Abstract
Background:
Near elimination of cervical cancer in the US is possible in coming decades, yet inequities will delay this achievement for some populations. We sought to explore the effects of human papillomavirus (HPV) vaccination on disparities in cervical cancer incidence between high- and low-poverty US counties.
Methods:
We calibrated a dynamic simulation model of HPV infection to reflect average counties in the highest and lowest quartile of poverty (percent of population below federal poverty level), incorporating data on HPV prevalence, cervical cancer screening, and HPV vaccination. We projected cervical cancer incidence through 2070, estimated absolute and relative disparities in incident cervical cancer for high- vs. low-poverty counties, and compared incidence to the near-elimination target (4 cases/100,000 women annually).
Results:
We estimated that, on average, low-poverty counties will achieve near-elimination targets an estimated 14 years earlier than high-poverty counties (2029 vs. 2043). Absolute disparities by county poverty will decrease, but relative differences are estimated to increase. We estimate 21,604 cumulative excess cervical cancer cases in high-poverty counties over the next 50 years. Increasing HPV vaccine coverage nationally to the Healthy People 2020 goal (80%) would reduce excess cancer cases, but not alter estimated time to reach the near-elimination threshold.
Conclusions:
High-poverty US counties will likely be delayed in achieving near-elimination targets for cervical cancer and as a result will experience thousands of potentially preventable cancers.
Impact:
Alongside vaccination efforts, it is important to address the role of social determinants and healthcare access in driving persistent inequities by area poverty.
Introduction
Geographic disparities in cancer are well-documented in the US, with those living in higher poverty areas experiencing higher morbidity and mortality from numerous preventable cancers.1–4 Among the largest disparities by area poverty are in cancers associated with human papillomavirus (HPV), including cervical, anal, and oropharyngeal cancers.1 Individuals in high-poverty areas are nearly twice as likely to be diagnosed with cervical cancer as those in low-poverty areas.1,2 Geographic inequities in cervical cancer are complex and attributed to multiple, overlapping risk factors, including prevalence of high-risk HPV types and lower provision of cervical cancer screening to detect pre-cancerous stages of disease.5–7
In 2006, the introduction of HPV vaccine created an important new opportunity for cancer prevention. The most recently licensed HPV vaccine in the US protects against seven oncogenic HPV types along with two types that cause approximately 90% of genital warts.8 The potential benefits of HPV vaccine are so promising that the near-elimination of cervical cancer is considered an achievable goal in the US.9–11 Previous simulation models of HPV have suggested that this goal could be achieved nationally as early as 2038, but have not examined how this timeline may vary geographically within in the US.12
Beyond reducing overall cancer burden, HPV vaccination has the potential to reduce geographic disparities in cancer outcomes by providing accessible prevention that has low out-of-pocket cost for patients.13 Studies examining HPV vaccine uptake by area poverty have suggested that HPV vaccination rates in high-poverty areas may be higher than in low-poverty areas14–17, but these observations vary by how areas are defined and better longitudinal data are needed, along with models that can explore the long-term implications of these patterns.
To date, studies have modeled the potential impact of HPV vaccination in the US, but the long-term effects of HPV vaccine have focused on the US as a whole, potentially missing geographic heterogeneity in HPV prevalence, cervical cancer screening rates, and underlying cervical cancer risk.18–20 To understand the long-term implication of current HPV vaccination patterns on disparities in cervical cancer incidence between high- and low-poverty counties in the US, we use a stratified dynamic HPV infection model, incorporating data on vaccination, screening, and HPV prevalence by county poverty.
Materials and Methods
HPV Transmission Model
We adapted a dynamic HPV transmission model to reflect composite high- and low-poverty US counties.21 Briefly, the compartmental model simulates the transmission of HPV through sexual partnerships, allowing for both direct and indirect effects of vaccination (i.e., herd protection), as well as simulating progression of HPV to cervical cancer (Supplemental Figure 1). Individuals are born, age in one-year increments, and die at age-varying mortality rates. Individuals begin the model susceptible and may acquire an HPV infection based on age-specific sexual activity, prevalence of HPV among opposite-sex sexual partners (as a model simplification, we do not include same-sex sexual partnerships), and transmissibility of HPV. We collapse HPV types to separately describe high-risk types protected by HPV vaccine (HPV 16, 18, 31, 33, 45, 52, 58) and high-risk types not protected by current vaccines (HPV 35, 39, 51, 56, 59, 66, 68). Women with a high-risk HPV infection can spontaneously clear infection, progress to pre-cancerous cervical lesions, have lesions be identified and treated through routine cervical cancer screening, or have lesions progress to incident cervical cancer, at which point they exit the model. Our model was constructed using R (version 4.0.3).
Model Inputs and Target Data by Poverty Quartile
Using the 2011-2015 American Community Survey, we classified US counties into quartiles based on percent of residents living below 100% of the Federal Poverty Level (FPL). 22 The lowest poverty quartile represents the approximately 84 million individuals living in counties where less than 11.9% of the population lives in poverty (Supplemental Table 1) while the highest poverty quartile represents approximately 45 million individuals living in counties with greater than 20.3% of the population living in poverty. We estimated age-specific all-cause mortality by poverty quartile using CDC Wonder.23
We obtained data on characteristics by poverty quartiles from three large nationally representative surveys; the National Health Interview Survey (NHIS), the National Health and Nutrition Examination Survey (NHANES), and the National Immunization Survey–Teen (NIS-Teen) through the National Center for Health Statistics which allowed for use of county identifiers not available in the public data. For each survey, respondents’ county of residence was matched to poverty quartile from the American Community Survey. Quartile-matched data were analyzed at a secure Federal Research Data Center. As the study team did not have direct access to the individual county identifiers (only the matched quartile data) the study was determined to be exempt by the University of North Carolina Institutional Review Board.
Input: Cervical Cancer Screening.
We obtained data on cervical cancer screening from the NHIS (2013-2015), including approximately 3,600 women living in high-poverty counties each year and 6,600 women living in low poverty counties in each year of data. We analyzed the proportion of women up to date on cervical cancer screening, by poverty quartile, using complex survey weights to account for sampling design. We assessed differences using an independent sample t-test with an alpha value of 0.05. All survey data were analyzed using Stata 16 (College Station, Tx).
