical, Pacheco, CA, USA). The negative control for each marker was mouse serum immunoglobulin G instead of primary antibody. Tonsil was used as positive control for Ki67, ovarian serous carcinoma was used as a positive control for p53 and colon cancer was used as a positive control for CEA according to the manufacturer’s instructions. All of the pathological sections were reviewed independently by two pathologists (F.Y. and F.S.) that were blinded to the clinical and follow-up status.
The results of the immunohistochemical staining were interpreted as follows: (i) positive CEA staining was defined as brown granular staining in the membrane and cytoplasm; (ii) positive Ki67 staining was defined as brown granular staining in the nucleus. Ki67 labelling index (LI) was calculated as ‘number of positive cells/total count cells ×100’ and it was divided into low (<50%) or high (≥50%); (iii) P53 expression status was scored into three groups: normal, negative and excessive immunostaining. ‘Normal’ referred to a weak focal nuclear staining (resembling that in normal epithelium) in <50% of tumour nuclei. ‘Negative’ indicated a complete lack of nuclear staining (even though the nuclei of normal epithelial and stromal cells showed a normal pattern). ‘Excessive’ was defined when strong nuclear staining was present in the majority of tumour cells (≥50%). Both negative and excessive staining were considered as an aberrant p53 pattern.14,15
Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 24.0 (IBM Corp., Armonk, NY, USA). Categorical data were compared using χ2-test or Fisher's exact test. The overall survival was calculated from the date of confirmed diagnosis to the date of last follow-up or death. Median survival time was estimated by Kaplan–Meier plots and Log-rank tests. Item-level model comparison was proposed for the Wald test based on the observed information matrix. Potential prognostic factors were evaluated by Cox proportional hazards regression models. A P-value of <0.05 was considered statistically significant.
Results
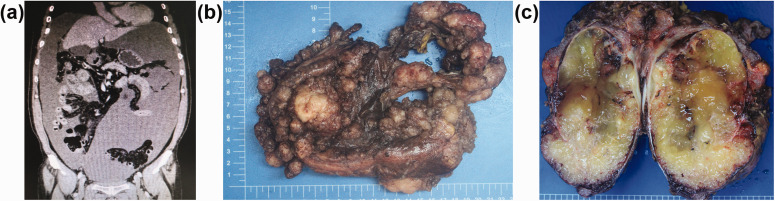
This retrospective study enrolled 141 patients with PMP of appendiceal origin (69 males [48.9%]; 72 females [51.1%]) with a median age of 54 years (range, 25–79 years). The follow-up duration ranged between 2 and 217 months. Of the 141 patients with PMP, 93 (66.0%) were diagnosed with low-grade mucinous carcinoma (LGMC), 43 (30.5%) with high-grade mucinous carcinoma (HGMC) and five (3.5%) with high-grade with signet ring cells (HGMC-S). The clinical manifestations of PMP were generally non-specific, such as abdominal discomfort, abdominal pain, distension, loss of appetite, nutritional compromise, weight loss and intestinal obstruction. Imaging indicated non-specific space-occupying lesions in the abdomen and pelvis, peritoneal effusion, as well as thickening and omental caking. Imaging examinations, including computed tomography, were used to predict and monitor recurrence and progression. Liquid density shadows and slightly more heterogeneous appearance were widely distributed in the abdominal cavity, surrounding the liver and stomach, and located in the intestinal space (Figure 1a).
Figure 1.
Representative clinical features of pseudomyxoma peritonei: (a) imaging indicated non-specific space-occupying lesions in the abdomen and pelvis, with peritoneal effusion; (b) multiple colloidal mucinous nodules; (c) destructive invasion of the intestinal canal, such as the spleen, from the serosa. The colour version of this figure is available at: http://imr.sagepub.com.
