Summary Sentence
We observed racial disparities in delays in the diagnostic process for screen detected breast cancer (BC) malignancies and total delay in diagnosis was associated with an increase in BC mortality.
Introduction
In the U.S, breast cancer (BC) mortality rates have been declining, with an average annual decrease of 1.4% over the last decade1. This decline is largely attributed to national screening programs, resulting in detection of BC at earlier, treatable stages, and the development of targeted therapies2. However, substantial barriers to primary care and screening programs still exist. These barriers to initial diagnosis can lead to delays in treatment and result in poor outcomes.
In addition, while overall mortality rates are decreasing, Black-White mortality disparities 30 years of national efforts to reduce the mortality gap3. Efforts to increase screening among Black women have resulted in similar mammography rates, with 77% of Black and 72% of White women reportedly receiving a mammogram in the last two years4. However, these data may represent only a select subset of women and fail to capture the type, frequency, and completeness of BC screening5–7.
The screening mammogram is only the first step in the BC diagnostic process. Abnormal findings on screening require further diagnostic evaluation and subsequent biopsy if deemed suspicious, each with an interval of time in between. At each stage of this multi-step process, there is potential for the patient to experience a prolongation of an interval (i.e., delay) that would impact their overall time to diagnosis. While there are no national guidelines or recommendations on follow-up time for abnormal screening results, the Breast Cancer Surveillance Consortium reports that approximately 90% of diagnostic imaging and 81% of biopsy or surgical consultation occur within 30 days of recommendation8. Previous studies have investigated the impact of patient characteristics, such as race, socioeconomic status, insurance type, and distance from screening facility, on diagnostic delay9–11. Still, others have estimated the association between screening and tumor characteristics (e.g., stage and subtype)12,13. However, there has been no study to date that has evaluated these collective factors in a cohort of women diagnosed with breast cancer, examining the impact of delay from screening to diagnosis at each interval beyond the initial screen. We sought to not only address this knowledge gap but understand how delays in diagnosis contribute to tumor characteristics and BC mortality among Black and White women diagnosed with BC in a metro-Atlanta hospital system.
Methods
Sample Population
Data for this study were obtained from the Emory University Breast Imaging Centers, which includes four sites in the Emory University Healthcare system that provide BC screening services throughout the metropolitan-Atlanta area. We first identified 806 women with a screening mammography date between 01 January 2010 and 31 December 2014, and who had a confirmed BC diagnosis. Race was self-reported. Of the 806 BC patients identified in the Emory University Healthcare screening database, we were able to validate BC diagnosis by linking to the Georgia Cancer Registry (GCR) for a final retrospective cohort14 of 730 (91%) BC patients. Reasons for not linking to the GCR included: BC diagnosis before initial screening date, diagnosed with BC outside of Georgia, not a first primary BC, or invalid patient identifier.
Diagnostic Delay
Delays leading up to a BC diagnosis were defined based on dates of mammography screening, diagnostic evaluation, and biopsy. We defined diagnostic delay as the number of days between screening mammography and diagnostic evaluation, biopsy delay as the number of days between diagnostic evaluation and biopsy, and total delay as the number of days between initial screening mammogram and biopsy. As no clinical guidelines exist on the recommended timeline from screening to diagnostic evaluation to biopsy for breast cancer, we established cut-points to define delay vs. no delay based on the distribution of the number of days in each of the intervals (screening to diagnostic evaluation, diagnostic evaluation to biopsy and screening to biopsy) along with clinical input by a breast radiologist (RS) and medical oncologist (KG). Diagnostic delay was categorized as ≥30 days vs. <30 days; biopsy delay was categorized as ≥15 days vs. <15 days, and total delay was categorized as ≥45 days vs. <45 days. The median and IQR were calculated for the total sample population for diagnostic evaluation delay, biopsy delay, and total delay (Supplemental Table 1) to confirm utility of the cut-points.
Predictors of Delay
Patient demographic information was collected from the GCR and was considered as possible predictors of delays in diagnosis. We included patient race (Black and White), age at BC diagnosis (<55, 55– <65, 65– <75, 75 or older), a census-derived area-based measure of socioeconomic status [SES] (0%– <10%, 10%– <20%, 20%–100% below poverty), insurance status (uninsured, private, Medicaid, Medicare, Military, unknown), and marital status (single, married, or other). Due to small sample size, we categorized insurance status as private vs. Medicare and marital status as partnered vs. other for the purposes of the analysis. We calculated geographical distance to screening facilities based on both the patient’s address at time of screening and the address of the screening hospital. Distance was derived based on the centroid of the census tract for both the patient and screening facility address using the SAS Macro Distance. The geographical distance to facility was subsequently categorized into quartiles (0 to 5.8 miles; 5.8 to 10.8 miles; 10.8 to 18.95 miles; ≥18.95). The quartiles for distance correspond to approximately 30 additional minutes of drive time, which could correspond to barriers in access to care. There were 6 women who lived >200 miles from the facility where they received screening. We excluded these women in a sensitivity analysis.
