Abstract
Objective:
The Nine Item Avoidant/Restrictive Food Intake Disorder (ARFID) Screen (NIAS) has three subscales aligned with ARFID presentations but clinically validated cutoff scores have not been identified. We aimed to examine NIAS subscale (picky eating, appetite, fear) validity to: (1) capture clinically-diagnosed ARFID presentations; (2) differentiate ARFID from other eating disorders (other-ED); and (3) capture ARFID symptoms among individuals with ARFID, individuals with other-ED, and nonclinical participants.
Method:
Participants included outpatients (ages 10–76 years; 75% female) diagnosed with ARFID (n=49) or other-ED (n=77), and nonclinical participants (ages 22–68 years; 38% female, n=40). We evaluated criterion-related concurrent validity by conducting receiver operating curve (ROC) analyses to identify potential subscale cutoffs and by testing if cutoffs could capture ARFID with and without use of the Eating Disorder Examination-Questionnaire (EDE-Q).
Results:
Each NIAS subscale had high AUC for capturing those who fit vs. do not fit each ARFID presentation, resulting in proposed cutoffs of ≥10 (sensitivity=.97, specificity=.63), ≥9 (sensitivity=.86, specificity=.70), and ≥10 (sensitivity=.68, specificity=.89) on the NIAS-picky eating, NIAS-appetite, and NIAS-fear subscales, respectively. ARFID vs. other-ED had high AUC on the NIAS-picky eating (≥10 proposed cutoff), but not NIAS-appetite or NIAS-fear subscales. NIAS subscale cutoffs had a high association with ARFID diagnosis, but only correctly classified other-ED in combination with EDE-Q Global <2.3.
Discussion:
To screen for ARFID, we recommend using a screening tool for other-ED (e.g., EDE-Q) in combination with a positive score on any NIAS subscale (i.e., ≥10, ≥9, and/or ≥10 on the NIAS-picky eating, NIAS-appetite, and NIAS-fear subscales, respectively).
Keywords: feeding and eating disorders, avoidant/restrictive food intake disorder, surveys and questionnaires, diagnosis
INTRODUCTION
Avoidant/restrictive food intake disorder (ARFID) is an eating disorder (ED) characterized by food avoidance or dietary restriction associated with at least one of four consequences––weight loss, nutritional deficiency, nutritional supplement dependence, and/or psychosocial impairment (APA, 2013). ARFID is differentiated from other-EDs (i.e., sub- and full-threshold anorexia nervosa, bulimia nervosa, binge-eating disorder, other specified feeding or eating disorders) in that restrictive eating is motivated by one or more of three overlapping presentations—sensitivity to sensory characteristics of food (i.e., sensory sensitivity presentation), lack of interest in eating/low appetite (i.e., lack of interest presentation), and fear of aversive consequences (i.e., fear of aversive consequences presentation) (APA, 2013). The Nine Item ARFID Screen (NIAS) was developed as a screening measure to detect ARFID symptoms and has nine items with three subscales that map onto symptoms of each ARFID presentation (Zickgraf & Ellis, 2018). The NIAS subscales were previously validated in a large community sample (Zickgraf & Ellis, 2018) and have been translated into multiple languages (e.g., He, Zickgraf, Ellis, Lin, and Fan, 2020), but have yet to be validated to detect ARFID using a clinical sample. The identification of cutoff scores on the NIAS subscales to detect ARFID could have significant implications for clinical practice and research (e.g., screening instrument for ARFID in medical settings, identification of ARFID presentation types, treatment selection).
There are growing calls for methods to understand the characteristics of ARFID presentations (Eddy et al., 2019; Norris et al., 2018; Reilly, Brown, Gray, Kaye, & Menzel, 2019; Sharp & Stubbs, 2019). Our group has previously described a three-dimensional model (Thomas et al., 2017) to account for commonly occurring ARFID presentations (e.g., Reilly et al., 2019). While a semi-structured diagnostic interview—the Pica, ARFID, and Rumination Disorder Interview (PARDI)—has initial data supporting its psychometric properties with a clinically diagnosed ARFID sample (Bryant-Waugh et al., 2019), no self-report measure has yet validated subscales with the three ARFID presentations.
There is also growing recognition of the potential of comorbidities and overlap between ARFID and other-EDs (Becker, Breithaupt, Lawson, Eddy, & Thomas, 2019). ARFID and other-EDs have been shown to have a similar severity of dietary restriction (Becker, Keshishian, et al., 2019), potentially making the detection of ARFID by self-report questionnaires difficult without assessing the motivations behind avoidant/restrictive eating. Preliminary research suggests that symptoms of the ARFID sensory presentation may be able to differentiate ARFID from some other-EDs. Compared to individuals with anorexia nervosa, individuals with ARFID have had higher food neophobia scores (i.e., difficulty trying new foods on the Food Neophobia Scale) (Becker, Keshishian, et al., 2019). The Food Neophobia Scale was originally created to measure picky eating issues in children (Pliner & Hobden, 1992), which are similar to the symptoms of the ARFID sensory presentation (APA, 2013). Thus, symptoms of sensory sensitivity in ARFID may be a particularly relevant distinguisher of ARFID versus other-ED. Further research is needed to understand the overlap and potential differentiation between ARFID and other-ED across all ARFID presentations. In fact, the ARFID lack of interest and fear of aversive consequences presentations may share symptoms with other-ED, as these presentations often involve gastrointestinal symptoms (Murray, Bailey, et al., 2020; Murray, Jehangir, Silvernale, Kuo, & Parkman, 2020), which are reported by 45–69% of outpatients with other-EDs (DeJong, Perkins, Grover, & Schmidt, 2011; Murray, Kuo, et al., 2020; Perkins, Keville, Schmidt, & Chalder, 2005).
The NIAS could serve as an informative tool clinically to further detect ARFID by its presentations and to distinguish between ARFID and other-ED symptoms. Given the absence of data supporting the NIAS’s subscale validation with ARFID presentations, we aimed to determine how well the NIAS subscales perform in a clinical sample, specifically the validity of the subscales to: (1) classify ARFID presentations; (2) differentiated ARFID from other-ED; and (3) capture ARFID symptoms as a screening tool in a community sample. For Aim 1, among an eating disorder outpatient sample of children, adolescents and adults diagnosed with ARFID, we examined the validity of the NIAS subscales in adequately classify presence of clinically-diagnosed ARFID presentations. For Aim 2, we examined if we could identify cutoff scores on each subscale to adequately differentiate individuals with clinically-diagnosed ARFID from individuals with clinically-diagnosed other-ED. Given previous findings that characteristics of the ARFID sensory presentation (i.e., food neophobia; Becker, Keshishian, et al., 2019) have distinguished ARFID from other-ED, we hypothesized that a cutoff on the NIAS picky eating subscale would be able to differentiate ARFID (vs. other-ED), but the fear and lack of interest subscales would not. For Aim 3, we examined if subscale cutoff scores could adequately capture ARFID among those with ARFID, those with other-ED, and those without ARFID or other-ED (i.e., nonclinical participants; NPs), with and without a self-report measure of other-ED symptoms.
METHODS
Participants and Procedure
ARFID and Other-ED groups.
