Abstract
Background:
The postpartum year is a vulnerable period for women with opioid use disorder, with increased rates of fatal and non-fatal overdose, yet the continuation of the use of medications to treat opioid use disorder (MOUD) on a population-level remains unknown.
Objectives:
To examine the discontinuation of methadone and buprenorphine among women with opioid use disorder in the year following delivery and determine the extent to which maternal and infant characteristics are associated with time to discontinuation of MOUD.
Study Design:
Population-based retrospective cohort study using linked administrative data of 211,096 deliveries in Massachusetts between 2011-2014 to examine MOUD receipt. Individuals receiving MOUD the month of delivery were included in the study. Demographic, psychosocial, prenatal, and delivery characteristics are described. Kaplan-Meier survival analysis and Cox regression modeling were used to examine factors associated with treatment discontinuation.
Results:
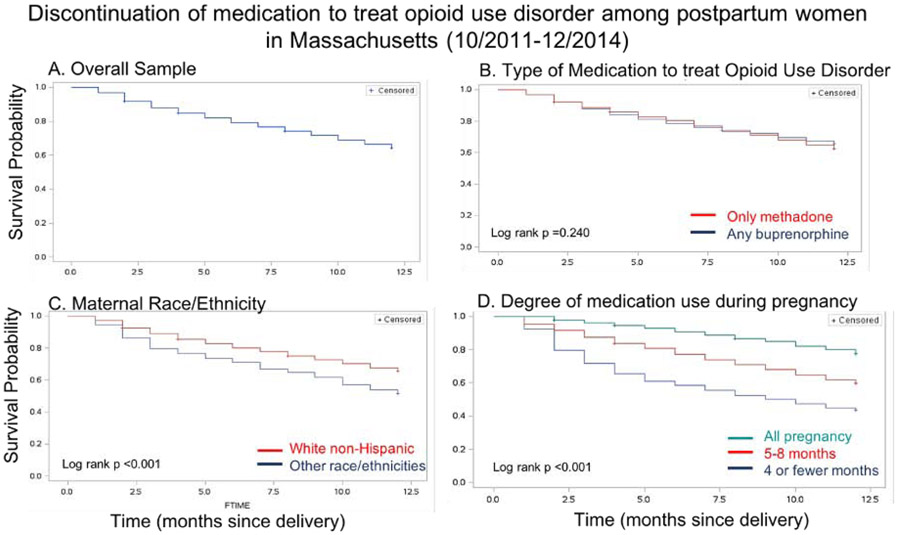
There were 2,314 women included who received MOUD at delivery; 64.1% (1,484) continued receiving MOUD for a full 12 months following delivery. The continuation rate varied from 34% if women started on medication the month before delivery to 80% if medications were used all of pregnancy. Kaplan-Meier survival curves differed by maternal race/ethnicity (white non-Hispanic 12-month continuation probability 0.65 compared with non-white women was 0.51, p<0.001) and duration of prenatal MOUD utilization (12-month continuation probability was 0.78 for full prenatal engagement compared with 0.60 and 0.44 for those receiving 5 or more months (but not all of pregnancy) and 4 or fewer months of MOUD prenatally, respectively p<0.001). In all multivariable models, duration of prenatal MOUD receipt (4 months or fewer v. all pregnancy: aHR 3.26, 95% CI 2.72-3.91) and incarceration (incarceration during pregnancy or postpartum period v. none: aHR 1.79 (95% CI 1.52-2.12) were most strongly associated with MOUD discontinuation.
Conclusions:
Almost two-thirds of women with OUD remained on MOUD for the full year postpartum, but rates varied significantly by race/ethnicity, degree of prenatal MOUD utilization, and incarceration status. Prioritizing treatment continuation across the perinatal continuum, enhancing gender-specific and family friendly recovery supports, and expanding access to MOUD while incarcerated can help improve postpartum treatment receipt.
Keywords: adherence, buprenorphine, disparities, discontinuation, medication for addiction treatment, methadone, opioid use disorder, perinatal continuum, postpartum, substance use
Condensation:
Among postpartum women with opioid use disorder in Massachusetts, methadone and buprenorphine continuation varied significantly by race/ethnicity, duration of prenatal MOUD utilization, and incarceration status.
Introduction:
The surge in the non-medical use of prescription opioids, heroin, and fentanyl has resulted in an overdose crisis in the United States, affecting women of child bearing age and their families.1-3 Multiple states have identified overdose as a major contributor to pregnancy-associated deaths, accounting for 11-20% deaths, with the majority occurring in the postpartum period.4-8 For pregnant women with opioid use disorder (OUD), first-line treatment includes medications for opioid use disorder (MOUD) methadone or buprenorphine, and behavioral therapy which have been shown to improve prenatal care attendance, reduce OUD relapse and overdose, and improve birth outcomes (higher birthweight, less preterm birth).8-14 For families involved with the child welfare system, MOUD utilization increases the likelihood of retaining parental custody.16
While pregnancy is a motivating time for women to engage in substance use treatment and initiate MOUD,.17-20 the period after delivery can present significant obstacles. In addition to the stresses of having a new baby, mothers with a history of OUD may face loss of access to special services designed for pregnant women, high rates of postpartum mood disorders, metabolism changes impacting MOUD dose, gaps in care transitioning from perinatal to primary care, and involvement with child welfare services.21-23 While the 6-12 months postpartum are a period of increased overdose risk,8 little is known about medication adherence during this time, as most studies stop at or a few months after delivery.19, 24, 25 Limited research from randomized trials or single sites has shown that MOUD discontinuation rates at six months postpartum range from 20% for buprenorphine26 to 56% for methadone,24,25 increasing the risk of relapse and overdose.24,26,27
In the general population, homelessness, daily injection drug use, incarceration, unemployment, lack of insurance, and non-white race/ethnicity have been associated with MOUD non-adherence.28-33 Yet during the postpartum year, treatment retention rates and the timing, reasons, and predictors of MOUD discontinuation are largely unknown at a population level.24 Understanding factors that contribute to postpartum discontinuation is critical to developing interventions to improve MOUD adherence for women with OUD. The primary aims of our study were to: 1) examine MOUD discontinuation in the first postpartum year using a population level cohort in Massachusetts; and 2) determine the extent to which maternal and infant characteristics are associated with time to MOUD discontinuation.
