Abstract
Background:
Cancer patients are recommended to follow cancer prevention guidelines due to inadequate evidence for specific recommendations for cancer survivors.
Methods:
We examined whether diet and lifestyle scores measuring adherence to the 2018 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) cancer prevention guidelines were associated with colorectal cancer-specific and overall mortality among 1,491 colorectal cancer (CRC) patients in two prospective cohorts. Cox proportional hazards regression models were used to calculate the multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs).
Results:
During a median follow-up of 7.92 years, there were 641 deaths (179 CRC-specific deaths). Patients in the highest quartile of the post-diagnostic WCRF/AICR lifestyle score including diet, body mass index (BMI), and physical activity had a 24% lower risk (HR=0.76, 95% CI: 0.49-1.18) of CRC-specific mortality and a 37% lower risk (HR=0.63, 95% CI: 0.50-0.78) of overall mortality compared with the lowest quartile. When BMI was not included in the lifestyle score due to potential disease-related weight loss, stronger inverse associations were observed for both CRC-specific and overall mortality for the same comparison (CRC-specific: HR=0.50, 95% CI: 0.32-0.79; overall: HR=0.59, 95% CI: 0.47-0.75). The post-diagnostic WCRF/AICR diet score was not statistically significantly associated with either CRC-specific or overall mortality.
Conclusions:
Greater adherence to the WCRF/AICR cancer prevention recommendations was associated with improved survival in CRC patients.
Impact:
This study provides support for CRC patients to follow cancer prevention recommendations after diagnosis. Future studies on cancer survivors will continue to contribute to evidence-based diet and lifestyle recommendations for cancer patients.
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and fourth leading cause of cancer death worldwide (1). By 2030, the global burden of CRC is estimated to increase by 60%, with 2.2 million new cases and 1.1 million deaths (1,2). In response to the need for improved survivorship among the growing number of CRC patients, it is important to identify dietary and lifestyle modifications that can improve prognosis.
Studies on recommendations of dietary and lifestyle changes among cancer patients are limited. Thus, patients are recommended to follow the general advice for cancer prevention (3). The World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) released updated cancer prevention recommendations in 2018 based on their comprehensive evaluation of the current evidence. It is unclear, however, whether following these recommendations after CRC diagnosis will improve the prognosis of CRC patients. Therefore, we evaluated associations between adherence to the current WCRF/AICR cancer prevention recommendations in relation to survival among 1,491 patients diagnosed with stage I-III CRC or CRC with unspecified stage in the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS), two prospective cohorts of U.S. women and men, respectively.
Materials and Methods
Study Population
The NHS was initiated in 1976 when 121,700 female registered nurses aged 30-55 years completed and returned a baseline questionnaire. The HPFS was initiated in 1986 when 51,529 male health professionals aged 40-75 years completed a similar self-administered baseline questionnaire. Biennial follow-up questionnaires have been mailed to participants in each cohort to collect updated information on demographic factors, lifestyle habits, medical history, and new disease diagnoses including CRC (4,5). The follow-up rate of both cohorts exceeds 90%. This study was approved by the Institutional Review Boards at Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required.
Our study population included NHS and HPFS participants who were diagnosed with a first primary incident CRC between 1986 and June 2010 for NHS and January 2010 for HPFS. After receiving permission to review relevant medical records and pathology reports, study physicians confirmed the reported cancer as a first primary incident CRC (International Classification of Diseases-9 codes of 153 and 154). In addition, data on age at diagnosis, year of diagnosis, and tumor stage, grade, and subsite were extracted from the medical records and pathology reports.
Ascertainment of Death
Outcomes of the study were CRC-specific death and overall death. Deaths were identified through review of the National Death Index, postal authorities, or the next-of-kin in response to the follow-up questionnaires. The cause of death was identified by study physicians blinded to exposure data through review of death certificates and medical records.
Assessment of Diet
Validated semi-quantitative food frequency questionnaires (FFQ) were mailed in 1980, 1984, and every four years since 1986 for the NHS and since 1986 for the HPFS. The FFQs contained a standard portion size and 9 possible frequency-of-consumption responses for each food ranging from never to six or more servings per day. Average daily nutrient intake was calculated by multiplying the frequency of intake by the nutrient content for each food and then summing nutrient values across all foods. The mean correlation coefficient between food intakes measured by the FFQ and multiple one-week dietary records was 0.66 in the NHS and 0.63 in the HPFS (6,7).
Assessment of Anthropometry and Physical Activity
Self-reported height and body weight were assessed at baseline; body weight was updated every 2 years thereafter. BMI was calculated as weight in kilograms divided by the square of height in meters (kg/m2). A list of leisure-time physical activities (e.g., walking, jogging, running, bicycling, and swimming) was first asked in the 1986 questionnaire in the NHS and HPFS and was repeated on most subsequent biennial questionnaires (except in 1990 and 2002 for NHS. In both cohorts, participants reported time spent per week (response categories ranged from zero to over 11 hours in the NHS, and zero to over 40 hours in the HPFS) participating in the different exercises and activities. Each activity was assigned a metabolic equivalent task (MET) score. Total MET-hours per week were calculated by summing values from the individual activities. We used time spent watching TV per week as our measure of sedentary behavior. Time spent watching TV per week was first assessed in 1990 in the NHS and updated in 1990, 1992, 2004, 2008, 2012, and 2014; response categories ranged from zero to over 90 hours per week. In the HPFS, TV watching time was first asked in the 1990 questionnaire and updated biennially thereafter; response categories ranged from zero to over 40 hours per week (8). A series of validation studies have demonstrated the validity of these self-reported measures (6,9-13).
Construction of the 2018 WCRF/AICR Diet and Lifestyle Scores
We created diet and lifestyle scores based on the 2018 WCRF/AICR cancer prevention recommendations (14). The diet score included seven components: 1) fruits and vegetables, 2) dietary fiber, 3) whole grains, nuts, and legumes, 4) refined grains and processed foods high in fat and sugar, 5) red and processed meat, 6) sugar-sweetened beverages, and 7) alcohol (Supplementary Table 1) (14). Each component was assigned a score of 0 (non-adherence), 0.5 (partial adherence), or 1 (adherence). For components 5 and 6, each of their respective sub-components was assigned a score of 0, 0.5, or 1; the sub-components were then averaged to construct the component score. The final WCRF/AICR diet score was calculated by averaging the seven diet component scores and ranged from 0 to 1.