The ARFID (n=49) and other-ED (n=77) groups were recruited consecutively upon seeking treatment at an outpatient ED facility between 2018–2020. Prior to clinic evaluation, patients completed a standard battery of questionnaires (see Measures section for questionnaire subset used in this study). Diagnoses were conferred by clinicians using DSM-5 (APA, 2013) criteria for ARFID (n=49), anorexia nervosa (n=27), bulimia nervosa (n=11), binge-eating disorder (n=11), and other specified feeding or eating disorder (n=28, including n=14 atypical anorexia nervosa, n=1 subthreshold bulimia nervosa, n=3 subthreshold binge-eating disorder, n=2 purging disorder, n=2 night eating syndrome, and n=6 other). For patients with a diagnosis of ARFID, clinicians used clinical judgment based on their intake evaluation to indicate any of the three presentations (sensory, lack of interest, and/or fear) the patient’s presentation most resembled (i.e., clinicians could select more than one for each patient, if appropriate). Each patient received only one eating disorder diagnosis, as recommended in DSM-5 (APA, 2013).
NP group.
The NP group was recruited as a part of a larger study on cognitive processes among those with eating pathology versus healthy individuals via Amazon Mechanical Turk (MTurk). Individuals were invited via an email message from MTurk to participate in the full set of surveys if they scored below a 2.3 on the Eating Disorder Examination Questionnaire, below a 44 on the State-Trait Anxiety Inventory trait scale, and below a 16 on the Center for Epidemiological Studies Depression Scale. These cutoffs were established based on prior work validating these cutoffs for determining presence or absence of other-ED (Mond, Hay, Rodgers, Owen, & Beumont, 2004), anxiety (Ercan et al., 2015), and depression (Radloff, 1991), respectively. The anxiety and depression cutoffs were based on the aims of the larger study. Participants were paid $1 for completing screening measures. If eligible after screening, participants were paid an additional $5 for completion of all study measures. For the current study, we included participants if they did not meet criteria for ARFID by the Pica, ARFID, & Rumination Disorder Questionnaire (PARDI-AR-Q; Thomas et al., 2020). We set our survey such that individuals could not participate twice. Additionally, we collected MTurk worker IDs to ensure no participants completed the study twice.
This study was approved by the Massachusetts General Hospital Institutional Review Board (protocol #2013P002614).
Measures
Demographics.
Participants reported their age, sex, race, and ethnicity.
State-Trait Anxiety Scale (STAI).
The STAI trait subscale is a 20-item self-report questionnaire used to measure anxiety (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983). We used the STAI only in our MTurk sample to screen out those with significant clinical anxiety. This measure was taken by NP only; McDonald’s omega coefficient (to account for skewed data) was .9, indicating high internal consistency.
Center for Epidemiological Studies Depression Scale (CES-D).
The CES-D is a 20-item self-report questionnaire used to measure depressive symptoms (Radloff, 1977). We used the CES-D only in our MTurk sample to screen out individuals with significant clinical depression. This measure was taken by NP only; McDonald’s omega coefficient was .87, indicating high internal consistency.
Pica, ARFID, & Rumination Disorder Questionnaire (PARDI-AR-Q).
The PARDI-AR-Q is a 32-item self-report questionnaire developed based on the Pica, ARFID, & Rumination Disorder Inventory (PARDI) to assess symptoms of ARFID (Bryant-Waugh et al., 2019). The PARDI-AR-Q uses an algorithm based on the four DSM-5 Criterion A subcriteria for ARFID (weight loss/failure to gain weight, nutritional deficiencies, dependence on nutritional supplements, psychosocial impairment). The algorithm indicates that an individual may meet criteria for ARFID if an individual endorses any of these four sub-criteria and endorses that they view eating to be a significant problem in their life (Thomas et al., 2020). We used the PARDI-AR-Q among our MTurk sample to ensure that our NP group did not exhibit significant avoidant/restrictive eating. Since we used only binary (yes/no) items in the current study, we calculated split-half reliability of these items. Split-half reliability was .94, indicating high internal consistency in our sample.
Eating Disorder Examination Questionnaire (EDE-Q).
The EDE-Q is a 28-item self-report questionnaire that assesses other-ED symptoms (i.e., non-ARFID symptoms) over the past 28 days (Fairburn & Beglin, 2008). Higher scores indicate greater levels of other-ED symptoms. We used a cutoff <2.3 on the EDE-Q Global score to indicate low other-ED symptoms (Mond et al., 2004). As other-EDs may include avoidant/restrictive eating, we used the EDE-Q to identify likely clinically significant other-ED symptoms. We used the global score of the EDE-Q because (1) the factor structure of the subscales has been shown to not replicate (Becker et al., 2010; Grilo, Reas, Hopwood, & Crosby, 2015); (2) the global score allows us to capture the full range of possible symptoms (i.e., shape/weight-motivated dietary restraint, body image disturbance); and (3) recently shortened versions (e.g., EDE-Q8) have been validated with a global cutoff score (Machado, Grilo, Rodrigues, Vaz, & Crosby, 2020a). In the current sample, Cronbach alpha of the Global score was .82.
The Nine Item ARFID Screen (NIAS).
The NIAS is a 9-item self-report questionnaire that assesses avoidant/restrictive eating patterns. The NIAS is comprised of three subscales: the picky eating subscale measures sensory aversion to food (e.g., “I dislike most foods that other people eat”), the appetite subscale measures a lack of interest in eating or food (e.g., “Even when I am eating foods I really like, it is hard for me to eat a large enough volume at meals”), and the fear subscale measures fear of aversive consequences as a consequence of eating (e.g., “I avoid or put off eating because I am afraid of GI discomfort, choking, or vomiting”). Individuals respond to each question on a scale from 0 (Strongly Disagree) to 5 (Strongly Agree). Subscales are each scored on a scale from 0–15, with higher scores indicating higher levels of each metric (picky eating, lack of interest, and fear). All items may also be summed to calculate a total score, ranging from 0–45, with higher scores indicating higher levels of avoidant/restrictive eating broadly (Zickgraf & Ellis, 2018). Cronbach alphas in the current sample (including all three groups) were .86, .91, and .90 for the picky eating, lack of interest, and fear subscales, respectively.
Statistical Analyses
Sample size.
This sample size was determined to be adequate after a power analysis using the pROC package in R (Team, 2018), accounting for 80% power, for detecting a statistically significant area under the curve (AUC) of .75 or greater, at an alpha of less than .05.
Validity of the NIAS Subscales to Classify ARFID Presentations.
All data were analyzed in R (Team, 2018). To assess the validity of the NIAS to classify each clinically-diagnosed ARFID presentation (sensory sensitivity, lack of interest, and fear), we conducted receiver operating curve (ROC) analyses using each corresponding NIAS subscale (NIAS-picky eating; NIAS-appetite; NIAS-fear). We conducted this procedure using the ARFID sample only. For each subscale, we computed AUC to assess whether we could extract an adequate cutoff on each subscale to differentiate those who were clinically diagnosed versus not clinically diagnosed with the corresponding presentation. For example, on the NIAS-picky eating we calculated AUC to assess whether we could differentiate between those who met versus did not meet criteria for the sensory sensitivity presentation. If the AUC value was greater than .70, we went further to propose a cutoff on that scale. To determine cutoff scores, we calculated sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for each possible cutoff score. Since the NIAS was developed as a screening tool, we prioritized sensitivity over specificity and PPV over NPV when proposing cutoff scores. In cases where sensitivity or PPV were lower than specificity or NPV, we holistically prioritized maximizing both sensitivity and PPV.
Validity of NIAS Subscales to Differentiate ARFID from Other-ED.
The above procedure was repeated using the ARFID and other-ED groups together to test whether the NIAS subscales could be used to differentiate between clinically-diagnosed ARFID and other-ED.