Materials and Methods:
Design
We performed a retrospective analysis using data from the Public Health Data Warehouse, a collection of linked statewide Massachusetts datasets overseen by the Massachusetts Department of Public Health. A detailed description of these datasets, data structure, and linkage rates has been described previously.34-37 The warehouse includes data from calendar years 2011-2015 linked at the individual level across administrative Massachusetts databases. This study used data linked across vital records, the All Payer Claims Database, state funded addiction treatment (detoxification, residential, outpatient treatment)38, acute care records (hospitalization, outpatient observation, and emergency department discharges), emergency medical services transport, prescription monitoring program (PMP), Medicaid (MassHealth) enrollment, corrections (jails and prisons), and homelessness data from the Department of Housing and Community Development.
Participants
We identified Massachusetts residents who delivered a live birth in Massachusetts with a documented gestational age ≥ 20 weeks using birth certificate data. Birth certificate linkage rates were 91.7%. We included all women who delivered between 10/2011-12/2014, to allow for full data during pregnancy and a year of treatment data after delivery. Both singletons and multiples were included with multiples treated as a single delivery episode. For each delivery, a pregnancy period was identified using the date of birth and recorded gestational age to estimate the date of conception. When a woman was linked to multiple deliveries during the study period, only the first delivery was included.
We restricted our sample to women who received methadone or buprenorphine during the month of, and/or prior to, delivery. Methadone receipt was defined as the presence of a claim for methadone treatment (Healthcare Common Procedure Coding System code H0020) or receipt of methadone from a state-funded treatment program. Buprenorphine utilization was defined as a filled prescription for either buprenorphine or buprenorphine/naloxone, identified from PMP data. Medication type was categorized as either “any buprenorphine” or “methadone only” given prior research showing disparities in buprenorphine receipt.39,40 Naltrexone was not included in our definition of MOUD as it is not currently standard of care for pregnant women with OUD.11
Variables
Our primary outcome was time in months to discontinuation of MOUD in the year following delivery. Discontinuation was defined as two consecutive months without receipt of MOUD, and women were censored at time of death from any cause or at 12 months following delivery.
Sociodemographic characteristics included maternal age, race/ethnicity (self-report on birth certificate records or across linked datasets if available), highest educational level, enrollment in Medicaid during delivery month, marital status, and rural v. urban residence at delivery.
Psychosocial characteristics included high utilization of unscheduled care (≥3 emergency and/or obstetric triage visits in pregnancy), maternal anxiety or depression (diagnosis during pregnancy), incarceration history (release from a Massachusetts prison or jail during pregnancy or postpartum period), and use of emergency housing assistance (shelter/hotel placement during pregnancy). Opioid use characteristics during pregnancy included opioid overdose events (claims diagnosis (ICD codes in Supplement) or ambulance encounter) and receipt of any opioid prescription (excluding buprenorphine) in the three months prior to the month of delivery. We classified prenatal MOUD treatment as the number of months of treatment during pregnancy (all of pregnancy, at least 5 months, or ≤4 months).
For obstetrical and birth characteristics, we included breastfeeding at discharge, infant preterm or low birthweight (< 37 weeks gestational age or < 2500 grams), adequacy of prenatal care (the Kotelchuck index from the birth certificate41), and infant diagnosis of neonatal abstinence syndrome (claims diagnosis ICD codes in Supplement), excluding deliveries less than 34 weeks to minimize misclassification of iatrogenic withdrawal from postnatal opioid receipt while mechanically ventilated).42
Statistical Analysis
We analyzed associations using Chi-square and Fisher tests to compare maternal characteristics across two MOUD treatment groups (full postpartum year vs. discontinued treatment). Next, we generated Kaplan-Meier survival curves of MOUD discontinuation by month from delivery and compared differences by: 1) type of MOUD, 2) prenatal MOUD treatment duration, and 3) maternal race/ethnicity using the log rank test. Finally, we used Cox regression modeling to assess factors associated with treatment discontinuation, adjusted for year of delivery. Using forward stepwise elimination (p <0.1 to enter, p <0.05 to remain), the multivariable model examined the independent associations of time varying (monthly evidence of overdose, homelessness, maternal anxiety/depression) and static covariates (remaining maternal and infant characteristics described above).
Sensitivity Analysis
We identified a potential seven-month gap in the reporting of methadone data in our dataset, affecting data between April 2014-December 2014 (see Supplementary Figure 1). There was an approximately 8% drop in the number of claims for methadone reported during this time, compared with months before and after, due to a transition in the way statewide data was reported. To explore the effect of these missing data on our results, we 1): ignored discontinuation among women receiving methadone during these months (i.e. all identified discontinuations were the result of missing records); 2): removed women receiving methadone who delivered after April 2013 and whose follow-up period fell within the range of the missing data; and 3): stratified our models by type of MOUD, as there was no comparable discrepancy in buprenorphine data.
Results:
Study Characteristics
Of the 211,096 deliveries to women in Massachusetts between October 1, 2011 and December 31, 2014, 2,497 (1.2%) women received MOUD during the month of or before delivery. Deliveries to women with missing demographic data (65) and repeat deliveries by the same woman during the study period (118) were excluded. There were 2,314 women in the final cohort; 35.9% (830) discontinued treatment in the postpartum year (Figure 1), and 64.1% (1,484) continued for a full 12 months following delivery.