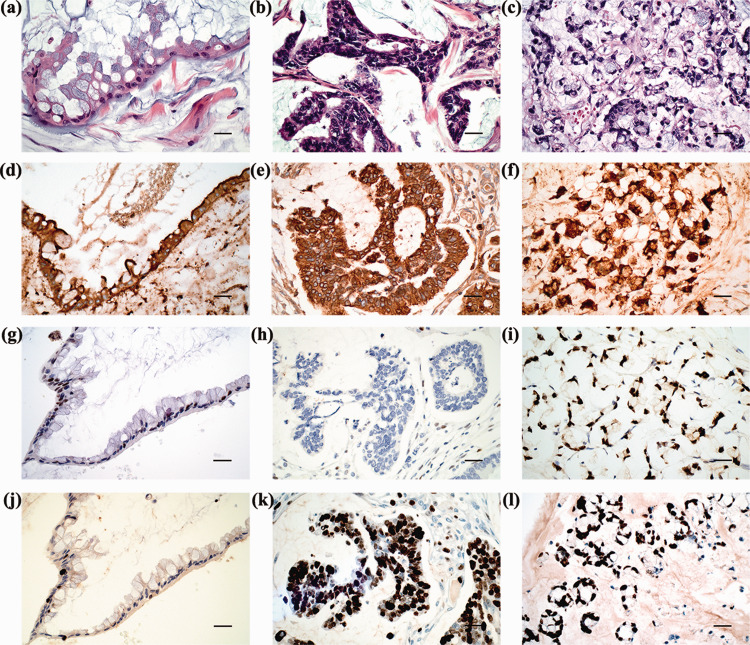
The intraperitoneal tumours consisted of multiple colloidal mucinous nodules (Figure 1b) or jelly-like mucous masses. Tumours often invaded the peritoneum and the surface of the abdominal viscera. Some tumours destroyed the pelvic and abdominal organs, such as the spleen (Figure 1c). Microscopically, mucous epithelial components were floating in the mucus lake and epithelial atypia could be identified to different extents between patients. LGMC presented as irregular mucous glandular structures, floating clusters or flat strips of neoplastic mucinous epithelium with lightly enlarged hyperchromatic nuclei and nuclear stratification (Figure 2a). HGMC presented as cribriform structures and enlarged, vesicular nuclei with full-thickness stratification, disappearance of nuclear polarity, prominent nucleoli and increased mitosis (Figure 2b) or destructive stromal invasion. HGMC with typical signet ring cells were defined as HGMC-S (Figure 2c).
Figure 2.
Representative histopathological features of pseudomyxoma peritonei: histological morphology of low-grade mucinous carcinoma (LGMC) (a), high-grade mucinous carcinoma (HGMC) (b) and high-grade with signet ring cells (HGMC-S) (c) (all haematoxylin and eosin; scale bar 50 µm); strong positive staining for carcinoembryonic antigen in LGMC (d), HGMC (e) and HGMC-S (f); weak focal nuclear staining of p53 in LGMC (g); complete lack of stained tumour nuclei for p53 in HGMC (h); strong positive staining for p53 in HGMC-S (i); low Ki67 LI in LGMC (j); high Ki67 LI in HGMC (k) and HGMC-S (l) (d–l, EnVision staining; scale bar 50 µm). The colour version of this figure is available at: http://imr.sagepub.com.
The CEA immunostaining exhibited ubiquitous immunopositivity (135 of 141 patients [95.7%]) in the membrane and cytoplasm of PMP tumour cells and there was no differential expression with increased histological grades. CEA immunoreactivity was also found in the mucus in some cases (Figures 2d–2f). Scattered expression of normal p53 with differential intensity of staining was observed more frequently in LGMC (Figure 2g). Aberrantly expressed p53 was more likely to have diffusely, consistently strong positive staining in HGMC and HGMC-S (Figure 2h and 2i). Ki67 was minimally expressed in LGMC, but highly expressed in HGMC and HGMC-S (Figures 2j–2l).