The lifestyle scores included three components: 1) the diet score, 2) adiposity, and 3) physical activity (Supplementary Table 1) (14). We constructed the adiposity component using two approaches. The primary approach included only BMI, whereas the alternative approach included BMI, weight gain, and waist circumference as sub-components. The physical activity component included two sub-components related to energy expenditure and sedentary activity. For both the adiposity and physical activity components, sub-components were assigned a score of 0, 0.5, or 1, which were averaged to form their respective component score. Based on a prior study reporting that the energy balance recommendations were the major drivers for a beneficial effect of adhering to the WCRF/AICR recommendations (15), the diet score (instead of each diet component separately), adiposity and physical activity were weighted equally in the construction of the lifestyle scores.
We evaluated two primary lifestyle scores. The first primary lifestyle score (ranging from 0-3), which included diet, adiposity, and physical activity, was used to assess adherence both pre- and post-diagnosis. Scores were constructed where the adiposity component contained either BMI only or BMI, weight gain, and waist circumference. The latter approach was only used to assess pre-diagnosis adherence because weight change could be a consequence of CRC treatment, and limited data on waist circumference after diagnosis were available. The second primary lifestyle score (ranging from 0-2) included only the diet and physical activity components to avoid inclusion of potential disease-related weight loss in the score and was used only for evaluating adherence after CRC diagnosis. Finally, for comparability with the recently published standardized scoring system for the WCRF/AICR cancer prevention recommendations (16), we also evaluated a lifestyle score (ranging from 0-9) that weighted each individual dietary factor, adiposity, and physical activity equally.
Diet and lifestyle scores were created for each questionnaire cycle. To avoid the influence of active cancer treatment, the first FFQ collected at least six months but no more than four years after diagnosis was used to create post-diagnostic scores. Pre-diagnostic scores were calculated using the last questionnaire collected before diagnosis. If the pre-diagnostic questionnaire just prior to diagnosis was missing, the most recently completed questionnaire from at most the two previous assessments was used; otherwise, the participant was not included in the analysis.
Statistical Analysis
Among the participants diagnosed with a first primary incident CRC between 1986-2010 (2,039 in the NHS, 1,441 in the HPFS), we applied the following exclusion criteria: diagnosis of stage IV CRC (325 in the NHS, 210 in the HPFS), missing post-diagnostic lifestyle scores between six-months and four-years of CRC diagnosis (777 in the NHS, 558 in the HPFS), and missing pre-diagnostic lifestyle score (78 in the NHS, 41 in the HPFS). After these exclusions, 1,491 participants (859 in the NHS, 632 in the HPFS) remained in the final analysis.
Cox proportional hazards regression was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) with time since diagnosis as the time scale. Person-time of follow-up was calculated from the return date of the FFQ used for post-diagnostic assessment to death or the end of the study period (January 31, 2014), whichever came first. We used cause-specific Cox proportional hazards analyses for CRC-specific mortality, where deaths from other causes were censored (17). Participants who were lost to follow-up were censored as well. Post-diagnostic scores were categorized into quartiles based on their distributions within each cohort. All models were stratified by age at diagnosis and tumor stage. Because no statistically significant differences were found between women and men (P-interaction=0.65 for CRC mortality and 0.30 for overall mortality), we combined the data from the two cohorts into a single dataset for all analyses and controlled for cohort (equivalent to controlling for sex).
The models used to evaluate the post-diagnostic lifestyle scores were further adjusted for age at diagnosis, tumor grade, tumor subsite, year of diagnosis, regular sigmoidoscopy/colonoscopy screening, post-diagnostic regular use of aspirin, post-diagnostic smoking status, and post-diagnostic energy intake. In models of the post-diagnostic lifestyle score that did not include the adiposity component, post-diagnostic BMI was additionally included as a covariate. In models of the post-diagnostic diet score, we further adjusted for post-diagnostic BMI and post-diagnostic physical activity. Models of post-diagnostic scores were also conducted with additional adjustment of pre-diagnostic scores. In models of pre-diagnostic scores, we included the same covariates as in the analyses of post-diagnostic scores, except that pre-diagnostic measures were obtained from the last questionnaire collected before diagnosis. The continuous analyses were conducted for an increment equivalent to the-interquartile-range of the lifestyle scores. The proportional hazards assumption was assessed by evaluating the interaction term between each score and follow-up time. No statistically significant violations of this assumption were detected (P>0.08 for all). A missing category was created for covariates with missing values. The percentage of missing data for any covariate was less than 3%.
We also evaluated the joint associations of the pre- and post-diagnostic lifestyle scores with CRC and overall mortality. Patients were dichotomized into low and high groups for each score based on the median score within each cohort. In addition, we assessed whether the associations between post-diagnostic lifestyle scores and overall mortality were modified by several demographic, clinical and lifestyle factors. Likelihood ratio tests were performed to calculate the p-values for interaction.
To address concerns that patients in very poor health were not able to follow some of the recommendations, we conducted sensitivity analyses in which we excluded the first six months (22 cases excluded, 1.5%), or the first three years of follow-up (187 cases excluded, 12.5%) after the post-diagnostic assessment. All statistical tests were performed using SAS 9.4 (SAS Institute, Cary, NC) and were two-sided.
Results
A total of 1,491 participants were diagnosed with stage I-III CRC or CRC with unspecified stage and completed both a pre- and post-diagnostic FFQ and lifestyle questionnaires. The median (10th-90th) time from CRC diagnosis to the post-diagnostic assessment was 2.25 (0.92-3.58) years for the NHS and 2.17 (0.75-3.58) years for the HPFS. The median (10th-90th) score for the post-diagnostic lifestyle score with BMI was 1.68 (0.89-2.43) for the NHS and 1.89 (1.18-2.46) for the HPFS. The median score for the post-diagnostic lifestyle score without BMI was approximately 0.6 points lower in both studies. Generally, the distribution of characteristics according to the post-diagnostic lifestyle score with or without BMI (Table 1) and the post-diagnostic diet score (Supplementary Table 2) were similar. The Pearson correlation coefficients were 0.70 in the NHS and 0.66 in the HPFS between the pre- and post-diagnostic lifestyle scores with BMI and 0.53 in the NHS and 0.51 in the HPFS between the pre- and post-diagnostic lifestyle scores without BMI.
Table 1.
