Abstract
Background and Aims
Cannabis use disorder (CUD) during pregnancy has increased dramatically in the United States (US). This study examined the associations between prenatal CUD and adverse neonatal outcomes and heterogeneities in the associations by mothers’ tobacco use status and race/ethnicity.
Design
Population-based, retrospective cohort study.
Setting
California, USA.
Participants
A total of 4.83 million mothers who delivered a live singleton birth during 2001 to 2012 and their paired infants. Data were obtained from mother–infant linked hospital discharge records and birth and death certificates. Identified by ICD-9 codes recorded at delivery, 20 237 mothers had prenatal CUD.
Measurements
Neonatal outcomes included length of gestation, preterm birth, birth weight, admission into neonatal intensive care unit, hospitalization within 1 year of birth, and death within 1 year of birth. Propensity score matching was used to balance maternal, paternal, and infant characteristics in the comparisons between infants exposed and unexposed to prenatal CUD.
Findings
CUD increased from 2.8 to 6.9 per 1000 deliveries during 2001 to 2012. Multivariable regressions in matched samples estimated that prenatal CUD was associated with greater odds of being small for gestational age (OR = 1.13, 95% CI = 1.08, 1.18), preterm birth (OR = 1.06, 95% CI = 1.01, 1.12), low birth weight (OR = 1.13, 95% CI = 1.07, 1.20), and death within 1 year of birth (OR = 1.35, 95% CI = 1.12, 1.62). Compared with infants whose mothers were tobacco non-users, infants whose mothers were tobacco users had greater odds of preterm birth, low birth weight, hospitalization, and death in association with prenatal CUD. Compared with infants whose mothers were non-Hispanic White, infants whose mothers were Hispanic had greater odds of hospitalization and death and infants whose mothers were non-Hispanic Black had greater odds of being small for gestational age in association with prenatal CUD.
Conclusion
Prenatal cannabis use disorder appears to be associated with escalated odds of major adverse neonatal outcomes, with heterogeneities in the associations by mothers’ tobacco use status and race/ethnicity.
Keywords: Cannabis use disorder, cohort study, hospital discharge, maternal health, neonatal outcomes, propensity score matching
INTRODUCTION
Cannabis is widely used during pregnancy in North American and European countries [1–4]. In the United States (US), self-reported past-month cannabis use among pregnant women more than doubled during 2002 to 2016 (3.4%–7.0%) [5]. Among those reporting cannabis use, 18.1% met criteria for cannabis use disorder (CUD) (i.e. continued use of cannabis despite impairments in physical) psychological, and social functioning [6]. From 1993 through 2014, the rate of CUD escalated from 1.8 to 9.4 per 1000 deliveries [7].
Cannabis is increasingly acceptable and available following the proliferation of cannabis liberalization, yet regulations and prevention programs targeting pregnant women remain inadequate. In the past two decades, over 30 states in the United States have approved medical cannabis use. Specifically relevant to pregnant women, nausea was approved in 25 states and vomiting was approved in 6 states as qualifying conditions [8]. Since 2012, 15 states and DC further legalized recreational cannabis use among adults, but only California, Colorado, and Michigan require warning labels to disclose pregnancy-related risks [9–11]. Studies suggested that the increase in cannabis use among pregnant women may be associated with medical and recreational cannabis legalization [12–14]. Across the United States, pregnant women receive insufficient cannabis-related screening and counseling from health professionals [15,16].
Pregnant women may justify cannabis use for treating nausea, vomiting, pain, and other symptoms [17–19]. However, there are growing public health concerns that the adverse health consequences on offspring may outweigh the potential therapeutic effects on mothers. As the main compound in cannabis, tetrahy-drocannabinol is highly lipophilic. It easily crosses many cell membranes (e.g. placenta and blood–brain barrier) and accumulates in fetal plasma with high concentration [20]. Animal models showed that high doses of cannabis use can result in growth retardation, malformations, and impaired neural development [21]. Some epidemiological studies suggested that cannabis use during pregnancy was associated with greater risks of small for gestational age, preterm birth, low birth weight, and admission to neonatal intensive care unit [2,22–29], but other studies found these associations statistically nonsignificant [29–34].
As pointed out by many researchers, most of the existing epidemiological studies had limitations such as small sample size, misclassification of cannabis use, and confounding factors [1,20,35,36]. Particularly, the co-use of cannabis and tobacco may confound the observed relationships. Approximately 80% of the pregnant women using cannabis also used tobacco [37]. A meta-analysis found that prenatal cannabis use no longer independently predicted adverse neonatal outcomes if co-use of tobacco was controlled for [38]. Very few studies explored heterogeneities in the associations among different racial and ethnic subgroups probably because of small sample size.
A recent study by Corsi et al. attempted to address some of these limitations [39]. Using health records of 0.6 million pregnant women and their births in Ontario, Canada, this study estimated the associations between self-reported prenatal cannabis use and neonatal outcomes. Coarsened exact matching and multivariable regressions were used to balance and control for confounding factors. It suggested that prenatal cannabis use was associated with greater risks of small for gestational age, placental abruption, preterm birth, admission to neonatal intensive care unit, and poor 5-mintue Apgar score. In subgroup analysis, the risk difference only differed in preterm birth between women reporting and not reporting tobacco use [39].
In this study, we aimed to add new US data to the relationships between prenatal cannabis use and adverse neonatal outcomes and identify heterogeneities in the relationships by mothers’ tobacco use status and race/ethnicity. Unlike all previous research focusing on use and non-use without considering dose and frequency [35], we assessed CUD as a proxy for heavy and/or long-term use. We examined birth outcomes that were commonly included in existing literature as well as infant outcomes after birth that were rarely assessed. Data on nearly 5 million mother–infant pairs were used to power the association detection for rare events and in subgroups. Matching techniques were used to explicitly account for the imbalanced characteristics between infants with and without prenatal CUD exposure.
METHODS
Data and sample
This is a population-based, retrospective cohort study of mother–infant pairs. Mothers who delivered a singleton birth between January 1, 2001 and December 31, 2012 in California, United States were included. We obtained the mother–infant linked hospital discharge records and infants’ birth and death certificates from the US California Office of Statewide Health Planning and Development. The data covered all live births delivered in a California hospital during 2001 to 2012 except for 2006 when prenatal tobacco use was inconsistently recorded on birth certificates.1 Data source for each variable is reported in Supporting information Table S1.