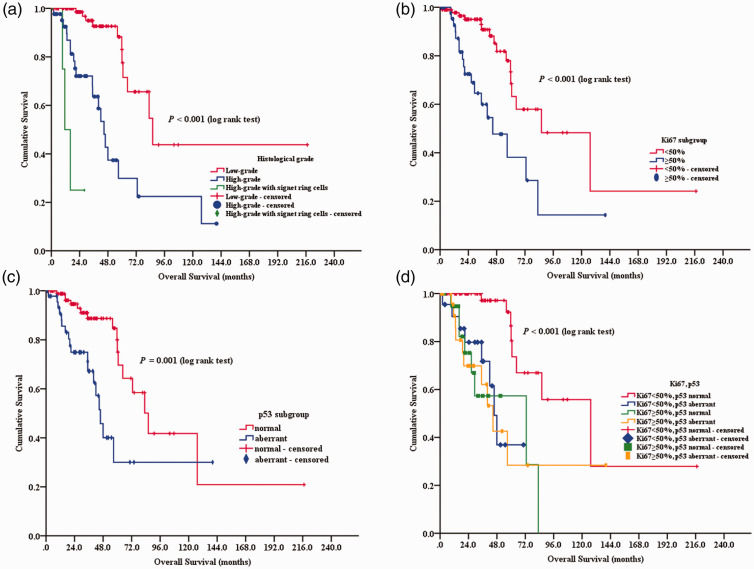
The median survival time of the cohort of 141 PMP patients was 72.8 months. A total of 34 patients died: 11 with LGMC, 20 with HGMC and three with HGMC-S. The median overall survival was longer in women than in men (not reached versus 59.7 months, respectively; P = 0.01) and in LGMC than in HGMC or HGMC-S (86.0 versus 45.7 or 11.4 months, respectively; P < 0.001). Patients with lymph node involvement or angiolymphatic invasion had reduced overall survival than those without these risk factors (20.0 versus 83.0 months, respectively, P < 0.001; 40.0 versus 83.0 months, respectively, P < 0.001). Univariate analysis identified pathological indicators affecting overall survival: histological grade (P < 0.001; Figure 3a), Ki67 (P < 0.001; Figure 3b), p53 (P = 0.001; Figure 3c), lymph node involvement (P < 0.001) and angiolymphatic invasion (P < 0.001) (Table 1). CEA expression and perineural invasion had no significant effect on overall survival.
Figure 3.
Kaplan–Meier survival curves of patients with pseudomyxoma peritonei stratified according to the following: (a) histological grade; (b) Ki67 immunostaining; (c) p53 status; (d) Ki67 and p53 subgroups. The colour version of this figure is available at: http://imr.sagepub.com.
Table 1.
Univariate analysis of overall survival of patients (n = 141) with pseudomyxoma peritonei.
| Index | n (%) | Median overall survival, months | 95% confidence interval | Statistical analysesa |
|---|---|---|---|---|
| Histological grade | P < 0.001 | |||
| LGMC | 93 (66.0) | 86.0 | 58.184, 113.816 | |
| HGMC | 43 (30.5) | 44.7 | 37.516, 51.884 | |
| HGMC-S | 5 (3.5) | 11.4 | 4.834, 17.966 | |
| CEA | NS | |||
| Positive | 135 (95.7) | 72.8 | 77.842, 138.056 | |
| Negative | 6 (4.3) | 127.3 | 38.249, 137.927 | |
| Ki67 LI | P < 0.001 | |||
| <50% | 95 (67.4) | 86.0 | 41.741, 130.258 | |
| ≥50% | 46 (32.6) | 44.7 | 23.753, 65.647 | |
| p53 | P = 0.001 | |||
| Normal | 94 (66.7) | 86.0 | 66.650, 105.350 | |
| Aberrant | 47 (33.3) | 45.7 | 38.545, 52.915 | |
| Lymph node involvement | P < 0.001 | |||
| Absent | 131 (92.9) | 83.0 | 59.567, 106.433 | |
| Present | 10 (7.1) | 20.0 | 17.434, 22.566 | |
| Angiolymphatic invasion | P < 0.001 | |||
| Absent | 130 (92.2) | 83.0 | 59.913, 106.087 | |
| Present | 11 (7.8) | 40.0 | 0.367, 79.633 | |
| Perineural invasion | NS | |||
| Absent | 137 (97.2) | 83.0 | 54.024, 111.976 | |
| Present | 4 (2.8) | 56.9 | – |
LGMC, low-grade mucinous carcinoma; HGMC, high-grade mucinous carcinoma; HGMC-S, high-grade with signet ring cells; CEA, carcinoembryonic antigen; LI, labelling index; NS, no significant association (P ≥ 0.05).
The CEA immunoreactivity was not related to histological grade (data not shown). The Ki67 LI was high in HGMC and HGMC-S but low in LGMC (P < 0.001) (Table 2). Aberrant staining of p53 was detected more frequently in HGMC and HGMC-S than in LGMC (P < 0.001). The p53 status was significantly associated with histological grade (P < 0.001), Ki67 LI (P = 0.001), lymph node involvement (P = 0.004) and angiolymphatic invasion (P = 0.001). The Ki67 LI was significantly associated with histological grade (P < 0.001), p53 (P < 0.001) and perineural invasion (P = 0.018).