Age-standardized characteristics of colorectal cancer patients by quartiles of post-diagnostic WCRF/AICR lifestyle scores.
| Quartile of post-diagnostic WCRF/AICR lifestyle score w/ BMI |
Quartile of post-diagnostic WCRF/AICR lifestyle score w/o BMI |
|||
|---|---|---|---|---|
| Q1 (n=367) |
Q4 (n=376) |
Q1 (n=374) |
Q4 (n=389) |
|
| Post-diagnostic lifestyle score w/ BMIa | 1.03 (0.30) | 2.41 (0.18) | - | - |
| Post-diagnostic lifestyle score w/o BMI | - | - | 0.66 (0.20) | 1.56 (0.12) |
| Male, % | 42.7 | 42.4 | 42.1 | 42.9 |
| Age at colorectal cancer diagnosis, yearsb | 68.5 (8.5) | 69.8 (8.8) | 69.5 (8.8) | 68.5 (8.5) |
| White, % | 98.0 | 97.7 | 97.7 | 98.4 |
| Post-diagnostic body mass index, kg/m2 | 30.6 (4.6) | 23.0 (2.0) | 27.1 (4.9) | 25.3 (4.1) |
| Post-diagnostic physical activity, MET-hours/week | 8.5 (15.8) | 27.9 (24.7) | 6.5 (15.4) | 30.3 (25.2) |
| Family history of colorectal cancer, % | 20.9 | 18.4 | 21.2 | 22.3 |
| Current smokers post diagnosis, % | 6.8 | 2.0 | 9.5 | 2.9 |
| Post-diagnostic diet | ||||
| Total energy intake, kcal/d | 1829 (582) | 1789 (623) | 1828 (601) | 1814 (647) |
| Dietary fiber, g/day | 19.1 (7.4) | 25.0 (11.2) | 18.4 (7.3) | 26.0 (10.5) |
| Fruits and vegetables, servings/day | 4.04 (2.27) | 5.60 (3.00) | 3.75 (2.10) | 6.05 (2.87) |
| Whole grains, servings/day | 1.28 (0.94) | 2.05 (1.44) | 1.20 (0.98) | 2.09 (1.35) |
| Refined grains, servings/day | 3.53 (2.27) | 2.84 (2.00) | 3.79 (2.28) | 2.65 (1.86) |
| Red meat, servings/week | 3.95 (2.76) | 2.51 (2.19) | 3.82 (2.69) | 2.70 (2.15) |
| Processed meat, g/day | 16.06 (21.36) | 7.15 (10.75) | 15.44 (21.02) | 8.50 (12.32) |
| Beverages with added sugars, drinks/day | 0.30 (0.56) | 0.19 (0.38) | 0.34 (0.64) | 0.16 (0.34) |
| Juices, drinks/day | 0.68 (0.75) | 0.84 (0.83) | 0.72 (0.86) | 0.76 (0.74) |
| Non-drinkers of alcohol, % | 38.4 | 38.8 | 38.2 | 42.9 |
| Alcohol consumption in drinkers, g/day | 13.2 (14.1) | 11.4 (13.0) | 14.6 (14.8) | 10.9 (11.6) |
| Post-diagnostic aspirin use (≥2 tablets/week), % | 42.0 | 30.0 | 38.9 | 31.6 |
| Regular screening, % | 37.4 | 38.8 | 35.9 | 41.8 |
| Tumor subsite | ||||
| - Proximal colon, % | 39.4 | 42.4 | 37.6 | 42.1 |
| - Distal colon, % | 32.8 | 27.4 | 31.3 | 28.1 |
| - Rectum, % | 22.1 | 24.5 | 24.4 | 23.9 |
| - Unspecified, % | 5.7 | 5.7 | 6.7 | 5.9 |
| Tumor grade | ||||
| - Grade 1, well differentiated, % | 14.7 | 13.4 | 13.7 | 15.5 |
| - Grade 2, moderately differentiated, % | 55.6 | 58.0 | 59.1 | 56.6 |
| - Grade 3, poorly differentiated, % | 14.2 | 12.9 | 11.9 | 11.7 |
| - Unspecified, % | 15.5 | 15.7 | 15.3 | 16.3 |
| Tumor stage | ||||
| - Stage I, % | 31.8 | 37.2 | 33.0 | 37.2 |
| - Stage II, % | 27.7 | 27.7 | 28.3 | 28.9 |
| - Stage III, % | 26.1 | 22.2 | 26.2 | 21.2 |
| - Unspecified, % | 14.4 | 13.0 | 12.6 | 12.8 |
Values are means (SD) or percentages and are standardized to the age distribution of the study population. Values of polytomous variables may not sum to 100% due to rounding.
Value is not age-adjusted.
Abbreviations: CRC, colorectal cancer; w/, with; w/o, without; Q, quartile; MET, metabolic equivalent.
Post-diagnostic scores and survival
During a median follow-up of 7.92 years, 179 CRC-specific deaths and 641 overall deaths occurred. Both post-diagnostic lifestyle scores were associated with lower CRC-specific and overall mortality, although the association for CRC-specific mortality was not statistically significant for the lifestyle score that included BMI. The multivariable HRs (Model 3) comparing the highest versus lowest quartile of the post-diagnostic lifestyle score without BMI were 0.50 (95% CI: 0.32-0.79) for CRC-specific death and 0.59 (95% CI: 0.47-0.75) for overall death (Table 2). There was little evidence of confounding by tumor characteristics and colorectal cancer risk factors compared with the model accounting for age at diagnosis and stage (Table 2 Model 1). Associations for the post-diagnostic lifestyle score without BMI were stronger and remained statistically significant after additionally adjusting for the pre-diagnostic lifestyle score (Table 2). Similar patterns were observed after excluding patients who died within the first six months or first three years of follow-up (Supplementary Table 3). Results also were similar after excluding the 201 cases with unspecified stage (Supplementary Table 4).
Table 2.