NHIS and other national US data sources have not shown changes over time in the proportion of women receiving timely cervical cancer screening;24–26 however, US recommendations for screening modality have shifted to recommend co-administering Pap smears with more sensitive HPV DNA testing.27,28 Our base case assumed the adoption of HPV DNA screening would increase from 0% in 2005 to 70% of tests by 2020 in both high- and low-poverty counties (Supplemental Figure 2). Because HPV DNA testing adoption varies geographically29; sensitivity analyses modeled delayed adoption of HPV DNA testing in high-poverty counties (taking until 2030 for these counties to reach 70% use of HPV DNA testing). We estimate 26% of women in a high-poverty setting and 15% of women in a low-poverty setting fail to return for subsequent follow-up from an abnormal screening test within one year.30–32
Input: HPV Vaccination.
HPV vaccination was obtained from the NIS-Teen (2008-2015), including provider-verified vaccination records from 5,000 adolescents living in high-poverty and 9,000 adolescents living in low-poverty counties each year. Data were available for girls starting in 2008 and boys starting in 2011. To facilitate comparison across settings, we present data on prevalent HPV vaccine coverage – percent of those age 11-17 with at least one dose and percent with all recommended doses by year, sex, and county poverty quartile. However, the inputs to our model are defined from these same data in a more specific way, as annual probability of incident HPV vaccine initiation (receiving first dose during the year) and completion (receiving final dose during the year) by sex, age group (11-12 vs. 13-17), and county poverty quartile. Model inputs for years before 2008 and after 2015 were projected, assuming stable uptake after 2020. (Supplemental Figure 3).
Calibration and Validation Targets: HPV Prevalence.
We obtained HPV prevalence data from NHANES, a nationally sampled survey that combines personal interviews with physical examination and laboratory testing data. We used 2003-2006 data for model calibration and 2011-2014 data for validation of model output, we had approximately 500 high-poverty and 900 low-poverty participants in each 4-year combined cycle. Female NHANES participants ages 18-60 were tested for HPV DNA using a self-collected vaginal swab.33 We report prevalence of vaccine-protected high-risk types and non-protected high-risk HPV types and prevalence of any high-risk type by age.
Model Calibration and Validation
The underlying causes of inequities in HPV prevalence and cervical cancer incidence are complex and likely mediated by differences in health care access, health behaviors, social determinants of health and underlying health status that may affect HPV acquisition and the natural history pathway. We used model calibration to approximate differences in model parameters that could not be estimated directly from the literature, including sexual behavior and natural history of infection. We calibrated parameters that could plausibly vary by county poverty, using a single base model and varying parameter values by +/−25% using a Latin hypercube sampling approach.34,35 We compared 10,000 possible parameter sets using a log-likelihood estimate against setting-specific calibration targets of HPV prevalence, separately by HPV type (all women ages 18-60) and by age (any high-risk type). The 50 best-fitting parameter sets for each quartile were then used to estimate pre-vaccine cervical cancer incidence, with a directed search algorithm identifying for each set an HPV progression multiplier which best matched 2006 age-adjusted cancer incidence by poverty quartile (5.9 and 8.4 cases per 100,000 for low- and high-poverty counties, respectively36).
The result of this process was 50 paired combinations of parameters describing possible variation in unobservable characteristics leading to the observable differences in high-poverty and low-poverty counties. We assessed validity of these parameter sets by comparing the model estimate of HPV prevalence in 2014 to observed 2011-2014 NHANES prevalence estimates by HPV type and for all high-risk HPV types by age. As there is no single standard for evaluating validity to external data37, we report the percent of model estimates that fall within two and within three standard deviations of their corresponding observed value.
Model Projections
Fully calibrated models were run starting in 2006 incorporating HPV vaccination rates by county poverty-quartile, repeating this process for each calibration set. We projected outcomes through 2070, as this covers the period of highest risk for cervical cancer among cohorts for which high-quality vaccination data are available. We compared a scenario assuming stable HPV vaccine rates after 2020 to a scenario with a 2020 increase to 80% HPV vaccine coverage. Our primary model outcome is projected annual age-adjusted cervical cancer incidence. We also report the year in which average high- and low-poverty counties would be projected to achieve targets for ‘near-elimination’ (annual incidence below 4 per 100,000). We measure disparities through comparing paired combinations of our high- and low-poverty models, producing 50 estimates of (1) the absolute number of excess cervical cancer cases (risk difference) and (2) the relative risk of cervical cancer in high vs. low-poverty counties. We estimated total excess cervical cancer cases by projecting the reduction in incident cervical cancers across the total female population of all high-poverty US counties (~24 million women) if annual incidence were matched to that of low-poverty counties.
Results
Empirical Data by County Poverty Quartile
Prevalence of high-risk HPV types covered by HPV vaccine did not significantly vary, at 12.9% in high-poverty and 10.2% in low-poverty counties in the pre-vaccine era (Figure 1A). Prevalence of one or more high-risk HPV types not protected against by current HPV vaccines was higher in high-poverty counties than low-poverty counties (18.3% vs. 8.9%, p<.01). Examining within age strata, prevalence of any high-risk HPV type was higher in high-poverty, compared to low-poverty counties, for those 45-54 (Figure 1B); other age groups showed the same direction of findings but were not statistically significant. Women ages 35-44 and 45-55 in high-poverty counties were less likely to be up to date on cervical cancer screening than for the same age groups in low-poverty counties (Supplemental Figure 4).
Figure 1. Prevalence of High-Risk HPV by County Poverty.