Tumor Characteristics
Information on the tumor characteristics at diagnosis were abstracted from the GCR. We collected information on stage (0–IV), tumor grade (I–III), lymph node involvement (none, 1–3, 3+, or unknown/unexamined), tumor size (≤1cm, 1–5cm, ≥5cm), and derived BC subtype (Luminal A, Luminal B, HER2-overexpressing, or triple negative breast cancer [TNBC]) based on estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2) status. We additionally collected information on BC mortality (ICD10-C50), which was determined using ICD-10 codes from death certificate data.
Statistical Analysis
Descriptive statistics were calculated as frequency and proportion for covariates of interest across delay categories.
We used multivariable-adjusted logistic regression models to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) associating the demographic predictors with diagnostic delay, biopsy delay, and total delay. We additionally explored the association between each predictor and delay using quantile regression, as the distribution of each category of delay was right-skewed. We report multivariable-adjusted estimates for the average diagnostic, biopsy and total delay days by each predictor with corresponding 95%CI at the 50th percentile of the distribution of the outcome.
To estimate the associations between delay and tumor characteristics, we used multivariable-adjusted ordinal logistic regression models to compute the ORs and 95%CIs associating diagnostic, biopsy and total delay with stage (I, II, III) and grade (I, II, III). We used logistic regression to compute the ORs and 95%CIs associating delay with lymph node involvement (positive vs negative), tumor size (≥1cm vs <1 cm), ER status (ER+ vs ER−), and BC mortality (BC mortality event vs. alive or other cause of death). Analyses examining the association between delay and tumor characteristics were restricted to those diagnosed with invasive, non-metastatic, BC (n=505).
For all analyses, potential confounders included in the models were based on a literature review and graphical assessment15. Graphical assessment for all models is provided in the online supplementary materials (Supplemental Figure 1). All analyses were carried out in SAS v9.4 (Cary, NC).
Results
We identified 730 women (362 White and 368 Black women) who underwent BC screening prior to a BC diagnosis at an Emory University Breast Imaging Center (Table 1). The median number of days for each time interval was approximately 60% greater for Black women than White women (for total delay: 42 vs. 26 days). Similarly, women residing in lower SES neighborhoods (20%–100% poverty) had longer median number of days of total delay to diagnosis (42 vs 28 days) compared with women residing in higher SES neighborhoods (0%–10% poverty). Uninsured women also experienced the longest median number of days of total delay (44.5 days). Approximately half of uninsured women experienced diagnostic, biopsy and total delays (Supplemental Table 1). In addition, compared with White BC patients, Black women were more likely to experience longer total delay to diagnosis (≥45 days: 28% vs 46%, respectively). With respect to tumor characteristics, women with later stage (stage IV: 90 days), larger tumor size (>5cm: 43 days), and triple-negative tumors (43 days) had longer median number of days of total delay to diagnosis (Table 1).
Table 1.
Demographic and tumor characteristics by diagnostic delay (median diagnostic evaluation, biopsy, and total delay days) among 730 Black and White women who underwent breast cancer screening prior to a breast cancer diagnosis at an Emory University Breast Imaging Center (2010–2014).
| Diagnostic Evaluation Delay | Biopsy Delay | Total Delay | |
|---|---|---|---|
| Demographic Characteristics | Median (IQR) | Median (IQR) | Median (IQR) |
| Total Patient Population | 22 (13, 38) | 7 (3, 15) | 33 (20, 59) |
| Race | |||
| White | 17 (9, 32) | 6 (2, 11) | 26 (15, 49) |
| Black | 27 (18, 42) | 9.5 (5, 19) | 42 (26, 67) |
| Age Category | |||
