Abstract
Activating receptor-tyrosine kinase rearranged during transfection (RET) mutations and fusions are potent drivers of oncogenesis. The recent FDA approvals of highly potent and selective RET inhibitors, selpercatinib and pralsetinib, has altered the therapeutic management of RET aberrant tumors. There is ample evidence of the role of RET signaling in certain cancers. RET aberrations as fusions or mutations occur in multiple cancers, however, there is considerable phenotypic diversity. There is emerging data on the lack of responsiveness of immunotherapy in RET-altered cancers. Herein, we review the registrational data from the selective RET-inhibitor trials, and comprehensively explore RET alterations in pan-cancer adult malignancies and their co-alterations. These co-occuring alterations may define the future of RET inhibition from specific selective targeting to customized combination therapies as data are rapidly emerging on both on-target and off-target acquired resistance mechanisms. Fascinatingly, oncogenic RET fusions have been reported to mediate resistance to EGFR inhibition and KRASG12C inhibition.
Introduction
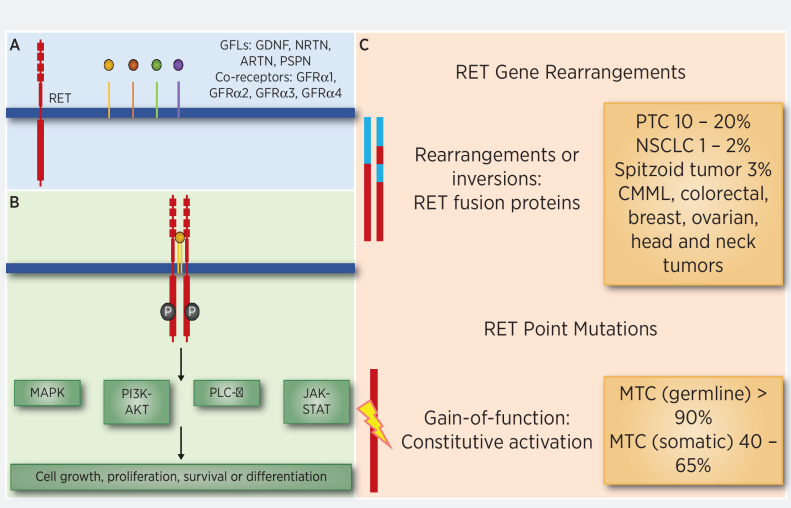
The RET gene is a proto-oncogene encoding for a receptor tyrosine kinase with three domains: extracellular, transmembrane, and intracellular (1). It was initially implicated in the pathogenesis of sporadic mutations found in papillary thyroid cancer (PTC) and germline mutations in multiple endocrine neoplasia syndromes 2A/B (2–4). More evidence suggests the role of RET in the development of other sporadic cancers through the activation of the MAPK, PIK3CA, and phospholipase C-γ pathways (Fig. 1; refs. 5, 6). RET rearrangements cause unregulated expression of the RET/PTC oncoprotein subsequently leading to constitutively activate the RET tyrosine kinase (5, 7).
Figure 1.
RET gene alterations and role on other cancer-causing pathways. RET mutations are seen as germline events in hereditary MEN syndrome and somatic RET mutations are seen in sporadic MTC. RET fusions are seen in NSCLC, papillary thyroid cancer, and many other cancers.
Prior to the advent of selective RET inhibitors, treatment of RET-altered cancers relied on multikinase inhibitors like vandetanib and cabozantinib with secondary RET activity. Given the off-target side effects such as hypertension, rash, and diarrhea arising from inhibition of VEGFR2 and other kinases, patients could not tolerate these drugs for extended durations, which led to dose reductions and drug discontinuations. Selpercatinib (RETEVMO) and pralsetinib (GAVRETO) were designed as highly potent and selective RET inhibitors to avoid the off-target toxicities. Rapid clinical translation and registrational trials have led to FDA approval of these drugs. Selpercatinib is FDA-approved for RET fusion-positive metastatic non–small cell lung cancer (NSCLC), RET-mutant medullary thyroid cancer (MTC), and RET fusion-positive metastatic thyroid cancer (8, 9). In the NSCLC trial, the objective response rate (ORR) for untreated MTC patients was 85% (n = 39) and in previously treated MTC patients 64% (n = 105; ref. 8). In the thyroid-cancer trial, the ORR for untreated patients was 73% (n = 88) and 69% (n = 55) for previously treated patients (9). Similarly, pralsetinib is also FDA-approved for patients with metastatic RET fusion-positive NSCLC and RET-mutant MTC, and RET fusion-positive metastatic thyroid cancer (10, 11). In the pralsetinib NSCLC trial, the ORR for treatment-naïve RET fusion-positive patients was 70% (n = 27) and for previously treated patients was 61% (n = 87; refs. 10, 12). In the MTC cohort ORR was 71% in treatment-naïve and 60% in patients previously treated with cabozantinib or vandetanib or both (12).
Previous efforts have analyzed over 30,000 cell-free DNA (cfDNA) patient samples and reported that activating RET alterations occur in 0.5% of cancer patients (13). Other efforts looking at over 4,000 samples, found that RET aberrations were present in 1.8% of diverse cancers (14). Roughly 43% to 71% (15–18) of sporadic MTCs harboring mutations in RET have been previously targeted with multikinase inhibitors (19). Additionally, 20% of sporadic papillary thyroid carcinomas harbor RET alterations (20, 21). Fusions in RET are found in NSCLC at a rate of roughly 1% to 2% (14). These efforts have led to the development of specific RET inhibitors, which have yielded meaningful clinical benefit for patients with advanced cancers (22–24). Large-scale analysis of nearly 100,000 patient-tumor samples in this study has shown that there may be utility in studying the effects of these and other RET inhibitors in patients with advanced cancers. This analysis also revealed genes that may be found to be co-altered in RET-aberrant cancers, which may lend itself potentially to combination treatment strategy approaches.