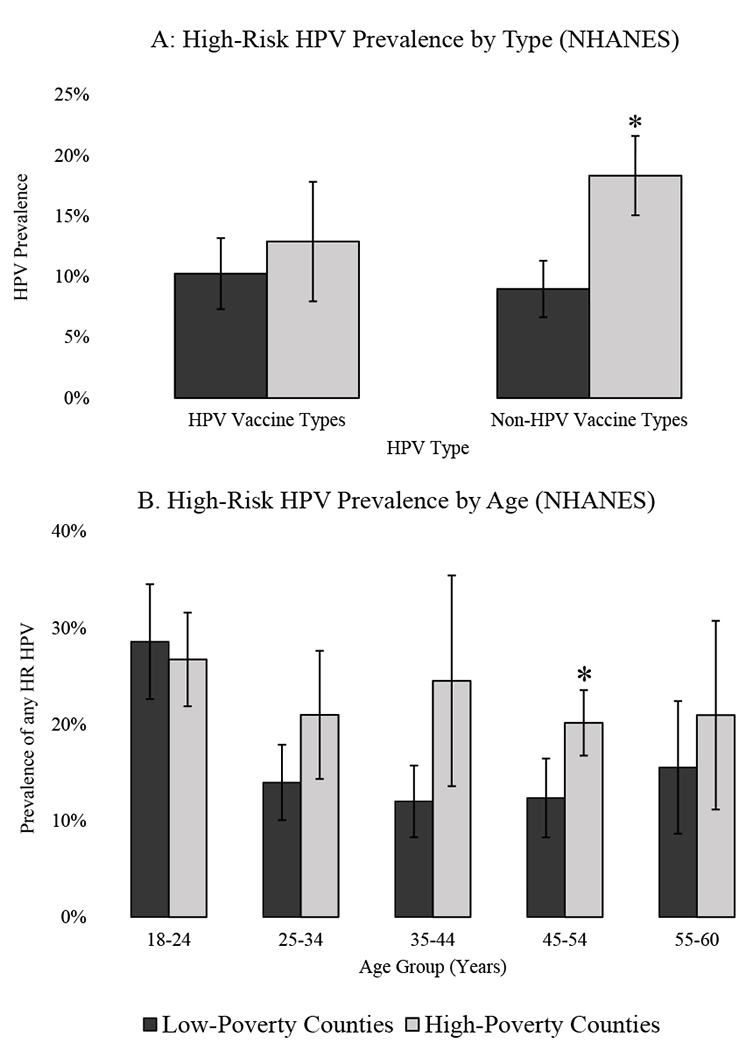
*Different by Wald test comparing high vs. low poverty counties (p <.05) ; Error bars represent 95% confidence interval; Data from National Health and Nutrition Examination Survey 2003-2006. Panel A includes women ages 18-60 by HPV type. Panel B includes prevalence of all high-risk (HR) HPV types by age group
High- and low-poverty counties showed similar patterns of HPV vaccine uptake across all years (Figure 2). By 2015, 69% of 11-17-year-old girls and 51% of 11-17-year-old boys in high-poverty counties had initiated HPV vaccination while 45% and 32% of girls and boys had completed the series. This was not different from low-poverty counties (initiation 63% and 53% for girls and boys, respectively; completion was 48% and 33%).
Figure 2. HPV Vaccine Initiation and Completion by Poverty Quartile.
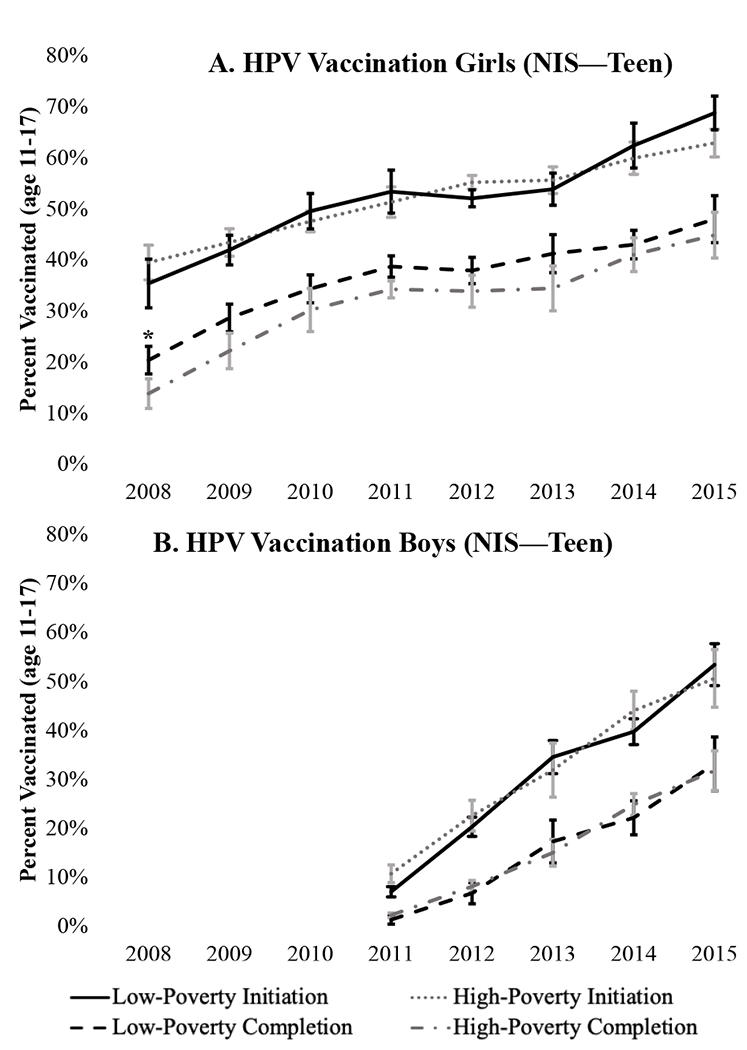
*Different by Wald test comparing high- vs. low-poverty counties (p <.05); error bars show 95% confidence intervals. Data from provider-verified records of adolescents 11-17 years of age in 2008-2015 National Immunization SurveyTeen. Initiation indicates receipt of at least one dose of HPV vaccine and completion indicates receipt of all recommended doses.
Model Calibration and Validation
The 50 best-fitting calibration sets for our high- and low-poverty models included values from across the search ranges (Supplemental Table 2), suggesting these sets represent a diversity of underlying drivers of disparities which each produce acceptable fits to pre-vaccine HPV prevalence data (Supplemental Figure 5). Our validation against 2011-2014 NHANES data showed that all parameter sets resulted in reasonable fits to empirical data by both age and HPV type. 91% of estimates were within two standard deviations of the corresponding NHANES value and 97% of estimates were within three standard deviations (Supplemental Figure 6).
Model Projections
Projecting model estimates forward to 2070, both low- and high-poverty county models see substantial reductions in cervical cancer incidence (Figure 3). In our low-poverty model, annual age-adjusted incidence rates were 5.9 per 100,000 in 2006 and were projected to fall to 0.7 per 100,000 by 2070, with parameter sets ranging from 0.5 to 1.2. In our high-poverty model, annual incidence declined from 8.4 per 100,000 in 2006 to 1.7 per 100,000 in 2070 [range: 1.1 to 2.5]. We estimated low-poverty counties will, on average, achieve the near-elimination target by 2030 [range: 2027-2032] while, on average, high-poverty counties are not projected to reach this goal for another fourteen years 2044 [2041 to 2048]. Over the 50 years from 2020-2070, we estimate a total of 21,604 excess cervical cancer cases in high-poverty counties, relative to the burden if incidence were identical to that of low-poverty counties.
Figure 3. Projected Cervical Cancer Incidence by Poverty Quartile.