During 2001 to 2012, approximately 5.68 million live births were successfully linked with all the data sources, representing approximately 96% of the total live births in California. Following previous literature [39], 173 234 births who were delivered as multiple births, 810 births whose mothers were out of the age range of 9 to 49, and 295 916 births whose recorded length of gestation were out of the range of 20 to 44 weeks2 were sequentially excluded. We further excluded 379 129 births with missing information on outcomes and/or covariates required in this study. The rate of missing values for each variable is presented in Supporting information Table S2. Compared to infants unexposed to prenatal CUD, those exposed to prenatal CUD were more likely to be excluded because of missing values. A total of 4 830 239 mother–infant pairs finally entered statistical analysis.
The California Health and Human Services Agency Committee for the Protection of Human Subjects and the University of California San Diego Human Research Protections Program approved this study.
Measures
Prenatal cannabis use disorder
We used the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) recorded in mothers’ hospital discharge records at delivery to identify CUD. Specifically, prenatal CUD was identified if ICD-9 codes related to CUD (cannabis dependence 304.30–304.33 or nondependent cannabis abuse 305.20–305.23) appeared in any of the up to 25 diagnostics in mothers’ discharge records at delivery.3
Neonatal outcomes
Primary neonatal outcomes included length of gestation (number of days), small for gestational age at birth (binary indicator for <10th percentile for a given week of gestation), preterm birth (binary indicator for <37 weeks), birth weight (grams), birth weight z score (calculated using gestational-age-specific birth weight medians and standard deviations by sex) [40], and low birth weight (binary indicator for weight at birth <2500 g).
Secondary neonatal outcomes included binary indicators for admission into neonatal intensive care unit, infant hospitalization within 1 year of birth, and infant death within 1 year of birth.
Maternal, paternal, and infant covariates
The following variables were considered potential confounding factors in previous research and controlled for in this study. Mothers’ demographics included age, educational attainment, race and ethnicity, health insurance, delivery mode, and birth history. Mothers’ physical health conditions included hypertension, diabetes, thyroid disease, anemia, cardiovascular disease, and pain. Mothers’ mental health conditions included major depressive disorder, anxiety disorder, and other mental disorders. Mothers’ behavioral health conditions included adequate prenatal care [41],4 tobacco use, alcohol use disorder, opioid use disorder, and other drug use disorders. Tobacco use during pregnancy was self-reported on birth certificate. ICD-9 codes for maternal health conditions are listed in Supporting information Table S3. Fathers’ demographic characteristics only included educational attainment. Infant demographic characteristics included sex, health insurance, and birth year.
Statistical analysis
Because a random assignment of prenatal CUD is unlikely, selection bias is a major challenge to draw a causal connection between prenatal CUD and neonatal outcomes [35]. Almost all previous research relied on conventional multivariable regressions to adjust for observed characteristics with very few exceptions [22,39]. Multivariable regressions may not address the situations where confounding factors do not adequately overlap between the treated and control groups. The validity of such between-group comparisons is threatened [42]. This is exactly the concern when we compare infants exposed to CUD (treated group) and unexposed to CUD (control group), because they had considerable differences in confounding factors particularly in mothers’ health conditions and drug use behaviors.
In this study, we adopted propensity score matching (PSM), a common approach in observational studies to alleviate selection bias. It allows us to mimic some of the characteristics of a randomized controlled trial by balancing the distribution of observed covariates in the treated and control groups [43–45]. We conducted PSM in accordance with published guidelines [42]. In the first step, we fitted logistic regressions with all the covariates described in ‘Maternal, Paternal, and Infant Covariates’ to estimate the propensity score of prenatal CUD exposure for each infant. In the second step, infants exposed to prenatal CUD were 1:2 matched to infants unexposed to prenatal CUD using nearest-neighbor matching with replacement, the most common implementation of PSM that minimizes bias in subsequent estimations [46]. In the last step, we computed standardized differences in covariates between the treated and control groups before and after matching to assess the improvement of balance on covariates (Supporting information Technical Note S1) [47]. A standardized difference of no more than 10% is considered an indicator of balance [47].
Following PSM, generalized linear mixed regressions (Gaussian family for continuous outcomes and binomial family for binary outcomes) were used to examine the associations between prenatal CUD and neonatal outcomes, accounting for the paring between the treated and control groups. All the covariates described in ‘Maternal, Paternal, and Infant Covariates’ were adjusted for in regressions. The final sample size for the regressions following PSM was 60 711. We also conducted subgroup analysis by mothers’ tobacco use status and race/ethnicity. Interaction terms in regressions were used to test the significance of subgroup differences.
In sensitivity analysis, we used different PSM algorithms to determine the robustness of results. In addition to the 1:2 nearest-neighbor matching with replacement in the main analysis, we used 1:1 nearest-neighbor matching with and without replacement and 1:1 and 1:2 nearest-neighbor matching with replacement and caliper 0.00005, 0.0001, and 0.0002. [48]
All statistical analyses were performed using Stata 15.1 (StataCorp LP). The analysis was not pre-registered and the results should be considered exploratory.
RESULTS
Sample characteristics before matching
Figure 1 depicts the time trend of diagnosed prenatal CUD in California. From 2001 through 2012, the rate of prenatal CUD increased from 2.8 to 6.9 per 1000 deliveries. A total of 20237 mother–infant pairs were exposed to prenatal CUD, constituting the treated group.
Figure 1.
Rates of diagnosed prenatal cannabis use disorder during 2001–2012 in California, US
Supporting information Table S4 reports descriptive statistics of mother–infant pairs exposed and unexposed to prenatal CUD before matching. Most covariates had standardized difference greater than 10% with only a few exceptions (Supporting information Table S5), indicating substantial differences between the treated and control groups before matching.
Propensity score matching
Using PSM, we matched the treated group with 40474 mother–infant pairs unexposed to prenatal CUD, which constituted the matched control group. Supporting information Table S4 reports their descriptive statistics.