RET and the Hallmarks of Cancer
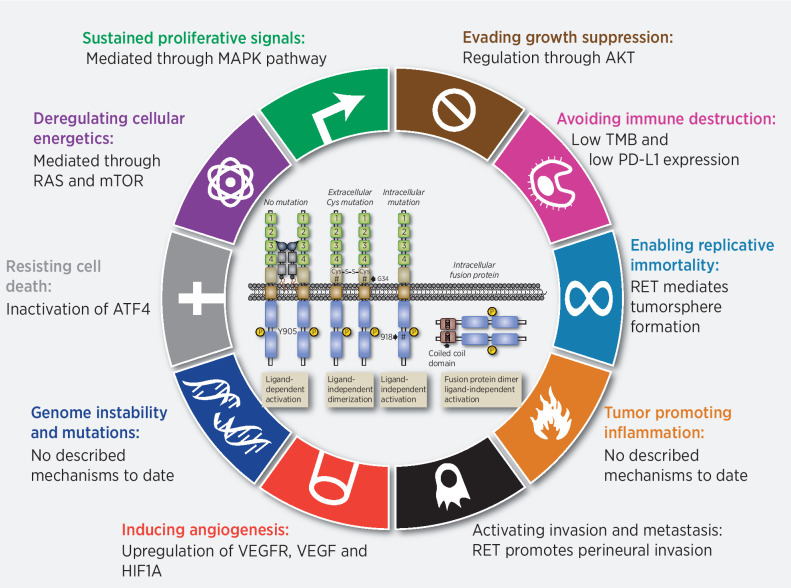
Like most oncogenes, aberrant RET mediates the ‘sustained proliferative signaling’ directed towards enhancing the cell division required for tumor formation (25–27). However, despite this ability aberrant RET signaling is not oncogenic in all cell types. In cell types where oncogenesis is observed there is a growing appreciation that RET's impact actually extends beyond the simple enhancing cellular proliferation, targeting additional “hallmarks” either directly or through targeting of cell-specific intracellular networks (COSMIC. RET and Hallmarks of Cancer, COSMIC project. 2021; Available from:https://cancer.sanger.ac.uk/cosmic/census-page/RET). While a comprehensive examination of potential mechanisms involved in all RET-associated cancers has not been performed, it is important to recognize examples where they exist. The role(s) of aberrant RET activation in the development of MTC have been broadly studied (28). Germline activating mutations of RET are associated with MTC, the primary tumor component of MEN2 (2). A similar role for RET is found in sporadic MTC through somatic mutation (28). There is correlative and in vitro evidence suggesting RET has roles in the 5 additional hallmarks: evading growth suppressors, resisting cell death, enabling replicative immortality (29), induction of angiogenesis (30), and activating invasion and metastasis (Fig. 2; refs. 29, 31–36). Roles for RET in the more recently proposed ‘emerging hallmarks’ and ‘enabling characteristics’ remain to be more directly examined, but at least early reports suggests RET-driven cancers (both MTC and NSCLC) have a low tumor-mutational burden and characterized by lower PD-L1 expression (37, 38). In addition studies have shown modifications of tumor microenvironment associated to RET-familial MTC/multiple endocrine neoplasia 2 and –associated oncoproteins (39) Herein, it is highly possible that RET-driven cancers are biologically ‘cold’ (40). Thus, the impact of RET on oncogenesis goes well beyond simply enhancing cell proliferation. Therefore, inhibition of RET is expected to have a broader impact on those tumor cells that employ aberrant RET activation for functions beyond proliferative signaling.
Figure 2.
Hallmarks of RET alterations in cancer. In cell types where oncogenesis is observed there is a growing appreciation that RET's impact actually extends beyond the simple enhancing cellular proliferation, targeting additional “hallmarks” either directly or through targeting of cell-specific intracellular networks.
Clinical Data
As discussed previously, the current landscape of RET inhibitors includes selpercatinib and pralsetinib, which were granted accelerated approval by the FDA in 2020. Selpercatinib is approved for adult patients with metastatic RET fusion-positive NSCLC. It is also approved for adult and pediatric patients ≥12 years of age with advanced or metastatic RET-mutant MTC who require systemic therapy.
In the first 105 consecutively enrolled patients with RET fusion-positive NSCLC who had previously received at least platinum-based chemotherapy, selpercatinib showed an objective response of 64% (95% CI, 54–73) and a median duration of response duration of response (DoR) of 17.5 months (8). Within the 39 previously-untreated patients, the ORR was 85% (95% CI, 70–94), 90% of the responses ongoing at 6 months. This study also demonstrated the evidence for intracranial activity of selpercatinib with 11 patients with central nervous system (CNS) metastasis showing an objective intracranial response of 91%. LIBRETTO-001 trial results also confirmed its CNS activity. In 22 patients with measurable intracranial disease at baseline, CNS ORR was 82% (95% CI, 60–95), including 23% with complete responses (41, 42).
Similarly, a real-world retrospective analysis of 50 patients from 27 centers across 12 countries with RET fusion-positive NSCLC were treated with selpercatinib (https://journals.sagepub.com/doi/full/10.1177/17588359211019675). In this population, the ORR was 68% (95% CI, 53–81) with a median progression-free survival (PFS) of 15.6 months (95% CI, 8.8–22.4) and a disease-control rate of 92% (26). Of note in the 8 patients with measurable brain metastatic disease, the intracranial ORR was 100% (26). These findings are congruent with the large-scale international reported studies. Among RET-altered thyroid cancers, selpercatinib led to an ORR of 69% (55%–81%) among 55 consecutively enrolled patients who had previously received vandetanib and/or cabozantinib with a 1-year PFS rate of 82% (69–90%; ref. 9). Furthermore, among the 88 treatment-naïve patients with RET-mutant MTC ORR was 73% (95% CI, 62%–82%) and 1-year PFS 92% (95% CI, 82–97). In the subset of RET fusion-positive patients (n = 19), ORR was 79% (95% CI, 54–94%) and 1-year PFS in 64% (95% CI, 37–82; ref. 9). Intracranial activity in RET-mutant thyroid cancer has been demonstrated as well (43).