Validity of the NIAS Subscales to Capture ARFID as a Screening Tool .
After determining cutoff scores on the subscales and total score using the entire sample, we ran two logistic regressions to investigate the criterion-related concurrent validity of cutoffs to capture presence versus absence of clinically-diagnosed ARFID, with age and sex as covariates. For this analysis, we use all three samples: those with ARFID, those with other-ED, and NP. The predictor in the first model was a binary coded variable indicating positive screen on the NIAS (i.e., above/below cutoffs on any of the NIAS subscales). In the second model, we added a binary coded variable indicating negative screen on both the NIAS and the EDE-Q Global score (i.e., EDE-Q Global <2.3). We calculated proportions of correctly classified ARFID cases for descriptive purposes. We also ran a chi-square difference test to assess difference in model fit between the logistic regression using NIAS subscales alone versus the logistic regression using the NIAS subscale and EDE-Q cutoffs. In addition, we re-ran analyses using a less strict EDE-Q Global cutoff of <4 and we re-ran analyses using an 8-item calculation for EDE-Q Global score (Machado, Grilo, Rodrigues, Vaz, & Crosby, 2020b). We re-ran analyses with an 8-item version of the EDE-Q since this shortened version could be more widely implemented in different settings (e.g., medical). Finally, we re-ran analyses with only participants ages 18 and older, given that the ARFID and other-ED groups had participants under age 18, but the NP group did not.
Sample Characteristics.
We used descriptive statistics to report demographic data and performance on the NIAS for each group. We also used ANOVAs to detect differences on these metrics between groups. For post-hoc tests, we used Tukey’s Honestly Significant Difference to adjust for multiple comparisons.
RESULTS
Sample characteristics
Summary statistics for all sample characteristics, including scores on the NIAS (subscale and total scores) and comparisons between groups are presented in Table 1. Both the ARFID and other-ED groups scored significantly higher on each NIAS subscale and on the total score than the NP group. The ARFID group scored significantly higher than the other-ED group on all NIAS scores except for the fear subscale, where the groups did not differ. In other words, aside from the fear subscale, the groups all significantly differed on NIAS scores, where the ARFID group scored highest, the other-ED group scored lower than the ARFID group, and the NP group scored lowest. Finally, our nonclinical participant group was significantly older than our ED sample; however, our results did not differ when we only included other-ED and ARFID group members ages 18 and older (in this subanalysis, n=142). Therefore, we included results using the full sample below (N=166) to maximize statistical power.
Table 1.
Sample characteristics.
| ARFID n=49 |
OTHER-ED n=77 |
NP n=40 |
F (df1, df2) |
η2 | Overall p-value | |
|---|---|---|---|---|---|---|
| Age, M (SD) | 22.00 (13.66) | 29.01 (14.35)† | 41.93 (13.23)† | 23.13 (2, 162) | 0.22 | < .001 |
| Sex, n female (%) | 31 (63.3) | 55 (79.7) | 15 (37.5) | .50 (2, 163) | .006b | .61 |
| Race, n (%)a | ||||||
| White | 48 (98%) | 74 (96%) | 36 (90%) | |||
| Black/African-American | 4 (8%) | 0 (0) | 4 (10%) | |||
| Pacific Islander/Native Hawaiian | 0 (0) | 0 (0) | 0 (0) | |||
| Native American/Native Alaskan | 0 (0) | 0 (0) | 0 (0) | |||
| Asian | 0 (0) | 5 (7%) | 1 (3%) | |||
| Ethnicity, n (%) | ||||||
| Hispanic/Latino | 4 (8%) | 3 (4%) | 1 (3%) | |||
| Not Hispanic/Latino | 45 (92%) | 74 (96%) | 39 (98%) | |||
| NIAS, M (SD) | ||||||
| Picky eating | 11.96 (3.46)† | 6.55 (4.23)† |
3.53 (3.19) | 58.67 (2, 162) | 0.42 | < .001 |
| Appetite | 8.12 (5.07)† |
5.24 (4.93)† |
2.55 (2.99) | 16.41 (2, 162) | 0.17 | < .001 |
| Fear | 5.87 (5.49) |
4.24 (4.46)† |
1.40 (2.13) | 11.62 (2, 162) | 0.13 | < .001 |
| ARFID Presentations (n, %)a | ||||||
| Sensory sensitivity only | 16 (32.7%) | N/A | N/A | |||
| Lack of interest only | 3 (6.1%) | N/A | N/A | |||
| Fear of aversive consequences only | 6 (12.2%) | N/A | N/A | |||
| Sensory sensitivity and lack of interest | 11 (22.4%) | N/A | N/A | |||
| Sensory sensitivity and fear of aversive consequences | 5 (10.2%) | N/A | N/A | |||
| Lack of interest and fear of aversive consequences | 7 (14.3%) | N/A | N/A | |||
| All three presentations | 1 (2.0%) | N/A | N/A | |||
| Body Mass Index, M (SD) | 21.67 (6.34) | 23.67 (6.58) | 26.85† (7.36) | 6.54 (2, 162) | 0.07 | .002 |
Note. ARFID=avoidant/restrictive food intake disorder; NIAS=Nine Item ARFID Screen; other-ED=eating disorder associated with shape/weight concerns; NP=nonclinical participant; M=mean; SD=standard deviation. For post-hoc tests, we used Bonferroni correction to adjust for multiple comparisons.
These items do not add to 100%, since participants could be associated with multiple categories
Represents R2 value because sex was a binary variable in the current sample and differences were computed using logistic regression.
Indicates this sample’s score was significantly higher than the sample(s) who scored lower on this measure (p<.05) (e.g., the ARFID group scored significantly higher on the NIAS than the other-ED group, and the other-ED group scored significantly higher on the NIAS than the NP group).
Validity of NIAS Subscales to Classify ARFID Presentations
Results from the ROC analysis to assess whether each NIAS subscale (NIAS-picky eating, NIAS-appetite, NIAS-fear) could classify ARFID presentations (i.e., sensory sensitivity, lack of interest, fear of aversive consequences) are in Table 2 and depicted in Figure 1. The AUC values on all three NIAS subscales were high as follows—NIAS-picky eating (detecting differences between those who met versus did not meet criteria for the sensory sensitivity presentation), AUC =.90, 95% CI = [.82,.99]. NIAS-appetite (detecting differences between those who met versus did not meet criteria for the lack of interest presentation), AUC = .83, 95% CI = [.72,.94]. NIAS-fear (detecting differences between those who met versus did not meet criteria for the fear of aversive consequences presentation), AUC = .83, 95% CI = [71,.95].
Table 2.