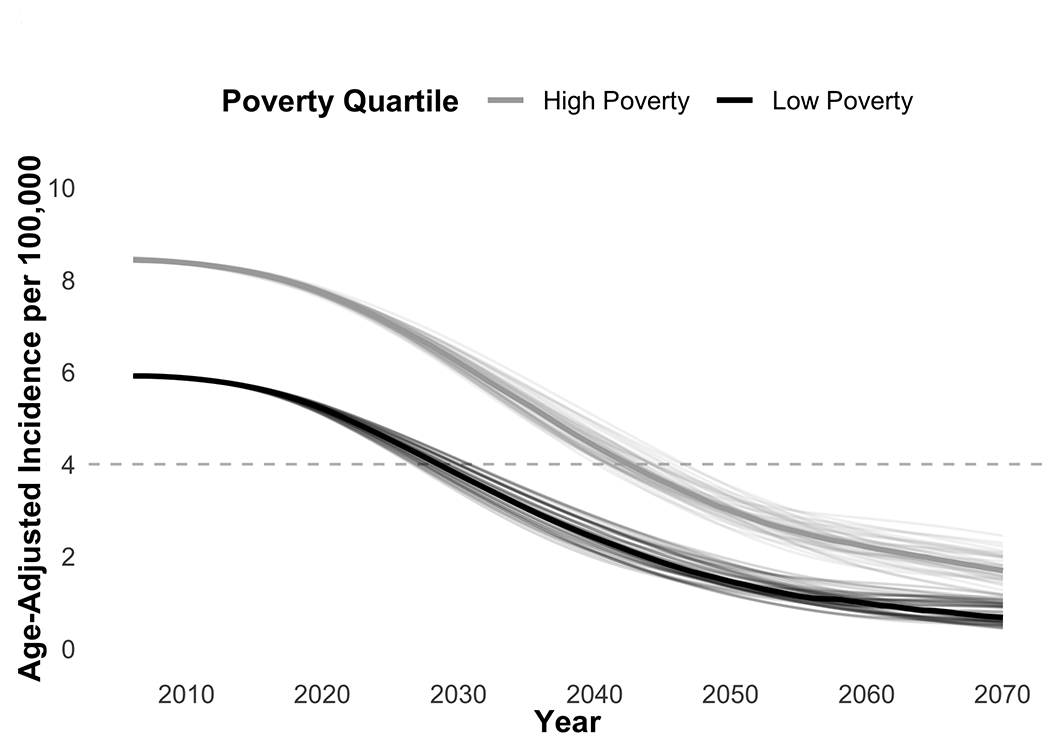
Shows 50 calibration sets each for high-poverty and low-poverty models. Lighter lines represent projected age-adjusted cervical cancer incidence for individual calibration sets, dark lines represent median value across all calibration sets for each month. Dashed line indicates target threshold of 4 cases per 100,000.
We estimate 2.5 excess incident cervical cancer cases per 100,000 women for average high-poverty counties relative to average low-poverty counties. We estimated this absolute disparity would shrink to 1.0 cases per 100,000 by 2070 (Figure 4A). The relative disparity is expected to increase (Figure 4B), with women in our high-poverty county at 1.4 times the risk of incident cancer vs. those in our low-poverty country in 2006 but 2.5 times the risk by 2070. These findings were consistent across paired calibration sets, with no comparisons showing a total disappearance of disparities by poverty quartile.
Figure 4. Projected Disparities in Cervical Cancer Incidence by Poverty Quartile.
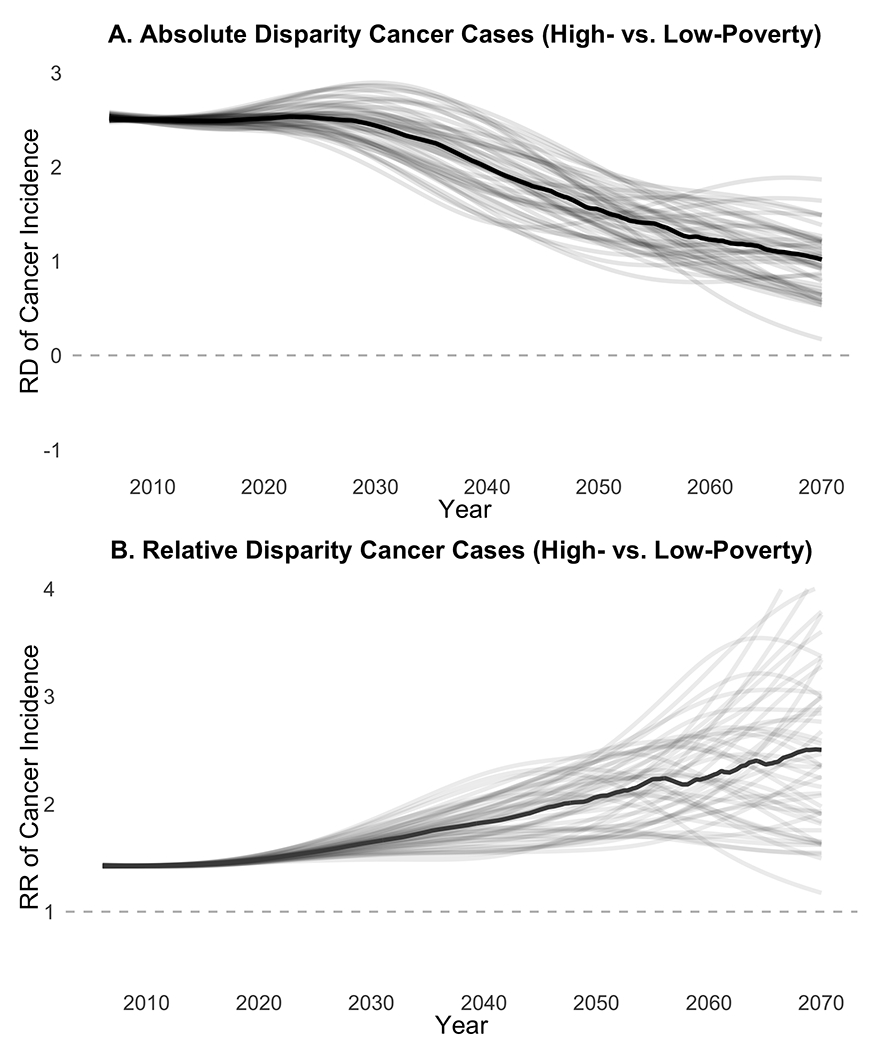
RD: Risk Difference, RR: Relative Risk; Shows paired comparisons of 50 calibration sets each for high-poverty and low-poverty models. Lighter lines represent individual pairs of calibration sets, dark lines represent median value across all comparisons in each month. Panel A shows risk difference (absolute disparity) between high-poverty and low-poverty counties. Panel B shows relative risk (relative disparity) between high-poverty and low-poverty counties. Dashed line indicates equity targets of (A) a 0.0 risk difference (B) a 1.0 relative risk.