Table 2.
The difference in clinicopathological characteristics between patients (n = 141) with pseudomyxoma peritonei stratified according to Ki67 and p53 immunostaining levels.
| p53, n (%) |
Ki67 LI, n (%) |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Normaln = 94 | Aberrantn = 47 | Statistical analysesa | <50%n = 95 | ≥50%n = 46 | Statistical analysesa |
| Sex | NS | NS | ||||
| Male | 48 (34.0) | 21 (14.9) | 48 (34.0) | 21 (14.9) | ||
| Female | 46 (32.6) | 26 (18.4) | 47 (33.3) | 25 (17.7) | ||
| Age, years | NS | NS | ||||
| <60 | 57 (40.4) | 34 (24.1) | 62 (44.0) | 29 (20.6) | ||
| ≥60 | 37 (26.2) | 13 (9.2) | 33 (23.4) | 17 (12.1) | ||
| Histological grade | P < 0.001 | P < 0.001 | ||||
| LGMC | 81 (57.4) | 12 (8.5) | 77 (54.6) | 16 (11.3) | ||
| HGMC | 12 (8.5) | 31 (22.0) | 18 (12.8) | 25 (17.7) | ||
| HGMC-S | 1 (0.7) | 4 (2.8) | 0 (0.0) | 5 (3.5) | ||
| CEA | NS | NS | ||||
| Positive | 90 (63.8) | 45 (31.9) | 90 (63.8) | 45 (31.9) | ||
| Negative | 4 (2.8) | 2 (1.4) | 5 (3.5) | 1 (0.7) | ||
| Ki67 LI | P = 0.001 | |||||
| <50% | 73 (51.8) | 22 (15.6) | – | – | ||
| ≥50% | 21 (14.9) | 25 (17.7) | – | – | ||
| p53 | P < 0.001 | |||||
| Normal | – | – | 73 (51.8) | 21 (14.9) | ||
| Aberrant | – | – | 22 (15.6) | 25 (17.7) | ||
| Lymph node involvement | P = 0.004 | NS | ||||
| Absent | 92 (65.2) | 39 (27.7) | 91 (64.5) | 40 (28.4) | ||
| Present | 2 (1.4) | 8 (5.7) | 4 (2.8) | 6 (4.3) | ||
| Angiolymphatic invasion | P = 0.001 | NS | ||||
| Absent | 92 (65.2) | 38 (27.0) | 91 (64.5) | 39 (27.7) | ||
| Present | 2 (1.4) | 9 (6.4) | 4 (2.8) | 7 (5.0) | ||
| Perineural invasion | NS | P = 0.018 | ||||
| Absent | 93 (66.0) | 44 (31.2) | 95 (67.4) | 42 (29.8) | ||
| Present | 1 (0.7) | 3 (2.1) | 0 (0.0) | 4 (2.8) | ||
Data presented as n of entire study cohort of 141 (%).
aCategorical data were compared using χ2-test or Fisher's exact test; NS, no significant association (P ≥ 0.05).
LGMC, low-grade mucinous carcinoma; HGMC, high-grade mucinous carcinoma; HGMC-S, high-grade with signet ring cells; CEA, carcinoembryonic antigen; LI, labelling index.
The histological grade was the only prognostic factor for overall survival of patients with PMP as determined by multivariate analysis (Table 3).
Table 3.
Multivariate analysis of overall survival of patients (n = 141) with pseudomyxoma peritonei.
| Characteristic | Wald | Hazard ratio | 95% confidence interval | Statistical analyses |
|---|---|---|---|---|
| Histological grade | 30.022 | P < 0.001 | ||
| HGMC versus LGMC | 17.591 | 4.895 | 2.330, 10.281 | P < 0.001 |
| HGMC-S versus LGMC | 23.061 | 29.005 | 7.338, 114.644 | P < 0.001 |
LGMC, low-grade mucinous carcinoma; HGMC, high-grade mucinous carcinoma; HGMC-S, high-grade with signet ring cells.
The patients were divided into four groups based on the Ki67 LI and p53 status: low Ki67 and normal p53; low Ki67 and aberrant p53; high Ki67 and normal p53; high Ki67 and aberrant p53. The patients with low Ki67 and normal p53 had a better prognosis compared with the other three subgroups (P ≤ 0.001 for all comparisons; Figure 3d, Table 4).