Receiver operating curve analyses to validate NIAS subscales with clinically diagnosed ARFID presentations (n=49) and identify cutoff scores to differentiate ARFID from other-ED.
| ARFID (n=49) | ARFID (n=49) vs. Other-ED (n=77) | |||||||
|---|---|---|---|---|---|---|---|---|
| (ARFID Sensory Sensitivity Presentation) | ||||||||
| Picky eating subscale |
Sensitivity | Specificity | PPV | NPV | Sensitivity | Specificity | PPV | NPV |
| 1 | 1.00 | 0.11 | 0.42 | 1.00 | ||||
| 2 | 1.00 | 0.14 | 0.43 | 1.00 | ||||
| 3 | 1.00 | 0.17 | 0.44 | 1.00 | ||||
| 4 | 1.00 | 0.28 | 0.47 | 1.00 | ||||
| 5 | 1.00 | 0.13 | 0.70 | 1.00 | 0.96 | 0.32 | 0.47 | 0.92 |
| 6 | 1.00 | 0.25 | 0.73 | 1.00 | 0.92 | 0.47 | 0.53 | 0.90 |
| 7 | 1.00 | 0.31 | 0.75 | 1.00 | 0.90 | 0.51 | 0.54 | 0.89 |
| 8 | 0.97 | 0.38 | 0.76 | 0.86 | 0.86 | 0.59 | 0.58 | 0.87 |
| 9 | 0.97 | 0.56 | 0.82 | 0.90 | 0.80 | 0.66 | 0.60 | 0.83 |
| 10 | 0.97 | 0.63 | 0.84 | 0.91 | 0.78 | 0.74 | 0.66 | 0.84 |
| 11 | 0.85 | 0.63 | 0.82 | 0.67 | 0.69 | 0.80 | 0.69 | 0.80 |
| 12 | 0.82 | 0.69 | 0.84 | 0.65 | 0.65 | 0.86 | 0.74 | 0.79 |
| 13 | 0.79 | 0.88 | 0.93 | 0.67 | 0.57 | 0.91 | 0.80 | 0.77 |
| 14 | 0.67 | 0.94 | 0.96 | 0.58 | 0.47 | 0.93 | 0.82 | 0.73 |
| 15 | 0.55 | 1.00 | 1.00 | 0.52 | 0.37 | 0.96 | 0.86 | 0.70 |
| (ARFID Lack of interest Presentation) | ||||||||
| Appetite Subscale | Specificity | Sensitivity | PPV | NPV | Specificity | Sensitivity | PPV | NPV |
| 1 | 1.00 | 0.23 | 0.22 | 1.00 | 0.94 | 0.28 | 0.46 | 0.88 |
| 2 | 0.95 | 0.32 | 0.23 | 0.97 | 0.84 | 0.34 | 0.45 | 0.76 |
| 3 | 0.95 | 0.36 | 0.24 | 0.97 | 0.82 | 0.38 | 0.46 | 0.76 |
| 4 | 0.95 | 0.49 | 0.28 | 0.98 | 0.67 | 0.47 | 0.45 | 0.69 |
| 5 | 0.95 | 0.51 | 0.29 | 0.98 | 0.67 | 0.50 | 0.46 | 0.70 |
| 6 | 0.95 | 0.58 | 0.32 | 0.98 | 0.67 | 0.59 | 0.52 | 0.74 |
| 7 | 0.91 | 0.64 | 0.35 | 0.97 | 0.61 | 0.66 | 0.54 | 0.72 |
| 8 | 0.86 | 0.67 | 0.36 | 0.96 | 0.59 | 0.68 | 0.55 | 0.72 |
| 9 | 0.86 | 0.70 | 0.38 | 0.96 | 0.55 | 0.70 | 0.54 | 0.71 |
| 10 | 0.68 | 0.73 | 0.35 | 0.92 | 0.47 | 0.74 | 0.53 | 0.68 |
| 11 | 0.59 | 0.76 | 0.34 | 0.90 | 0.43 | 0.78 | 0.55 | 0.68 |
| 12 | 0.59 | 0.84 | 0.43 | 0.91 | 0.39 | 0.86 | 0.63 | 0.68 |
| 13 | 0.36 | 0.90 | 0.44 | 0.87 | 0.22 | 0.91 | 0.61 | 0.64 |
| 14 | 0.23 | 0.92 | 0.38 | 0.85 | 0.14 | 0.92 | 0.54 | 0.63 |
| 15 | 0.14 | 0.95 | 0.38 | 0.84 | 0.10 | 0.96 | 0.63 | 0.62 |
| (ARFID Fear of Aversive Consequences Presentation) | ||||||||
| Fear subscale |
Sensitivity | Specificity | PPV | NPV | Sensitivity | Specificity | PPV | NPV |
| 1 | 1.00 | 0.39 | 0.23 | 1.00 | 0.78 | 0.39 | 0.45 | 0.73 |
| 2 | 0.95 | 0.40 | 0.22 | 0.98 | 0.73 | 0.39 | 0.44 | 0.70 |
| 3 | 0.89 | 0.45 | 0.22 | 0.96 | 0.67 | 0.43 | 0.43 | 0.67 |
| 4 | 0.79 | 0.58 | 0.25 | 0.94 | 0.53 | 0.55 | 0.43 | 0.65 |
| 5 | 0.74 | 0.64 | 0.26 | 0.93 | 0.43 | 0.58 | 0.40 | 0.61 |
| 6 | 0.74 | 0.69 | 0.30 | 0.94 | 0.41 | 0.64 | 0.43 | 0.63 |
| 7 | 0.74 | 0.73 | 0.33 | 0.94 | 0.39 | 0.68 | 0.44 | 0.63 |
| 8 | 0.68 | 0.78 | 0.35 | 0.93 | 0.33 | 0.72 | 0.43 | 0.63 |
| 9 | 0.68 | 0.86 | 0.46 | 0.94 | 0.29 | 0.82 | 0.50 | 0.64 |
| 10 | 0.68 | 0.89 | 0.52 | 0.94 | 0.29 | 0.86 | 0.56 | 0.65 |
| 11 | 0.63 | 0.91 | 0.55 | 0.93 | 0.27 | 0.88 | 0.59 | 0.65 |
| 12 | 0.58 | 0.93 | 0.58 | 0.93 | 0.24 | 0.91 | 0.63 | 0.65 |
| 13 | 0.42 | 0.94 | 0.57 | 0.90 | 0.18 | 0.93 | 0.64 | 0.64 |
| 14 | 0.42 | 0.97 | 0.73 | 0.90 | 0.18 | 0.97 | 0.82 | 0.65 |
| 15 | 0.37 | 0.98 | 0.78 | 0.90 | 0.16 | 0.99 | 0.89 | 0.65 |
Note. ARFID=avoidant/restrictive food intake disorder; NIAS=Nine Item ARFID Screen; other-ED=eating disorder associated with shape/weight concerns; PPV=positive predictive value; NPV=negative predictive value.
Figure 1. Receiver operating curves for NIAS subscales with clinically diagnosed ARFID presentations (n=49).
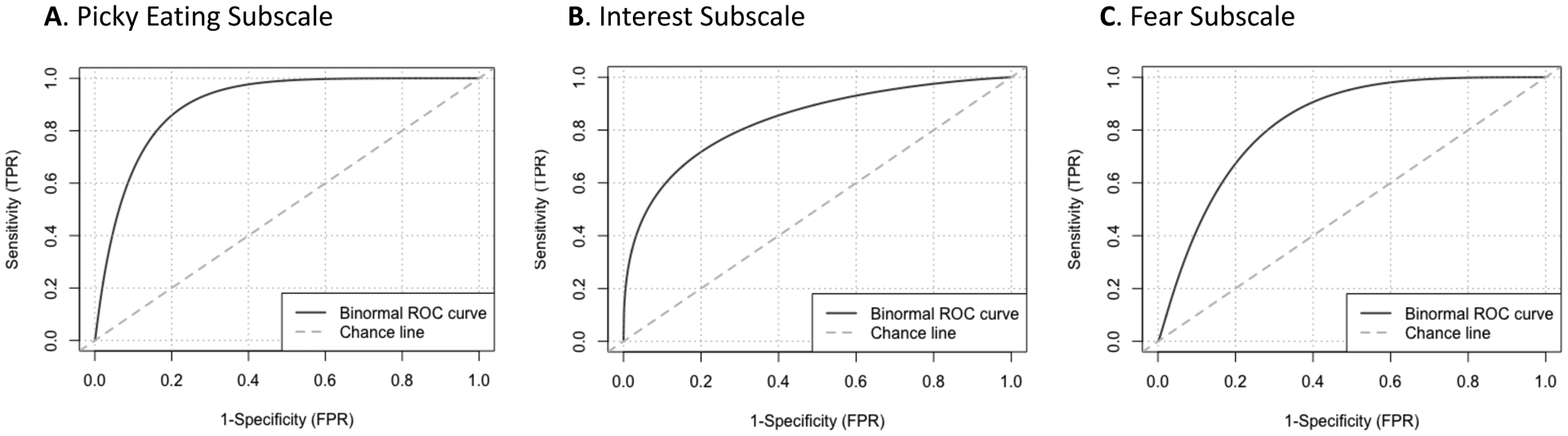
Note. ARFID=avoidant/restrictive food intake disorder; NIAS=Nine Item ARFID Screen.