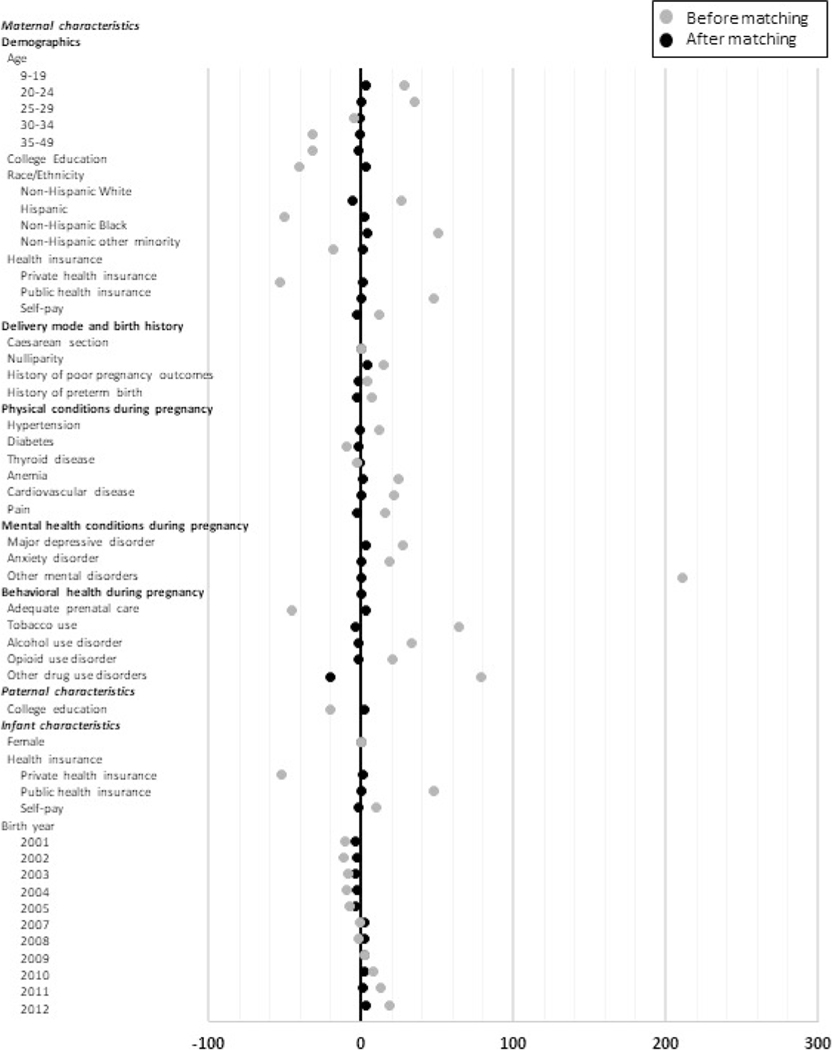
Figure 2 presents the standardized differences between the treated and control groups before and after matching (details in Supporting information Table S5). The standardized differences in all the covariates were considerably reduced after matching; only one covariate (other drug use disorders) still had difference >10% and most covariates had differences reduced to 3% or less. PSM considerably improved the comparability between the treated and control groups.
Figure 2.
Standardized differences between the treated and control groups before and after propensity score matching. The gray dots represent standardized differences before matching, and the black dots represent standardized differences after matching. The 1:2 nearest-neighbor matching with replacement was used. Standardized differences in individual covariates (in percentage points) are reported for the comparisons between infants exposed and unexposed to prenatal cannabis use disorder (treated group vs. control group). Birth cohort in year 2006 was not included in this study because of missing information on prenatal tobacco use. Details in this figure are reported in Supporting information Table S5
Association estimation after matching
Table 1 reports descriptive statistics of neonatal outcomes in the treated and matched control groups without regression adjustments.
Table 1.
Unadjusted neonatal outcomes between treated and matched control groups after propensity score matching.
| Treated (n = 20237) |
Matched control (n = 40474) |
t test on between-group difference |
|||||
|---|---|---|---|---|---|---|---|
| Outcome | Mean or % | 95% CI | Mean or % | 95% CI | Mean or % | 95% CI | P value |
| Length of gestation, continuous | 272.32 | 272.03, 272.60 | 271.88 | 271.69, 272.97 | 0.43 | 0.099, 0.77 | 0.011 |
| Small for gestational age, binary | 19.12% | 18.58%, 19.66% | 17.72% | 17.35%, 18.10% | 1.39% | 0.74%, 2.04% | <0.001 |
| Preterm birth, binary | 12.99% | 12.53%, 13.45% | 13.16% | 12.83%, 13.49% | −0.17% | −0.74%, 0.39% | 0.55 |
| Birth weight, continuous | 3153.71 | 3144.96, 3162.47 | 3184.59 | 3178.46, 3190.72 | −30.87 | −41.52, −20.22 | <0.001 |
| Birth weight z-score, continuous | −0.20 | −0.22, −0.18 | −0.12 | −0.14, −0.11 | −0.075 | −0.10, −0.051 | <0.001 |
| Low birth weight, binary | 11.71% | 11.27%, 12.15% | 11.20% | 10.89%, 11.51% | 0.50% | −0.26%, 1.04% | 0.062 |
| Admission into neonatal intensive care unit, binary | 9.20% | 8.80%, 9.60% | 10.02% | 9.73%, 10.31% | −0.81% | −0.13%, −0.31% | 0.0014 |
| Hospitalization within a year, binary | 11.37% | 10.93%, 11.81% | 12.42% | 12.09%, 12.74% | −1.04% | −1.59%, −0.49% | <0.001 |
| Death within a year, binary | 0.98% | 0.85%, 1.12% | 0.75% | 0.66%, 0.83% | 0.23% | 0.081%, 0.38% | 0.0027 |
Treated group included all mother–infant pairs exposed to prenatal cannabis use disorder. Matched control group included propensity-score-matched mother– infant pairs unexposed to prenatal cannabis use disorder using 1:2 nearest-neighbor matching with replacement method.