Hazard ratios and 95% CIs for associations between post-diagnostic WCRF/AICR lifestyle scores and mortality among colorectal cancer patients.
| Quartile of post-diagnostic WCRF/AICR lifestyle scores | P- continuous |
per IQR increasea |
||||
|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |||
| Post-diagnostic WCRF/AICR lifestyle score with BMI | ||||||
| Median score (NHS) | 0.93 | 1.50 | 1.89 | 2.36 | ||
| Median score (HPFS) | 1.29 | 1.71 | 2.04 | 2.39 | ||
| Colorectal cancer specific mortality | ||||||
| No. of events | 48 | 46 | 47 | 38 | 179 | |
| Model 1 HR (95% CI)b | Referent | 0.97 (0.64-1.46) | 0.96 (0.64-1.43) | 0.78 (0.51-1.20) | 0.16 | 0.88 (0.73-1.05) |
| Model 2 HR (95% CI)c | Referent | 0.99 (0.66-1.49) | 0.98 (0.65-1.47) | 0.80 (0.52-1.23) | 0.29 | 0.91 (0.75-1.09) |
| Model 3 HR (95% CI)d | Referent | 0.97 (0.65-1.46) | 0.94 (0.63-1.42) | 0.76 (0.49-1.18) | 0.18 | 0.86 (0.70-1.07) |
| Model 4 HR (95% CI)e | Referent | 1.02 (0.67-1.56) | 1.03 (0.65-1.62) | 0.86 (0.51-1.46) | 0.53 | 0.91 (0.69-1.21) |
| Overall mortality | ||||||
| No. of events | 179 | 155 | 167 | 140 | 641 | |
| Model 1 HR (95% CI)b | Referent | 0.75 (0.60-0.93) | 0.79 (0.63-0.97) | 0.61 (0.49-0.76) | 0.05 | 0.91 (0.83-1.00) |
| Model 2 HR (95% CI)c | Referent | 0.75 (0.61-0.94) | 0.79 (0.64-0.98) | 0.62 (0.49-0.77) | 0.01 | 0.88 (0.80-0.97) |
| Model 3 HR (95% CI)d | Referent | 0.77 (0.62-0.95) | 0.78 (0.63-0.97) | 0.63 (0.50-0.78) | <0.001 | 0.80 (0.72-0.90) |
| Model 4 HR (95% CI)e | Referent | 0.82 (0.65-1.03) | 0.87 (0.68-1.11) | 0.74 (0.56-0.98) | 0.08 | 0.88 (0.76-1.02) |
| Post-diagnostic WCRF/AICR lifestyle score without BMI | ||||||
| Median score (NHS) | 0.59 | 0.96 | 1.21 | 1.50 | ||
| Median score (HPFS) | 0.86 | 1.14 | 1.32 | 1.61 | ||
| Colorectal cancer specific mortality | ||||||
| No. of events | 57 | 51 | 40 | 31 | 179 | |
| Model 1 HR (95% CI)b | Referent | 0.92 (0.63-1.34) | 0.69 (0.46-1.04) | 0.51 (0.33-0.78) | 0.002 | 0.73 (0.61-0.89) |
| Model 2 HR (95% CI)c | Referent | 0.98 (0.67-1.44) | 0.75 (0.50-1.13) | 0.54 (0.35-0.84) | 0.01 | 0.77 (0.64-0.94) |
| Model 3 HR (95% CI)d | Referent | 0.97 (0.66-1.43) | 0.72 (0.47-1.09) | 0.50 (0.32-0.79) | 0.003 | 0.72 (0.59-0.89) |
| Model 4 HR (95% CI)e | Referent | 1.01 (0.68-1.50) | 0.76 (0.49-1.18) | 0.54 (0.33-0.88) | 0.02 | 0.76 (0.60-0.96) |
| Overall mortality | ||||||
| No. of events | 194 | 162 | 144 | 141 | 641 | |
| Model 1 HR (95% CI)b | Referent | 0.83 (0.67-1.02) | 0.68 (0.55-0.85) | 0.58 (0.46-0.72) | <0.001 | 0.82 (0.74-0.91) |
| Model 2 HR (95% CI)c | Referent | 0.85 (0.69-1.05) | 0.70 (0.56-0.87) | 0.58 (0.47-0.73) | <0.001 | 0.81 (0.73-0.89) |
| Model 3 HR (95% CI)d | Referent | 0.88 (0.71-1.09) | 0.70 (0.56-0.87) | 0.59 (0.47-0.75) | <0.001 | 0.76 (0.67-0.85) |
| Model 4 HR (95% CI)e | Referent | 0.92 (0.74-1.15) | 0.76 (0.60-0.96) | 0.66 (0.52-0.85) | <0.001 | 0.80 (0.71-0.91) |
IQR (interquartile range) of the post-diagnostic lifestyle score with BMI in NHS=0.89 and in HPFS=0.64. IQR of the post-diagnostic lifestyle score without BMI in NHS=0.54 and in HPFS=0.46.
Model 1: Cox model stratified by age at diagnosis (<65, ≥65 years) and tumor stage (I, II, III, and unspecified), and adjusted for age at diagnosis (continuous).
Model 2: Model 1 + further adjustment for cohort (NHS, HPFS), tumor grade (well differentiated, moderately differentiated, poorly differentiated, and unspecified), tumor subsite (proximal colon, distal colon, rectum, and unspecified), year of diagnosis (continuous), regular sigmoidoscopy/colonoscopy screening (yes, no), and post-diagnostic total energy intake (continuous).
Model 3: Model 2 + further adjustment for post-diagnostic regular use of aspirin (yes, no) and post-diagnostic smoking status (current, non-current) for models of post-diagnostic score with BMI. Post-diagnostic BMI (<23, 23-29.9, ≥30 kg/m2) was additionally adjusted in the models of post-diagnostic lifestyle scores without BMI.
Model 4: Model 3 + further adjustment for pre-diagnostic WCRF/AICR lifestyle score (continuous).
Abbreviations: CRC, colorectal cancer; NHS, Nurses’ Health Study; HPFS: Health Professionals Follow-up Study.
In sensitivity analyses using different weighting schemes to construct the scores, the post-diagnostic lifestyle score that weighted each dietary factor equally with the adiposity and physical activity components showed a statistically nonsignificant association with CRC-specific mortality and a significant but slightly weaker association for overall mortality than the primary lifestyle score with BMI that weighted all dietary factors as one component (Supplementary Table 5).
The post-diagnostic diet score was generally not statistically significantly associated with either CRC-specific or overall mortality (Supplementary Table 6).