Assessment of sensitivity, specificity, positive predictive value, and negative predictive value indicated that cutoffs of 10 on the picky eating subscale, 9 on the NIAS-appetite, and 10 on the NIAS-fear can discern between those who meet versus do not meet criteria for each corresponding ARFID presentation. On the NIAS-fear subscale, we chose a cutoff of 10, despite sensitivity being lower than specificity at that cutoff in order to ensure PPV was adequate.
Validity of the NIAS Subscales to Differentiate ARFID from Other-ED
Results from the ROC analysis using each NIAS subscale to differentiate between ARFID and other-ED are in Table 2. The AUC value on the picky eating subscale was high, AUC = .84[95% CI =.76,.92]. Subsequent ROC analysis indicates that a cutoff of 10 on the picky eating subscale could discern between those with ARFID versus those with other-ED. As expected, AUC values on the NIAS-appetite and NIAS-fear were lower, AUC = .66, 95% CI = [.56,.76] and AUC = .59, 95% CI = [.49,.70], respectively. Given that these values were lower, ROC analyses were not interpreted to determine cutoffs on these subscales to discern between ARFID and other-ED.
Validity of the NIAS Subscales to Capture ARFID as a Screening Tool
To examine if the NIAS subscale cutoffs could adequately capture ARFID, we used logistic regression to assess the criterion-related concurrent validity of screening positively on any NIAS subscale, with age and sex as covariates, in association with diagnostic group (ARFID, other-ED, and NP). Given that we were unable to identify cutoff scores on the NIAS-appetite and NIAS-fear subscales, we used the cutoff scores that we identified accurately captured each ARFID presentation—NIAS-picky eating ≥10, NIAS-appetite ≥9, and NIAS-fear ≥10. Results indicated that screening positively on NIAS subscales alone yielded high association with ARFID diagnosis (OR=39.90, Z=5.03, p <.001, 95% CI=[11.55, 251.90]), but incorrectly classified 51% of participants with other-ED as having ARFID. Combining the NIAS subscales and the EDE-Q still yielded high association with ARFID diagnosis, (OR=17.40, Z=4.98 , p< .001, 95% CI=[6.06, 59.39]), and improved correct classification of other-ED. Frequencies for accurately capturing ARFID using the NIAS subscales alone and using both the NIAS subscales and the EDE-Q cutoffs is in Table 3. A chi-square difference test between these two logistic regression models demonstrated that the model using both NIAS subscale and EDE-Q cutoffs fit the data better than the model that used the NIAS subscale cutoffs alone (χ2 (1, N=166)=35.01, p < .001). Furthermore, the model using the NIAS and EDE-Q correctly classified 140 of our 166 participants as ARFID, other-ED, or NP, whereas the model using the NIAS alone only correctly classified 118 of our 166 participants. Of the n=49 clinically diagnosed with ARFID, only n=2 did not meet a NIAS subscale cutoff. Of note, n=5 in the ARFID group did not score below the EDE-Q cutoff (i.e., they screened positively for other-ED). Results remained the same when we used an EDE-Q cutoff <4, as well as cutoffs applied with only the 8 items from the EDE-Q proposed in Machado et al. (2020). The NP group did not include participants under age 18 years old, but results remained the same when we only included participants ages 18 and older in all three groups.
Table 3.
Proportions classified as having ARFID.
| ARFID n=49 |
Other-ED n=77 |
NP n=40 |
|
|---|---|---|---|
| NIAS Subscales1 | |||
| Classified having ARFID (%) | 47 (96%) | 34 (49%) | 4 (10%) |
| Classified not having ARFID (%) | 2 (4%) | 35 (51%) | 36 (90%) |
| NIAS Subscales1 and EDE-Q2 | |||
| Classified having ARFID (%) | 42 (86%) | 9 (13%) | 4 (10%) |
| Classified not having ARFID (%) | 7 (14%) | 62 (87%) | 36 (90%) |
Note. ARFID=avoidant/restrictive food intake disorder; NIAS=Nine Item ARFID Screen; EDE-Q=Eating Disorder Examination-Questionnaire; other-ED=eating disorder associated with shape/weight concerns; NP=nonclinical participant.
NIAS subscales cutoffs—picky eating ≥ 10, appetite ≥ 9, OR fear ≥ 10.
EDE-Q Global score cutoff < 2.3. Results were similar when EDE-Q Global score cutoff <4.0 was used and when EDE-Q 8-item version (Machado et al., 2020a) at these cutoffs was used.
DISCUSSION
The NIAS was created as a brief screening tool to detect ARFID and its three presentations (Zickgraf & Ellis, 2018). Among a sample of outpatients presenting for eating disorder treatment evaluation and nonclinical participants, we found that the NIAS subscales classified ARFID presentations well among a clinical sample of patients with ARFID (i.e., high AUC values). We also found that using both NIAS subscale cutoffs and an other-ED measure (EDE-Q) captured ARFID well (i.e., large odds ratio) among a sample of patients with ARFID, patients with other-ED, and NP participants from the community. Therefore, we recommend identifying possible cases of ARFID by a positive screen on any NIAS subscale (≥10 NIAS-picky eating, ≥9 NIAS-appetite, and ≥10 NIAS-fear) in the presence of a negative screen on a measure of other-ED symptoms (e.g., EDE-Q or EDE-Q8 <2.3). As we predicted, there was high overlap between the ARFID and other-ED groups on the NIAS-appetite and NIAS-fear subscales, providing further evidence for shared transdiagnostic characteristics in avoidant/restrictive eating across EDs.
Among patients with ARFID, we found that the NIAS subscales could classify clinically diagnosed ARFID presentations. Both the sensory and fear subscales had high accuracy in classifying their respective presentations at a cutoff score of 10 and the NIAS-appetite subscale had high accuracy in classifying its presentation at a cutoff score of 9. Thus, we recommend these cutoffs for classifying presentations among individuals who have been identified to have ARFID. Multiple studies have shown that patients often have symptoms of more than one presentation (Norris et al., 2018; Reilly et al., 2019; Zickgraf, Lane‐Loney, Essayli, & Ornstein, 2019; Zickgraf, Murray, Kratz, & Franklin, 2019), thus use of the NIAS subscales could help clinicians identify which presentations are present for a patient to inform treatment targets (Thomas & Eddy, 2019; Thomas, Wons, & Eddy, 2018).