Figure 1:
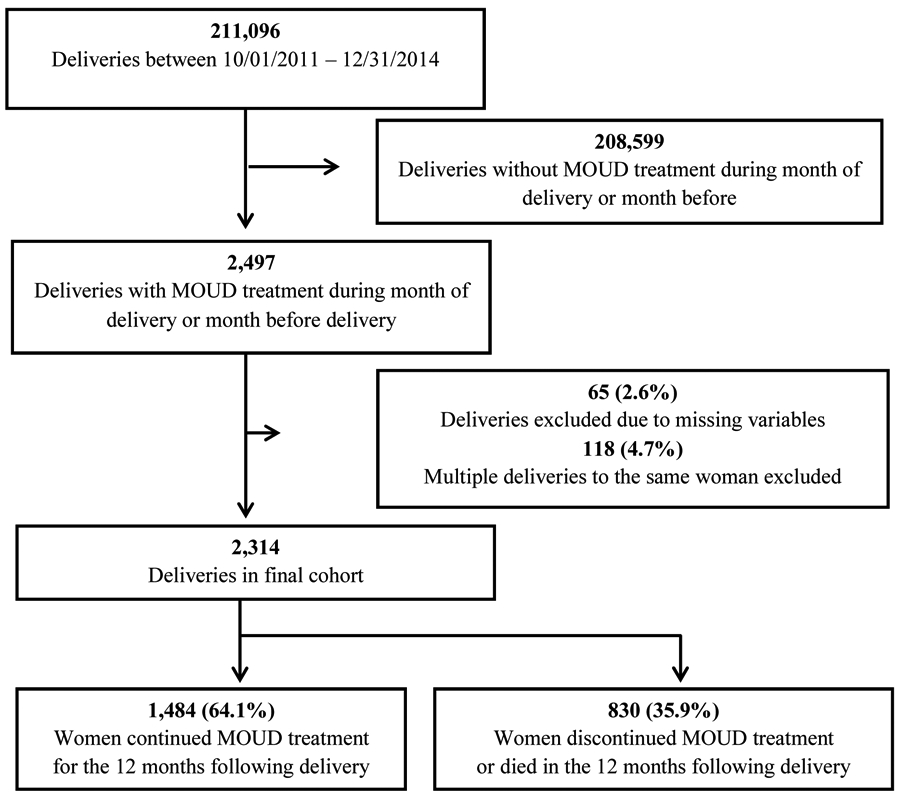
Study Schema
Women who were <25 years at delivery, were not white non-Hispanic race, did not have public insurance, had fewer months of prenatal MOUD treatment, had three or more unscheduled emergency department visits during pregnancy, had anxiety, had evidence of incarceration, or received emergency housing assistance were more likely to discontinue MOUD after delivery. (Table 1) Women who delivered an infant preterm or low birthweight, had less adequate prenatal care utilization, and delivered an infant with a diagnosis of neonatal abstinence syndrome after delivery were more likely to discontinue MOUD treatment.
Table 1:
Characteristics of mothers continuing and discontinuing treatment (n=2,314)
| Continued treatment for 1 year postpartum (n=1,484) |
Discontinued treatment during 1 year postpartum (n = 826)* |
P-Value | |
|---|---|---|---|
| Demographics | |||
| Maternal Age | 0.001 | ||
| ≤ 25 years old | 367 (24.7%) | 265 (32.1%) | |
| 26-34 years old | 951 (64.1%) | 480 (58.1%) | |
| ≥ 35 years old | 166 (11.2%) | 81 (9.8%) | |
| Maternal Race/Ethnicity | <0.001 | ||
| White non-Hispanic | 1,368 (92.2%) | 717 (86.8%) | |
| Black non-Hispanic | 24 (1.6%) | 32 (3.9%) | |
| Hispanic | 73 (4.9%) | 57 (6.9%) | |
| Other | 19 (1.3%) | 20 (2.4%) | |
| Maternal Education | 0.427 | ||
| High school or less | 810 (54.6%) | 465 (56.3%) | |
| Some college or more | 674 (45.4%) | 361 (43.7%) | |
| MassHealth Delivery (%Yes) | 1,412 (95.2%) | 756 (91.5%) | 0.001 |
| Marital Status (% Married) | 220 (14.8%) | 126 (15.3%) | 0.782 |
| Rural Residence | 166 (11.2%) | 99 (12.0%) | 0.563 |
| Psychosocial, Opioid Use, and Health Care Utilization (in pregnancy unless noted) | |||
| ED Visits (%3 or More) | 250 (16.9%) | 174 (21.1%) | 0.012 |
| Anxiety Diagnosis (%Yes) | 305 (20.6%) | 205 (24.8%) | 0.018 |
| Depression Diagnosis (%Yes) | 345 (23.3%) | 222 (26.9%) | 0.052 |
| Incarceration in pregnancy or postpartum (%Yes) | 158 (10.7%) | 188 (22.8%) | <0.001 |
| Homelessness (claims data) (%Yes) | 76 (5.1%) | 61 (7.4%) | 0.027 |
| Overdose Event (%Yes) | 19 (1.3%) | 18 (2.2%) | 0.099 |
| Type of MOUD | 0.142 | ||
| Any buprenorphine† | 891 (60.0%) | 470 (56.9%) | |
| Exclusively methadone | 593 (40.0%) | 356 (43.1%) | |
| MOUD Treatment Months | <0.001 | ||
| All pregnancy | 770 (51.9%) | 220 (26.6%) | |
| 5 to 8 months | 511 (34.4%) | 345 (41.8%) | |
| 4 or fewer months | 203 (13.7%) | 261 (31.6%) | |
| Any Opioid Prescription (Excluding Bup) (3MB)(%Yes) | 33 (2.2%) | 33 (4.0%) | 0.014 |
| Obstetric and Birth Characteristics | |||
| Breastfeeding at Discharge (%Yes) | 698 (47.0%) | 359 (43.5%) | 0.099 |
| Preterm or Low Birth Weight (%Yes) | 290 (19.5%) | 198 (24.0%) | 0.012 |
| Adequacy of Prenatal Care | 0.001 | ||
| Less than Adequate | 577 (38.9%) | 388 (47.0%) | |
| Adequate | 419 (28.2%) | 203 (24.6%) | |
| Intensive | 488 (32.9%) | 235 (28.5%) | |
| Multiple pregnancy (%Yes) | 26 (1.8%) | 20 (2.4%) | 0.270 |
| Infant NAS Diagnosis (%Yes) | 656 (44.2%) | 412 (49.9%) | 0.009 |
(3MB = 3 months before delivery; Bup = Buprenorphine; CI = Confidence Interval; ED = Emergency Department; HR = Hazards Ratio; MOUD = Medication for Opioid Use Disorder; NAS = Neonatal Abstinence Syndrome)
4 women died during the postpartum year, they are not included in the discontinued group
Individuals receiving both methadone and buprenorphine are classified in “any buprenorphine” group
Postpartum Monthly MOUD Discontinuation
Kaplan Meier curves of the monthly probability of MOUD continuation in the overall sample and stratified by type of MOUD, maternal race/ethnicity, and degree of prenatal medication use are shown in Figure 2. There were no differences in the probability of medication continuation at 12 months postpartum between the “methadone only” and “any buprenorphine” groups (p=0.240). There were significant differences stratified by maternal race/ethnicity, with a probability of 0.65 for white non-Hispanic women compared with 0.51 for women of other races for continuing MOUD for a full postpartum year (p<0.001). Additionally, among women who received MOUD their entire pregnancy, the probability of continuation was 0.78 if engaged for all of pregnancy compared to 0.44 for those who received <4 months of MOUD during pregnancy (p<0.001).