Hypersensitivity reactions to selpercatinib in patients with RET fusion-positive NSCLC following immune checkpoint inhibition (ICI) is an adverse event of special interest. It is defined as a constellation of events in the initial treatment weeks: maculopapular rash, often preceded by fever, with associated arthralgias or myalgias followed by thrombocytopenia and/or AST/ALT increase (common) and/or blood pressure decrease, tachycardia, and/or creatinine increase (less common). About 11% (17/152) in previously treated patients with ICI and 3% (5/177) in ICI-naïve patients were found to have treatment-related hypersensitivity reactions, with most patients being successfully treated with dose modification and concomitant steroids (44).
Pralsetinib has been granted accelerated approval for adult patients with metastatic RET fusion-positive NSCLC. This was based on the results of a multicenter, open-label, multi-cohort ARROW clinical trial, which showed that among 87 patients with RET fusion-positive NSCLC (previously treated with platinum-chemotherapy), an ORR of 53% (95% CI, 50%–71%), with a complete response (CR) rate of 6% (11). The median time to first response was 1.8 months, with a median DoR not reached, after a median follow-up of 12.9 months (11). The median PFS was 17.1 months (95% CI, 8.3–22.1) with a median overall survival (OS) that was not reached (11). Notably, in all patients with measurable intracranial metastases shrinkage of tumor was seen. Furthermore, among 27 treatment-naïve patients, ORR was found to 70% (50%–86%), with a CR rate of 11%. The median DoR was 9.0 months with a median follow-up of 10.2 months (11). In treatment-naïve patients, the median PFS in was 9.1 months and OS was not reached (11).
An update on the clinical activity of pralsetinib in other RET fusion-positive solid-tumor types other than NSCLC (16 PTC, 1 undifferentiated thyroid, 3 pancreatic, 3 colon, 6 others) showed an ORR of 75% (9/12) with median DoR of 14.5 (range, 3.7–16.8) months, and 67% of responding patients continuing treatment (10).
Lastly, pralsetinib use in RET-mutant MTCs for patients with prior cabozantinib and/or vandetanib, the ORR was 60% (95% CI, 46%–73%) and CR rate of 2% (12). The median time to first response was 3.7 months and the median DoR was not reached, with median follow-up of 11.2 months (12). The median ongoing response was 6 months was 92% (95% CI, 82–100; ref. 12). In treatment-naïve RET-mutant MTCs, the ORR was 71% (95% CI, 48%–89%) and CR rate of 5% (12). The median time to first response was 5.6 months and the median DoR was not reached, with median follow-up of 10.8 months (12). The median ongoing response was 6 months was 93% (95% CI, 81–100) (12). The median PFS and OS in both subsets of patients were not reached (12). The ORR for patients with RET fusion-positive thyroid cancer was 89% (95% CI, 52–100), with median time to first response of 1.9 months (12). The duration of response was not reached with median follow-up of 9.5 months (12). The median PFS and OS were not reached in these patients, as well (12).
Acquired Resistance Mechanisms to RET Inhibition
Despite the exciting efficacy data, long-term RET inhibitor activity can be hampered by acquired resistance as with other tyrosine kinase inhibitors (TKI). Although selective RET inhibitors are effective against most RET mutants and the gatekeeper RET V804M mutations, there is emerging data on nongatekeeper mutations as resistance mechanisms. RET G810 solvent front mutations found on circulating tumor DNA and patient-xenograft model analysis in patients with disease progression on selpercatinib have been described (45). Although these solvent front mutations are significant, they occur at relatively lower frequencies as studied on analysis of posttreatment tissue and/or plasma biopsies of 18 patients with RET fusion-positive who received an RET-selective inhibitor (45). However, more importantly, the majority of the resistance was found to be driven by RET-independent resistance such as acquired MET or KRAS amplification. Thus, it is clear that next-generation RET inhibitors developed must have potency against RET resistance mutations and maintain activity against RET gatekeeper mutations.
Another novel resistance mechanism that has been reported is that in a patient with RET fusion-positive high-grade neuroendocrine carcinoma who was being treated with selpercatinib who initially responded to therapy (46). At 10 months of treatment with selpercatinib and now presumed progression, NGS of a progressing lesion was sent for analysis revealing a novel NTRK3 fusion in addition to the RET fusion (46). Preclinical models showed resistance to NTRK3-expressing cells to selpercatinib (46). This reveals another potential mechanism for resistance to selective RET inhibition, which could hypothetically be overcome by adding an NTRK inhibitor agent such as larotrectinib or entrectinib (46).
Furthermore, given the co-alterations, combination strategies may be used effectively to overcome resistance in these patients. Rosen and colleagues demonstrated that increased MET overexpression in RET fusion-positive tumor cells causes resistance to selpercatinib (47). Subsequently, they showed that this could be overcome partially by combining selpercatinib with crizotinib in patients who developed MET amplification as a resistance mechanism to selpercatinib with responses lasting as long as 10 months (47). These data suggest the potential for combination therapies of targeted agents to overcome resistance pathways.
RET Cancers and Immunotherapy
The role of ICI in RET-altered cancers has also been of interest. In 74 patients with RET-mutated NSCLC, PD-L1 expression was absent or below 50% in over 80% (n = 21/26) tumors (37). In addition, tumor mutation burden (TMB) was also significantly lower (P < 0.0001) in RET-altered tumors than in RET wild-type samples (37). In the patients who did receive ICI, which included pembrolizumab, nivolumab, atezolizumab, durvalumab, or ipilimumab with nivolumab, there were no objective responses (37). These patients also had no association between PD-L1 expression, TMB, and PFS (37).