| <55 | 21 (15, 41) | 7 (3, 13) | 34 (23, 56) |
| 55–64 | 21 (14, 39) | 6 (2, 15) | 30 (19, 56) |
| 65–74 | 24 (13, 40.5) | 7 (3, 15) | 37 (20, 63) |
| >=75 | 21 (12, 37) | 8 (4, 15) | 33 (20, 59) |
| Socioeconomic Status | |||
| 0% – <10% poverty | 20 (13, 31) | 6 (2, 13) | 28 (18, 51) |
| 10% – <20% poverty | 22 (13, 38) | 7 (3, 15) | 34 (19, 59) |
| 20% – 100% poverty | 27 (14, 43) | 10 (4, 18) | 42 (24, 69) |
| Insurance Status | |||
| Uninsured | 28.5 (24, 49) | 13.5 (9, 27) | 44.5 (33, 62) |
| Private | 22 (13, 39) | 7 (2, 14) | 32 (19, 58) |
| Medicaid | 29 (24, 47) | 11 (6, 17) | 39 (29, 84) |
| Medicare | 21 (12, 36) | 8 (3, 16) | 33 (19, 16) |
| Military | 28 (20, 48) | 6 (3, 21) | 43 (26, 54) |
| Unknown | 13 (10, 37.5) | 6.5 (3.5, 12.5) | 21 (14, 50) |
| Geographical Distance to Screening | |||
| Facilities | |||
| Range 1 (0–5.8 miles) | 21 (12, 40) | 7 (2, 16) | 33 (19, 16) |
| Range 2 (5.8–10.8) | 25 (16, 37) | 8 (5, 15) | 37 (25, 60) |
| Range 3(10.8–18.95) | 26 (13, 35.5) | 7 (3, 17) | 35 (20, 57.5) |
| Range 4(18.95–2180.5) | 18 (8, 42) | 6 (3, 13) | 26 (15, 58) |
| Marital Status | |||
| Single | 24 (14, 37) | 9 (4, 21) | 40.5 (21, 63) |
| Married (common law and unmarried domestic) | 21 (13, 36) | 7 (3, 16) | 31 (19.58) |
| Other (divorced, widowed, separated | 25 (13, 42) | 7 (3, 16) | 35.5 (20, 60) |
| Unknown | 15 (9, 28.5) | 6.5 (1, 9) | 23 (17, 38.5) |
| Tumor Characteristics | |||
| Stage | |||
| 0 | 25 (14, 37) | 10 (6, 20) | 37 (22, 61) |
| I | 20 (12, 32.5) | 7 (3, 14) | 29 (17, 49.5) |
| II | 26 (13, 68) | 6 (2, 13) | 39 (21, 109) |
| III | 21 (14, 62) | 6 (1, 10) | 29.5 (20, 87) |
| IV | 52 (19, 203) | 6 (0, 11) | 90 (19, 273) |
| Unknown | 63.5 (47, 179.5) | 9 (2.5, 16.5) | 80 (56, 189.5) |
| Grade | |||
| I | 21 (13, 39) | 7 (3, 17) | 34.5 (19, 56) |
| II | 22 (13, 36) | 7 (3, 15) | 33 (20, 58) |
| III | 22 (13, 42) | 7 (2, 14) | 33.5 (20, 62) |
| Other/unknown | 25 (13, 45) | 6 (2, 18) | 36 (18, 61) |
| Lymph Node Involvement | |||
| 1–3 positive | 25 (13, 52) | 5 (2, 9) | 32 (17, 87) |
| 3+ | 20 (15, 47) | 7 (3, 11) | 28 (22, 58) |
| Negative | 22 (13, 37) | 7 (3, 14) | 32 (20, 58) |
| Unknown/no nodes examined | 22 (12, 37) | 8 (5, 19) | 37 (20, 62) |
| Tumor Size | |||
| ≤1 cm | 22 (13, 34) | 8 (4, 15) | 34 (20, 54) |
| 1–5 cm | 21 (13, 40) | 6 (2,13) | 31 (19, 58) |
| ≥5 cm | 27 (13, 45) | 10.5 (6, 20) | 43 (21, 72) |
| Subtype | |||
| Luminal A | 21 (12, 37) | 6.5 (3, 13) | 31 (18, 53.5) |
| Luminal B | 22.5 (16, 53) | 6.5 (2, 11) | 34.5 (22, 89) |
| HER2-overexpressing | 21 (18, 71) | 7 (2, 14) | 31 (20, 93) |
| TNBC | 28 (17, 54) | 7 (1, 14) | 43 (21, 68) |
| Unknown | 23 (13, 36) | 10 (6, 20) | 36 (22, 61) |
| ER Status | |||
| ER+ | 21 (13, 38) | 6.5 (3, 13) | 31 (18, 56) |
| ER− | 28 (17. 56) | 7 (1, 14) | 42 (21, 69) |
Patient Characteristics
Associations between patient demographic characteristics and diagnostic delay are provided in Table 2. In quantile regression models, we observed that Black women experienced an average 10-day delay in diagnosis compared with White women (β=10.1; 95%CI=7.62, 12.6). Black women were also more likely to experience a biopsy delay (β=3.38; 95%CI=2.10, 4.67), and total delay ≥45 days (β=16.3; 95%CI=11.6, 21.0). Neighborhood-level SES was also associated with delays in diagnosis. Women residing in the lowest SES neighborhoods had a slight increase in delays in biopsy (β=1.63; 95%CI=0.10, 3.17) and total delay of ≥45 days (β=6.79; 95%CI=0.22, 13.4), although the estimates were imprecise. In logistic regression models, we observed that Black women were at least twice as likely to experience diagnostic delays (OR=1.98; total 95%CI=1.45, 2.71), biopsy delays (OR=2.41; 95%CI=1.67, 3.41), and delays ≥45 days (OR=2.22; 95%CI=1.63, 3.02) compared with White women [Supplemental Table 2]. Among women who resided in neighborhoods with low SES, we observed a 1.69 times increase in the odds of diagnostic delay compared with women who resided in neighborhoods with high SES (OR=1.69; 95%CI=1.12, 2.57).