Figure 2:
Kaplan Meier survival analysis curves
Cox Regression Models
Factors associated with MOUD discontinuation in the final adjusted model after using stepwise elimination for the overall sample are shown in Table 2. A shorter duration of prenatal MOUD utilization (<4 months MOUD aHR 3.26; 95% CI 2.72-3.91 compared to receipt all of pregnancy) and incarceration during pregnancy or postpartum (aHR 1.79; 95% CI 1.52-2.12 compared with women not incarcerated) most strongly impacted treatment discontinuation. Additionally, compared with women who were white non-Hispanic, women of other race/ethnicities were more likely to discontinue MOUD following delivery (aHR 1.48; 95% CI 1.21-1.81). Women receiving Medicaid were less likely to discontinue compared with individuals receiving other insurance or no insurance (aHR 0.58; 95% CI 0.45-0.75), and those receiving an opioid prescription other than MOUD in the three months before delivery had increased likelihood of discontinuation compared with those without a prenatal opioid prescription (aHR 1.60; 95% 1.13-2.28). Finally, women receiving any buprenorphine had a decreased likelihood of discontinuation (aHR 0.84; 95% 0.72-0.97) compared with those receiving methadone.
Table 2.
Risk factors for discontinuation of medications to treat opioid use disorder (MOUD) in the year after delivery (N=2,314)
| Characteristics | Any MOUD Treatment | Any Buprenorphine (n=1,362) | Methadone Only (n=952) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude HR* 95% CI | Adj. HR** 95% CI | Crude HR* 95% CI | Adj. HR** 95% CI | Crude HR* 95% CI | Adj. HR** 95% CI | |||||||
| Maternal Race/Ethnicity | ||||||||||||
| White non-Hispanic | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) |
| Other | 1.53 | (1.25-1.87) | 1.48 | (1.21-1.81) | 1.45 | (1.10-1.93) | 1.33 | (1.00-1.77) | 1.60 | (1.20-2.14) | 1.80 | (1.34-2.41) |
| Maternal age | ||||||||||||
| ≤ 25 years old | 1.30 | (1.12-1.51) | 1.38 | (1.13-1.68) | 1.27 | (1.04-1.55) | 1.21 | (0.96-1.52) | ||||
| 26-34 years old | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | ||||
| ≥ 35 years old | 0.95 | (0.75-1.20) | 0.91 | (0.67-1.23) | 0.89 | (0.66-1.21) | 1.07 | (0.74-1.55) | ||||
| MassHealth delivery | ||||||||||||
| Yes | 0.65 | (0.51-0.83) | 0.58 | (0.45-0.75) | 0.60 | (0.46-0.77) | 0.56 | (0.43-0.73) | 0.98 | (0.37-2.63) | ||
| No | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | ||
| Rural Residence | ||||||||||||
| Yes | 1.04 | (0.84-1.28) | 0.95 | (0.72-1.24) | 1.21 | (0.87-1.69) | 1.41 | (1.01-1.98) | ||||
| No | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | ||||
| Overdose event† | ||||||||||||
| Yes | 2.10 | (1.48-2.99) | 1.61 | (0.86-3.03) | 2.44 | (1.58-3.76) | 1.79 | (1.15-2.78) | ||||
| No | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | ||||
| MOUD treatment in pregnancy | ||||||||||||
| All pregnancy | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) |
| 5-8 months | 2.06 | (1.74-2.44) | 1.98 | (1.67-2.35) | 2.53 | (1.98-3.23) | 2.38 | (1.86-3.04) | 1.77 | (1.38-2.27) | 1.53 | (1.18-1.97) |
| 1-4 months | 3.50 | (2.93-4.20) | 3.26 | (2.72-3.91) | 4.37 | (3.36-5.69) | 4.11 | (3.15-5.36) | 2.91 | (2.26-3.76) | 2.66 | (2.05-3.46) |
| Other opioid prescription (3MB) | ||||||||||||
| Yes | 1.63 | (1.16-2.32) | 1.60 | (1.13-2.28) | 1.44 | (0.96-2.18) | 3.07 | (1.58-5.97) | 3.01 | (1.54-5.87) | ||
| No | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | ||
| Incarceration in pregnancy/postpartum | ||||||||||||
| Yes | 2.01 | (1.71-2.36) | 1.79 | (1.52-2.12) | 1.93 | (1.53-2.44) | 1.88 | (1.49-2.38) | 2.10 | (1.68-2.64) | 1.75 | (1.38-2.21) |
| No | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) |
| Medication for MOUD | ||||||||||||
| Any Buprenorphine | 0.91 | (0.79-1.04) | 0.84 | (0.72-0.97) | ||||||||
| Methadone Only | 1.00 | (Reference) | 1.00 | (Reference) | ||||||||
3MB = 3 months before delivery, MOUD = medication for opioid use disorder
Adjusted for year of birth only
Adjusted for year of birth and all other variables in the final model (stepwise selection - p < 0.1 to enter model, p < 0.05 to remain)
Time dependent. Yes after first occurrence during pregnancy or the follow up period.