These findings are further corroborated in a case series of 2 patients with NSCLC (48). One of the patients initially received an ICI and had significant clinical deterioration before starting pralsetinib (48). After initiation of pralsetinib in this patient, the patient had an improvement in all of the metastatic sites and primary NSCLC (48).
Similarly, 70 patients with RET-altered cancers were analyzed for responsiveness to immunotherapy in a single institutional study from a large clinical trials unit (49). This study found that approximately 78% of patients had PD-L1 expression was absent or below 50% and in the 15 patients with TMB analyzed all were TMB-low (49). Among all patients who received ICI had a significantly shorter time to treatment discontinuation (TTD; 18.0 vs. 5.2 months, P = 0.00045; ref. 49). In the 29 patients with NSCLC who received ICI the TTD was shorter, but did not reach statistical significance (9.3 vs. 3.4 months, P = 0.16; ref. 49).
In another study where 16 of 551 patients had RET-altered NSCLC all patients received either pembrolizumab or nivolumab (50). The ORR for the patients with RET-alterations was 6% where 75% of the patients had disease progression (50). This study showed poor survival for patients with RET-altered NSCLC receiving single-agent ICIs with median PFS of 2.1 months and median OS of 21.3 months (50).
An additional study of 59 patients with RET-fusion NSCLC reported the ORR to ICI to be 7.7% in 13 patients where 11 of the 13 patients had no response. The median PFS in patients with RET-fusion NSCLC who received ICI was 2.1 months and the median OS was 12.4 months (51).
Further, in a study of 233 patients with RET fusion-positive NSCLC 64 patients had received ICI (52). This study found that TTD for frontline ICI in these patients was median 5.8 months and as second-line median TTD was 5.1 months (52). This appears to be similar to the previous findings and limited utility of single-agent ICI in patients with RET-altered NSCLC.
These studies share a common theme of lack of response and clinical benefit seen in patients with RET-altered cancers receiving single-agent ICIs. There also appears to be minimal to no concern for patients with RET-altered cancers to experience hyperprogression of disease from ICIs, despite lack of clinical benefit (53, 54). It is unclear if upon progression of disease on a selective RET inhibitor tumor-genomic landscape and proteomic landscape may change in regard to PD-L1 and TMB expression, but with lack of evidence there remains to be no derived benefit from using ICI in patients with RET-altered cancers.
RET Cancer and co-occuring Alterations
Ninety-six thousand, three hundred and twenty-four samples from 89,754 patients available from American Association for Cancer Research (AACR) Project Genie database version 8 (55) were analyzed for the prevalence of RET fusions, mutations, and copy-number alterations in diverse cancer types accessed July 21, 2020. The mutations were further characterized by the tissue of origin, mutations (excluding fusions), and fusions. Fusions in NSCLC were also analyzed to see if there was an association with other co-altered genes, and Benjamini–Hockberg Procedure was applied to eliminate the false discovery rate (56). Analysis of amplification within all tumors was conducted and stratified by copy-number variants.
In the 96,324 tumor samples analyzed, there were 2,706 (2.81%) RET alterations within the cohort. The median age at the time of sequencing was 61 years old, with 52,579 (54.6%) samples from women and 43,635 (45.3%) from men [110 (0.1%) unknown genders]. There were 56,382 (58.5%) samples from primary tumors, 24,204 (25.1%) samples from unspecified metastasis sites, 4,798 (5.0%) from distant-organ metastasis, 1,379 (1.4%) from lymph node metastasis, 1,279 (1.3%) from local recurrence, and 8,282 (8.6%) unknown.
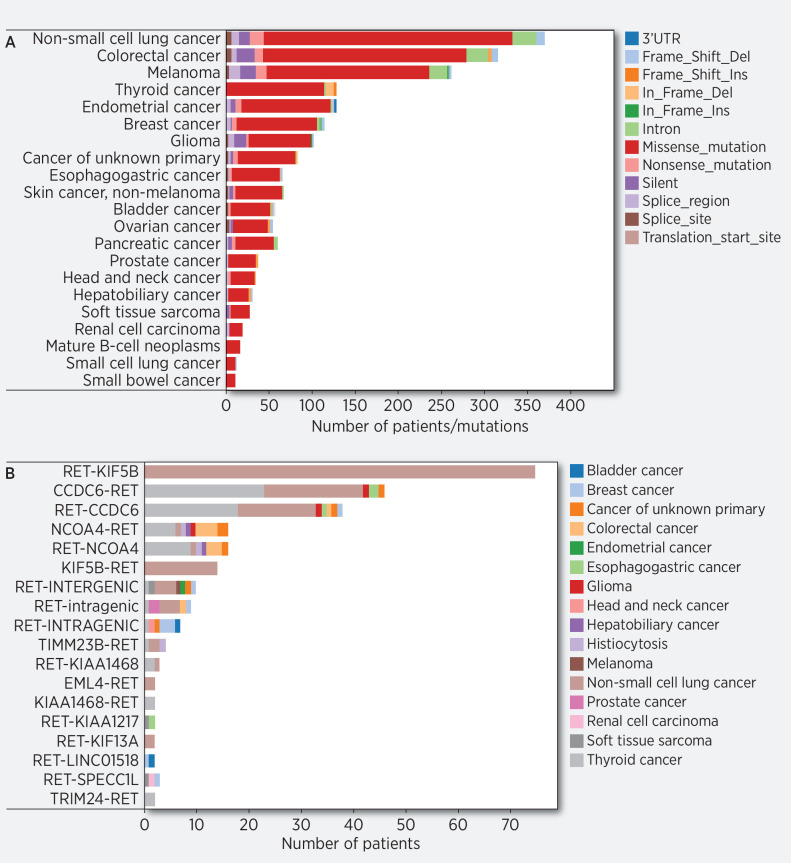
There were 223 (0.23%) fusions, 1,689 RET mutations found (1.75%) in 21 tumor histologies, and 794 (0.82%) RET amplifications identified. These amplifications were most commonly in breast cancer (n = 143), NSCLC (n = 104), and endometrial cancer (n = 84; Supplementary Table S1). The mutations were composed of 1,541 (1.60%) missense mutations, 78 (0.08%) truncating mutations, 70 (0.07%) frame-shift mutations. The RET missense mutations were found most abundantly in NSCLC (n = 355), colorectal cancer (n = 292), melanoma (n = 236), and thyroid cancer (n = 127; Fig. 3A, Supplementary Table S2).