Table 2:
Age and multivariable-adjusted Quantile Regression β coefficient and 95% confidence intervals for diagnostic delay (diagnostic evaluation, biopsy, and total delay) according to patient demographic characteristics among 730 Black and White women who underwent breast cancer screening prior to a breast cancer diagnosis at an Emory University Breast Imaging Center (2010–2014).
| Diagnostic Evaluation Delay | Biopsy Delay | Total Delay | |
|---|---|---|---|
| (30≥ vs <30) | (15≥ vs <15) | (45≥ vs <45) | |
| Demographic Characteristics | β (95% CI) | β (95% CI) | β (95% CI) |
| Race * | |||
| White | Reference | Reference | Reference |
| Black | 10.10 (7.62, 12.60) | 3.38 (2.10, 4.67) | 16.30 (11.6,21.00) |
| Age Category | |||
| <55 | Reference | Reference | Reference |
| 55–65 | 0.00 (−6.09, 6.09) | −1.00 (−3.03, 1.03) | −4.00 (−14.4, 6.39) |
| 66–75 | 3.00 (−2.96, 8.96) | 0.00 (−1.99, 1.99) | 2.00 (−8.17, 12.20) |
| >75 | 0.00 (−5.93, 5.93) | 1.00 (−0.98, 2.98) | −1.00 (−11.1, 9.1) |
| Socioeconomic Status ** | |||
| 0% – <10% poverty | Reference | Reference | Reference |
| 10% – <20% poverty | 1.69 (−1.48, 4.87) | −0.39 (−1.84, 1.05) | 2.89 (−3.27, 9.06) |
| 20% – 100% poverty | 1.64 (−1.74, 5.02) | 1.63 (0.10, 3.17) | 6.79 (0.22, 13.40) |
| Insurance Status *** | |||
| Private(BLUE CROSS, HMO PPO) | Reference | Reference | Reference |
| MEDICARE | −2.26 (−6.40, 1.88) | 1.00 (−0.40, 2.4) | −3.31 (−9.82, 3.60) |
| Geographical Distance to Screening Facilities **** | |||
| Range 1 (0–5.8 miles) | Reference | Reference | Reference |
| Range 2 (5.8–10.8) | 1.00 (−2.43, 4.43) | 0.40 (−1.46, 2.26) | 3.07 (−3.85, 10.00) |
| Range 3(10.8–18.95) | 0.00 (−3.56, 3.56) | 0.10 (−1.83, 2.03) | 0.85 (−6.33, 8.04) |
| Range 4(18.95–2180.5) | −3.00 (−6.50, 0.50) | −0.85 (−2.76, 1.06) | −3.93 (−11.0, 3.16) |
| Marital Status **** | |||
| Married (common law and unmarried domestic) | Reference | Reference | Reference |
| Other (single, divorced, widowed, separated) | 0.30 (−2.36, 2.96) | 0.50 (−0.87, 1.87) | 1.36(−4.09, 6.81) |
Adjustments
age
age, race
age, SES
age, SES, race
Tumor Characteristics
When considering the association between delay and tumor characteristics, we observed that women with a total delay of ≥45 days were more likely to have a later stage tumor compared with women who did not experience a total delay (OR=1.72; 95%CI=1.17, 2.53) (Figure 1). In multivariable-adjusted models, women who experienced a diagnostic delay were less likely to be diagnosed with ER+ disease compared with women who did not experience a diagnostic delay (OR=0.61; 95%CI=0.36, 0.99).
Figure 1.