Sensitivity Analyses
When we assumed that either all women receiving methadone continued on medication during the poor reporting months or removed deliveries for women receiving methadone during the months with poor reporting, the Kaplan Meier curves showed no changes to the general magnitude and significance of the survival curves. In the alternate cohorts, differences in probabilities of continuation at 12 months ranged between 4-6% depending on the model (Supplementary Figure 2). In the Cox regression models, we found that across all three analyses, maternal non-white race, non-public payer insurance, less prenatal MOUD treatment, and incarceration significantly increased the likelihood of discontinuation (Supplementary Table 2).
We then stratified the main model by medication type and key differences between buprenorphine and methadone emerged (Table 2). First, differences by maternal overdose, rural residence, and history of prenatal opioid prescription remained significant in the adjusted model for methadone only. Age and insurance were significant for those receiving buprenorphine, but not for methadone. Across all three models (the entire cohort and stratified by medication type), maternal race/ethnicity, the degree of prenatal MOUD utilization, and incarceration remained significant.
Structured Discussion/Comment:
Principal Findings
Across a statewide comprehensive sample of more than 2,300 pregnant women in Massachusetts who received MOUD at delivery, we identified that approximately two thirds of women continued receiving medication for a full postpartum year. The probability of continuation varied significantly by maternal race/ethnicity and degree of prenatal MOUD utilization. In our final multivariable model, less prenatal MOUD utilization and evidence of incarceration during pregnancy or the postpartum period strongly increased the risk of postpartum MOUD discontinuation.
Results
We found a higher rate of continuation during the year following pregnancy (64.1%) compared with other studies that report 50-60% continuation at one year after initiating treatment.30,43,44 Prior studies reporting postpartum MOUD adherence have ended by 3-6 months postpartum, and most have reported adherence in the context of a clinical trial.19,24,25,45 Our study builds on this literature, using population level data that contains both methadone and buprenorphine treatment, and extends out a full 12 months following delivery. We required two months without treatment receipt to be considered as discontinuation, which may explain our higher continuation rates compared to more restrictive studies. This finding is not surprising, as individuals receiving MOUD throughout pregnancy likely represented a group of women who were in recovery when their pregnancy began. However, it is notable that for women who received five or more months of treatment during pregnancy, there was a significantly reduced risk of discontinuing treatment in our adjusted models compared to those receiving ≤4 months.
Incarceration status significantly impacted MOUD discontinuation. In Massachusetts, it is standard practice for pregnant women with OUD who become incarcerated to receive methadone. During our study period (2011-2015), however, among incarcerated women a rapid taper postpartum was routine and non-pregnant women would not have had access to MOUD.46 Although MOUD treatment availability in the U.S. criminal justice system has increased, including in Massachusetts, MOUD access remains uncommon nationally.47,48 In a cross sectional survey of 26 jails and prisons across the country in 2016-17, a third of pregnant women with OUD were not offered MOUD and few continue to receive medications past three months post-partum.49 Jails and prison systems must limit the logistical, cost, and regulatory barriers and overcome negative attitudes towards use of MOUD.47
Clinical Implications
Our findings suggest that if we can more successfully engage women in MOUD before delivery, we can promote better OUD treatment retention outcomes after delivery, reducing the risk of postpartum overdose and custody loss.8,16 Further, for women who were newly engaged in treatment shortly prior to delivery, additional postpartum supports like home visiting nurse, parental-infant mental health, and peer support programs may be necessary to improve MOUD continuation following delivery. Additionally, improved access to preconception counseling, shared-decision making around contraception choices, and access to long-acting reversible contraception within addiction treatment settings are needed to support reproductive aged women with OUD.
While the literature on MOUD adherence for postpartum women is limited, similarities exist between postpartum HIV medication adherence and MOUD continuation; a history of sexual abuse or recurrent trauma,50 internalized stigma,51 lack of disclosure of diagnosis, depression and anxiety, and limited social support52 reduce HIV medication adherence and may present common barriers to MOUD adherence. We hypothesize that interventions that have been used to promote postpartum maternal HIV medication adherence including enhanced care coordination, co-located maternal and pediatric care, peer support, mobile technology, integrated maternal mental health services, and promotion of self-efficacy may yield improved MOUD adherence.53
We identified maternal racial/ethnic disparities in postpartum MOUD treatment continuation, consistent with disparities found in MOUD use during pregnancy.40,54 This analysis adds to literature on disparities in maternal morbidity and mortality and in addiction treatment by identifying the persistence of racial/ethnic disparities across the perinatal continuum. Patient-centered, culturally responsive interventions and structural changes are urgently needed to ensure equitable care for all women with OUD.
Receipt of public payer insurance at delivery was associated with a reduced odds of medication discontinuation compared to no or private insurance. We hypothesize that the expanded options for public insurance for all individuals in Massachusetts contributed to this finding. Further, not all private insurance options during the study period covered MOUD. These findings may not be generalizable outside of Massachusetts as many women lose access to insurance at 8-12 weeks postpartum, resulting in significant barriers to continuing in care.55
We were surprised to find that obstetric and/or birth characteristics including adequacy of prenatal care, an index based on the gestational age at prenatal care initiation and total number of visits, 41 were not significantly associated with medication discontinuation in our multivariable model. It may be that for pregnant women with OUD, who often have more frequent appointments linked to addiction and/or behavioral treatment visits, the prenatal care adequacy metric, is not the most useful for assessing the impact of health utilization during pregnancy.
Research Implications
Future research should explore the treatment experiences of women starting MOUD during pregnancy, particularly the views of Black, Indigenous, and people of color. Additionally, research should assess how clinical care models impact addiction outcomes like postpartum continuation of MOUD and how patterns in postpartum MOUD adherence affect maternal and child health outcomes.