Figure 3.
A, Distribution of RET aberrations (excluding fusions) in AACR Genie database. Distribution of frequency of RET alterations in AACR distributed by tumor histology, correlates with Supplementary Table S2. B, Distribution of RET fusions in AACR Genie database. Distribution of frequency of RET fusions in AACR distributed by type of fusion.
In this cohort, 54.3% of RET fusions were found in patients with NSCLC (n = 121), with papillary thyroid cancer (n = 53) being the next most abundant tumor type with 22.8% of RET fusions (Fig. 3B). Breast cancer (n = 8), colorectal cancer (n = 7), esophagogastric cancer (n = 6), and carcinoma of unknown primary (n = 5) were the other tissues that had RET fusions.
In addition, 121 of the NSCLC tumors with RET fusions had significantly co-altered KRAS (n = 2; adj. P = 1.38E-09), SETD2 (n = 22; adj. P = 4.60E-09), PVRL4 (n = 3; adj. P = 0.000169), EZH1 (adj. P = 0.00772; n = 3), and RRAGC (n = 2; adj. P = 0.00772). Further, there were strong associations with RET fusions and TP53 alterations (n = 45; adj. P = 0.0634), BCL2L12 alterations (n = 1; adj. P = 0.0638), and EGFR alterations (n = 13; adj. P = 0.0893).
RET and Resistance Mechanisms and RET as Resistance Mechanism
Despite the deep and durable responses that patients have had from RET inhibitors various on-target and off-target resistance mechanisms are emerging (57). In patients with RET-fusion NSCLC and RET-mutated MTC, mutations within the RET gene were implicated in resistance to selpercatinib (45). Specifically, RET mutations G810R, G810S, and G810C in the solvent-front domain were found in the patients who progressed on selpercatinib and these findings were validated in a xenograft model (45).
In another cohort of patients who progressed on selpercatinib, MET amplifications were identified as causing resistance (47). Interestingly, in these patients the addition of crizotinib, a MET inhibitor, was sufficient to overcome this resistance and provide for response (47). The combination of RET inhibitors with a MET inhibitor or employing a TKI that targets both alterations may be beneficial in these patients (58–60).
A unique feature of the FDA-approved RET inhibitors, selpercatinib and pralsetinib, is that their mechanism of wrapping around the tyrosine kinase which allows them to evade resistance of gatekeeper mutations. In doing so however, this leaves the drugs susceptible to nongatekeeper mutations as resistance mechanisms. The mutations that were identified included RET V738A, RETY806C/N, and RETG810C/S, which were located within the β-2 strand, solvent front, and hinge regions of the kinase domain (61).
Additional studies have found recurrent mutations in the solvent-front part of the kinase domain with resistance mutations in RET G810 residue and MET amplifications. Interestingly, in the patients with identified MET amplifications, there were no concomitant mutations in RET domain and the MET amplifications were the only implicated cause for resistance further strengthening the notion that MET inhibition could overcome this resistance mechanism (62).
Distinguishing various mechanisms for resistance to RET inhibitors allows for the ability to find and develop drugs to overcome the resistance. Resistance mechanisms for RET inhibitors include both on-target and off-target alterations, some of which can be targeted with other therapeutics.
Interestingly, acquired RET fusions have been implicated as a resistance mechanism to EGFR inhibitor–targeted therapy with osimertinib. Moreover, it was also demonstrated preclinically and clinically that dual inhibition with EGFR and RET with osimertinib and pralsetinib can overcome the resistance mechanism and may be a safe and effective treatment strategy for such patients (63). Fascinatingly, activating RET M918T mutation and oncogenic CCDC6-RET fusion were reported as acquired resistance mechanism to KRAS G12 C inhibition (64). Combination therapy strategies using RET inhibition to overcome these unique resistance mechanisms may be required.
Conclusions
NSCLC and thyroid cancer contribute to the majority of cases with RET fusions and RET mutations where patients have clinically meaningful benefit from selective RET inhibition–directed therapy. Beyond NSCLC and thyroid cancer while RET fusions comprise an infrequent event (0.23%) within multiple malignancies, RET mutations occur in approximately 2% of tumors. It is unclear whether these mutations are clinically significant and actionable. However, the addition of two FDA-approved treatments may provide value to select patients whose tumors harbor some actionable mutations. In patients harboring RET fusions, selective RET inhibition has already shown clinical benefit in case reports and the basket cohorts of the selective RET trials (65, 66).
The presence of significantly co-altered genes within RET fusion-positive NSCLC samples may also provide insight into future directions in overcoming treatment resistance and a combination approach to improve outcomes in this patient population (58, 59, 61). It will also be important to follow the significance of RET amplifications regarding response to RET inhibition for various cancers. Roughly 6% of RET amplifications were found in samples defined as carcinoma of unknown primary (CUP), which have previously been shown to benefit from tumor-agnostic treatment strategies (67). For example, the addition of either selpercatinib or pralsetinib to treatment combination could play a role in improving outcomes for patients with CUP.
The identification of highly targetable genomic events within cancers and the ability for novel agents to inhibit the consequence of the genomic insult has proven to be beneficial in a multitude of tumor histologies. The presence of RET alterations within a diverse cohort of tumors as well as co-altered mutations in RET fusion-positive NSCLC may further inform rational treatment strategies and clinical trial design.