Assuming our high- and low-poverty county each achieved target thresholds of 80% vaccine completion in 2020, near-elimination timelines improved by less than a year (Table 1) and we found a small improvement in absolutely and relative disparities by 2070 compared to current practice. Assuming delayed adoption of HPV DNA testing in the high-poverty county, disparities initially widened in all analyses, but by 2070 absolute and relative disparities were similar to those in the base case. Alternative assumptions about protection from partial-series completion had only small effects, with a small increase in both absolute and relative disparities if protection was lowered (50% of full series) and a slight improvement if fully protective (equivalent to completing the full series).
Table 1.
Sensitivity Analysis
| Base Case | Partial series efficacy (relative to full series) | 80% HPV Vaccine Coverage | Low HPV DNA Uptake in High-Poverty Countya | ||
|---|---|---|---|---|---|
| Median [Min-Max] | 100% | 50% | |||
| Near-elimination year: low-poverty county |
2029 [2027-2031] |
2027 [2026-2030] |
2029 [2027-2033] |
2028 [2027-2031] |
2029 [2027-2031] |
| Near-elimination year: high-poverty county |
2043 [2040-2046] |
2041 [2039-2045] |
2044 [2041-2048] |
2042 [2040-2046] |
2044 [2042-2048] |
| Absolute disparity: 2070 (risk difference) |
1.0 [0.2-1.9] |
0.8 [0.2-1.8] |
1.1 [0.3-1.8] |
0.7 [−0.1-1.6] |
1.1 [0.3-2.0] |
| Relative disparity: 2070 (risk ratio) |
2.5 [1.2-4.7] |
1.9 [1.2-4.5] |
2.7 [1.4-4.6] |
1.9 [1.0-4.4] |
2.7 [1.3-5.0] |
| Cumulative excess cases (2020 to 2070)b |
21,604 [16,585-25,854] |
20,741 [16,029-25,347] |
22,075 [16,971- 26,729 |
20,545 [16,225-24,690] |
24,368 [19,363-28,606] |
High-poverty county reaches 70% uptake of HPV DNA testing by 2030
Estimates scaled to total population of all high-poverty counties
Discussion
Our dynamic HPV transmission model suggests current uptake of HPV vaccination is likely to dramatically reduce cervical cancer burden in both low- and high-poverty US counties. Absolute disparities (i.e., number of excess cancers per 100,000) will decline as overall burden decreases in both high- and low-poverty counties. However, it is likely that relative differences will remain, with incident cervical cancer burden in high-poverty counties 1.5 to 3 times that of low-poverty counties. We found these conclusions robust to changes in model inputs and across calibrated parameter sets.
Differential access to advancements in cancer treatment and prevention often reduces overall cancer burden but widens inequity.38 Disparate access has not been generally seen in HPV vaccination, where uptake is similar or higher among multiple traditionally underserved groups.39,40 While we did not find higher average uptake of HPV vaccine in high-poverty areas, as some studies have reported14,16, we found comparable uptake in high- and low-poverty counties, which is nonetheless promising. Differences in conclusions across studies are likely due to differences in the definition of geographies (e.g., states, counties, census tracts, and zip codes) as well as the socioeconomic variables selected for comparisons.17 A geospatial approach may help prioritize areas where improved HPV vaccination could have the highest benefits for equity, but as uptake remains below coverage goals in nearly all areas of the US, broad approaches to improving HPV vaccine uptake are still urgently needed.41
Additionally, we found higher prevalence of high-risk HPV types that are not vaccine-protected in high-poverty counties. Histological studies attribute only a small portion of invasive cervical cancers to these types, but differences in type-distribution and co-infection may further disadvantage those already experiencing lower access to screening and preventive care.42 Better characterizing cancer attribution by HPV type, particularly among high-burden populations, is an important step for understanding disparities.
Our findings are comparable with previous modeling and empirical studies evaluating likely changes for the full US population. Two microsimulation models projected near-elimination in the US by 2038 and 2046. While studies on area poverty are limited, studies focusing on populations of color echo the pattern of our findings. For example, Black women have higher cervical cancer incidence3and higher HPV prevalence- particularly for high-risk types not protected by HPV vaccine43, but higher HPV vaccine initation.40 A study modeling the potential impact of HPV vaccine on racial disparities found that current HPV vaccination patterns were likely to decrease, but not eliminate, cervical cancer disparities by race.44
As high-poverty counties in the US have, on average, higher proportions of Black, American Indian and Latinx populations than low-poverty counties and are more likely to be rural, the disparities we explore here reflect many of the same structural inequities that lead to racial disparities and rural/urban disparities.45 This includes lower access to cervical cancer screening, an important method of secondary prevention.46 Those living in high-poverty settings are also more likely to experience certain risk factors for HPV cancers, including higher smoking rates, higher parity, and co-occurring sexually transmitted infection.47–49
Identifying multiple calibration sets allowed us to explore results across different combinations of uncertain variables. Our conclusions were largely robust to differences across calibrated parameter sets, but a better understanding of the underlying causes of observed disparities could be important for informing specific policy. We found that while increasing HPV vaccination to the target of 80% US-wide would reduce total number of incidence cervical cancers in the next 50 years, it will not improve the timeline for a high-poverty county to reach near-elimination and is unlikely to completely eliminate disparities. This suggests additional consideration of social determinants and other prevention strategies are important for understanding and reducing cancer disparities by area poverty in the near and long-term.
We did not construct our model to evaluate cervical cancer screening in detail and thus did not incorporate fine-grained detail on screening or surveillance patterns. Future work should examine cervical cancer screening by county poverty in greater detail to understand whether higher rates of, diagnosis, and treatment could further reduce disparities. This is particularly important given evolving recommendations regarding screening frequency and modality. The shift to HPV testing, including new self-collection methods, may improve access to screening among traditionally underserved populations, including those living in poverty.50–52 However, disruptions in preventive care resulting from the COVD-19 pandemic have produced delays or omissions of both cervical cancer screening and HPV vaccination that are likely to result in thousands of excess cervical cancers nationwide.53–55 While no data are yet available on whether these delays differ by county poverty, it is likely that the disproportionate impact of COVID-19 along socioeconomic gradients may set back progress made by HPV vaccination and cervical cancer screening innovation. To continue meaningful gains in cancer prevention equity, it will be important to understand and address multilevel barriers to HPV vaccination, screening, follow-up, and treatment of pre-cancerous diseases among traditionally underserved groups.56
We note several limitations and strengths of our modeling approach. As the field’s understanding of cervical carcinogenesis continues to grow, granularity and accuracy of simulation models will continue to improve. Using a compartmental model limited our ability to incorporate concurrent infections with multiple genotypes or genotype replacement, potentially important sources of uncertainty in modeling vaccine impact.57,58 We include only heterosexual partnerships, a simplification that reduces heterogeneity in mixing and excludes some populations with high burden of HPV disease.59,60 Further, recent work has proposed a new framework that moves away from histology-based classification (CIN1, CIN2,3) to a more parsimonious model that can be explicitly informed by time since HPV appearance, HPV genotype, and other disease biomarkers.61 As these approaches continue to evolve, we hope future work will be able to adapt them to better understand structural, behavioral, and biological causes of existing cervical cancer disparities.