Strengths and Limitations
Our analyses add population-level information surrounding MOUD utilization, particularly in the 6-12 months following delivery, yet limitations exist. First, we found evidence from non-parametric tests that discontinuation differed by month of delivery overall and for methadone patients, which limits our ability to draw definitive conclusions around discontinuation rates from women on methadone across our study period. We tried to control for these differences by including year of birth in Cox regression modeling. Additionally, our post-hoc sensitivity analyses reassures us that the main findings are robust, and we do not expect there to be differential missingness in the unavailable data. Second, we did not assess medication dose, which is important because some women may have discontinued MOUD by preference following desired and monitored weaning plans with their clinician post-partum. Future analyses should investigate changes in medication dose and clinical discontinuation decisions. Third, limitations of administrative data sources generally include reporting errors and under-coding of both diagnoses and treatment.56 Fourth, we were only able to identify medication treatment received in Massachusetts, and may have miscategorized women as being off treatment who had moved or transferred care across states. Fifth, with greater availability of substance treatment, insurance access, and lower rate of rural dwellings in Massachusetts our findings may be less generalizable to other states. Finally, our study period from 2011-2015 may not be reflective of improved treatment access over the last five years.
Conclusion
Prioritizing treatment continuation across the perinatal continuum, expanding gender-specific and family friendly recovery supports, addressing key disparities in maternal health, reducing punitive incarcerations for drug-related crimes, and expanding access to life-saving medications to treat OUD while incarcerated are needed to improve postpartum treatment adherence.
Supplementary Material
AJOG at a Glance:
A. Why was the study conducted?
The postpartum year can be a vulnerable period for women with opioid use disorder (OUD). We examined methadone and buprenorphine utilization among women with OUD in the year following delivery and determined factors associated with medication discontinuation.
B. What are the key findings?
Almost two-thirds of women with OUD remained on medications to treat OUD for the full year postpartum although rates varied significantly by degree of prenatal medication utilization. In our multivariable models, receiving 4 or fewer months of medication treatment prenatally, maternal non-white race, and a history of incarceration increased likelihood of postpartum medication discontinuation.
C. What does this study add to what is already known?
This study adds the first population-level analysis of postpartum utilization of medications to treat OUD, expanding prior literature from clinical trials or single site programs that often was limited to only 3-6 months postpartum. Prioritizing gender-specific and family friendly recovery supports across the perinatal continuum and expanding access to MOUD while incarcerated can help improve postpartum treatment receipt.
Acknowledgements:
The authors thank Dr. Mardge Cohen (Boston Health Care for the Homeless) and Ms. Erin Work (Division of General Academic Pediatrics, MassGeneral Hospital for Children) for her editorial review of an earlier version of this manuscript. Neither Dr. Cohen or Ms. Work did not receive compensation for her review.
Funding Support:
Dr. Schiff was supported by NIDA (K12 DA043490 and K23DA048169). Dr. Hadland was supported by NIDA (K23DA045085 and L40DA042434). Dr. Kelly was supported by NIAAA (K24AA022136); Dr. Taveras was supported by NIDDK (K24 DK105989). Dr. Wilens was supported by NIDA (R01 DA050252). The funding agencies had no involvement in study the design, data collection, analysis, interpretation of data, or in the writing of the reports.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Wilens reported received support from the Cambridge University Press, Guilford Press, Ironshore, KemPharm, Otsuka Pharmaceutical, and Vallon Pharmaceuticals; personal fees from Bay Cove Human Services, the Gavin Foundation, US Major League Baseball, US Minor League Baseball, and the US National Football League outside the submitted work; and receiving royalties as a co-owner (with Ironshore) of the patent for the Before School Functioning Questionnaire, a copyrighted diagnostic questionnaire. No other disclosures were reported.
Literature Cited
- 1.Compton WM, Jones CM, Baldwin GT. Relationship between Nonmedical Prescription-Opioid Use and Heroin Use. Longo DL, ed. N Engl J Med. 2016;374(2):154–163. doi: 10.1056/NEJMra1508490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal Abstinence Syndrome and Associated Health Care Expenditures. JAMA. 2012;307(18):1934–1940. doi: 10.1001/jama.2012.3951 [DOI] [PubMed] [Google Scholar]
- 3.Ko JY, Patrick SW, Tong VT, Patel R, Lind JN, Barfield WD. Incidence of Neonatal Abstinence Syndrome - 28 States, 1999-2013. MMWR Morb Mortal Wkly Rep. 2016;65(31):799–802. doi: 10.15585/mmwr.mm6531a2 [DOI] [PubMed] [Google Scholar]
- 4.Hogan L, Rutherford BK, Schrader DR. Maryland Maternal Mortality Review 2016 Annual Report Health – General Article 13-1203–1207. [Google Scholar]
- 5.Kavanaugh V Pregnancy-Associated Deaths From Drug Overdose in Virginia, 1999-2007: A Report from the Virginia Maternal Mortality Review Team.; 2015. [Google Scholar]
- 6.Metz TD, Rovner P, Hoffman MC, Allshouse AA, Beckwith KM, Binswanger IA. Maternal Deaths From Suicide and Overdose in Colorado, 2004-2012. Obstet Gynecol. 2016;128(6):1233–1240. doi: 10.1097/AOG.0000000000001695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta PK, Bachhuber MA, Hoffman R, Srinivas SK. Deaths From Unintentional Injury, Homicide, and Suicide During or Within 1 Year of Pregnancy in Philadelphia. Am J Public Health. 2016;106(12):2208–2210. doi: 10.2105/AJPH.2016.303473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiff DM, Nielsen T, Terplan M, et al. Fatal and Nonfatal Overdose Among Pregnant and Postpartum Women in Massachusetts. Obstet Gynecol. 2018;132(2):466–474. doi: 10.1097/AOG.0000000000002734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peles E, Schreiber S, Bloch M, Dollberg S, Adelson M. Duration of Methadone Maintenance Treatment During Pregnancy and Pregnancy Outcome Parameters in Women With Opiate Addiction. J Addict Med. 2012;6(1):18–23. doi: 10.1097/ADM.0b013e318229bb25 [DOI] [PubMed] [Google Scholar]