Authors' Disclosures
A.Y. Andreev-Drakhlin reports other support from Genentech outside the submitted work. V. Subbiah reports grants from Eli Lilly/LOXO Oncology, Blueprint Medicines Corporation, Turning Point Therapeutics, Boston Pharmaceuticals; and grants from Helsinn Pharmaceuticals during the conduct of the study; in addition, V. Subbiah reports a grant and advisory board/consultant position with Eli Lilly/Loxo Oncology during the conduct of the study; research grants from Roche/Genentech, Bayer, GlaxoSmithKline, Nanocarrier, Vegenics, Celgene, Northwest Biotherapeutics, Berghealth, Incyte, Fujifilm, D3, Pfizer, Multivir, Amgen, Abbvie, Alfa-sigma, Agensys, Boston Biomedical, Idera Pharma, Inhibrx, Exelixis, Blueprint Medicines, Altum, Dragonfly Therapeutics, Takeda, National Comprehensive Cancer Network, NCI-CTEP, University of Texas MD Anderson Cancer Center, Turning Point Therapeutics, Boston Pharmaceuticals, Novartis, Pharmamar, Medimmune; an advisory board/consultant position with Helsinn, Incyte, QED Pharma, Daiichi-Sankyo, Signant Health, Novartis, Relay therapeutics, Roche, Medimmune; travel funds from Pharmamar, Incyte, ASCO, ESMO; other support from Medscape; all outside the submitted work. No disclosures were reported by the other authors.
Acknowledgments
V. Subbiah is an Andrew Sabin Family Foundation Fellow at The University of Texas MD Anderson Cancer Center. V. Subbiah acknowledges support of The Jacquelyn A. Brady Fund. V. Subbiah is supported by NIH grant R01CA242845. MD Anderson Cancer Center Department of Investigational Cancer Therapeutics is supported by the Cancer Prevention and Research Institute of Texas (RP1100584), the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy (1U01 CA180964), NCATS Grant UL1 TR000371 (Center for Clinical and Translational Sciences), and the MD Anderson Cancer Center Support Grant (P30 CA016672).
Footnotes
Note: Supplementary data for this article are available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
References
- 1. Ishizaka Y, Itoh F, Tahira T, Ikeda I, Sugimura T, Tucker J, et al. Human ret proto-oncogene mapped to chromosome 10q11.2. Oncogene 1989;4:1519–21. [PubMed] [Google Scholar]
- 2. Mulligan LM, Kwok JB, Healey CS, Elsdon MJ, Eng C, Gardner E, et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 1993;363:458–60. [DOI] [PubMed] [Google Scholar]
- 3. Hofstra RM, Landsvater RM, Ceccherini I, Stulp RP, Stelwagen T, Luo Y, et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature 1994;367:375–6. [DOI] [PubMed] [Google Scholar]
- 4. Donis-Keller H, Dou S, Chi D, Carlson KM, Toshima K, Lairmore TC, et al. Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum Mol Genet 1993;2:851–6. [DOI] [PubMed] [Google Scholar]
- 5. Jhiang SM. The RET proto-oncogene in human cancers. Oncogene 2000;19:5590–7. [DOI] [PubMed] [Google Scholar]
- 6. Subbiah V, Roszik J. Towards precision oncology in RET-aberrant cancers. Cell Cycle 2017;16:813–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arighi E, Borrello MG, Sariola H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev 2005;16:441–67. [DOI] [PubMed] [Google Scholar]
- 8. Drilon A, Oxnard GR, Tan DSW, Loong HHF, Johnson M, Gainor J, et al. Efficacy of selpercatinib in RET fusion-positive non-small-cell lung cancer. N Engl J Med 2020;383:813–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med 2020;383:825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Subbiah V, Hu MI-N, Gainor JF, Mansfield AS, Alonso G, Taylor MH, et al. Clinical activity of the RET inhibitor pralsetinib (BLU-667) in patients with RET fusion+ solid tumors. J Clin Oncol 2020;38:109-. [Google Scholar]
- 11. Gainor JF, Curigliano G, Kim D-W, Lee DH, Besse B, Baik CS, et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol 2021;22:959–69. [DOI] [PubMed] [Google Scholar]
- 12. Subbiah V, Hu MI, Wirth LJ, Schuler M, Mansfield AS, Curigliano G, et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol 2021;9:491–501. [DOI] [PubMed] [Google Scholar]
- 13. Rich TA, Reckamp KL, Chae YK, Doebele RC, Iams WT, Oh M, et al. Analysis of cell-free DNA from 32,989 advanced cancers reveals novel co-occurring activating RET alterations and oncogenic signaling pathway aberrations. Clin Cancer Res 2019;25:5832–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kato S, Subbiah V, Marchlik E, Elkin SK, Carter JL, Kurzrock R. RET aberrations in diverse cancers: next-generation sequencing of 4,871 patients. Clin Cancer Res 2017;23:1988–97. [DOI] [PubMed] [Google Scholar]
- 15. Moura MM, Cavaco BM, Pinto AE, Domingues R, Santos JR, Cid MO, et al. Correlation of RET somatic mutations with clinicopathological features in sporadic medullary thyroid carcinomas. Br J Cancer 2009;100:1777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dvorakova S, Vaclavikova E, Sykorova V, Vcelak J, Novak Z, Duskova J, et al. Somatic mutations in the RET proto-oncogene in sporadic medullary thyroid carcinomas. Mol Cell Endocrinol 2008;284:21–7. [DOI] [PubMed] [Google Scholar]
- 17. Elisei R, Cosci B, Romei C, Bottici V, Renzini G, Molinaro E, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab 2008;93:682–7. [DOI] [PubMed] [Google Scholar]
- 18. Heilmann AM, Subbiah V, Wang K, Sun JX, Elvin JA, Chmielecki J, et al. Comprehensive genomic profiling of clinically advanced medullary thyroid carcinoma. Oncology 2016;90:339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kurzrock R, Sherman SI, Ball DW, Forastiere AA, Cohen RB, Mehra R, et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol 2011;29:2660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Santoro M, Melillo RM, Fusco A. RET/PTC activation in papillary thyroid carcinoma: European Journal of Endocrinology Prize Lecture. Eur J Endocrinol 2006;155:645–53. [DOI] [PubMed] [Google Scholar]