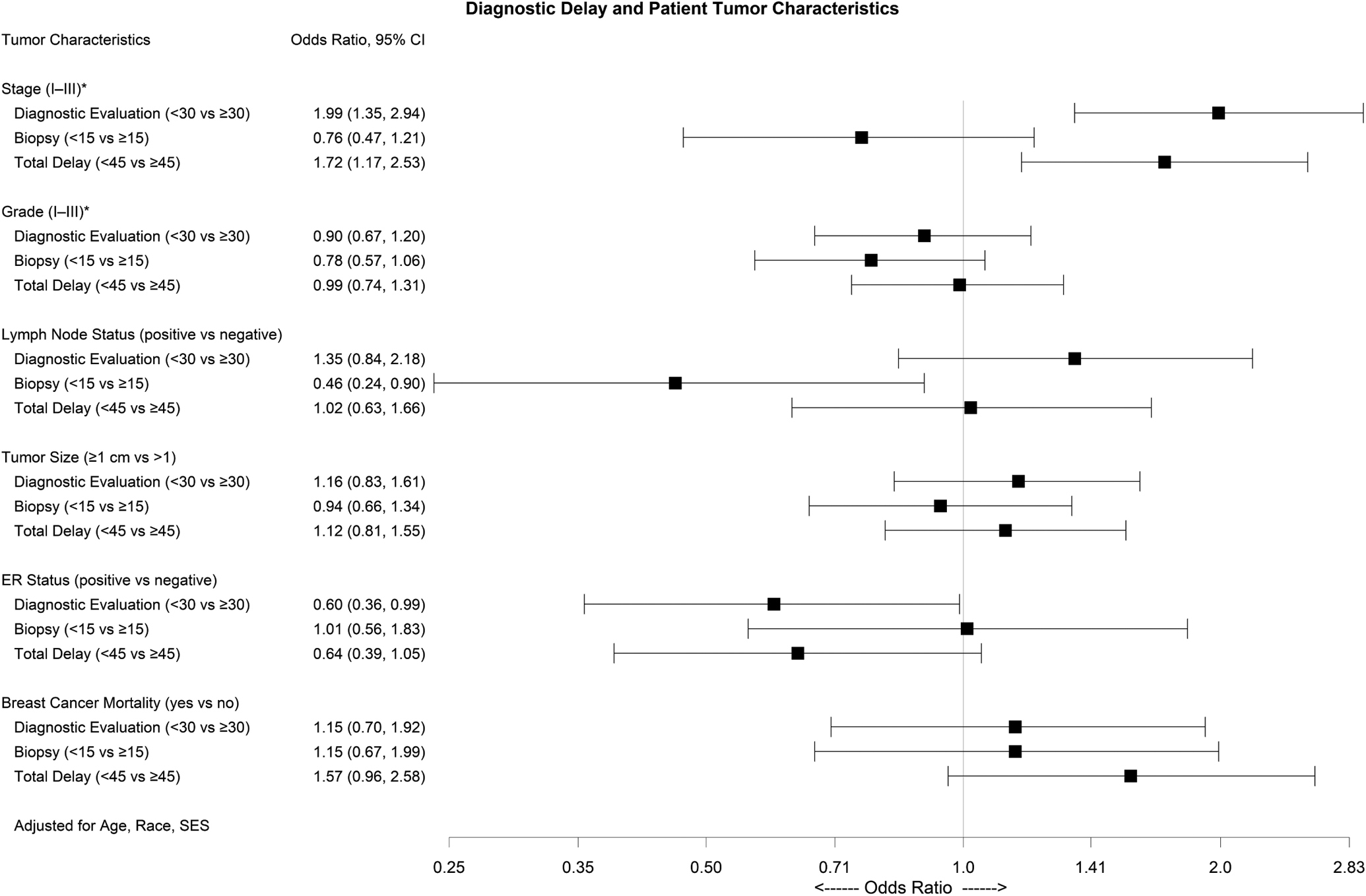
Multivariable-adjusted odds ratios (OR) and 95% confidence intervals (95% CI) for the association between diagnostic delay and patient tumor characteristics among 730 Black and White women with screen-detected breast cancer at an Emory University Breast Imaging Center (2010–2014). *Ordinal logistic regression
Breast Cancer Mortality
Among women who experienced a total diagnostic delay of ≥45 days, we observed 1.57 times increase in the odds of BC mortality compared with women who did not experience a delay (OR=1.57, 95%CI=0.96, 2.58) (Figure 1). We additionally explored how delay contributed to racial disparities in BC mortality. Among women who experienced a total diagnostic delay, Black women had a 1.6 times increased odds of BC mortality compared with White women (OR=1.61, 95%CI=0.77, 3.37). Among women who did not experience a total diagnostic delay the estimate was attenuated (OR=1.22, 95%CI=0.63, 2.34).
Discussion
In our study, we examined delays in diagnosis of screen detected malignancies including delays in diagnostic evaluation and biopsy, ultimately leading to a total delay to diagnosis. We explored the contribution of patient demographic characteristics to delay, and the contribution of delay to tumor characteristics and BC mortality, potentially identifying stages of the diagnostic process that may benefit from intervention. Our results showed the most pronounced associations with delay among Black women and women living in lower SES neighborhoods. For Black women, association with delay was observed in each stage of the diagnostic process. In contrast, associations with delay among women residing in lower SES neighborhoods were driven by delay to diagnostic evaluation. Delay was associated with more advanced stage of BC diagnosis, with the most pronounced association for delay to diagnostic evaluation. Additionally, we observed an association between total delay and BC mortality, underscoring the importance of early detection.