The NIAS-picky eating also adequately differentiated clinically-diagnosed ARFID from other-ED at a cutoff score of 10, indicating that the sensory presentation may be a diagnostic distinguisher between ARFID and other-ED. This finding aligns with our previous research showing that the Food Neophobia Scale was significantly higher in ARFID versus anorexia nervosa (Becker, Keshishian, et al., 2019). However, as indicated in a previous case report of a patient with ARFID and binge-eating disorder (Becker, Breithaupt, et al., 2019), it is possible for patients with ARFID sensory presentation to have comorbid other-ED, and perhaps even to develop other-ED secondary to ARFID avoidant/restrictive eating. Thus, when using the NIAS-picky eating subscale, clinicians should consider using other-ED screening tools in addition to the NIAS to detect other-ED symptoms.
We found that to capture ARFID using a self-report tool such as the NIAS, other-ED symptoms needed to be systematically ruled out, highlighting the overlap between ARFID and other-ED. Scoring above the NIAS subscale cutoffs in combination with the EDE-Q Global score (either the full version— Fairburn and Beglin, 2008; or an abbreviated version like the 8-item version—Machado et al., 2020b), was better than using the NIAS subscales alone. Other studies have included both ARFID and other-ED assessments (Murray, Jehangir, et al., 2020; Murray et al., under review), but there was not yet data with clinically diagnosed ARFID and other-ED samples to support the validity of such an approach. This study adds important data to support the use of ARFID and other-ED self-report measures concurrently to detect possible ARFID, particularly given the overlap between the lack of interest and fear of aversive consequences presentations between ARFID and other-ED. However, it is possible for patients who meet criteria for ARFID to also have body shape/weight concerns (whether or not they are partially connected or not at all connected to ARFID symptoms; e.g., Becker, Breithaupt, et al., 2019). In fact, 5 participants with ARFID scored above the EDE-Q cutoff. While in clinical practice, scale cutoffs are useful to detect likely presence of ARFID and other-EDs, clinicians should use positive questionnaire responses to inform further assessment (e.g., in the case of medical settings, referral to a psychology provider for further assessment).
Given that we were not able to determine a cutoff score on either the ARFID-appetite or NIAS-fear subscales to distinguish ARFID from other-ED, it is possible that the motivations behind ARFID lack of interest and fear of aversive consequences presentations may be diagnostically similar with avoidant/restrictive eating in other-ED. In fact, prior to DSM-5, patients with the ARFID lack of interest or fear of aversive consequences presentations may have been diagnosed with an other-ED. For example, patients now diagnosed with ARFID lack of interest who report a desire to eat but an inability to eat due to aversive physical sensations (e.g., nausea, abdominal pain, extreme fullness; e.g., Zucker et al., 2019) may have previously been diagnosed with anorexia nervosa (Thomas et al., 2015) or eating disorder not otherwise specified and identified as having a not-fat phobic presentation (Izquierdo et al., 2019; Thomas, Hartmann, & Killgore, 2013). Similarly, patients now diagnosed with ARFID fear of aversive consequences who report a desire to eat more food variety or volume but who have an extreme fear of eating may have previously been diagnosed with eating disorder not otherwise specified (Fisher et al., 2014; Ornstein et al., 2013).
Although ARFID lack of interest and fear of aversive consequences presentations underlying avoidant/restrictive eating may have diagnostic similarities to other-ED, presence of these presentations could inform transdiagnostic treatment targets. Rather than distinguishing ARFID from other-ED, the NIAS-appetite and NIAS-fear subscales could be used in patients with ARFID and patients with other-ED to identify potential interventions. If patients score a 10 or higher on the fear subscale or a 9 or higher on the NIAS-appetite subscale, they may benefit from specific behavioral interventions used for ARFID (Thomas & Eddy, 2019; Zucker et al., 2019) that are not part of standard care for other-ED (e.g., as we and others have proposed; Murray, Kuo, et al., 2020; Zucker and Bulik, 2020). For example, interoceptive exposure can be an effective tool that may facilitate increased volume intake for ARFID lack of interest (Thomas & Eddy, 2019; Zucker et al., 2019) and behavioral exposures (mostly with food stimuli) is the core treatment technique for ARFID fear of aversive consequences (Thomas & Eddy, 2019; Zucker et al., 2019). While some researchers have suggested the incorporation of interoceptive and food exposures into other-ED (Reilly, Anderson, Gorrell, Schaumberg, & Anderson, 2017; Romano et al., 2020; Zucker & Bulik, 2020), these are still not part of standard care. Future research is needed to determine if comorbid ARFID lack of interest and fear of aversive consequences presentations may be behavioral phenotypes that could serve as indicators for exposure-based techniques in the context of other-ED treatment. It is also possible that NIAS could be used not just as a screening tool but a tool to examine mechanisms of change during the course of treatment.
A major strength of our study was the inclusion of a pediatric and adult clinical sample of patients with ARFID and other-ED, particularly the inclusion of patients with diagnosed ARFID presentations. However, our sample may not be fully generalizable to other groups, including more racially and ethnically diverse samples and to samples of individuals in a higher level of care (e.g., inpatient). The nature of our nonclinical participant group may similarly have limitations for generalizability, given they were individuals from the community recruited by MTurk, had a higher proportion of males than the eating disorder sample (38% vs. 75%, respectively), were older on average, and represent a subset of nonclinical participants that were identified without any eating disorder pathology. For example, the cutoffs used in this study for other-ED symptoms (EDE-Q <2.3) may not be generalizable, especially since this cutoff was determined in a female-only sample (Mond et al., 2004). Although, in our sample 83% (10/12) of males in the other-ED group met the cut off for a positive screen on the EDE-Q Global score (≥2.3), those who score below 2.3 may still have eating disorder symptoms (Smith et al., 2017). Moreover, there is a need for future research to examine the best screening cutoffs for different demographic and cultural contexts (Kelly, Cotter, & Mazzeo, 2012; Nagata et al., 2020; Serier, Smith, & Yeater, 2018; Smith et al., 2017; Unikel Santoncini et al., 2018). Additionally, our cutoffs and AUC values may have been influenced by uneven base rates of ARFID vs. other-ED in our sample, which may partially explain why no cutoff could be determined for the fear and lack of interest profiles between these two groups. On the other hand, in population screening setting, such as primary care, there would likely be a higher rate of non-ARFID patients than ARFID patients. Therefore, a cutoff ascertained from a sample with more non-ARFID than ARFID patients may be more clinically useful (Robinson, Boissoneault, Sevel, Letzen, & Staud, 2016). Additionally, all three aims of this study measured criterion-related concurrent validity; however, conclusions should be interpreted with caution, since our data are cross-sectional, and, although data for the ARFID and other-ED groups came from two sources (i.e., self-report and clinical interview), data for the NP group came only from one source (i.e., MTurk). Next, although our clinic documents DSM-5 eating disorder diagnoses as routine practice, we did not capture diagnoses by validated semi-structured interviews. Furthermore, we included a spectrum of other-EDs, including diagnoses in which overvaluation of body shape/weight is not diagnostic (by DSM-5); for example, not all patients with BED in the community have significant overvaluation of shape/weight (Forrest, Jacobucci, & Grilo, 2020) and in fact some research findings support shape/weight overvaluation presence as a diagnostic specifier for BED (Coffino, Udo, & Grilo, 2019). Finally, although we checked to ensure no MTurk workers completed our study twice and invited individuals to participate via an email from MTurk only if they had qualified based on the screener, we did not implement other checks to ensure quality of MTurk participants. Although attention check questions may not be an effective method to ensure attention in professional survey workers like those on MTurk (Chandler & Shapiro, 2016; Thomas & Clifford, 2017), future research should employ other methods, such as hidden timers on each survey page.