While the field’s understanding of the natural history in cervical cancer is evolving, it is relatively well-described among HPV cancers. Poverty disparities exist for all HPV cancer sites, yet the drivers of these differences are unclear.1 Future work should explore the implication of current vaccination patterns in other HPV cancer sites, including oropharyngeal cancer which has recently surpassed cervical cancer in annual incidence in the US.62 Finally, we modeled a composite high- and low-poverty county using average data that may obscure heterogeneity between counties and we do not model migration between counties. As vaccination rates are similar in high- and low-poverty settings, migration would likely only influence our primary model conclusions if differential by vaccination status, but better data on migration between high- and low-poverty areas would also be valuable for improving our understanding of current disparities and assessing other strategies to improve equity.
Our study is strengthened by the use of quartile-matched data from multiple large national surveys to inform model inputs, as well as validation of our model estimates to empirical data and comparability to other cervical modeling studies.12,19 Our study takes a nuanced approach to modeling cancer disparities through incorporating novel empirical data on HPV burden and HPV vaccination for both low- and high-poverty US counties and we hope it will help to guide priority setting for national and regional cancer prevention efforts.
In addition to having potential for cancer prevention, HPV vaccine offers an unprecedented opportunity to reduce the large and persistent disparities in HPV cancer between high- and low-poverty counties in the US. Current vaccination rates are projected to reduce, but not eliminate, the higher incidence of HPV cancers in high-poverty areas relative to low-poverty areas and may increase relative disparities. HPV vaccination alone is unlikely to achieve equity in HPV cancer in the near term; therefore, policymakers and advocates should continue broad efforts to increase HPV vaccination alongside more targeted efforts to improve social determinants of health potentially including improving access to preventive, screening, and diagnostic care for communities with disproportionate burden from HPV and HPV cancers.
Supplementary Material
Funding Source
Jennifer Spencer received support for this work from the University of North Carolina’s Cancer Care Quality Training Program (NCI: T32CA11633) and Dana Farber’s Training in Oncology Population Sciences Program (NCI: T32CA092203).
The research in this paper was conducted while the lead author was a Special Sworn Status researcher of the U.S. Census Bureau at the Center for Economic Studies. Research results and conclusions expressed are those of the authors and do not necessarily reflect the views of the Census Bureau. This paper has been screened to ensure that it reveals no confidential data.
Footnotes
Financial Disclosure Dr. Brewer has served as a paid advisory board member for Merck, the Centers for Disease Control and Prevention, and the World Health Organization. Other authors have no financial disclosures or potential conflicts of interest to report.
References
- 1.Boscoe FP, Johnson CJ, Sherman RL, Stinchcomb DG, Lin G, Henry KA. The relationship between area poverty rate and site-specific cancer incidence in the United States. Cancer. 2014;120(14):2191–2198. doi: 10.1002/cncr.28632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh GK, Jemal A. Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950-2014: Over Six Decades of Changing Patterns and Widening Inequalities. J Environ Public Health. 2017;2017. doi: 10.1155/2017/2819372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Simard EP, Dorell C, et al. Annual report to the nation on the status of cancer, 1975-2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105(3):175–201. doi: 10.1093/jnci/djs491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh GK, Miller BA, Hankey BF, Edwards BK. Persistent area socioeconomic disparities in U.S. incidence of cervical cancer, mortality, stage, and survival, 1975-2000. Cancer. 2004;101(5):1051–1057. doi: 10.1002/cncr.20467 [DOI] [PubMed] [Google Scholar]
- 5.Horner M-J, Altekruse SF, Zou Z, Wideroff L, Katki HA, Stinchcomb DG. U.S. geographic distribution of prevaccine era cervical cancer screening, incidence, stage, and mortality. Cancer Epidemiol Biomarkers Prev. 2011;20(4):591–599. doi: 10.1158/1055-9965.EPI-10-1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benard VB, Johnson CJ, Thompson TD, et al. Examining the association between socioeconomic status and potential human papillomavirus-associated cancers. Cancer. 2008;113(S10):2910–2918. doi: 10.1002/cncr.23742 [DOI] [PubMed] [Google Scholar]
- 7.Kahn JA, Lan D, Kahn RS. Sociodemographic Factors Associated With High-Risk Human Papillomavirus Infection. Obstet Gynecol. 2007;110(1):87–95. doi: 10.1097/01.AOG.0000266984.23445.9c [DOI] [PubMed] [Google Scholar]
- 8.Dunne EF, Park IU. HPV and HPV-Associated Diseases. Infect Dis Clin North Am. 2013;27(4):765–778. doi: 10.1016/j.idc.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 9.Garland S, Giuliano A, Brotherton J, et al. IPVS statement moving towards elimination of cervical cancer as a public health problem. Papillomavirus Res. 2018;5:87–88. doi: 10.1016/j.pvr.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Association of Cancer Research. AACR Foundation Eradicating Cervical Cancer. https://www.aacrfoundation.org/Pages/cervical-cancer-awareness-month.aspx. Published 2018. Accessed May 9, 2018.