Table 4.
Multivariate analysis of the association between Ki67/p53 status and the overall survival of patients (n = 141) with pseudomyxoma peritonei.
| Wald | Hazard ratio | 95% confidence interval | Statistical analyses | |
|---|---|---|---|---|
| Ki67/p53 subgroup | 17.996 | P < 0.001 | ||
| Ki67 < 50%/p53 normal | ||||
| Versus Ki67 < 50%/p53 aberrant | 11.134 | 5.646 | 2.043, 15.604 | P = 0.001 |
| Versus Ki67 ≥ 50%/p53 normal | 12.683 | 6.128 | 2.260, 16.620 | P < 0.001 |
| Versus Ki67 ≥ 50%/p53 aberrant | 12.658 | 5.491 | 2.149, 14.032 | P < 0.001 |
Discussion
The serum marker CEA is commonly used in gastrointestinal tumours as it is valuable for monitoring tumour progression or remission.6 However, serum CEA might not be an ideal marker of the aggressiveness and progression of PMP tumours.16 Immunohistochemical staining of CEA may help to distinguish a benign tumour from a malignant tumour and it might be involved in predicting prognosis.17,18 In general, Ki67 is associated with the prognosis of multiple tumours by reflecting the level of tumour cell proliferation, but Ki67 alone might not predict the aggressiveness and progression as demonstrated by previous studies in breast and other cancers.19,20 P53 is an important tumour suppressor gene and it plays multifaceted roles in the development and progression of malignancies.21 Usually, wild-type p53 is maintained at a low level, while in response to cellular stress, p53 is activated through multiple mechanisms.22 Activated wild-type p53 inhibits tumorigenesis, whereas mutant p53 loses its suppressive functions.22 Strong and diffuse expression of p53 is generally interpreted as an immunohistochemical marker for TP53 gene mutations.23 The result of immunohistochemical staining of p53 protein was consistent with gene mutations.23 High Ki67 LI and aberrant p53 have synergistic effects on tumorigenesis in other tumours such as breast cancer and gastroenteropancreatic neuroendocrine carcinoma.24,25 The expression patterns of CEA, Ki67 and p53 have not been previously reported in PMP specimens of appendiceal origin.
In this current study, CEA exhibited ubiquitous immunopositivity in the membrane and cytoplasm of epithelium cells of different histological grades. In some cases, CEA displayed immunoreactivity in the mucinous island, implying that this glycoprotein was probably shed from the surface of the PMP tumour cells into the mucinous ascites. However, the expression of CEA was not related to overall survival. Histological grade is an independent prognostic parameter for overall survival.26 Aberrant p53 or high Ki67 LI correlated with high histological grade in the current study. Patients with a lower level of Ki67 and normal p53 had better prognosis that those patients in the other three subgroups in the current study. High histological grade, high expression of Ki67 and aberrant p53 conferred worse overall survival of patients with PMP in the current study. These current findings were consistent with a previous report.15 Based on the current analysis, it was clear that some patients with aberrant p53 have low Ki67 and vice versa, so looking at just one of the markers would not be adequate. Combined analysis of high Ki67 LI and aberrant p53 may provide the basis for evaluating the biological behaviour of PMP and predicting clinical outcome.
In addition, considering the results of the immunohistochemical staining of Ki67 and p53 might contribute to a more precise histological classification of PMP, a comprehensive understanding of tumorigenesis and biological behaviour and a personalized treatment plan. Previous studies have shown that the mutation rate of the KRAS and GNAS genes were 58–100% and 40–77% in patients with PMP, respectively.27–31 In addition, the P53, SMAD4 and PIK3CA genes had mutations consistent with the polygenic effects of tumorigenesis.32 Although a number of studies have looked at genetic mutations and molecular markers,27–31 the mechanisms of PMP development remain unclear. PMP has traditionally been considered as a benign tumour, with a wide spectrum of progression, from slow-growing, moderate lesions to rapidly progressive infiltrative disease.33 After treatment with CRS and HIPEC, PMP tumours remain prone to recurrence and progression, extensively invading and infiltrating the adjacent tissues/organs.34,35
In conclusion, the biological characteristics of PMP need to be further investigated, so that recurrence may be prevented or delayed after operation and overall survival might be improved without increasing the incidence of postoperative complications. The histological grade in addition to the combined detection of p53 and Ki67 may provide the basis for the assessment of development, progression and prognosis of PMP in the future.