Pre-diagnostic scores and survival
The multivariable HRs comparing the highest versus lowest quartile of the pre-diagnostic lifestyle score with BMI were 0.70 (95% CI: 0.46-1.08) for CRC-specific death and 0.66 (95% CI: 0.53-0.83) for overall death (Table 3). The results for the pre-diagnostic lifestyle score also containing weight gain and waist circumference as adiposity sub-components were slightly stronger for both outcomes (Supplementary Table 7) than results for the pre-diagnostic lifestyle score with BMI as the only measure of adiposity, indicating an improvement in the pre-diagnostic lifestyle score when including other measures of adiposity. The results for the pre-diagnostic diet score (Supplementary Table 8) were generally nonsignificant.
Table 3.
Hazard ratios and 95% CIs for the associations between pre-diagnostic WCRF/AICR lifestyle scores and mortality among colorectal cancer patients.
| Quartile of pre-diagnostic WCRF/AICR lifestyle score | P- continuous |
per IQR increasea |
||||
|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |||
| Median score (NHS) | 0.96 | 1.54 | 1.96 | 2.50 | ||
| Median score (HPFS) | 1.21 | 1.71 | 2.04 | 2.46 | ||
| Colorectal cancer specific mortality | ||||||
| No. of events | 53 | 43 | 46 | 37 | 179 | |
| Model 1 HR (95% CI)b | Referent | 0.77 (0.51-1.15) | 0.85 (0.57-1.26) | 0.72 (0.47-1.09) | 0.25 | 0.89 (0.73-1.08) |
| Model 2 HR (95% CI)c | Referent | 0.80 (0.53-1.20) | 0.88 (0.59-1.31) | 0.73 (0.48-1.11) | 0.39 | 0.92 (0.75-1.12) |
| Model 3 HR (95% CI)d | Referent | 0.78 (0.52-1.18) | 0.85 (0.57-1.28) | 0.70 (0.46-1.08) | 0.35 | 0.90 (0.72-1.12) |
| Overall mortality | ||||||
| No. of events | 192 | 156 | 153 | 140 | 641 | |
| Model 1 HR (95% CI)b | Referent | 0.84 (0.68-1.03) | 0.72 (0.58-0.89) | 0.64 (0.51-0.79) | 0.01 | 0.87 (0.79-0.97) |
| Model 2 HR (95% CI)c | Referent | 0.86 (0.69-1.06) | 0.72 (0.58-0.90) | 0.63 (0.51-0.79) | 0.002 | 0.85 (0.77-0.94) |
| Model 3 HR (95% CI)d | Referent | 0.87 (0.70-1.07) | 0.74 (0.59-0.92) | 0.66 (0.53-0.83) | <0.001 | 0.81 (0.72-0.91) |
IQR (interquartile range) of the pre-diagnostic lifestyle score in NHS = 0.93 and in HPFS = 0.70.
Model 1: Cox model stratified by age at diagnosis (<65, ≥65 years) and tumor stage (I, II, III, and unspecified), and adjusted for age at diagnosis (continuous).
Model 2: Model 1 + further adjustment for cohort (NHS, HPFS), tumor grade (well differentiated, moderately differentiated, poorly differentiated, and unspecified), tumor subsite (proximal colon, distal colon, rectum, and unspecified), year of diagnosis (continuous), regular sigmoidoscopy/colonoscopy screening (yes, no), and pre-diagnostic total energy intake (continuous).
Model 3: Model 2 + further adjustment for pre-diagnostic regular use of aspirin (yes or no) and pre-diagnostic smoking (current, non-current).
Abbreviations: CRC, colorectal cancer; NHS, Nurses’ Health Study; HPFS: Health Professionals Follow-up Study.
Joint association of pre- and post-diagnostic lifestyle scores
When participants were classified jointly by pre- and post-diagnostic lifestyle scores with BMI, those with consistently high lifestyle scores had a nonsignificant lower risk of CRC-specific mortality (HR=0.85, 95% CI: 0.60-1.22) and significantly lower risk of overall mortality (HR=0.73, 95% CI: 0.60-0.88) compared with the group having consistently low lifestyle scores (Table 4). However, when using the lifestyle score without BMI for the post-diagnostic classification, patients with consistently high lifestyle scores had significantly lower risk of both CRC-specific mortality and overall mortality compared with those having consistently low lifestyle scores (CRC-specific mortality: HR=0.62, 95% CI: 0.42-0.91; overall mortality: HR=0.59, 95% CI: 0.48-0.73) (Table 4).
Table 4.
Hazard ratiosa and 95% CIs for the joint associations between WCRF/AICR lifestyle scores before and after colorectal cancer diagnosis and mortality.
| Pre-diagnostic WCRF/AICR lifestyle scoreb | P-interaction | ||
|---|---|---|---|
| Low score | High score | ||
| Post-diagnostic WCRF/AICR lifestyle score with BMI c | |||
| Colorectal cancer specific mortality | |||
| No. of events | 76 | 18 | |
| Low score | referent | 0.98 (0.60-1.59) | 0.24 |
| No. of events | 20 | 65 | |
| High score | 1.00 (0.64-1.59) | 0.85 (0.60-1.22) | |
| Overall mortality | |||
| No. of events | 266 | 68 | |
| Low score | referent | 0.79 (0.60-1.02) | 0.07 |
| No. of events | 82 | 225 | |
| High score | 0.94 (0.74-1.20) | 0.73 (0.60-0.88) | |
| Post-diagnostic WCRF/AICR lifestyle score without BMI d | |||
| Colorectal cancer specific mortality | |||
| No. of events | 71 | 37 | |
| Low score | referent | 1.12 (0.75-1.67) | 0.82 |
| No. of events | 25 | 46 | |
| High score | 0.68 (0.43-1.10) | 0.62 (0.42-0.91) | |
| Overall mortality | |||
| No. of events | 240 | 116 | |
| Low score | referent | 0.86 (0.69-1.08) | 0.19 |
| No. of events | 108 | 177 | |
| High score | 0.75 (0.59-0.95) | 0.59 (0.48-0.73) | |
Cox models were stratified by age at diagnosis (<65, ≥65 years) and tumor stage (I, II, III, and unspecified), with additional adjustment for age at diagnosis (continuous), cohort (NHS, HPFS), tumor grade (well differentiated, moderately differentiated, poorly differentiated, and unspecified), tumor subsite (proximal colon, distal colon, rectum, and unspecified), year of diagnosis (continuous), regular sigmoidoscopy/colonoscopy screening (yes, no), post-diagnostic total energy intake (continuous), post-diagnostic regular use of aspirin (yes, no), and post-diagnostic smoking status (current, non-current).