In sum, this study provides support for the use of the NIAS subscales as a screening tool for ARFID. For general detection of potential ARFID cases, we recommend a positive screen on any NIAS subscale (≥10 NIAS-picky eating, ≥9 NIAS-appetite, and ≥10 NIAS-fear) with a negative screen on a measure of other-ED symptoms (e.g., EDE-Q or EDE-Q8 <2.3). Future research is needed to understand ARFID symptoms of the lack of interest and fear of aversive consequences presentations which may be particularly comorbid among some patients with other-ED.
Footnotes
Conflicts of Interest: HBM, LB, and MJD have no personal or financial conflicts to declare. JJT, KTE, and KRB receive royalties from Cambridge University Press for the sale of their books on ARFID.
Data availability statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- APA. (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (5th ed.): American Psychiatric Publishing, Arlington, VA. [Google Scholar]
- Becker AE, Thomas JJ, Bainivualiku A, Richards L, Navara K, Roberts AL, … Striegel-Moore RH (2010). Validity and reliability of a Fijian translation and adaptation of the Eating Disorder Examination Questionnaire. International Journal of Eating Disorders, 43(2), 171–178. doi: 10.1002/eat.20675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KR, Breithaupt L, Lawson EA, Eddy KT, & Thomas JJ (2019). Co-occurrence of Avoidant/Restrictive Food Intake Disorder and Traditional Eating Psychopathology. Journal of the American Academy of Child and Adolescent Psychiatry, 59(2), 209–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KR, Keshishian AC, Liebman RE, Coniglio KA, Wang SB, Franko DL, … Thomas JJ (2019). Impact of expanded diagnostic criteria for avoidant/restrictive food intake disorder on clinical comparisons with anorexia nervosa. International Journal of Eating Disorders, 52(3), 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant-Waugh R, Micali N, Cooke L, Lawson EA, Eddy KT, & Thomas JJ (2019). The Pica, ARFID, and Rumination Disorder Interview, a multi-informant, semi-structured interview of feeding disorders across the lifespan: A pilot study for ages 10–22. International Journal of Eating Disorders, 52, 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler J, & Shapiro D (2016). Conducting Clinical Research Using Crowdsourced Convenience Samples. Annual Review of Clinical Psychology, 12, 53–81. doi: 10.1146/annurev-clinpsy-021815-093623 [DOI] [PubMed] [Google Scholar]
- Coffino JA, Udo T, & Grilo CM (2019). The significance of overvaluation of shape or weight in binge‐eating disorder: Results from a National Sample of US adults. Obesity, 27(8), 1367–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJong H, Perkins S, Grover M, & Schmidt U (2011). The prevalence of irritable bowel syndrome in outpatients with bulimia nervosa. International Journal of Eating Disorders, 44(7), 661–664. [DOI] [PubMed] [Google Scholar]
- Eddy KT, Harshman SG, Becker KR, Bern E, Bryant‐Waugh R, Hilbert A, … Menzel J (2019). Radcliffe ARFID Workgroup: Toward operationalization of research diagnostic criteria and directions for the field. International Journal of Eating Disorders, 52(4), 361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan I, Hafizoglu S, Ozkaya G, Kirli S, Yalcintas E, & Akaya C (2015). Examining cut-off values for the state-trait anxiety inventory. Revista Argentina de Clinica Psicologica, 24(II), 143. [Google Scholar]
- Fairburn CG, & Beglin S (2008). Eating Disorder Examination Questionnaire (EDE-Q 6.0). In Fairburn CG (Ed.), Cognitive Behavior Therapy and Eating Disorders (pp. 309–313). New York, NY: Guilford Press. [Google Scholar]
- Fisher MM, Rosen DS, Ornstein RM, Mammel KA, Katzman DK, Rome ES, … Walsh BT (2014). Characteristics of avoidant/restrictive food intake disorder in children and adolescents: a “new disorder” in DSM-5. Journal of Adolescent Health, 55(1), 49–52. [DOI] [PubMed] [Google Scholar]
- Forrest LN, Jacobucci RC, & Grilo CM (2020). Empirically determined severity levels for binge-eating disorder outperform existing severity classification schemes. Psychological Medicine, 1–11. doi: 10.1017/s0033291720002287 [DOI] [PubMed] [Google Scholar]
- Grilo CM, Reas DL, Hopwood CJ, & Crosby RD (2015). Factor structure and construct validity of the eating disorder examination‐questionnaire in college students: Further support for a modified brief version. International Journal of Eating Disorders, 48(3), 284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Zickgraf HF, Ellis JM, Lin Z, & Fan X (2020). Chinese Version of the Nine Item ARFID Screen: Psychometric Properties and Cross-Cultural Measurement Invariance. Assessment, 1073191120936359. doi: 10.1177/1073191120936359 [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Plessow F, Becker KR, Mancuso CJ, Slattery M, Murray HB, … Eddy KT (2019). Implicit attitudes toward dieting and thinness distinguish fat‐phobic and non‐fat‐phobic anorexia nervosa from avoidant/restrictive food intake disorder in adolescents. International Journal of Eating Disorders, 52(4), 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly NR, Cotter EW, & Mazzeo SE (2012). Eating Disorder Examination Questionnaire (EDE-Q): norms for Black women. Eat Behav, 13(4), 429–432. doi: 10.1016/j.eatbeh.2012.09.001 [DOI] [PubMed] [Google Scholar]
- Machado PP, Grilo CM, Rodrigues TF, Vaz AR, & Crosby RD (2020a). Eating Disorder Examination–Questionnaire short forms: A comparison. International Journal of Eating Disorders, 53(6), 937–944 [DOI] [PubMed] [Google Scholar]
- Machado PP, Grilo CM, Rodrigues TF, Vaz AR, & Crosby RD (2020b). Eating Disorder Examination–Questionnaire short forms: A comparison. International Journal of Eating Disorders. [DOI] [PubMed] [Google Scholar]
- Mond JM, Hay PJ, Rodgers B, Owen C, & Beumont P (2004). Validity of the Eating Disorder Examination Questionnaire (EDE-Q) in screening for eating disorders in community samples. Behaviour Research and Therapy, 42(5), 551–567. [DOI] [PubMed] [Google Scholar]
- Murray HB, Bailey AP, Keshishian AC, Silvernale CJ, Staller K, Eddy KT, … Kuo B (2020). Prevalence and Characteristics of Avoidant/Restrictive Food Intake Disorder in Adult Neurogastroenterology Patients. Clinical Gastroenterology and Hepatology, 18(9), 1995–2002. e1991. [DOI] [PubMed] [Google Scholar]
- Murray HB, Jehangir A, Silvernale CJ, Kuo B, & Parkman HP (2020). Avoidant/Restrictive Food Intake Disorder Symptoms are Frequent in Patients Presenting for Symptoms of Gastroparesis. Neurogastroenterology and Motility, 32(12), e13931. [DOI] [PubMed] [Google Scholar]
- Murray HB, Kuo B, Eddy KT, Breithaupt L, Becker KR, Dreier MJ, … Staller K (2020). Disorders of gut-brain interaction common among outpatients with eating disorders including avoidant/restrictive food intake disorder. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray HB, Riddle M, Rao F, McCann BS, Staller K, Heitkemper M, & Zia J (under review). Eating disorder symptoms, including avoidant/restrictive food intake disorder, in patients with disorders of gut-brain interaction. [DOI] [PubMed] [Google Scholar]