Footnotes
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Beijing Municipal Natural Science Foundation, China (no. 7172108), the Youth Research Fund of Beijing Shijitan Hospital (no. 2019-q11), the Beijing Municipal Administration of Hospitals’ Ascent Plan (no. DFL20180701), the Special Fund for the Capital Characteristic Clinical Medicine Development Project (no. Z161100000516077) and the Beijing Municipal Grant for Medical Talents Group on Peritoneal Surface Oncology (no. 2017400003235J007).
ORCID iDs: Fengcai Yan https://orcid.org/0000-0002-8815-1692
References
- 1.Carr NJ, Cecil TD, Mohamed F, et al. A Consensus for Classification and Pathologic Reporting of Pseudomyxoma Peritonei and Associated Appendiceal Neoplasia: The Results of the Peritoneal Surface Oncology Group International (PSOGI) Modified Delphi Process. Am J Surg Pathol 2016; 40: 14–26. [DOI] [PubMed] [Google Scholar]
- 2.Mittal R, Chandramohan A, Moran B.Pseudomyxoma peritonei: natural history and treatment. Int J Hyperthermia 2017; 33: 511–519. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett DJ, Thacker PG, Grotz TE, et al. Mucinous appendiceal neoplasms: classification, imaging, and HIPEC. Abdom Radiol (NY) 2019; 44: 1686–1702. [DOI] [PubMed] [Google Scholar]
- 4.Rana SS, Bhasin DK, Srinivasan R, et al. Endoscopic ultrasound-guided fine needle aspiration of peritoneal nodules in patients with ascites of unknown cause. Endoscopy 2011; 43: 1010–1013. [DOI] [PubMed] [Google Scholar]
- 5.Tan GHC, Shamji T, Mehta A, et al. Diagnostic and therapeutic laparoscopy in assessment and management of patients with appendiceal neoplasms. Int J Hyperthermia 2018; 34: 336–340. [DOI] [PubMed] [Google Scholar]
- 6.Duffy MJ, Lamerz R, Haglund C, et al. Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers 2014 guidelines update. Int J Cancer 2014; 134: 2513–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodríguez-Alonso A, Pita-Fernández S, González-Carreró J, et al. P53 and ki67 expression as prognostic factors for cancer-related survival in stage T1 transitional cell bladder carcinoma. Eur Urol 2002; 41: 182–188. [DOI] [PubMed] [Google Scholar]
- 8.Tzanakis NE, Peros G, Karakitsos P, et al. Prognostic significance of p53 and Ki67 proteins expression in Greek gastric cancer patients. Acta Chir Belg 2009; 109: 606–611. [DOI] [PubMed] [Google Scholar]
- 9.Kamal CK, Simionescu CE, Mărgăritescu C, et al. P53 and Ki67 immunoexpression in mucinous malignant ovarian tumors. Rom J Morphol Embryol 2012; 53: 799–803. [PubMed] [Google Scholar]
- 10.Jemaa M, Galluzzi L, Kepp O, et al. Preferential killing of p53-deficient cancer cells by reversine. Cell Cycle 2012; 11: 2149–2158. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Liu Z, Fisher KW, et al. Prognostic value of programmed death ligand 1, p53, and Ki-67 in patients with advanced-stage colorectal cancer. Hum Pathol 2018; 71: 20–29. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Zhang X, Qiu J, et al. Comparisons of p53, KI67 and BRCA1 expressions in patients with different molecular subtypes of breast cancer and their relationships with pathology and prognosis. J BUON 2019; 24: 2361–2368. [PubMed] [Google Scholar]
- 13.Temraz S, Shamseddine A, Mukherji D, et al. Ki67 and P53 in relation to disease progression in metastatic pancreatic cancer: a single institution analysis. Pathol Oncol Res 2019; 25: 11059–1066. [DOI] [PubMed] [Google Scholar]
- 14.Vartiainen J, Lassus H, Lehtovirta P, et al. Combination of serum HCG beta and p53 tissue expression defines distinct subgroups of serous ovarian carcinoma. Int J Cancer 2008; 122: 2125–2129. [DOI] [PubMed] [Google Scholar]
- 15.Nummela P, Saarinen L, Thiel A, et al. Genomic profile of pseudomyxoma peritonei analyzed using next-generation sequencing and immunohistochemistry. Int J Cancer 2015; 136: E282–E289. [DOI] [PubMed] [Google Scholar]
- 16.Nummela P, Leinonen H, Jarvinen P, et al. Expression of CEA, CA19-9, CA125, and EpCAM in pseudomyxoma peritonei. Hum Pathol 2016; 54: 47–54. [DOI] [PubMed] [Google Scholar]