The cutpoint for the pre-diagnostic WCRF/AICR lifestyle score categories was 1.75 in NHS and 1.86 in HPFS.
The cutpoint for the post-diagnostic WCRF/AICR lifestyle score with BMI categories was 1.68 in NHS and 1.89 in HPFS.
The cutpoint for the post-diagnostic WCRF/AICR lifestyle score without BMI categories was 1.11 in NHS and 1.25 in HPFS.
Post-diagnostic lifestyle score and survival within subgroups
We found no statistically significant modification of the associations between post-diagnostic lifestyle scores with or without BMI and overall mortality by several clinical and lifestyle factors (Figures 1 and 2).
Figure 1.
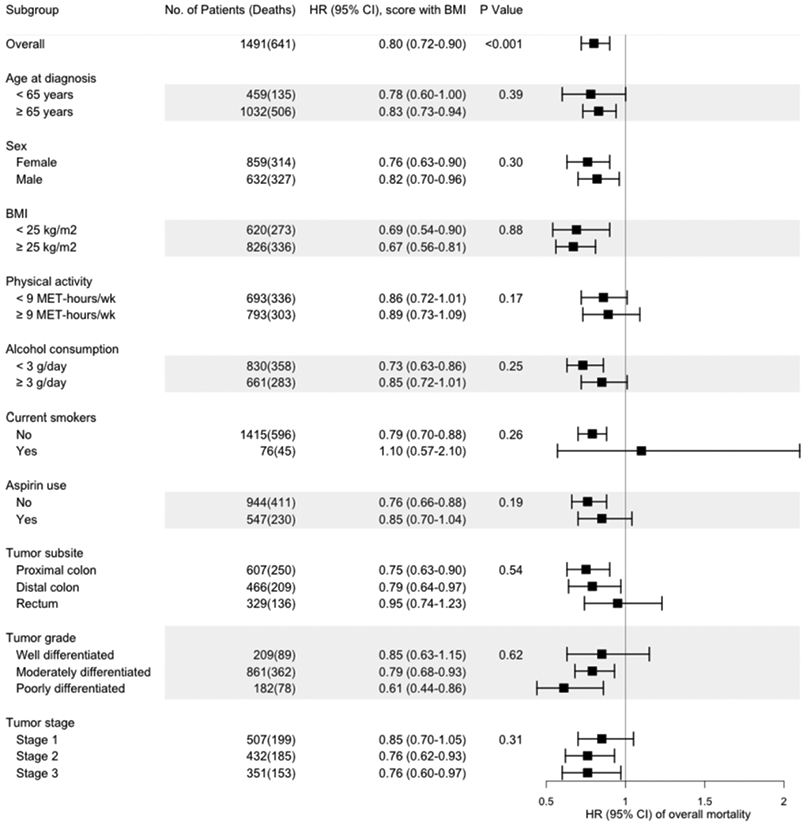
Multivariable hazard ratios (HRs) and 95% confidence intervals (95% CIs) of overall mortality per IQRa increase in the post-diagnostic WCRF/AICR lifestyle score with BMI, stratified by demographic, lifestyle, and other factors and tumor characteristics.
The hazard ratios (HRs) for the post-diagnostic WCRF/AICR lifestyle score with BMI are indicated by solid squares; the 95% CIs are indicated by horizontal lines. Models were stratified by age at diagnosis (<65, ≥65 years) and tumor stage (I, II, III, and unspecified), with adjustment for age at diagnosis (continuous), cohort (NHS, HPFS), tumor grade (well differentiated, moderately differentiated, poorly differentiated, and unspecified), tumor subsite (proximal colon, distal colon, rectum, and unspecified), year of diagnosis (continuous), regular sigmoidoscopy/colonoscopy screening (yes, no), post-diagnostic total energy intake (continuous), post-diagnostic regular use of aspirin (yes, no), and post-diagnostic smoking status (current, non-current), except in models stratified by these variables. P-values for interaction were calculated by the likelihood ratio test.
aIQR (interquartile range) of the post-diagnostic WCRF/AICR lifestyle score with BMI in NHS=0.89 and in HPFS=0.64.
Figure 2.
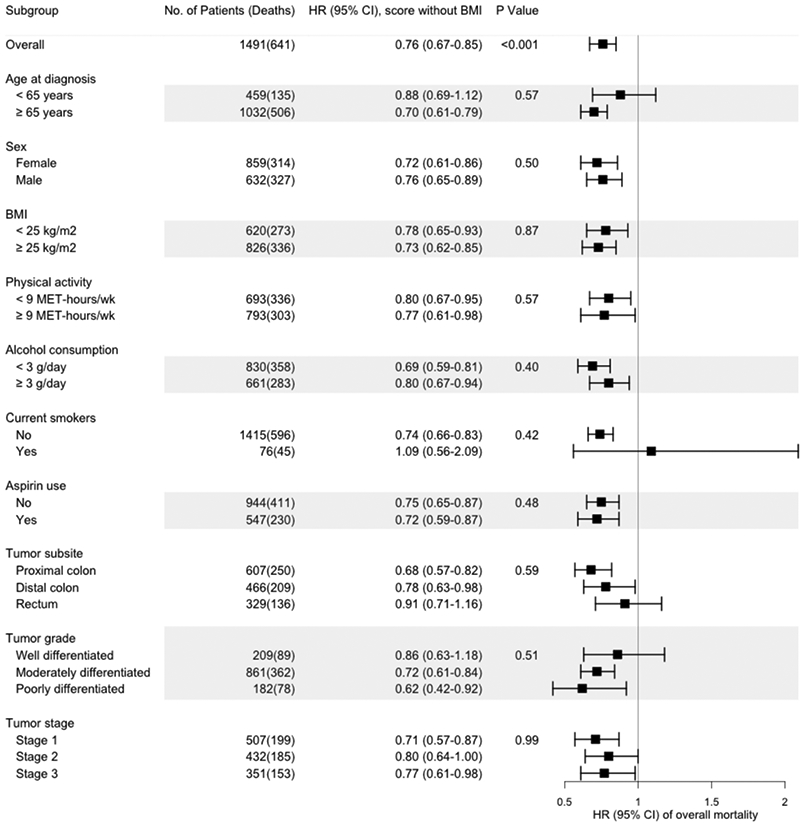
Multivariable hazard ratios (HRs) and 95% confidence intervals (95% CIs) of overall mortality per IQRa increase in the post-diagnostic WCRF/AICR lifestyle score without BMI, stratified by demographic, lifestyle, and other factors and tumor characteristics.