- Nagata JM, Murray SB, Compte EJ, Pak EH, Schauer R, Flentje A, … Obedin-Maliver J (2020). Community norms for the Eating Disorder Examination Questionnaire (EDE-Q) among transgender men and women. Eat Behav, 37, 101381. doi: 10.1016/j.eatbeh.2020.101381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris ML, Spettigue W, Hammond NG, Katzman DK, Zucker N, Yelle K, … Obeid N (2018). Building evidence for the use of descriptive subtypes in youth with avoidant restrictive food intake disorder. International Journal of Eating Disorders, 51(2), 170–173. [DOI] [PubMed] [Google Scholar]
- Ornstein RM, Rosen DS, Mammel KA, Callahan ST, Forman S, Jay MS, … Walsh BT. (2013). Distribution of eating disorders in children and adolescents using the proposed DSM-5 criteria for feeding and eating disorders. Journal of Adolescent Health, 53(2), 303–305. [DOI] [PubMed] [Google Scholar]
- Perkins S, Keville S, Schmidt U, & Chalder T (2005). Eating disorders and irritable bowel syndrome: is there a link? Journal of Psychosomatic Research, 59(2), 57–64. [DOI] [PubMed] [Google Scholar]
- Pliner P, & Hobden K (1992). Development of a scale to measure the trait of food neophobia in humans. Appetite, 19(2), 105–120. [DOI] [PubMed] [Google Scholar]
- Radloff L (1977). The CED-S scale: A self-report depression scale for research in the general population. Applied Psychosocial Measurement, 1, 385–401. [Google Scholar]
- Radloff LS (1991). The use of the Center for Epidemiologic Studies Depression Scale in adolescents and young adults. Journal of Youth and Adolescence, 20(2), 149–166. [DOI] [PubMed] [Google Scholar]
- Reilly EE, Anderson LM, Gorrell S, Schaumberg K, & Anderson DA (2017). Expanding exposure‐based interventions for eating disorders. International Journal of Eating Disorders, 50(10), 1137–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly EE, Brown TA, Gray EK, Kaye WH, & Menzel JE (2019). Exploring the cooccurrence of behavioural phenotypes for avoidant/restrictive food intake disorder in a partial hospitalization sample. European Eating Disorders Review, 27(4), 429–435. [DOI] [PubMed] [Google Scholar]
- Robinson M, Boissoneault J, Sevel L, Letzen J, & Staud R (2016). The Effect of Base Rate on the Predictive Value of Brain Biomarkers. Journal of Pain, 17(6), 637–641. doi: 10.1016/j.jpain.2016.01.476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano K, Heron KE, Smith KE, Crosby RD, Engel SG, Wonderlich SA, … Mason TB (2020). Somatic symptoms and binge eating in Women’s daily lives. Journal of Psychosomatic Research, 110161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serier KN, Smith JE, & Yeater EA (2018). Confirmatory factor analysis and measurement invariance of the Eating Disorder Examination Questionnaire (EDE-Q) in a non-clinical sample of non-Hispanic White and Hispanic women. Eat Behav, 31, 53–59. doi: 10.1016/j.eatbeh.2018.08.004 [DOI] [PubMed] [Google Scholar]
- Sharp WG, & Stubbs KH (2019). Avoidant/restrictive food intake disorder: a diagnosis at the intersection of feeding and eating disorders necessitating subtype differentiation. International Journal of Eating Disorders, 52(4), 398–401. [DOI] [PubMed] [Google Scholar]
- Smith KE, Mason TB, Murray SB, Griffiths S, Leonard RC, Wetterneck CT, … Lavender JM (2017). Male clinical norms and sex differences on the Eating Disorder Inventory (EDI) and Eating Disorder Examination Questionnaire (EDE-Q). International Journal of Eating Disorders, 50(7), 769–775. doi: 10.1002/eat.22716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R, Vagg P, & Jacobs G (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Team RC (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Thomas JJ, & Eddy K (2019). Cognitive-behavioral therapy for avoidant/restrictive food intake disorder: children, adolescents, and adults. Cambridge: UK: Cambridge University Press. [Google Scholar]
- Thomas JJ, Eddy KT, Micali N, Kuhnle M, Dreier M, Lawson EA, … Bryant-Waugh R (2020). Preliminary validation of a self-report questionnaire version of the pica, ARFD, and rumination disorder interview (PARDI-ARQ). Paper presented at the Eating Disorders Research Society Meeting, Sitges, Spain; held vritually due to COVID-19. [Google Scholar]
- Thomas JJ, Eddy KT, Murray HB, Tromp MD, Hartmann AS, Stone MT, … Becker AE (2015). The impact of revised DSM-5 criteria on the relative distribution and inter-rater reliability of eating disorder diagnoses in a residential treatment setting. Psychiatry Research, 229(1), 517–523. [DOI] [PubMed] [Google Scholar]
- Thomas JJ, Hartmann AS, & Killgore WD (2013). Non‐fat‐phobic eating disorders: Why we need to investigate implicit associations and neural correlates. International Journal of Eating Disorders, 46(5), 416–419. [DOI] [PubMed] [Google Scholar]
- Thomas JJ, Lawson EA, Micali N, Misra M, Deckersbach T, & Eddy KT (2017). Avoidant/Restrictive Food Intake Disorder: a Three-Dimensional Model of Neurobiology with Implications for Etiology and Treatment. Current psychiatry reports, 19(8), 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JJ, Wons OB, & Eddy KT (2018). Cognitive-behavioral treatment of avoidant/restrictive food intake disorder. Current Opinion in Psychiatry, 31(6), 425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KA, & Clifford S (2017). Validity and Mechanical Turk: An assessment of exclusion methods and interactive experiments. Computers in Human Behavior, 77, 184–197. [Google Scholar]
- Unikel Santoncini C, Bojorquez Chapela I, Díaz de León Vázquez C, Vázquez Velázquez V, Rivera Márquez JA, Galván Sánchez G, & Rocha Velis I (2018). Validation of eating disorders examination questionnaire in Mexican women. International Journal of Eating Disorders, 51(2), 146–154. doi: 10.1002/eat.22819 [DOI] [PubMed] [Google Scholar]
- Zickgraf HF, & Ellis JM (2018). Initial validation of the Nine Item Avoidant/Restrictive Food Intake Disorder Screen (NIAS): A measure of three restrictive eating behaviors. Appetite(123), 32–42. [DOI] [PubMed] [Google Scholar]
- Zickgraf HF, Lane‐Loney S, Essayli JH, & Ornstein RM (2019). Further support for diagnostically meaningful ARFID symptom presentations in an adolescent medicine partial hospitalization program. International Journal of Eating Disorders, 52(4), 402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zickgraf HF, Murray HB, Kratz HE, & Franklin ME (2019). Characteristics of outpatients diagnosed with the selective/neophobic presentation of avoidant/restrictive food intake disorder. International Journal of Eating Disorders, 52(4), 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker NL, & Bulik CM (2020). On bells, saliva, and abdominal pain or discomfort: Early aversive visceral conditioning and vulnerability for anorexia nervosa. International Journal of Eating Disorders, 53(4), 508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker NL, LaVia MC, Craske MG, Foukal M, Harris AA, Datta N, … Maslow GR (2019). Feeling and body investigators (FBI): ARFID division—An acceptance‐based interoceptive exposure treatment for children with ARFID. International Journal of Eating Disorders, 52(4), 466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]