Our results are consistent with studies that have reported pronounced racial disparities in delays between an abnormal screening mammogram to definitive BC diagnosis9,16–19. When compared with White women, Black women had longer times between a provider recognized abnormality to BC diagnosis, with one study reporting delays up to twice that of White women20. Consistent with these studies, we found that Black women were at least twice as likely to experience delays at each stage of the diagnostic process compared with White women. Interestingly, in our study, race was the only patient characteristic associated with delay to biopsy; Black women had a 2.4 times increase in the odds of delay to biopsy compared with White women [OR=2.41; 95%CI=1.67, 3.41].
Our results also suggest that area-level SES is an important factor driving delays in BC diagnostic evaluation. Previous studies have reported that lower income is associated with both incomplete diagnostic work-up and delays from abnormal mammogram to diagnosis21,22. In addition to SES as a contributor to delay in BC diagnosis, type of insurance has also been considered10,23,24. We observed that half of uninsured patients experienced diagnostic and total screening delays, but numbers were too few to estimate associations in multivariable models. A previous study among uninsured women <65 years of age in North Carolina reported that women experienced a longer time to initial diagnostic evaluation after positive screening mammography [HR=0.47; 95%CI=0.25–0.89] compared with women who had private insurance10. Another study found that having private health insurance did not eliminate race-associated delays in BC diagnosis, with insured Black women having more than twice as many days between a suspicious finding during a physical exam, mammography, or ultrasound and diagnosis than insured White women24. These findings may suggest that systemic inequities (i.e., provider biases, discrimination, social and physical environments), rather than race itself, are fundamental drivers of delay, as observed in the present study. Understanding what barriers contribute to Black women receiving delays in diagnostic evaluation requires further study and is necessary for determining what interventions are required to reduce this disparity.
Tumor characteristics are an important consideration in this study, as prognosis for certain aggressive tumors may be more sensitive to delays, and still other tumor characteristics (e.g., stage) are directly related to timeliness of diagnosis. Women diagnosed with late stage BC (III and IV) have worse prognosis compared with women diagnosed with early stage BC4. Black women are more likely to present with late stage tumors at diagnosis25. In our study, we found that there was an increased odds of being diagnosed with a late-stage tumor among women who experienced a total delay >45 days compared with women who did not experience a total delay to diagnosis. Our findings are supported by other studies that have shown an association between diagnostic delay and late stage BC diagnosis20,26,27. Women diagnosed with TNBC were more likely to have a diagnostic delay, compared with women diagnosed with hormone receptor positive disease. In multivariable models we report a 40% lower odds of ER+ disease among women with a diagnostic delay after adjusting for age, race, and area-level SES. Few studies have examined the relationship between delays and tumor markers but, given that ER− tumors (particularly TNBC) are more likely to have an aggressive phenotype and poorer prognosis compared with ER+ tumors, it is imperative to ensure women at highest risk for aggressive tumor types (namely Black and young women) are evaluated in a timely manner.
The deleterious impact of delaying BC diagnosis and subsequent treatment, including neoadjuvant and adjuvant systemic therapy, surgery, and radiation on mortality has been well-documented28–35. After adjusting for age, race, and area-level SES, we found a positive association between total delay and BC-specific mortality. While we were underpowered to perform formal mediation analyses, it is likely that the associations between delay and BC mortality are driven primarily by tumor stage and reinforces that efforts to reduce overall BC mortality also include strategies to reduce delays in diagnosis.
The major strengths of our study include the comprehensive evaluation of patient characteristics, our diverse study population, ability to study multiple delay intervals, the inclusion of important tumor characteristics, and long-term follow-up for mortality. However, our study was restricted to looking at women who were diagnosed with BC within one healthcare system in the metropolitan Atlanta area. The selected healthcare system services the diverse population of metro-Atlanta and, while the proportion of patients varied by race, each center reflected the surrounding neighborhood composition. The advantage of using a single hospital system is that the processes for screening and follow up are consistent across sites, with each site having adequate capacity for care. We acknowledge that women in our study may not represent the larger population of women that are receiving screening mammograms without abnormal results or women that receive abnormal results but are not diagnosed with BC. However, the primary aim of our study was to understand the diagnostic process, both with assessment of patient characteristics related to delay and assessment of delay on tumor characteristics and BC mortality, we believe our population was appropriate for this study. Additionally, we were unable to assess all potential contributors of delay, primarily the role of family history of BC36, BRCA1/2 status, screening mammography usage, or reason for diagnostic evaluation, for example self-detected or system detected18,37. Furthermore, our study population received their screening and diagnosis at private care facilities and the distribution of insurance types was limited and did not allow us to look at the 3-way interaction between race, screening, insurance and BC disparities. As the expansion of Medicaid in recent years has contributed to a decrease in uninsured patients and a reduction in late-stage breast cancer diagnosis38, it is important to include Medicaid coverage in future analyses. Finally, due to our limited sample size, some of our estimates were imprecise and we were unable to fully explore the association between diagnostic delay and racial disparities in BC mortality. Future studies may benefit from a formal mediation analysis to understand the contribution of diagnostic delay on racial disparities in BC mortality. Our results suggest that race is the most pronounced driver of delays in the diagnosis of screen detected breast cancers. As such, necessary steps must be taken to identify the personal and structural barriers that influence timely receipt of care for Black women. Further understanding the contribution of delays in BC diagnosis to racial disparities in BC outcomes is needed to inform strategies to reduce the mortality gap.