- 21. Ciampi R, Nikiforov YE. RET/PTC rearrangements and BRAF mutations in thyroid tumorigenesis. Endocrinology 2007;148:936–41. [DOI] [PubMed] [Google Scholar]
- 22. Subbiah V, Yang D, Velcheti V, Drilon A, Meric-bernstam F. State-of-the-art strategies for targeting RET-dependent cancers. J Clin Oncol 2020;38:1209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Subbiah V, Cote GJ. Advances in targeting RET-dependent cancers. Cancer Discov 2020;10:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Belli C, Anand S, Gainor JF, Penault-Llorca F, Subbiah V, Drilon A, et al. Progresses toward precision medicine in RET-altered solid tumors. Clin Cancer Res 2020;26:6102–11. [DOI] [PubMed] [Google Scholar]
- 25. Mulligan LM. RET revisited: expanding the oncogenic portfolio. Nat Rev Cancer 2014;14:173–86. [DOI] [PubMed] [Google Scholar]
- 26. Illini O, Hochmair MJ, Fabikan H, et al. Selpercatinib in RET fusion-positive non-small-cell lung cancer (SIREN): a retrospective analysis of patients treated through an access program. Therapeutic Advances in Medical Oncology. January2021. doi: 10.1177/17588359211019675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ban K, Feng S, Shao L, Ittmann M. RET signaling in prostate cancer. Clin Cancer Res 2017;23:4885–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hedayati M, Zarif Yeganeh M, Sheikholeslami S, Afsari F. Diversity of mutations in the RET proto-oncogene and its oncogenic mechanism in medullary thyroid cancer. Crit Rev Clin Lab Sci 2016;53:217–27. [DOI] [PubMed] [Google Scholar]
- 29. Lin C, Lu W, Ren Z, Tang Y, Zhang C, Yang R, et al. Elevated RET expression enhances EGFR activation and mediates EGFR inhibitor resistance in head and neck squamous cell carcinoma. Cancer Lett 2016;377:1–10. [DOI] [PubMed] [Google Scholar]
- 30. Verrienti A, Tallini G, Colato C, Boichard A, Checquolo S, Pecce V, et al. RET mutation and increased angiogenesis in medullary thyroid carcinomas. Endocr Relat Cancer 2016;23:665–76. [DOI] [PubMed] [Google Scholar]
- 31. Lian EY, Hyndman BD, Moodley S, Maritan SM, Mulligan LM. RET isoforms contribute differentially to invasive processes in pancreatic ductal adenocarcinoma. Oncogene 2020;39:6493–510. [DOI] [PubMed] [Google Scholar]
- 32. Kim D, Dressler GR. PTEN modulates GDNF/RET mediated chemotaxis and branching morphogenesis in the developing kidney. Dev Biol 2007;307:290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luo Y, Tsuchiya KD, Il Park D, Fausel R, Kanngurn S, Welcsh P, et al. RET is a potential tumor suppressor gene in colorectal cancer. Oncogene 2013;32:2037–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bordeaux MC, Forcet C, Granger L, Corset V, Bidaud C, Billaud M, et al. The RET proto-oncogene induces apoptosis: a novel mechanism for Hirschsprung disease. EMBO J 2000;19:4056–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bagheri-Yarmand R, Sinha KM, Gururaj AE, Ahmed Z, Rizvi YQ, Huang SC, et al. A novel dual kinase function of the RET proto-oncogene negatively regulates activating transcription factor 4-mediated apoptosis. J Biol Chem 2015;290:11749–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Amit M, Na'ara S, Leider-Trejo L, Binenbaum Y, Kulish N, Fridman E, et al. Upregulation of RET induces perineurial invasion of pancreatic adenocarcinoma. Oncogene 2017;36:3232–9. [DOI] [PubMed] [Google Scholar]
- 37. Offin M, Guo R, Wu SL, Sabari J, Land JD, Ni A, et al. Immunophenotype and response to immunotherapy of RET-rearranged lung cancers. JCO Precis Oncol 2019:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nelkin B. Recent advances in the biology and therapy of medullary thyroid carcinoma. F1000Res 2017;6:2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Castellone MD, Melillo RM. RET-mediated modulation of tumor microenvironment and immune response in multiple endocrine neoplasia type 2 (MEN2). Endocr Relat Cancer 2018;25:T105–T19. [DOI] [PubMed] [Google Scholar]
- 40. Addeo A, Passaro A, Malapelle U, Luigi Banna G, Subbiah V, Friedlaender A. Immunotherapy in non-small cell lung cancer harbouring driver mutations. Cancer Treat Rev 2021;96:102179. [DOI] [PubMed] [Google Scholar]
- 41. Subbiah V, Gainor JF, Oxnard GR, Tan DS-W, Owen DH, Cho BC, et al. Intracranial activity of selpercatinib (LOXO-292) in RET fusion-positive non-small cell lung cancer (NSCLC) patients on the LIBRETTO-001 trial. J Clin Oncol 2020;38:9516-. [Google Scholar]
- 42. Subbiah V, Gainor JF, Oxnard GR, Tan DSW, Owen DH, Cho BC, et al. Intracranial efficacy of selpercatinib in RET fusion-positive non-small cell lung cancers on the LIBRETTO-001 trial. Clin Cancer Res 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Andreev-Drakhlin A, Cabanillas M, Amini B, Subbiah V. Systemic and CNS activity of selective RET inhibition with selpercatinib (LOXO-292) in a patient with RET-mutant medullary thyroid cancer with extensive CNS metastases. JCO Precis Oncol 2020;4:PO.20.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McCoach C, Tan DSWBB, et al. 1291P Hypersensitivity reactions (HR) to selpercatinib in RET fusion+ non-small cell lung cancer (NSCLC) patients (pts) following immune checkpoint inhibition (CPI). ESMO; 2020;2020.