- 11.Aranda S, Berkley S, Cowal S, et al. Ending cervical cancer: A call to action. Int J Gynecol Obstet. 2017;138:4–6. doi: 10.1002/ijgo.12182 [DOI] [PubMed] [Google Scholar]
- 12.Burger EA, Smith MA, Killen J, et al. Projected time to elimination of cervical cancer in the USA: a comparative modelling study. Lancet Public Heal. 2020;5(4):e213–e222. doi: 10.1016/S2468-2667(20)30006-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGhee E, Harper H, Ume A, et al. Elimination of Cancer Health Disparities through the Acceleration of HPV Vaccines and Vaccinations: A Simplified Version of the President’s Cancer Panel Report on HPV Vaccinations. J Vaccines Vaccin. 2017;08(03). doi: 10.4172/2157-7560.1000361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pruitt SL, Schootman M. Geographic Disparity, Area Poverty, and Human Papillomavirus Vaccination. Am J Prev Med. 2010;38(5):525–533. doi: 10.1016/j.amepre.2010.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moss JL, Reiter PL, Brewer NT. Correlates of human papillomavirus vaccine coverage: A state-level analysis. Sex Transm Dis. 2015;42(2):71–75. doi: 10.1097/OLQ.0000000000000225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henry KA, Swiecki-Sikora AL, Stroup AM, Warner EL, Kepka D. Area-based socioeconomic factors and Human Papillomavirus (HPV) vaccination among teen boys in the United States. BMC Public Health. 2018;18(1):19. doi: 10.1186/s12889-017-4567-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Do EK, Rossi B, Miller CA, et al. Area-Level Variation and Human Papillomavirus Vaccination among Adolescents and Young Adults in the United States: A Systematic Review. Cancer Epidemiol Biomarkers Prev. 2021;30(1):13–21. doi: 10.1158/1055-9965.epi-20-0617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med. 2008;359(8):821–832. doi: 10.1056/NEJMsa0707052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brisson M, Bénard É, Drolet M, et al. Population-level impact, herd immunity, and elimination after human papillomavirus vaccination: a systematic review and meta-analysis of predictions from transmission-dynamic models. Lancet Public Heal. 2016;1(1):e8–e17. doi: 10.1016/S2468-2667(16)30001-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van de Velde N, Boily M-C, Drolet M, et al. Population-level impact of the bivalent, quadrivalent, and nonavalent human papillomavirus vaccines: a model-based analysis. J Natl Cancer Inst. 2012;104(22):1712–1723. doi: 10.1093/jnci/djs395 [DOI] [PubMed] [Google Scholar]
- 21.Spencer JC, Brewer NT, Trogdon JG, Weinberger M, Coyne-Beasley T, Wheeler SB. Cost-effectiveness of Interventions to Increase HPV Vaccine Uptake. Pediatrics November2020:e20200395. doi: 10.1542/peds.2020-0395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.SocialExplorer. 2019. http://www.socialexplorer.com/pub/reportdata/HtmlResults.aspx?reportid=R12026676.Accessed October 16, 2018.
- 23.CDC WONDER Online. Compressed. http://wonder.cdc.gov/cmf-icd10.html.
- 24.Watson M, Benard V, King J, Crawford A, Saraiya M. National assessment of HPV and Pap tests: Changes in cervical cancer screening, National Health Interview Survey. Prev Med (Baltim). 2017;100:243–247. doi: 10.1016/j.ypmed.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacLaughlin KL, Jacobson RM, Radecki Breitkopf C, et al. Trends over time in pap and pap-HPV cotesting for cervical cancer screening. J Women’s Heal. 2019;28(2):244–249. doi: 10.1089/jwh.2018.7380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garrido CO, Coşkun RA, Lent AB, Calhoun E, Harris RB. Use of cervical cancer preventive services among US women aged 21–29: an assessment of the 2010 Affordable Care Act rollout through 2018. Cancer Causes Control. 2020;31(9):839–850. doi: 10.1007/s10552-020-01325-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schiffman M, Herrero R, Hildesheim A, et al. HPV DNA testing in cervical cancer screening: Results from women in a high risk province of Costa Rica. J Am Med Assoc. 2000;283(1):87–93. doi: 10.1001/jama.283.1.87 [DOI] [PubMed] [Google Scholar]
- 28.Burger EA, Ortendahl JD, Sy S, Kristiansen IS, Kim JJ. Cost-effectiveness of cervical cancer screening with primary human papillomavirus testing in Norway. Br J Cancer. 2012;106(9):1571–1578. doi: 10.1038/bjc.2012.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamineni A, Tiro JA, Beaber EF, et al. Cervical cancer screening research in the PROSPR I consortium: Rationale, methods and baseline findings from a US cohort. Int J Cancer. 2019;144(6):1460–1473. doi: 10.1002/ijc.31940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eggleston KS, Coker AL, Das IP, Cordray ST, Luchok KJ. Understanding Barriers for Adherence to Follow-Up Care for Abnormal Pap Tests. J WOMEN’S Heal. 2007;16(3). doi: 10.1089/jwh.2006.0161 [DOI] [PubMed] [Google Scholar]
- 31.Peterson NB, Han J, Freund KM. Inadequate follow-up for abnormal pap smears in an urban population. J Natl Med Assoc. 2003;95(9):825–832. /pmc/articles/PMC2594474/?report=abstract.Accessed June 27, 2020. [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JJ, Campos NG, Sy S, et al. Inefficiencies and high-value improvements in U.S. cervical cancer screening practice: A cost-effectiveness analysis. Ann Intern Med. 2015. doi: 10.7326/M15-0420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.NHANES - National Health and Nutrition Examination Survey. National Center for Health Statistics. https://www.cdc.gov/nchs/nhanes/index.htm. Published 2006. Accessed February 13, 2019.