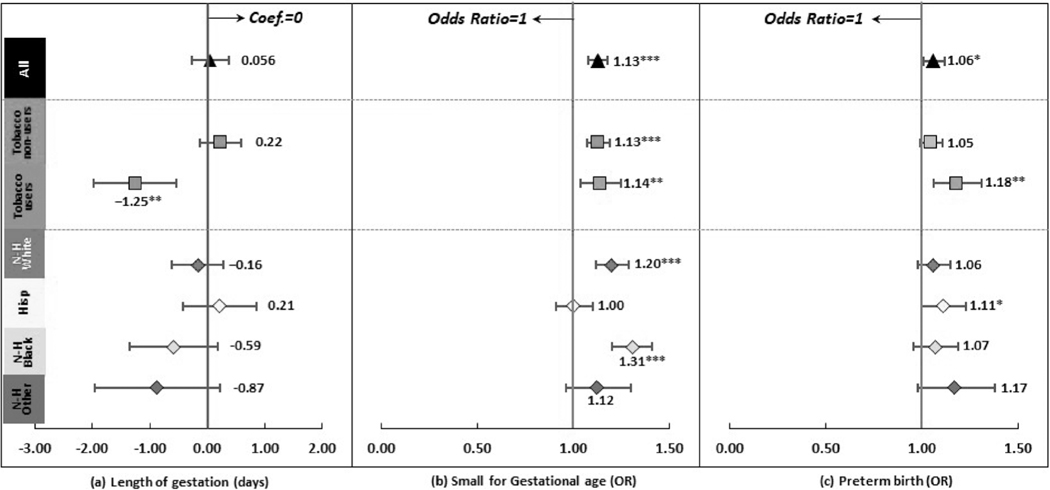
Figure 3 reports the associations between prenatal CUD and gestational age related outcomes(details in Supporting information Table S6). The association between prenatal CUD and length of gestation was nonsignificant. Prenatal CUD was associated with higher odds of small for gestational age (OR = 1.13, 95% CI = 1.08, 1.18) and preterm birth (OR = 1.06, 95% CI = 1.01, 1.12).
Figure 3.
Regression results after propensity score matching: gestational age related outcomes *P < 0.05, **P < 0.01, ***P < 0.001. ‘Hisp’ short for ‘Hispanic’, ‘N-H’ short for ‘Non-Hispanic’. Dots and lines represent means and 95% confidence intervals for coefficients or odds ratios estimated from generalized linear mixed regressions. Maternal, paternal, and infant characteristics were also included in regressions but not reported. Details are reported in Supporting information Table S6
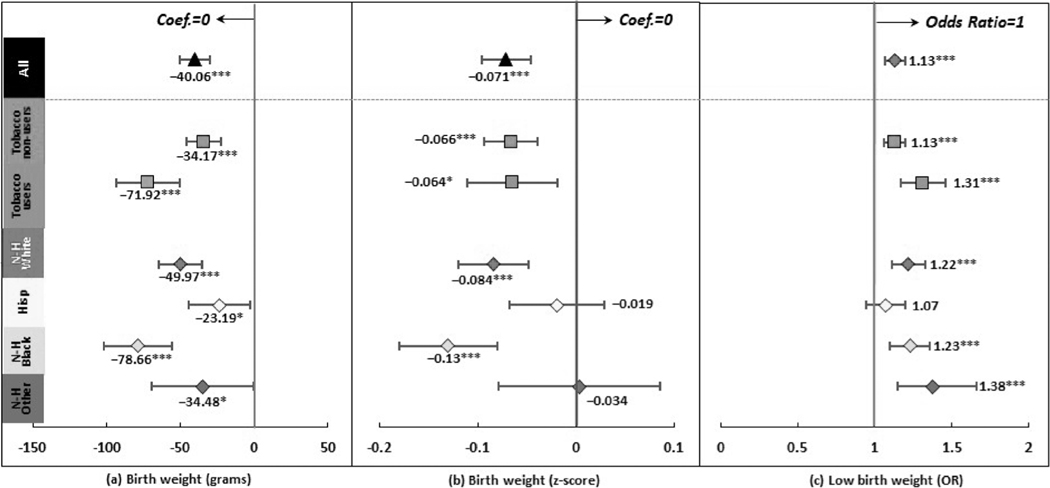
Figure 4 reports the associations between prenatal CUD and birth weight related outcomes, which were significant regardless of how birth weight was measured (details in Supporting information Table S6). Prenatal CUD was associated with a smaller birth weight by 40.06 grams (95% CI = −50.34, −29.77) and a smaller birth weight z score by 0.071 (95% CI = −0.096, −0.047). It was also associated with a higher odds of low birth weight (OR = 1.13, 95% CI = 1.07, 1.20).
Figure 4.
Regression results after propensity score matching: birth weight related outcomes *P < 0.05, ***P < 0.001. ‘Hisp’ short for ‘Hispanic’, ‘N-H’ short for ‘Non-Hispanic’. Dots and lines represent means and 95% CI for coefficients or OR estimated from generalized linear mixed regressions. Maternal, paternal, and infant characteristics were also included in regressions but not reported. Details are reported in Supporting information Table S6
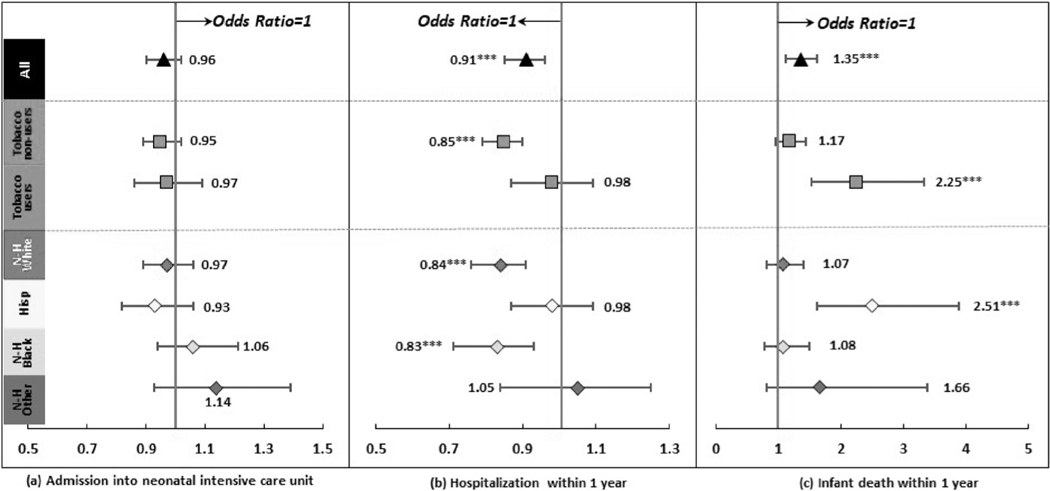
Figure 5 reports the associations between prenatal CUD and other neonatal outcomes (details in Supporting information Table S6). The association between prenatal CUD and admission into neonatal intensive care unit was nonsignificant. Prenatal CUD was associated with a higher odds of death within 1 year of birth (OR = 1.35, 95% CI = 1.12, 1.62) but a lower odds of hospitalization with 1 year of birth (OR = 0.91, 95% CI = 0.86, 0.96).