- 17.Suenaga Y, Kanda M, Ito S, et al. Prognostic significance of perioperative tumor marker levels in stage II/III gastric cancer. World J Gastrointest Oncol 2019; 11: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flatmark K, Davidson B, Kristian A, et al. Exploring the peritoneal surface malignancy phenotype – a pilot immunohistochemical study of human pseudomyxoma peritonei and derived animal models. Hum Pathol 2010; 41: 1109–1119. [DOI] [PubMed] [Google Scholar]
- 19.Brown DC, Gatter KC.Ki67 protein: the immaculate deception? Histopathology 2002; 40: 2–11. [DOI] [PubMed] [Google Scholar]
- 20.Yerushalmi R, Woods R, Ravdin PM, et al. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol 2010; 11: 174–183. [DOI] [PubMed] [Google Scholar]
- 21.Oren M, Rotter V.Mutant p53 gain-of-function in cancer. Cold Spring Harb Perspect Biol 2010; 2: a001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato S, Han SY, Liu W, et al. Understanding the function-structure and function-mutation relationships of p53 tumor suppressor protein by high-resolution missense mutation analysis. Proc Natl Acad Sci U S A 2003; 100: 8424–8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfister NT, Prives C.Transcriptional regulation by wild-type and cancer-related mutant forms of p53. Cold Spring Harb Perspect Med 2017; 7: a026054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali AS, Gronberg M, Federspiel B, et al. Expression of p53 protein in high-grade gastroenteropancreatic neuroendocrine carcinoma. PLoS One 2017; 12: e0187667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen K, Binderup T, Langer SW, et al. P53, somatostatin receptor 2a and chromogranin a immunostaining as prognostic markers in high grade gastroenteropancreatic neuroendocrine neoplasms. BMC Cancer 2020; 20: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan F, Lin Y, Zhou Q, et al. Pathological prognostic factors of pseudomyxoma peritonei: comprehensive clinicopathological analysis of 155 cases. Hum Pathol 2020; 97: 9–18. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Mody K, de Abreu FB, et al. Molecular profiling of appendiceal epithelial tumors using massively parallel sequencing to identify somatic mutations. Clin Chem 2014; 60: 1004–1011. [DOI] [PubMed] [Google Scholar]
- 28.Alakus H, Babicky ML, Ghosh P, et al. Genome-wide mutational landscape of mucinous carcinomatosis peritonei of appendiceal origin. Genome Med 2014; 6: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davison JM, Choudry HA, Pingpank JF, et al. Clinicopathologic and molecular analysis of disseminated appendiceal mucinous neoplasms: identification of factors predicting survival and proposed criteria for a three-tiered assessment of tumor grade. Mod Pathol 2014; 27: 1521–1539. [DOI] [PubMed] [Google Scholar]
- 30.Sio TT, Mansfield AS, Grotz TE, et al. Concurrent MCL1 and JUN amplification in pseudomyxoma peritonei: a comprehensive genetic profiling and survival analysis. J Hum Genet 2014; 59: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saarinen L, Nummela P, Thiel A, et al. Multiple components of PKA and TGF-beta pathways are mutated in pseudomyxoma peritonei. PLoS One 2017; 12: e0174898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noguchi R, Yano H, Gohda Y, et al. Molecular profiles of high-grade and low-grade pseudomyxoma peritonei. Cancer Med 2015; 4: 1809–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fallis SA, Moran BJ.Management of pseudomyxoma peritonei. J BUON 2015; 20: S47–S55. [PubMed] [Google Scholar]
- 34.Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 2012; 30: 2449–2456. [DOI] [PubMed] [Google Scholar]
- 35.Blackham AU, Swett K, Eng C, et al. Perioperative systemic chemotherapy for appendiceal mucinous carcinoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Surg Oncol 2014; 109: 740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]