- 10.Burns L, Mattick RP, Lim K, Wallace C. Methadone in pregnancy: treatment retention and neonatal outcomes. Addiction. 2007;102(2):264–270. doi: 10.1111/j.1360-0443.2006.01651.x [DOI] [PubMed] [Google Scholar]
- 11.American College of Obstetricians and Gynecologists (ACOG). Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 711 Summary. Obstet Gynecol. 2017;130(2):488–489. doi: 10.1097/AOG.0000000000002229 [DOI] [PubMed] [Google Scholar]
- 12.Zedler BK, Mann AL, Kim MM, et al. Buprenorphine compared with methadone to treat pregnant women with opioid use disorder: a systematic review and meta-analysis of safety in the mother, fetus and child. Addiction. 2016; 111(12):2115–2128. doi: 10.1111/add.13462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2). doi: 10.1002/14651858.CD002207.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ordean A, Wong S, Graves L. No. 349-Substance Use in Pregnancy. J Obstet Gynaecol Canada. 2017;39(10):922–937.e2. doi: 10.1016/j.jogc.2017.04.028 [DOI] [PubMed] [Google Scholar]
- 15.Schuckit MA. Treatment of Opioid-Use Disorders. Longo DL, ed. N Engl J Med. 2016;375(4):357–368. doi: 10.1056/NEJMra1604339 [DOI] [PubMed] [Google Scholar]
- 16.Hall MT, Wilfong J, Huebner RA, Posze L, Willauer T. Medication-assisted treatment improves child permanency outcomes for opioid-using families in the child welfare system. J Subst Abuse Treat. 2016;71:63–67. doi: 10.1016/j.jsat.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 17.Forray A, Merry B, Lin H, Ruger JP, Yonkers KA. Perinatal substance use: A prospective evaluation of abstinence and relapse. Drug Alcohol Depend. 2015;150:147–155. doi: 10.1016/j.drugalcdep.2015.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forray A, Foster D. Substance Use in the Perinatal Period. Curr Psychiatry Rep. 2015;17(11):91. doi: 10.1007/s11920-015-0626-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo-Ciganic W-H, Donohue JM, Kim JY, et al. Adherence trajectories of buprenorphine therapy among pregnant women in a large state Medicaid program in the United States. Pharmacoepidemiol Drug Saf 2019;28(1):80–89. doi: 10.1002/pds.4647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hadland SE, Bagley SM, Rodean J, et al. Receipt of Timely Addiction Treatment and Association of Early Medication Treatment With Retention in Care Among Youths With Opioid Use Disorder. JAMA Pediatr. 2018;172(11):1029. doi: 10.1001/jamapediatrics.2018.2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapman SLC, Wu L-T. Postpartum substance use and depressive symptoms: a review. Women Health. 2013;53(5):479–503. doi: 10.1080/03630242.2013.804025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benningfield MM, Dietrich MS, Jones HE, et al. Opioid dependence during pregnancy: relationships of anxiety and depression symptoms to treatment outcomes. Addiction. 2012;107Suppl:74–82. doi: 10.1111/j.1360-0443.2012.04041.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klaman SL, Isaacs K, Leopold A, et al. Treating Women Who Are Pregnant and Parenting for Opioid Use Disorder and the Concurrent Care of Their Infants and Children: Literature Review to Support National Guidance. J Addict Med. 2017;11(3):178–190. doi: 10.1097/adm.0000000000000308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilder C, Lewis D, Winhusen T. Medication assisted treatment discontinuation in pregnant and postpartum women with opioid use disorder. Drug Alcohol Depend. 2015;149:225–231. doi: 10.1016/j.drugalcdep.2015.02.012 [DOI] [PubMed] [Google Scholar]
- 25.Ellis JD, Cairncross M, Struble CA, Carr MM, Ledgerwood DM, Lundahl LH. Correlates of Treatment Retention and Opioid Misuse Among Postpartum Women in Methadone Treatment. J Addict Med. 2019;13(2):153–158. doi: 10.1097/ADM.0000000000000467 [DOI] [PubMed] [Google Scholar]
- 26.O’Connor AB, Uhler B, O’Brien LM, Knuppel K. Predictors of treatment retention in postpartum women prescribed buprenorphine during pregnancy. J Subst Abuse Treat. 2018;86:26–29. doi: 10.1016/j.jsat.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 27.Peles E, Schreiber S, Bloch M, Dollberg S, Adelson M. Duration of Methadone Maintenance Treatment During Pregnancy and Pregnancy Outcome Parameters in Women With Opiate Addiction. J Addict Med. 2012;6(1):18–23. doi: 10.1097/ADM.0b013e318229bb25 [DOI] [PubMed] [Google Scholar]
- 28.Socías ME, Dong H, Wood E, et al. Trajectories of retention in opioid agonist therapy in a Canadian setting. Int J Drug Policy. 2020;77. doi: 10.1016/j.drugpo.2020.102696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo A, Kerr T, Hayashi K, et al. Factors associated with methadone maintenance therapy discontinuation among people who inject drugs. J Subst Abuse Treat. 2018;94:41–46. doi: 10.1016/j.jsat.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinstein ZM, Kim HW, Cheng DM, et al. Long-term retention in Office Based Opioid Treatment with buprenorphine. J Subst Abuse Treat. 2017;74:65–70. doi: 10.1016/j.jsat.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Godersky ME, Saxon AJ, Merrill JO, Samet JH, Simoni JM, Tsui JI. Provider and patient perspectives on barriers to buprenorphine adherence and the acceptability of video directly observed therapy to enhance adherence. Addict Sci Clin Pract. 2019; 14(1): 11. doi: 10.1186/s13722-019-0139-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manhapra A, Agbese E, Leslie DL, Rosenheck RA. Three-year retention in buprenorphine treatment for opioid use disorder among privately insured adults. Psychiatr Serv. 2018;69(7):768–776. doi: 10.1176/appi.ps.201700363 [DOI] [PubMed] [Google Scholar]
- 33.Samples H, Williams AR, Olfson M, Crystal S. Risk factors for discontinuation of buprenorphine treatment for opioid use disorders in a multi-state sample of Medicaid enrollees. J Subst Abuse Treat. 2018;95:9–17. doi: 10.1016/j.jsat.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massachusetts Department of Public Health. Data Brief: Opioid-Related Overdose Deaths among Massachusetts Residents. Boston, MA; 2017. [Google Scholar]
- 35.Massachusetts Department of Public Health. An Assessment of Opioid-Related Deaths in Massachusetts (2013-2014).; 2016. https://www.mass.gov/files/documents/2016/09/pg/chapter-55-report.pdf.Accessed April 11, 2018.