Supplementary Material
Take-home points.
Race is the most pronounced driver of delays in the diagnosis of screen detected breast cancers.
Total delay to diagnosis is associated with an increase in breast cancer mortality
Intervention strategies are needed to reduce delays among Black women at all stages of the diagnostic process.
Additional research is required to understand whether these delays contribute to racial disparities in BC mortality.
Acknowledgements:
The authors acknowledge the contribution of Dr. Kevin Ward for his assistance in validating cancer incidence data and patient characteristics. This work was supported in part by CCR19608510, awarded to L McCullough. J Miller-Kleinhenz was supported by NIH/NIGMS (2K12GM000680). L Collin was supported in part by the National Cancer Institute (F31CA239566) and the National Center for Advancing Translational Sciences of the National Institutes of Health (TL1TR002540).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors declare no conflicts of interest.
Statement of data access and integrity: The authors declare that they had full access to all of the data in this study and the authors take complete responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Institute., N. C. Cancer Stat Facts: Female Breast Cancer., <Available at: https://seer.cancer.gov/statfacts/html/breast.html> (
- 2.Berry DA et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 353, 1784–1792, doi: 10.1056/NEJMoa050518 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Miller JW, Plescia M & Ekwueme DU Public health national approach to reducing breast and cervical cancer disparities. Cancer 120 Suppl 16, 2537–2539, doi: 10.1002/cncr.28818 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Society AC (American Cancer Society, Inc., Atlanta, GA, 2017).
- 5.Allgood KL, Rauscher GH, Whitman S, Vasquez-Jones G & Shah AM Validating self-reported mammography use in vulnerable communities: findings and recommendations. Cancer Epidemiol Biomarkers Prev 23, 1649–1658, doi: 10.1158/1055-9965.EPI-13-1253 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Njai R, Siegel PZ, Miller JW & Liao Y Misclassification of survey responses and black-white disparity in mammography use, Behavioral Risk Factor Surveillance System, 1995–2006. Prev ChronicDis 8, A59 (2011). [PMC free article] [PubMed] [Google Scholar]
- 7.Cronin KA et al. Bias associated with self-report of prior screening mammography. Cancer Epidemiol Biomarkers Prev 18, 1699–1705, doi: 10.1158/1055-9965.EPI-09-0020 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg RD et al. Timeliness of follow-up after abnormal screening mammogram: variability of facilities. Radiology 261, 404–413, doi: 10.1148/radiol.11102472 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George P et al. Diagnosis and surgical delays in African American and white women with early-stage breast cancer. J Womens Health (Larchmt) 24, 209–217, doi: 10.1089/jwh.2014.4773 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durham DD et al. Insurance-Based Differences in Time to Diagnostic Follow-up after Positive Screening Mammography. Cancer Epidemiol Biomarkers Prev 25, 1474–1482, doi: 10.1158/1055-9965.EPI-16-0148 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khang L et al. Travel distance to screening facilities and completion of abnormal mammographic follow-up among disadvantaged women. Ann Epidemiol 27, 35–41, doi: 10.1016/j.annepidem.2016.08.013 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Dianatinasab M et al. Socioeconomic Factors, Health Behavior, and Late-Stage Diagnosis of Breast Cancer: Considering the Impact of Delay in Diagnosis. Clin Breast Cancer 18, 239–245, doi: 10.1016/j.clbc.2017.09.005 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Elfgen C et al. The impact of distinct triple-negative breast cancer subtypes on misdiagnosis and diagnostic delay. Breast Cancer Res Treat 177, 67–75, doi: 10.1007/s10549-019-05298-6 (2019). [DOI] [PubMed] [Google Scholar]
- 14.von Elm E et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology 18, 800–804, doi: 10.1097/EDE.0b013e3181577654 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Rothman KJ, G. S, Lash TL. Modern Epidemiology. 3rd ed. (Wolters Kluwer, 2008). [Google Scholar]