- 45. Solomon BJ, Tan L, Lin JJ, Wong SQ, Hollizeck S, Ebata K, et al. RET Solvent front mutations mediate acquired resistance to selective RET inhibition in RET-Driven Malignancies. J Thorac Oncol 2020;15:541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Subbiah V, Shen T, Tetzlaff M, Weissferdt A, Byers LA, Cascone T, et al. Patient-driven discovery and post-clinical validation of NTRK3 fusion as an acquired resistance mechanism to selpercatinib in RET fusion-positive lung cancer. Ann Oncol 2021;32:817–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rosen EY, Johnson ML, Clifford SE, Somwar R, Kherani JF, Son J, et al. Overcoming MET-dependent resistance to selective RET inhibition in patients with RET fusion-positive lung cancer by combining selpercatinib with crizotinib. Clin Cancer Res 2021;27:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Baglivo S, Ludovini V, Moretti R, Bellezza G, Sidoni A, Roila F, et al. RET rearrangement as a predictor of unresponsiveness to immunotherapy in non-small cell lung cancer: report of two cases with review of the literature. Oncol Ther 2020;8:333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hegde A, Andreev-Drakhlin AY, Roszik J, Huang L, Liu S, Hess K, et al. Responsiveness to immune checkpoint inhibitors versus other systemic therapies in RET-aberrant malignancies. ESMO Open 2020;5:e000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 2019;30:1321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lee J, Ku BM, Shim JH, La Choi Y, Sun JM, Lee SH, et al. Characteristics and outcomes of RET-rearranged Korean non-small cell lung cancer patients in real-world practice. Jpn J Clin Oncol 2020;50:594–601. [DOI] [PubMed] [Google Scholar]
- 52. Sireci A, Morosini D, Rothenberg S. P1.01-101 efficacy of immune checkpoint inhibition in RET fusion positive non-small cell lung cancer patients. J Thorac Oncol 2019;14:S401. [Google Scholar]
- 53. Adashek JJ, Kato S, Ferrara R, Lo Russo G, Kurzrock R. Hyperprogression and immune checkpoint inhibitors: hype or progress? Oncologist 2020;25:94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Adashek JJ, Subbiah IM, Matos I, Garralda E, Menta AK, Ganeshan DM, et al. Hyperprogression and immunotherapy: fact, fiction, or alternative fact? Trends Cancer 2020;6:181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Consortium APG. AACR Project GENIE: powering precision medicine through an international consortium. Cancer Discov 2017;7:818–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995;57:289–300. [Google Scholar]
- 57. Adashek JJ, Kato S, Parulkar R, Szeto CW, Sanborn JZ, Vaske CJ, et al. Transcriptomic silencing as a potential mechanism of treatment resistance. JCI Insight 2020;5:e134824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kato S, Okamura R, Adashek JJ, Khalid N, Lee S, Nguyen V, et al. Targeting G1/S phase cell-cycle genomic alterations and accompanying co-alterations with individualized CDK4/6 inhibitor-based regimens. JCI Insight 2021;6:e142547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kato S, Kim KH, Lim HJ, Boichard A, Nikanjam M, Weihe E, et al. Real-world data from a molecular tumor board demonstrates improved outcomes with a precision N-of-One strategy. Nat Commun 2020;11:4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Adashek JJ, Subbiah V, Kurzrock R. From tissue-agnostic to N-of-one therapies: (R)evolution of the precision paradigm. Trends Cancer 2021;7:15–28. [DOI] [PubMed] [Google Scholar]
- 61. Subbiah V, Shen T, Terzyan SS, Liu X, Hu X, Patel KP, et al. Structural basis of acquired resistance to selpercatinib and pralsetinib mediated by non-gatekeeper RET mutations. Ann Oncol 2021;32:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lin JJ, Liu SV, McCoach CE, Zhu VW, Tan AC, Yoda S, et al. Mechanisms of resistance to selective RET tyrosine kinase inhibitors in RET fusion-positive non-small-cell lung cancer. Ann Oncol 2020;31:1725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Piotrowska Z, Isozaki H, Lennerz JK, Gainor JF, Lennes IT, Zhu VW, et al. Landscape of acquired resistance to osimertinib in EGFR-Mutant NSCLC and clinical validation of combined EGFR and RET inhibition with osimertinib and BLU-667 for Acquired RET fusion. Cancer Discov 2018;8:1529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Awad MM, Liu S, Rybkin II, Arbour KC, Dilly J, Zhu VW, et al. Acquired resistance to KRAS(G12C) inhibition in cancer. N Engl J Med 2021;384:2382–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ortiz MV, Gerdemann U, Raju SG, Henry D, Smith S, Rothenberg SM, et al. Activity of the highly specific RET inhibitor selpercatinib (LOXO-292) in pediatric patients with tumors harboring RET gene alterations. JCO Precis Oncol 2020;4:PO.19.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Watanabe S, Takeda M, Otani T, Yoshida T, Sakai K, Olek E, et al. Complete response to selective RET inhibition with selpercatinib (LOXO-292) in a patient with RET fusion–positive breast cancer. JCO Precis Oncol 2021;5:PO.20.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Adashek JJ, Kato S, Gumas S, Lee S, Okamura R, Sicklick J, et al. 86MO Personalized molecularly matched therapies for carcinomas of unknown primary is associated with improved outcomes. Ann Oncol 2020;31:S275–S6. [Google Scholar]