Figure 5.
Regression results after propensity score matching: other neonatal outcomes ***P < 0.001. ‘Hisp’ short for ‘Hispanic, ‘N-H’ short for ‘Non-Hispanic’. Dots and lines represent means and 95% CI for coefficients or OR estimated from generalized linear mixed regressions. Maternal, paternal, and infant characteristics were also included in regressions but not reported. Details are reported in Supporting information Table S6
Subgroup analysis
Figures 3–5 also report regression results in subgroups by mothers’ tobacco use status and race/ethnicity (details in Supporting information Table S6). Supporting information Table S7 reports likelihood ratio tests results on the significance of adding interaction terms in regressions for subgroup differences. Supporting information Table S8 reports the estimations on the interaction terms.
Compared to infants whose mothers were tobacco nonusers, infants whose mothers were tobacco users had greater odds of preterm birth, low birth weight, hospitalization within 1 year of birth, and death within 1 year of birth and lower length of gestation and birth weight in association with prenatal CUD. Compared to infants whose mothers were non-Hispanic Whites, infants whose mothers were Hispanics had greater odds of hospitalization and death within 1 year of birth, lower odds of small for gestational age and low birth weight, and higher birth weight and birth weight z score in association with prenatal CUD. Infants whose mothers were non-Hispanic Blacks had a greater odds of small for gestational age and lower birth weight and birth weight z score in association with prenatal CUD. Infants whose mothers were non-Hispanic other minorities had a greater odds of hospitalization within 1 year of birth in association with prenatal CUD.
Sensitivity analysis
The estimated associations were overall robust to different matching algorithms (Supporting information Table S9).
DISCUSSION
Using a large retrospective cohort in California, United States, this study found significant associations between prenatal CUD exposure and major adverse neonatal outcomes. In accordance with Corsi et al. [39] we found that prenatal CUD was associated with greater odds of small for gestational age and preterm birth. However, we did not find a significant association with admission to neonatal intensive care unit as Corsi et al. [39]. We also examined a series of birth weight measures and reported negative associations between prenatal CUD and birth weight.
Unlike most previous studies centering on birth outcomes at delivery, this study added new data on outcomes after birth. The most notable observation is that exposed infants were 35% more likely to die within 1 year of birth than unexposed infants. Another finding, yet counterintuitive, was that prenatal CUD was associated with a lower odds of hospitalization within 1 year of birth. A possible explanation is that, the most severely ill infants died as a result of prenatal CUD exposure, so the remaining exposed infants might be relatively healthier than infants unexposed to CUD. Future studies are needed to provide additional evidence on this seemingly ‘protective’ effect of prenatal CUD.
Previous research discussed the potentially greater risks of adverse neonatal outcomes imposed by the co-use of cannabis and tobacco during pregnancy [37,49]. This study demonstrated that the odds of adverse outcomes in association with prenatal CUD indeed differed between infants whose mothers did and did not use tobacco. In addition to the similar results on preterm birth as Corsi et al. [39], we found that the odds of low birth weight and hospitalization and death within 1 year of birth in association with prenatal CUD were greater among infants whose mothers used tobacco. These findings demonstrated the importance of accounting for the confounding from tobacco use, a common pattern among women using cannabis during pregnancy [37]. Meanwhile, prenatal CUD was a risk factor regardless of tobacco co-use; it was associated with greater odds of small for gestational age and low birth weight even in infants whose mothers were tobacco nonusers. This finding conflicted with some smaller-scale studies that found prenatal cannabis use alone did not predict adverse neonatal outcomes after tobacco co-use was controlled for [36].
We also revealed heterogeneities in the relationships by mothers’ race and ethnicity. Relative to non-Hispanic Whites, Hispanics had greater whereas non-Hispanic Blacks had smaller birth weight outcomes in association with prenatal CUD. Nonetheless, Hispanics were more likely to be hospitalized or dead within 1 year of birth as a result related to prenatal CUD. Racial and ethnic differences in infant outcomes have been documented in previous literature [50,51], but little research has been conducted to understand the moderating role of maternal drug use. Future research is warranted to explore the mechanisms underlying these heterogeneities.
It should be noted that prenatal CUD in this study was identified from hospital records with ICD codes. In the United States, it is not yet a standard care to screen cannabis use among pregnant women and the diagnosis primarily relies on self-reporting. Toxicology tests could alleviate measurement errors, but to what extent and under what circumstances they have been conducted in healthcare systems are unknown. Previous research suggested that approximately 60% pregnant women in California having a positive toxicology test on cannabis also had a positive self-report, indicating an underestimation of CUD diagnosis in this population [52,53]. The mothers diagnosed with CUD were therefore likely those who had the most severe symptoms. We expect that in recent years self-reporting bias might become less concerning in California where recreational cannabis has been legalized.
The findings call for special consideration of prenatal CUD in prevention, treatment, and policies. Because medical cannabis use during pregnancy may have health benefits, there are ongoing debates regarding whether informed decision of cannabis use is justifiable [17,35,54]. Nonetheless, problem cannabis use (CUD in this study) presumably has no known health benefits; even if there are any, the adverse consequences clearly outweigh the benefits. The American College of Obstetricians and Gynecologists committee has recommended that physicians encourage pregnant women to discontinue cannabis use including medical use [55]. Given the adverse neonatal consequences associated with prenatal CUD, we further recommend CUD screening among pregnant women using cannabis along with appropriate education, counseling, or referral to substance abuse treatment services. In states approving medical and/or recreational cannabis, regulatory approaches targeting pregnant women could be also considered, such as developing guidelines for physicians to appropriately recommend medical cannabis and communicating potential risks of prenatal cannabis use and CUD via point-of-sale warning signs and product warning labels [56].
This study has limitations. First, even though we used matching techniques with a wide range of covariates to balance the measured confounding factors, we were not able to eliminate bias from unobserved confounding factors. The findings cannot be interpreted as causal, a limitation common to almost all previous epidemiological studies.