- 16.Molina Y, Silva A & Rauscher GH Racial/Ethnic Disparities in Time to a Breast Cancer Diagnosis: The Mediating Effects of Health Care Facility Factors. Med Care 53, 872–878, doi: 10.1097/MLR.0000000000000417 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stuver SO et al. Identifying women at risk of delayed breast cancer diagnosis. Jt Comm J Qual Patient Saf 37, 568–575, doi: 10.1016/s1553-7250(11)37073-0 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Maly RC et al. What influences diagnostic delay in low-income women with breast cancer? J Womens Health (Larchmt) 20, 1017–1023, doi: 10.1089/jwh.2010.2105 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Press R, Carrasquillo O, Sciacca RR & Giardina EG Racial/ethnic disparities in time to follow-up after an abnormal mammogram. J Womens Health (Larchmt) 17, 923–930, doi: 10.1089/jwh.2007.0402 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams DL, Tortu S & Thomson J Factors associated with delays to diagnosis and treatment of breast cancer in women in a Louisiana urban safety net hospital. Women Health 50, 705–718, doi: 10.1080/03630242.2010.530928 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Perez-Stable EJ et al. Factors influencing time to diagnosis after abnormal mammography in diverse women. J Womens Health (Larchmt) 22, 159–166, doi: 10.1089/jwh.2012.3646 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian N, Goovaerts P, Zhan FB, Chow TE & Wilson JG Identifying risk factors for disparities in breast cancer mortality among African-American and Hispanic women. Womens Health Issues 22, e267–276, doi: 10.1016/j.whi.2011.11.007 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang B, Dignan M, Han D & Johnson O Does distance matter? Distance to mammography facilities and stage at diagnosis of breast cancer in Kentucky. J Rural Health 25, 366–371, doi: 10.1111/j.1748-0361.2009.00245.x (2009). [DOI] [PubMed] [Google Scholar]
- 24.Hoffman HJ et al. Having health insurance does not eliminate race/ethnicity-associated delays in breast cancer diagnosis in the District of Columbia. Cancer 117, 3824–3832, doi: 10.1002/cncr.25970 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeSantis CE, Miller KD, Goding Sauer A, Jemal A & Siegel RL Cancer statistics for African Americans, 2019. CA Cancer J Clin 69, 211–233, doi: 10.3322/caac.21555 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Warner ET et al. Time to diagnosis and breast cancer stage by race/ethnicity. Breast Cancer Res Treat 136, 813–821, doi: 10.1007/s10549-012-2304-1 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unger-Saldaña K et al. Health system delay and its effect on clinical stage of breast cancer: Multicenter study. Cancer 121, 2198–2206, doi: 10.1002/cncr.29331 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin DW et al. Delay to curative surgery greater than 12 weeks is associated with increased mortality in patients with colorectal and breast cancer but not lung or thyroid cancer. Ann Surg Oncol 20, 2468–2476, doi: 10.1245/s10434-013-2957-y (2013). [DOI] [PubMed] [Google Scholar]
- 29.Richards MA, Westcombe AM, Love SB, Littlejohns P & Ramirez AJ Influence of delay on survival in patients with breast cancer: a systematic review. Lancet 353, 1119–1126, doi: 10.1016/s0140-6736(99)02143-1 (1999). [DOI] [PubMed] [Google Scholar]
- 30.Murchie P et al. Effect of longer health service provider delays on stage at diagnosis and mortality in symptomatic breast cancer. Breast 24, 248–255, doi: 10.1016/j.breast.2015.02.027 (2015). [DOI] [PubMed] [Google Scholar]
- 31.McLaughlin JM et al. Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J Clin Oncol 30, 4493–4500, doi: 10.1200/jco.2012.39.7695 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green AK et al. Racial disparities in chemotherapy administration for early-stage breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 172, 247–263, doi: 10.1007/s10549-018-4909-5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gagliato Dde M et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol 32, 735–744, doi: 10.1200/jco.2013.49.7693 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flores-Balcázar CH, Flores-Luna L, Villarreal-Garza C, Mota-García A & Bargalló-Rocha E Impact of Delayed Adjuvant Radiotherapy in the Survival of Women with Breast Cancer. Cureus 10, e3071, doi: 10.7759/cureus.3071 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flores-Balcázar CH, Flores-Luna ML, Villarreal-Garza CM & Bargalló-Rocha JE Provider delay in treatment initiation and its influence on survival outcomes in women with operable breast cancer. Rep Pract Oncol Radiother 25, 271–275, doi: 10.1016/j.rpor.2020.02.002 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wernli KJ, Aiello Bowles EJ, Haneuse S, Elmore JG & Buist DS Timing of follow-up after abnormal screening and diagnostic mammograms. Am J Manag Care 17, 162–167 (2011). [PMC free article] [PubMed] [Google Scholar]
- 37.Wujcik D et al. Delay in diagnostic testing after abnormal mammography in low-income women. Oncol Nurs Forum 36, 709–715, doi: 10.1188/09.ONF.709-715 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Blanc JM, Heller DR, Friedrich A, Lannin DR & Park TS Association of Medicaid Expansion Under the Affordable Care Act With Breast Cancer Stage at Diagnosis. JAMA Surg 155, 752–758, doi: 10.1001/jamasurg.2020.1495 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