The hazard ratios (HRs) for the post-diagnostic WCRF/AICR lifestyle score without BMI are indicated by solid squares; the 95% CIs are indicated by horizontal lines. Models were stratified by age at diagnosis (<65, ≥65 years) and tumor stage (I, II, III, and unspecified), with adjustments for age at diagnosis (continuous), cohort (NHS, HPFS), tumor grade (well differentiated, moderately differentiated, poorly differentiated, and unspecified), tumor subsite (proximal colon, distal colon, rectum, and unspecified), year of diagnosis (continuous), regular sigmoidoscopy/colonoscopy screening (yes, no), post-diagnostic total energy intake (continuous), post-diagnostic regular use of aspirin (yes, no), post-diagnostic smoking status (current, non-current), and post-diagnostic BMI (<23, 23-29.9, ≥30 kg/m2), except in models stratified by these variables. P-values for interaction were calculated by the likelihood ratio test.
aIQR (interquartile range) of the post-diagnostic WCRF/AICR lifestyle score without BMI in NHS=0.54 and in HPFS=0.46.
Discussion
Our findings from two prospective cohorts suggest that a lifestyle consistent with the 2018 WCRF/AICR cancer prevention recommendations after CRC diagnosis was associated with lower CRC-specific and overall mortality with stronger associations observed for the score without a BMI component. The inverse association between the post-diagnostic lifestyle scores with or without BMI and overall mortality did not differ among several subgroups defined by clinical and lifestyle factors. In addition, when examining the post-diagnostic lifestyle score without BMI jointly with the pre-diagnostic lifestyle score, individuals with low pre- but high post-diagnostic lifestyle scores had significantly lower overall mortality compared with those with low lifestyle scores before and after diagnosis, indicating a potential benefit of making lifestyle improvements adhering to the WCRF/AICR recommendations after CRC diagnosis.
Our findings on the post-diagnostic lifestyle score with BMI are similar to those reported in a recent study which found a nonsignificant 8% reduction in overall mortality per a one standard deviation increment in their post-diagnostic WCRF/AICR score including diet, BMI, and physical activity (18). A challenge in evaluating the WCRF/AICR lifestyle score after a CRC diagnosis is that the component on post-diagnostic body fatness reflects both pre-diagnostic body weight and post-diagnostic weight change which may be due to unintentional weight loss as a result of complications from CRC or treatment (19). In a meta-analysis of 5 studies, modest weight loss after a CRC diagnosis was associated with a 69% higher risk of CRC-specific death and a 78% higher risk of overall mortality compared to stable weight. Larger post-diagnostic weight loss showed stronger positive associations (20). Thus, post-diagnostic body fatness may not be a good predictor for survival in CRC patients. As expected, in our study, we observed a stronger association with the post-diagnostic lifestyle score without BMI compared with the post-diagnostic lifestyle score including BMI, especially for CRC-specific mortality which might be more prone to disease-related weight loss. The stronger association for the post-diagnostic lifestyle score without BMI is likely due to the increased weighting of physical activity in the construct of the score because the post-diagnostic diet score alone was not strongly associated with CRC-specific or overall mortality. Thus, post-diagnostic physical activity may play an important role in survival for CRC patients as documented previously (8,21-23). The different results observed for the post-diagnostic lifestyle score with and without BMI indicate that different components may need to be considered in studies of cancer survival than those of cancer incidence. For cancer survival, the post-diagnostic lifestyle score without BMI appeared to be more relevant than the score with BMI.
Several studies have previously investigated associations between post-diagnostic diet and survival among CRC patients in the NHS and HPFS. In these cohorts, a plant-rich, low-carbohydrate diet after CRC diagnosis was associated with lower CRC-specific mortality, but not overall mortality (24). A borderline significant reduction of CRC-specific mortality and a significant decrease in overall mortality has also been reported for higher post-diagnostic scores for the Alternate Healthy Eating Index-2010 (25). As the findings for the WCRF/AICR diet score were nonsignificant for both CRC and overall mortality in the current analyses, the WCRF/AICR diet score, which was formulated for cancer prevention in general, may not optimally reflect dietary factors specific for CRC survivorship.
A standardized scoring system was published recently for operationalizing the 2018 WCRF/AICR cancer prevention recommendations in epidemiologic studies (16). The most substantial difference between our primary operationalization and the standardized scoring system is how diet was weighted in the lifestyle score: the current study weighted the totality of diet equally to adiposity and physical activity, whereas the standardized scoring system weights each individual dietary factor, adiposity, and physical activity equally. The downweighting of potential disease-related weight loss (as measured by BMI) in the score applying equal weights for all components likely resulted in the stronger association observed for CRC-specific mortality compared to our primary lifestyle score with BMI while the upweighting of the dietary components in the post-diagnostic lifestyle score using equal weights for each component likely resulted in its weaker association with overall mortality.
The strengths of this study included its prospective design, the use of validated, comprehensive FFQs, assessment of both pre-and post-diagnostic diet and lifestyle factors, and long-term follow-up. However, there were some limitations. First, a large number of CRC patients (n=1018) identified in these cohorts did not have a post-diagnostic lifestyle score assessment. Compared to patients included in the study, those who were missing a post-diagnostic lifestyle score assessment were older, were less likely to have received regular screening, were more likely to have stage III and unspecified stage tumors, and had shorter median survival time (Supplementary Table 9). Thus, our results are most applicable to those with good prognosis at the time of diagnosis who survived to complete the cohort questionnaire scheduled after their diagnosis. Second, the study used self-reported data on diet, body fatness, and physical activity, which are subject to measurement error. Third, we could not control for cancer treatment. However, the post-diagnostic assessment was conducted after six months of diagnosis to avoid the influence of active cancer treatment. In addition, we controlled for tumor stage, grade, subsite, and year of diagnosis as a proxy for treatment. Fourth, cancer recurrence data were not available. However, given the relatively short survival time for metastatic CRC, CRC-specific mortality can be considered as a surrogate of cancer recurrence. Another limitation was that we had a relatively small number of CRC-specific deaths. While the sample size did not affect our ability to detect associations with our main outcomes, it may have limited our ability to detect associations within subgroups. Finally, the study population was predominantly white and highly educated, leading to limited generalizability.