Second, this study focused on CUD, a proxy for heavy and/or long-term use of cannabis. The findings hence represented roughly 20% cannabis users in pregnant women [6], whose infants presumably had the highest risks of adverse outcomes. The findings may not generalize to mothers who used cannabis but did not meet the criteria for CUD. Further, as we discussed above, prenatal CUD identified through ICD codes could be considerably underestimated. The underestimation would attenuate the observed associations toward null; therefore our estimations likely represented lower bounds of the true associations. We solely relied on records at delivery for CUD identification. Some mothers may be misclassified to the control group if they had CUD during pregnancy but had no CUD at delivery. We were not able to examine cannabis use patterns such as frequency, dose, duration, and routes of administration, which may be associated with varying health consequences.
Third, measures from birth certificates had caveats. Although a good agreement in parents’ demographics and neonatal outcomes was found between birth certificates and hospital and claims records [57–60], maternal medical conditions and complications tended to be under-reported on birth certificates [58,59,61–63].Particularly concerning in this study, maternal tobacco use were potentially under-reported, even though our prevalence estimate was on a par with that estimated from previous literature [64]. In addition, ~7% mother–infant pairs were dropped primarily because of missing information on birth certificates. Paternal characteristics were only available on birth certificates, which were very limited with considerable missing values. We were unable to control for many confounding factors from fathers such as paternal cannabis use.
Fourth, we examined a comprehensive list of neonatal outcomes including those rarely studied, but the outcome list was by no means exhaustive because of data limitations. For instance, we did not assess cause of deaths in different stages of infancy although it is critical for our understanding of a higher death rate among infants exposed to prenatal CUD. We did not examine stillbirth or long-term neurodevelopmental outcomes. We were also not able to account for infant confounding factors that were revealed after birth because only a small portion of infants had hospital discharge records after birth.
Last, the findings may not generalize to mothers who delivered outside of hospital setting or outside of California. The higher rate of missing values in mother–infant pairs exposed to prenatal CUD may also affect the generalizability of the findings.
Notwithstanding the limitations, this study contributed to the literature by using nearly 5 million mother–infant pairs in the United States in a 10-year period when cannabis was increasingly liberalized. To our knowledge, this was the largest cohort in prenatal cannabis use research. The unprecedentedly large cohort allowed us to assess rare outcomes such as infants’ hospitalization and death as well as heterogeneities in subgroups. The application of PSM considerably improved the validity of comparisons. Centralized healthcare records also provided diverse samples and improved data accuracy [36,65]. Tobacco users and nonusers were explicitly compared, contributing to the debate on the confounding role of tobacco use.
CONCLUSION
This study added new data to the associations between cannabis use during pregnancy and adverse neonatal outcomes in the United States. We found that prenatal CUD was associated with major adverse neonatal outcomes, including greater odds of small for gestational age, preterm birth, low birth weight, and death within 1 year of birth. Heterogeneities in the associations were revealed by mothers’ tobacco use status and race/ethnicity.
Supplementary Material
Table S1 Data sources.
Table S2 Proportion of mother–infant pairs with missing data.
Table S3 ICD-9 codes for identifying maternal medical conditions at delivery.
Table S4 Descriptive statistics of the study sample.
Table S5 Standardized differences in covariates, Fig. 2 details.
Table S6 Detailed regression results after propensity score matching.
Table S7 Likelihood ratio tests on adding interaction terms for detecting subgroup differences.
Table S8 Regression results on subgroup differences by adding interaction terms.
Table S9 Sensitivity analyses: regression results with different propensity score matching algorithms.
Acknowledgements
This research was supported by grant R01DA042290 (PI: Shi) from the US National Institute on Drug Abuse. This article is the sole responsibility of the authors and does not reflect the views of the National Institute on Drug Abuse.
Footnotes
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Declaration of interests
None.
Throughout the manuscript, we described the study period as 2001–2012 with the understanding that 2006 was excluded.
Length of gestation smaller than 20 weeks or greater than 44 weeks were considered invalid data.
Prenatal CUD was only identified at delivery because most of the pregnant mothers did not have hospital discharge records before delivery.
Adequate prenatal care was identified if the mother had one prenatal care visit every month through 28 weeks’ gestation and one visit every 2 weeks during 28–36 weeks’ gestation for a pregnancy shorter than 36 weeks or the mother had nine or more prenatal care visits for a pregnancy of 36 weeks or longer.
References
- 1.El Marroun H, Brown QL,Lund IO,Coleman-Cowger VH, Loree AM, Chawla D, et al. An epidemiological, developmental and clinical overview of cannabis use during pregnancy. Prev Med 2018; 116: 1–5. [DOI] [PubMed] [Google Scholar]
- 2.Luke S, Hutcheon J, Kendall T. Cannabis use in pregnancy in British Columbia and selected birth outcomes. J Obstet Gynaecol Can 2019; 41: 1311–7. [DOI] [PubMed] [Google Scholar]
- 3.Corsi DJ, Hsu H, Weiss D, Fell DB, Walker M. Trends and correlates of cannabis use in pregnancy: a population-based study in Ontario, Canada from 2012 to 2017. Can J Public Health 2019; 110: 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saurel-Cubizolles MJ, Prunet C, Blondel B. Cannabis use during pregnancy in France in 2010. BJOG 2014; 121: 971–7. [DOI] [PubMed] [Google Scholar]
- 5.Volkow ND, Han B, Compton WM, McCance-Katz EF Self-reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA 2019; 322: 167–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko JY, Farr SL, Tong VT, Creanga AA, Callaghan WM Prevalence and patterns of marijuana use among pregnant and nonpregnant women of reproductive age. Am J Obstet Gynecol 2015; 213: 201 e201–201 e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Y, Zhong S. Trends in cannabis use disorder among pregnant women in the U.S., 1993–2014. J Gen Intern Med 2018; 33: 245–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leafly. Qualifying conditions for medical marijuana by state. Leafly.com; 2020. Available at: https://www.leafly.com/news/health/qualifying-conditions-for-medical-marijuana-by-state. (accessed 13 June 2020).
- 9.MichiganState. House bill no. 4126. 2019. Available at: http://www.legislature.mi.gov/documents/2019-2020/billintroduced/House/htm/2019-HIB-4126.htm. (accessed 14 June 2020).