- 36.Commonwealth of Massachusetts. An Act Requiring Certain Reports for Opiate Overdoses.; 2015. https://malegislature.gov/Laws/SessionLaws/Acts/2015/Chapter55. Accessed October 8, 2017.
- 37.MA Department of Public Health. An Assessment of Fatal and Nonfatal Opioid Overdoses in Massachusetts (2011 – 2015). Boston; 2017. https://www.mass.gov/files/documents/2017/08/31/legislative-report-chapter-55-aug-2017.pdf. Accessed October 8, 2017. [Google Scholar]
- 38.Bureau of Substance Addiction Services ∣ Mass.gov. https://www.mass.gov/orgs/bureau-of-substance-addiction-services.Accessed November 22, 2020. [Google Scholar]
- 39.Krans EE, Kim JY, James AE, Kelley D, Jarlenski MP. Medication-Assisted Treatment Use Among Pregnant Women With Opioid Use Disorder. Obstet Gynecol. 2019;133(5):943–951. doi: 10.1097/AOG.0000000000003231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schiff DM, Nielsen T, Hoeppner BB, et al. Assessment of Racial and Ethnic Disparities in the Use of Medication to Treat Opioid Use Disorder Among Pregnant Women in Massachusetts. JAMA Netw open. 2020;3(5):e205734. doi: 10.1001/jamanetworkopen.2020.5734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kotelchuck M An Evaluation of the Kessner Adequacy of Prenatal Care Index and a Proposed Adequacy of Prenatal Care Utilization Index. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1615177/pdf/amjph00460-0056.pdf.Accessed December 10, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: {United} {States}, 2000-2009. JAMA. 2012;307(18):1934–1940. doi: 10.1001/jama.2012.3951 [DOI] [PubMed] [Google Scholar]
- 43.Hser YI, Saxon AJ, Huang D, et al. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2014;109(1):79–87. doi: 10.1111/add.12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shulman M, Wai JM, Nunes EV. Buprenorphine Treatment for Opioid Use Disorder: An Overview. CNS Drugs. 2019;33(6):567–580. doi: 10.1007/s40263-019-00637-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crane D, Marcotte M, Applegate M, et al. A statewide quality improvement (QI) initiative for better health outcomes and family stability among pregnant women with opioid use disorder (OUD) and their infants. J Subst Abuse Treat. 2019;102:53–59. doi: 10.1016/j.jsat.2019.04.010 [DOI] [PubMed] [Google Scholar]
- 46.Gray J, Saia K, Walley AY. 28-year-old Woman With Opioid Use Disorder Delivers Healthy Baby While in Custody: Addressing Forced Detox. J Addict Med. 2019;13(3):237–240. doi: 10.1097/ADM.0000000000000468 [DOI] [PubMed] [Google Scholar]
- 47.Fiscella K, Wakeman SE, Beletsky L. Implementing opioid agonist treatment in correctional facilities. JAMA Intern Med. 2018;178(9): 1157–1158. doi: 10.1001/jamainternmed.2018.3504 [DOI] [PubMed] [Google Scholar]
- 48.Nunn A, Zaller N, Dickman S, Trimbur C, Nijhawan A, Rich JD. Methadone and buprenorphine prescribing and referral practices in US prison systems: results from a nationwide survey.[Erratum appears in Drug Alcohol Depend. 2011 Jan 15;113(2-3):252]. Drug Alcohol Depend. 2009;105(1-2):83–88. doi: 10.1016/j.drugalcdep.2009.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sufrin C, Sutherland L, Beal L, Terplan M, Latkin C, Clarke JG. Opioid Use Disorder Incidence and Treatment Among Incarcerated Pregnant People in the U.S.: Results from a National Surveillance Study. Addiction. March2020:add.15030. doi: 10.1111/add.15030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dale S, Cohen M, Weber K, Cruise R, Kelso G, Brody L. Abuse and Resilience in Relation to HAART Medication Adherence and HIV Viral Load Among Women with HIV in the United States. AIDS Patient Care STDS. 2014;28(3):136–143. doi: 10.1089/apc.2013.0329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turan B, Smith W, Cohen MH, et al. Mechanisms for the Negative Effects of Internalized HIV-Related Stigma on Antiretroviral Therapy Adherence in Women. JAIDS J Acquir Immune Defic Syndr. 2016;72(2):198–205. doi: 10.1097/QAI.0000000000000948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adams JW, Brady KA, Michael YL, Yehia BR, Momplaisir FM. Postpartum Engagement in HIV Care: An Important Predictor of Long-term Retention in Care and Viral Suppression. Clin Infect Dis. 2015;61(12):1880–1887. doi: 10.1093/cid/civ678 [DOI] [PubMed] [Google Scholar]
- 53.Momplaisir FM, Storm D, Nkwihoreze H, Jayeola O, Jemmott JB. Improving postpartum retention in care for women living with HIV in the United States. AIDS. 2017;32(2):1. doi: 10.1097/QAD.0000000000001707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peeler M, Gupta M, Melvin P, et al. Racial and Ethnic Disparities in Maternal and Infant Outcomes Among Opioid-Exposed Mother–Infant Dyads in Massachusetts (2017–2019). Am J Public Health. 2020;Epub ahead. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mcmorrow S, Dubay L, Kenney GM, Johnston EM, Caraveo CA. Uninsured New Mothers’ Health and Health Care Challenges Highlight the Benefits of Increasing Postpartum Medicaid Coverage.; 2020. [Google Scholar]
- 56.Howell BA, Abel EA, Park D, Edmond SN, Leisch LJ, Becker WC. Validity of Incident Opioid Use Disorder (OUD) Diagnoses in Administrative Data: a Chart Verification Study. J Gen Intern Med. 2020. doi: 10.1007/s11606-020-06339-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.