- 34.Carnell R lhs: Latin Hypercube Samples. [Google Scholar]
- 35.Stein M Large sample properties of simulations using latin hypercube sampling. Technometrics. 1987;29(2):143–151. doi: 10.1080/00401706.1987.10488205 [DOI] [Google Scholar]
- 36.Surveillance, Epidemiology, and End Results Program. National Cancer Institute. https://seer.cancer.gov/explorer/application.php?site=47&data_type=1&graph_type=2&compareBy=race&chk_sex_1=1&chk_race_5=5&chk_race_4=4&chk_race_3=3&chk_race_6=6&chk_race_2=2&chk_age_range_1=1&chk_data_type_1=1&advopt_precision=1&advopt_display=1&showDataF. Published 2010. Accessed February 13, 2019. [Google Scholar]
- 37.Eddy DM, Hollingworth W, Caro JJ, Tsevat J, Mcdonald KM, Wong JB. Model Transparency and Validation: A Report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7 Background to the Task Force. 2011. doi: 10.1016/j.jval.2012.04.012 [DOI] [Google Scholar]
- 38.Rubin MS, Clouston S, Link BG. A fundamental cause approach to the study of disparities in lung cancer and pancreatic cancer mortality in the United States. Soc Sci Med. 2014;100:54–61. doi: 10.1016/j.socscimed.2013.10.026 [DOI] [PubMed] [Google Scholar]
- 39.Staras SAS, Vadaparampil ST, Livingston MD, Thompson LA, Sanders AH, Shenkman EA. Increasing Human Papillomavirus Vaccine Initiation Among Publicly Insured Florida Adolescents. J Adolesc Heal. 2015;56(5):S40–S46. doi: 10.1016/j.jadohealth.2014.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spencer JC, Calo WA, Brewer NT. Disparities and reverse disparities in HPV vaccination: A systematic review and meta-analysis. Prev Med (Baltim). 2019;123:197–203. doi: 10.1016/j.ypmed.2019.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elam-Evans LD, Yankey D, Singleton JA, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years — United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(33):1109–1116. doi: 10.15585/mmwr.mm6933a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hopenhayn C, Christian A, Christian WJ, et al. Prevalence of human papillomavirus types in invasive cervical cancers from 7 us cancer registries before vaccine introduction. J Low Genit Tract Dis. 2014;18(2):182–189. doi: 10.1097/LGT.0b013e3182a577c7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niccolai LM, Russ C, Julian PJ, et al. Individual and geographic disparities in human papillomavirus types 16/18 in high-grade cervical lesions. Cancer. 2013;119(16):3052–3058. doi: 10.1002/cncr.28038 [DOI] [PubMed] [Google Scholar]
- 44.Burger EA, Lee K, Saraiya M, et al. Racial and ethnic disparities in human papillomavirus-associated cancer burden with first-generation and second-generation human papillomavirus vaccines. Cancer. 2016;122(13):2057–2066. doi: 10.1002/cncr.30007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, Rural-Urban, and Racial Inequalities in US Cancer Mortality: Part I—All Cancers and Lung Cancer and Part II—Colorectal, Prostate, Breast, and Cervical Cancers. J Cancer Epidemiol. 2011;2011:1–27. doi: 10.1155/2011/107497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coughlin SS, King J, Richards TB, Ekwueme DU. Cervical Cancer Screening among Women in Metropolitan Areas of the United States by Individual-Level and Area-Based Measures of Socioeconomic Status, 2000 to 2002. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2154–2159. doi: 10.1158/1055-9965.EPI-05-0914 [DOI] [PubMed] [Google Scholar]
- 47.Dreyer G Clinical implications of the interaction between HPV and HIV infections. Best Pract Res Clin Obstet Gynaecol. 2018;47:95–106. doi: 10.1016/j.bpobgyn.2017.08.011 [DOI] [PubMed] [Google Scholar]
- 48.Eldridge RC, Pawlita M, Wilson L, et al. Smoking and subsequent human papillomavirus infection: a mediation analysis. Ann Epidemiol. 2017;27(11):724–730.e1. doi: 10.1016/j.annepidem.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramachandran B Functional association of oestrogen receptors with HPV infection in cervical carcinogenesis. Endocr Relat Cancer. 2017;24(4):R99–R108. doi: 10.1530/ERC-16-0571 [DOI] [PubMed] [Google Scholar]
- 50.Perkins RB, Guido RL, Saraiya M, et al. Summary of Current Guidelines for Cervical Cancer Screening and Management of Abnormal Test Results: 2016-2020. J Women’s Heal. 2021;30(1):5–13. doi: 10.1089/jwh.2020.8918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campos NG, Scarinci IC, Tucker L, et al. Cost-effectiveness of offering cervical cancer screening with HPV self-sampling among African-American women in the Mississippi Delta. Cancer Epidemiol Biomarkers Prev. March2021:cebp.1673.2020. doi: 10.1158/1055-9965.epi-20-1673 [DOI] [PubMed] [Google Scholar]
- 52.Biddell CB, Spees LP, Smith JS, et al. Perceived Financial Barriers to Cervical Cancer Screening and Associated Cost Burden Among Low-Income, Under-Screened Women. J Women’s Heal. April2021:jwh.2020.8807. doi: 10.1089/jwh.2020.8807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burger EA, Jansen EEL, Killen J, et al. Impact of COVID-19-related care disruptions on cervical cancer screening in the United States. J Med Screen. 2021. doi: 10.1177/09691413211001097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castanon A, Rebolj M, Burger EA, et al. Cervical screening during the COVID-19 pandemic: optimising recovery strategies. Lancet Public Heal. April2021. doi: 10.1016/S2468-2667(21)00078-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daniels V, Saxena K, Roberts C, et al. Impact of reduced human papillomavirus vaccination coverage rates due to COVID-19 in the United States: A model based analysis. Vaccine. 2021;39(20). doi: 10.1016/j.vaccine.2021.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fuzzell LN, Perkins R, Christy S, Lake PW, Vadaparampil ST. Hard to reach populations in cervical cancer screening in high income countries. Prev Med (Baltim). 2021;144:106400. doi: 10.1016/j.ypmed.2020.106400 [DOI] [PubMed] [Google Scholar]
- 57.Choi YH, Chapman R, Gay N, Jit M. Potential overestimation of HPV vaccine impact due to unmasking of non-vaccine types: Quantification using a multi-type mathematical model. Vaccine. 2012;30(23):3383–3388. doi: 10.1016/j.vaccine.2012.03.065 [DOI] [PubMed] [Google Scholar]
- 58.Pons-Salort M, Letort V, Favre M, et al. Exploring individual HPV coinfections is essential to predict HPV-vaccination impact on genotype distribution: A model-based approach. Vaccine. 2013;31(8):1238–1245. doi: 10.1016/j.vaccine.2012.11.098 [DOI] [PubMed] [Google Scholar]
- 59.Marra E, Lin C, Clifford GM. Type-Specific Anal Human Papillomavirus Prevalence Among Men, According to Sexual Preference and HIV Status: A Systematic Literature Review and Meta-Analysis. J Infect Dis. 2019;219(4):590–598. doi: 10.1093/infdis/jiy556 [DOI] [PubMed] [Google Scholar]
- 60.Nyitray AG, Carvalho Da Silva RJ, Baggio ML, et al. The prevalence of genital HPV and factors associated with oncogenic HPV among men having sex with men and men having sex with women and men: The HIM study. Sex Transm Dis. 2011;38(10):932–940. doi: 10.1097/OLQ.0b013e31822154f9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Campos NG, Demarco M, Bruni L, et al. A proposed new generation of evidence-based microsimulation models to inform global control of cervical cancer. Prev Med (Baltim). 2021;144:106438. doi: 10.1016/j.ypmed.2021.106438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cancers Associated with Human Papillomavirus, United States—2013–2017 | CDC. https://www.cdc.gov/cancer/uscs/about/data-briefs/no18-hpv-assoc-cancers-UnitedStates-2013-2017.htm.Accessed September 27, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