- 10.DrugPolicyAlliance. Labeling requirements for marijuana products in Colorado. Drug Policy Alliance; 2014. Available at: https://www.drugpolicy.org/sites/default/files/Labeling_Requirements_for_Marijuana_Products_in_Colorado.pdf. (accessed 14 June 2020). [Google Scholar]
- 11.OEHHA. The Proposition 65 list. California Office of Environmental Health Hazard Assessment; 2020. Available at: https://oehha.ca.gov/proposition-65/proposition-65-list. (accessed 26 June 2020).
- 12.Gnofam M, Allshouse AA, Stickrath EH, Metz TD Impact of marijuana legalization on prevalence of maternal marijuana use and perinatal outcomes. Am J Perinatol 2020; 37: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meinhofer A, Witman A, Murphy SM, Bao Y. Medical marijuana laws are associated with increases in substance use treatment admissions by pregnant women. Addiction 2019; 114: 1593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee E, Pluym ID, Wong D, Kwan L, Varma V, Rao R. The impact of state legalization on rates of marijuana use in pregnancy in a universal drug screening population. J Matern Fetal Neonatal Med 20201–8. [DOI] [PubMed] [Google Scholar]
- 15.Mark K, Terplan M. Cannabis and pregnancy: maternal child health implications during a period of drug policy liberalization. Prev Med 2017; 104: 46–9. [DOI] [PubMed] [Google Scholar]
- 16.Polen MR, Whitlock EP, Wisdom JP, Nygren P, Bougatsos C. Screening in primary care settings for illicit drug use: staged systematic review for the United States Preventive Services Task Force: Agency for Healthcare Research and Quality. US; 2008. [PubMed] [Google Scholar]
- 17.Takakuwa KM, Schears RM Why pregnant women may justifiably choose to use cannabis. JAMA Intern Med 2019; 179: 120. [DOI] [PubMed] [Google Scholar]
- 18.Young-Wolff KC, Sarovar V, Tucker LY, Avalos LA, Conway A, Armstrong MA, et al. Association of nausea and vomiting in pregnancy with prenatal marijuana use. JAMA Intern Med 2018; 178: 1423–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Postonogova T, Xu C, Moore A. Marijuana during labour: a survey of maternal opinions. J Obstet Gynaecol Can 2020; 42: 774–8. [DOI] [PubMed] [Google Scholar]
- 20.Thompson R, DeJong K, Lo J. Marijuana use in pregnancy: are view. Obstet Gynecol Surv 2019; 74: 415–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet 2009; 374: 1383–91. [DOI] [PubMed] [Google Scholar]
- 22.Bailey BA, Wood DL, Shah D. Impact of pregnancy marijuana use on birth outcomes: results from two matched population-based cohorts. J Perinatol 2020; 40: 1477–82. [DOI] [PubMed] [Google Scholar]
- 23.Straub HL, Mou J, Drennan KJ, Pflugeisen BM Maternal marijuana exposure and birth weight: an observational study surrounding recreational marijuana legalization. Am J Perinatol 2019. [DOI] [PubMed] [Google Scholar]
- 24.Howard DS, Dhanraj DN, Devaiah CG, Lambers DS Cannabis use based on urine drug screens in pregnancy and its association with infant birth weight. J Addict Med 2019; 13: 436–41. [DOI] [PubMed] [Google Scholar]
- 25.Metz TD, Borgelt LM Marijuana use in pregnancy and while breastfeeding. Obstet Gynecol 2018; 132: 1198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leemaqz SY, Dekker GA, McCowan LM, Kenny LC,Myers JE, Simpson NA, et al. Maternal marijuana use has independent effects on risk for spontaneous preterm birth but not other common late pregnancy complications. Reprod Toxicol 2016; 62: 77–86. [DOI] [PubMed] [Google Scholar]
- 27.Gunn JK, Rosales CB, Center KE, Nunez A, Gibson SJ, Christ C, et al. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open 2016; 6: e009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown SJ, Mensah FK, Ah Kit J, Stuart-Butler D, Glover K, Leane C, et al. Use of cannabis during pregnancy and birth outcomes in an aboriginal birth cohort: a cross-sectional, population-based study. BMJ Open 2016; 6: e010286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kharbanda EO, Vazquez-Benitez G, Kunin-Batson A, Nordin JD, Olsen A, Romitti PA Birth and early developmental screening outcomes associated with cannabis exposure during pregnancy. J Perinatol 2020; 40: 473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klebanoff MA, Wilkins DG, Keim SA Marijuana use during pregnancy and preterm birth: a prospective cohort study. Am J Perinatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko JY, Tong VT, Bombard JM, Hayes DK, Davy J, Perham-Hester KA Marijuana use during and after pregnancy and association of prenatal use on birth outcomes: a population-based study. Drug Alcohol Depend 2018; 187: 72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metz TD, Allshouse AA, Hogue CJ, Goldenberg RL, Dudley DJ, Varner MW, et al. Maternal marijuana use, adverse pregnancy outcomes, and neonatal morbidity. Am J Obstet Gynecol 2017; 217: 478 e471–478 e478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conner SN, Carter EB, Tuuli MG, Macones GA, Cahill AG Maternal marijuana use and neonatal morbidity. Am J Obstet Gynecol 2015; 213: e421–e424. [DOI] [PubMed] [Google Scholar]
- 34.Chabarria KC, Racusin DA, Antony KM, Kahr M, Suter MA, Mastrobattista JM, et al. Marijuana use and its effects in pregnancy. Am J Obstet Gynecol 2016; 215: e501–e507. [DOI] [PubMed] [Google Scholar]
- 35.Silverstein M, Howell EA, Zuckerman B. Cannabis use in pregnancy: a tale of 2 concerns. JAMA 2019; 322: 121–2. [DOI] [PubMed] [Google Scholar]
- 36.Goler N, Conway A, Young-Wolff KC Data are needed on the potential adverse effects of marijuana use in pregnancy. Ann Intern Med 2018; 169: 492–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coleman-Cowger VH, Schauer GL, Peters EN Marijuana and tobacco co-use among a nationally representative sample of US pregnant and non-pregnant women: 2005–2014 National Survey on drug use and health findings.Drug Alcohol Depend 2017; 177: 130–5. [DOI] [PubMed] [Google Scholar]
- 38.Conner SN, Bedell V, Lipsey K, Macones GA, Cahill AG, Tuuli MG Maternal marijuana use and adverse neonatal outcomes: a systematic review and meta-analysis. Obstet Gynecol 2016; 128: 713–23. [DOI] [PubMed] [Google Scholar]