In conclusion, this study indicated that better adherence to the 2018 WCRF/AICR cancer prevention recommendations on diet, body fatness, and physical activity after CRC diagnosis was associated with better survival among CRC patients, with the best survival found among patients who adhered to the recommendations both before and after diagnosis. Of the three main components in the score, adherence to the physical activity recommendation may be the primary driver of the reduced risks we observed; conversely, post-diagnostic body fatness may bias associations because of the challenge of distinguishing intentional from disease-related weight loss. We only found weak, nonsignificant associations for the diet score, suggesting that dietary recommendations formulated for cancer prevention may not optimally reflect dietary factors for improving CRC survivorship. More studies evaluating post-diagnostic dietary and lifestyle factors are needed to provide solid support for nutritional recommendations for cancer survivors.
Supplementary Material
Acknowledgements
We would like to thank the participants and staff of the NHS and HPFS for their valuable contributions and the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data. This work was supported by the National Cancer Institute of the National Institutes of Health under award numbers UM1 CA186107 (MPI: Meir Stampfer and A. Heather Eliassen; NHS), U01 CA167552 (MPI: Walter C. Willett and Lorelei Mucci; HPFS), and R03 CA249027 (PI: Dong Hoon Lee). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer 2015;136(5):E359–86 doi 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66(4):683–91 doi 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 3.Survivors of breast and other cancers. World Cancer Research Fund/American Institute for Cancer Research Diet, Nutrition, Physical Activity and Cancer: A Global Perspective Continuous Update Project Expert Report. 2018.
- 4.Colditz GA, Hankinson SE. The Nurses' Health Study: lifestyle and health among women. Nature reviews Cancer 2005;5(5):388–96 doi 10.1038/nrc1608.. [DOI] [PubMed] [Google Scholar]
- 5.Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet (London, England) 1991;338(8765):464–8. [DOI] [PubMed] [Google Scholar]
- 6.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. Journal of the American Dietetic Association 1993;93(7):790–6 doi 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 7.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. International journal of epidemiology 1989;18(4):858–67 doi 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 8.Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, et al. Physical activity and survival after colorectal cancer diagnosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2006;24(22):3527–34 doi 10.1200/jco.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 9.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. American journal of epidemiology 1986;123(5):894–900. [DOI] [PubMed] [Google Scholar]
- 10.Giovannucci E, Colditz G, Stampfer MJ, Rimm EB, Litin L, Sampson L, et al. The assessment of alcohol consumption by a simple self-administered questionnaire. American journal of epidemiology 1991;133(8):810–7. [DOI] [PubMed] [Google Scholar]
- 11.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology (Cambridge, Mass) 1990;1(6):466–73. [DOI] [PubMed] [Google Scholar]
- 12.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. International journal of epidemiology 1994;23(5):991–9. [DOI] [PubMed] [Google Scholar]
- 13.Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology (Cambridge, Mass) 1996;7(1):81–6 doi 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Petimar J, Smith-Warner SA, Rosner B, Chan AT, Giovannucci EL, Tabung FK. Adherence to the World Cancer Research Fund/American Institute for Cancer Research 2018 Recommendations for Cancer Prevention and Risk of Colorectal Cancer. Cancer Epidemiol Biomarkers Prev 2019;28(9):1469–79 doi 10.1158/1055-9965.Epi-19-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabung FK, Fung TT, Chavarro JE, Smith-Warner SA, Willett WC, Giovannucci EL. Associations between adherence to the World Cancer Research Fund/American Institute for Cancer Research cancer prevention recommendations and biomarkers of inflammation, hormonal, and insulin response. International journal of cancer 2017;140(4):764–76 doi 10.1002/ijc.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shams-White MM, Brockton NT, Mitrou P, Romaguera D, Brown S, Bender A, et al. Operationalizing the 2018 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Cancer Prevention Recommendations: A Standardized Scoring System. Nutrients 2019;11(7) doi 10.3390/nu11071572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prentice RL, Kalbfleisch JD, Peterson AV Jr., Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics 1978;34(4):541–54. [PubMed] [Google Scholar]
- 18.van Zutphen M, Boshuizen HC, Kenkhuis MF, Wesselink E, Geijsen A, de Wilt JHW, et al. Lifestyle after colorectal cancer diagnosis in relation to recurrence and all-cause mortality. The American journal of clinical nutrition 2021. doi 10.1093/ajcn/nqaa394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winkels RM, Snetselaar T, Adriaans A, van Warmerdam LJC, Vreugdenhil A, Slooter GD, et al. Changes in body weight in patients with colorectal cancer treated with surgery and adjuvant chemotherapy: An observational study. Cancer Treatment and Research Communications 2016;9:111–5 doi 10.1016/j.ctarc.2016.09.002. [DOI] [Google Scholar]
- 20.Jaspan V, Lin K, Popov V. The impact of anthropometric parameters on colorectal cancer prognosis: A systematic review and meta-analysis. Crit Rev Oncol Hematol 2021;159:103232 doi 10.1016/j.critrevonc.2021.103232. [DOI] [PubMed] [Google Scholar]
- 21.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Annals of oncology : official journal of the European Society for Medical Oncology 2014;25(7):1293–311 doi 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- 22.Meyerhardt JA, Giovannucci EL, Ogino S, Kirkner GJ, Chan AT, Willett W, et al. Physical activity and male colorectal cancer survival. Arch Intern Med 2009;169(22):2102–8 doi 10.1001/archinternmed.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao Y, Meyerhardt JA, Chan AT, Wu K, Fuchs CS, Giovannucci EL. Television watching and colorectal cancer survival in men. Cancer causes & control : CCC 2015;26(10):1467–76 doi 10.1007/s10552-015-0645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song M, Wu K, Meyerhardt JA, Yilmaz O, Wang M, Ogino S, et al. Low-Carbohydrate Diet Score and Macronutrient Intake in Relation to Survival After Colorectal Cancer Diagnosis. JNCI cancer spectrum 2018;2(4):pky077 doi 10.1093/jncics/pky077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fung TT, Kashambwa R, Sato K, Chiuve SE, Fuchs CS, Wu K, et al. Post diagnosis diet quality and colorectal cancer survival in women. PLoS One 2014;9(12):e115377 doi 10.1371/journal.pone.0115377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