- 39.Corsi DJ, Walsh L, Weiss D, Hsu H, El-Chaar D, Hawken S,et al. Association between self-reported prenatal cannabis use and maternal, perinatal, and neonatal outcomes. JAMA 2019; 322: 145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Talge NM, Mudd LM, Sikorskii A, Basso O. United States birth weight reference corrected for implausible gestational age estimates. Pediatrics 2014; 133: 844–53. [DOI] [PubMed] [Google Scholar]
- 41.Kotelchuck M. An evaluation of the Kessner adequacy of prenatal care index and a proposed adequacy of prenatal care utilization index. Am J Public Health 1994; 84: 1414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao XI, Wang X, Speicher PJ, Hwang ES, Cheng P, Harpole DH, et al. Reporting and guidelines in propensity score analysis: a systematic review of cancer and cancer surgical studies. J Natl Cancer Inst 2017; 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Austin PC, Grootendorst P, Anderson GM A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med 2007; 26: 734–53. [DOI] [PubMed] [Google Scholar]
- 44.Thoemmes FJ, Kim ES A systematic review of propensity score methods in the social sciences. Multivar Behav Res 2011; 46: 90–118. [DOI] [PubMed] [Google Scholar]
- 45.Austin PC An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res 2011; 46: 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Austin PC Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am J Epidemiol 2010; 172: 1092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Austin PC Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat-Simul C 2009; 38: 1228–34. [Google Scholar]
- 48.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 2009; 20: 512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Passey ME, Sanson-Fisher RW, D’Este CA, Stirling JM Tobacco, alcohol and cannabis use during pregnancy: clustering of risks. Drug Alcohol Depend 2014; 134: 44–50. [DOI] [PubMed] [Google Scholar]
- 50.Rossen LM, Schoendorf KC Trends in racial and ethnic disparities in infant mortality rates in the United States, 1989–2006. Am J Public Health 2014; 104: 1549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donahue SM, Kleinman KP, Gillman MW, Oken E.Trends in birth weight and gestational length among singleton term births in the United States: 1990–2005. Obstet Gynecol 2010; 115: 357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Young-Wolff KC, Tucker LY, Alexeeff S, Armstrong MA,Conway A, Weisner C, et al. Trends in self-reported and biochemically tested marijuana use among pregnant females in California from 2009–2016. JAMA 2017; 318: 2490–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young-Wolff KC, Sarovar V, Tucker L-Y, Goler N, Conway A, Weisner C, et al. Validity of self-reported cannabis use among pregnant females in northern California. J Addict Med 2020; 14: 287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Young-Wolff KC, Conway A, Goler N. Why pregnant women may justifiably choose to use cannabis-reply. JAMA Intern Med 2019; 179: 120–1. [DOI] [PubMed] [Google Scholar]
- 55.Committee on Obstetric P Committee opinion no. 722: marijuana use during pregnancy and lactation. Obstet Gynecol 2017; 130: e205–e209. [DOI] [PubMed] [Google Scholar]
- 56.Chasnoff IJ Medical marijuana laws and pregnancy: implications for public health policy. Am J Obstet Gynecol 2017; 216: 27–30. [DOI] [PubMed] [Google Scholar]
- 57.Andrade SE, Scott PE, Davis RL, Li DK, Getahun D, Cheetham TC, et al. Validity of health plan and birth certificate data for pregnancy research. Pharmacoepidemiol Drug Saf 2013; 22: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Northam S, Knapp TR The reliability and validity of birth certificates. J Obstet Gynecol Neonatal Nurs 2006; 35: 3–12. [DOI] [PubMed] [Google Scholar]
- 59.Zollinger TW, Przybylski MJ, Gamache RE Reliability of Indiana birth certificate data compared to medical records. Ann Epidemiol 2006; 16: 1–10. [DOI] [PubMed] [Google Scholar]
- 60.Vinikoor LC, Messer LC, Laraia BA, Kaufman JS Reliability of variables on the North Carolina birth certificate: a comparison with directly queried values from a cohort study. Paediatr Perinat Epidemiol 2010; 24: 102–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ananth CV Perinatal epidemiologic research with vital statistics data: validity is the essential quality. Am J Obstet Gynecol 2005; 193: 5–6. [DOI] [PubMed] [Google Scholar]
- 62.Haghighat N, Hu M, Laurent O, Chung J, Nguyen P, Wu J. Comparison of birth certificates and hospital-based birth data on pregnancy complications in Los Angeles and Orange County, California. BMC Pregnancy Childbirth 2016; 16: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Howland RE, Mulready-Ward C, Madsen AM, Sackoff J, Nyland-Funke M, Bombard JM, et al. Reliability of reported maternal smoking: comparing the birth certificate to maternal worksheets and prenatal and hospital medical records, New York City and Vermont, 2009. Matern Child Health J 2015; 19: 1916–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Drake P, Driscoll AK, Mathews T. Cigarette smoking during pregnancy: United States, 2016. NCHS Data Brief, No. 305: National Center for Health Statistics, Centers for Disease Control and Prevention; 2018. [PubMed] [Google Scholar]
- 65.Lydon-Rochelle MT, Holt VL, Cardenas V, Nelson JC, Easterling TR, Gardella C, et al. The reporting of pre-existing maternal medical conditions and complications of pregnancy on birth certificates and in hospital discharge data. Am J Obstet Gynecol 2005; 193: 125–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Data sources.
Table S2 Proportion of mother–infant pairs with missing data.
Table S3 ICD-9 codes for identifying maternal medical conditions at delivery.
Table S4 Descriptive statistics of the study sample.
Table S5 Standardized differences in covariates, Fig. 2 details.
Table S6 Detailed regression results after propensity score matching.
Table S7 Likelihood ratio tests on adding interaction terms for detecting subgroup differences.
Table S8 Regression results on subgroup differences by adding interaction terms.
Table S9 Sensitivity analyses: regression results with different propensity score matching algorithms.